Abstract
A complete understanding of how parasites influence marine ecosystem functioning requires characterizing a broad range of parasite-host interactions while determining the effects of parasitism in a variety of habitats. In deep-sea fishes, the prevalence of parasitism remains poorly understood. Knowledge of ectoparasitism, in particular, is limited because collection methods often cause dislodgment of ectoparasites from their hosts. High-definition video collected during 43 remotely operated vehicle surveys (2013–2014) provided the opportunity to examine ectoparasitism on fishes across habitats (open slope, canyon, seamount, cold seep) and depths (494–4689 m) off the northeastern U.S., while providing high-resolution images and valuable observations of fish behavior. Only 9% (n = 125 individuals) of all observed fishes (25 species) were confirmed with ectoparasites, but higher percentages (∼33%) were observed for some of the most abundant fish species (e.g., Antimora rostrata). Ectoparasites included two copepod families (Lernaeopodidae, Sphyriidae) that infected four host species, two isopod families (Cymothoidae, Aegidae) that infected three host species, and one isopod family (Gnathiidae) that infected 19 host species. Hyperparasitism was also observed. As host diversity declined with depth, ectoparasite diversity declined; only gnathiids were observed at depths down to 3260 m. Thus, gnathiids appear to be the most successful group to infect a diversity of fishes across a broad depth range in the deep sea. For three dominant fishes (A. rostrata, Nezumia bairdii, Synaphobranchus spp.), the abundance and intensity of ectoparasitism peaked in different depths and habitats depending on the host species examined. Notably, gnathiid infections were most intense on A. rostrata, particularly in submarine canyons, suggesting that these habitats may increase ectoparasite infections. Although ectoparasitism is often overlooked in deep-sea benthic communities, our results demonstrate that it occurs widely across a variety of habitats, depths, and locations and is a significant component of deep-sea biodiversity.
Keywords: Ectoparasite, Deep sea, Demersal, Fish, Remotely-operated vehicle, Submarine canyon, Visual based surveys
Graphical abstract

Highlights
-
•
Ectoparasitism occurs across a wide range of depths, habitats, and localities.
-
•
Host specificity was exhibited by 4 families of ectoparasites.
-
•
Gnathiidae infected a wide diversity of fishes.
-
•
Ectoparasite diversity and host specificity declined with depth.
-
•
ROV surveys provide valuable observations of ectoparasitism and fish behavior.
1. Introduction
The importance of parasites in shaping community structure and influencing ecosystem functioning in the marine environment has gained considerable recognition over the past few decades (Dobson and Hudson, 1986, Poulin, 1999, Poulin et al., 2016). Parasites have complex roles in community ecology by influencing population sizes and shifting patterns in both biodiversity and community structure. Parasites can also alter the outcome of competitive interactions, either by enabling rare species to coexist with dominant ones or by helping to eliminate competitors. Additionally, parasites have become increasingly recognized as important components of trophic pathways (see Demopoulos and Sikkel, 2015). The inclusion of parasites in food webs has revealed higher connections among species (Amundsen et al., 2009) and higher trophic efficiency (Arias-González and Morand, 2006). Although the importance of parasites in marine ecosystems is clear, there is still much to be learned regarding the multiple effects that parasites have in different ecosystems throughout the marine realm.
A recent review regarding the synergy of marine ecology and parasitology highlighted seven key areas to further increase our understanding of the importance of parasites in marine ecosystem functioning (Poulin et al., 2016). Poulin et al. (2016) emphasized the need to discover and identify key parasite species that play pivotal roles in ecosystems, while adding new model systems to broaden perspectives on marine parasitism. Because the majority of marine parasitology studies have been conducted in coastal and coral reef ecosystems, it was also suggested that research should be expanded to additional marine habitats. Focusing on a narrow range of habitats can constrain generalizations regarding parasitism in the marine environment (Poulin et al., 2016).
The deep sea is one such understudied ecosystem in which data on parasitism remains limited. For fishes inhabiting the deep sea, knowledge of parasitism is limited to <10% (Klimpel et al., 2006). The few studies on parasitism in deep-sea fishes have focused mainly on the prevalence of endoparasitism (Noble, 1973, Campbell et al., 1980, Klimpel et al., 2006, Palm and Klimpel, 2008), revealing the importance of temperature, depth, and habitat (such as submarine canyons) in influencing the prevalence of endoparasite infections in the deep sea (Manter, 1934, Campbell et al., 1980, Gartner and Zwerner, 1989, Marcogliese, 2002, Klimpel et al., 2006). However, deep-sea fishes are also hosts to ectoparasites, which can adversely affect fishes by causing anemia (Adlard and Lester, 1995, Lester et al., 1995), tissue damage (Adlard and Lester, 1995, Lester et al., 1995), scarring (Ross et al., 2001), and behavioral changes (e.g. Welicky and Sikkel, 2014, Artim et al., 2015), while transmitting other diseases [e.g., blood parasites, (Davies and Smit, 2001), viruses (Lawler et al., 1974)]. Ectoparasitism may thus influence population dynamics of deep-sea fishes and may be important in trophic ecology through direct consumption by other organisms (Johnson et al., 2010, Demopoulos and Sikkel, 2015). Yet, ectoparasitism remains understudied, partly because prior data have been obtained opportunistically from trawling and dredging efforts. These types of gear can dislodge ectoparasites from their hosts during collection (Ross et al., 2001).
To investigate ectoparasitism in the deep sea, direct observations using remotely operated vehicles (ROVs) provide an alternative method to trawling. Visual based surveys have provided a considerable amount of information on ectoparasite-host interactions in shallow waters, while revealing effects of parasitism on fish behavior (e.g., swimming behavior, site fidelity, Barber et al., 2000, Sikkel et al., 2004). Trophic connections have also been determined from in situ observations (i.e., cleaner stations on coral reefs, Sikkel et al., 2004). Thus, the value of visual analysis in parasite studies, from shallow waters to the deep-sea, is clear.
Recent expeditions to survey various seafloor features along the continental margin of the northeastern United States (NEUS) provided an opportunity to increase knowledge of ectoparasites infecting demersal fishes in the deep sea. Visual observations from ROV surveys were used in the present study to identify ectoparasites and their hosts and examine whether ectoparasite diversity declines with increasing depth. We also examined whether ectoparasite-host interactions and intensity of infections differ among depths and habitats in each of three common fish species [Antimora rostrata (family Moridae), Nezumia bairdii (family Macrouridae), and Synaphobranchus (family Synaphobranchidae)]. The high-definition video obtained from these expeditions enabled in situ observations of host-parasite interactions while providing unparalleled, high-resolution images of ectoparasites infecting fishes in the deep sea.
2. Material and methods
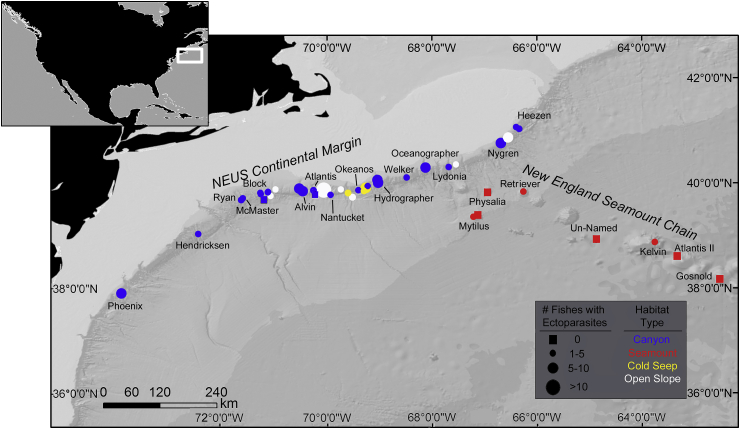
Forty-three remotely operated vehicle (ROV) dives were conducted with the ROV Deep Discoverer (D2) along the NEUS continental margin and New England Seamount Chain during two expeditions (9 July to 16 Aug 2013 and 19 Sep to 6 Oct 2014) aboard the NOAA Ship Okeanos Explorer (Fig. 1). These expeditions were telepresence-enabled, with live video feeds transmitted back to shore in real time (http://oceanexplorer.noaa.gov/okeanos/media/exstream/exstream.html), allowing scientists on shore and on the ship to interact during the dives via an Internet chat room and satellite teleconference line.
Fig. 1.
Locations of 43 ROV dives conducted along the northeastern U.S. continental margin and New England Seamount Chain. Circle Size = Number of ectoparasite-host interactions per dive. Square = No ectoparasites observed. Colors denote habitats.
The ROV D2 was equipped with two high-definition cameras and 16,600 lumens of hydraulically positioned LED lights. A Sea-bird 911+ conductivity-temperature-depth (CTD) logger with a dissolved oxygen (DO) sensor was also attached to the ROV. Paired lasers (10 cm apart) were positioned on the ROV to approximate field of view and sizes of fishes and ectoparasites. The Okeanos Explorer followed the vehicles using dynamic positioning and tracked vehicle position with an ultra-short baseline tracking system.
Each ROV dive traversed one broad-scale habitat feature at depths ranging from 494 to 4689 m (Fig. 1). These habitat features included: submarine canyons (25 dives), cold seeps (three dives), open slope/intercanyon areas (seven dives), and seamounts (eight dives). No fishes were observed during one dive at the deepest seamount surveyed (un-named Seamount, 4552–4689 m). As the ROV traversed a habitat feature (∼0.1–0.2 knots, 1 knot = 0.514 m s−1), the cameras were generally set on wide-angle view, but zooms were frequently conducted to obtain detailed imagery of each previously undocumented species encountered during a given dive survey. The over-ground distance covered by the ROV [measured in ArcGIS v9 (ESRI)] varied across dives (300–2200 m), but the observation time on bottom was approximately the same (5–7 h per dive).
During each dive, video clips (103–191clips) from the high-definition camera mounted on the ROV D2 were contiguously acquired as part of the mission of the expeditions. These video clips ranged in length from approximately 30 s to 5 min. Frame grabs (112–351 per dive) were subsequently taken from video clips. Sixty-nine demersal fish taxa and three mesopelagic taxa were identified using both frame grab and video observations (see Quattrini et al., 2015). Ectoparasites were identified to the lowest taxonomic level on fishes from all available frame grabs. Ectoparasite type, placement, number and size also ensured that individuals were counted only once. Because we restricted this analysis to using frame grabs only, we calculated frequency of ectoparasite-host interactions to examine general patterns across the region.
Three species of fishes (Antimora rostrata, N. bairdii and Synaphobranchus spp.) that were dominant in the region and had ectoparasites were further enumerated using all video clips. The average intensity of infection (number per one side) was estimated for these species using individuals imaged during times when the camera was positioned to permit accurate counts. Although gnathiid parasites were common, these could not be consistently identified on all individuals due to the wide camera view. Thus, estimates provided herein for this taxonomic group are conservative and many parasites labeled as “unknown” may in fact be gnathiids.
For each dominant fish species, abundances of ectoparasite-host interactions were estimated by taking the total number of hosts observed with at least one ectoparasite during a dive and dividing by the product of the total over the ground distance covered by the ROV and the estimated field of view (4.3 m). Abundances were also calculated within particular depth zones per dive. Depth zones were binned into 300 m depth intervals from 500 to 3200 m, except the last depth zone ranged from 2900 to 3262 m. A single dive may have traversed across two depth zones, but only across one broad-scale habitat feature. A Kruskal-Wallis (K-W) test was used to determine if hosts or ectoparasite-host interactions were significantly more abundant within a particular depth range or habitat. Following other deep-sea studies (e.g., Davies et al., 2008, Doughty et al., 2014), only dives in which each of the dominant species was present were included in these tests. All statistical tests were conducted in R v 3.1 (R Core Team., 2015; http://www.R-project.org).
3. Results
3.1. Ectoparasite-host interactions
A total of 125 adult fishes [out of 1429 individuals confirmed with or without ectoparasites] representing at least 25 species [out of 69 demersal species (Quattrini et al., 2015) and three mesopelagic species] from 18 families were observed hosting at least five families of ectoparasites (Table 1). The Isopoda (Aegidae, Cymothoidae, Gnathiidae) was the most common group of ectoparasites observed, infecting 74 individual hosts. Siphonostomatoid (Lernaeopodidae, Sphyriidae) copepods infected 22 individual hosts. An additional 29 individual hosts were infected with ectoparasites that could not be identified; however, many were possible gnathiids. Gnathiids were the most common ectoparasite observed across species, infecting at least 19 species (Table 1). Amblyraja radiata and N. bairdii hosted aegid isopods. Hoplostethus mediterraneus hosted a cymothoid isopod (Table 1, Table 2). Siphonostomatoid copepod parasites were observed on at least four host species: A. rostrata, Diaphus sp., N. bairdii, and Synaphobranchus spp.
Table 1.
Number of individuals per species observed with ectoparasites. * indicates species for which parasites were counted using video clips. Total number of hosts present in video clips but too distant to confirm ectoparasite infections are in parentheses.
| # Hosts |
Siphonostomatoid Copepoda |
Isopoda |
Unknown | # |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Examined | Lernaeopodidae | Sphyriidae | Unknown | Cymothoidae | Aegidae | Gnathiidae | Parasites | ||
| Etmopteridae | |||||||||
| Centroscyllium fabricii | 9 | 4 | 5 | 9 | |||||
| Scyliorhinidae | |||||||||
| Apristurus manis | 3 | 1 | 1 | 2 | |||||
| Rajiidae | |||||||||
| Amblyraja radiata | 3 | 2 | 2 | ||||||
| Chimaeridae | |||||||||
| Hydrolagus pallidus | 3 | 2 | 2 | ||||||
| Halosauridae | |||||||||
| Halosauropsis macrochir | 3 | 1 | 1 | 2 | |||||
| Notacanthidae | |||||||||
| Notacanthus chemnitzii | 2 | 2 | 2 | ||||||
| Synaphobranchidae | |||||||||
| Synaphobranchus spp.* | 1241 (1785) | 8 | 5 | 12 | 25 | ||||
| Myctophidae | |||||||||
| Diaphus sp. | 2 | 2 | 2 | ||||||
| Unidentified | 4 | 4 | 4 | ||||||
| Bathysauridae | |||||||||
| Bathysaurus ferox | 2 | 2 | 2 | ||||||
| Ophidiidae | |||||||||
| ?Lamprogrammus sp. | 1 | 1 | 1 | ||||||
| Monomitopus agassizi | 1 | 1 | 1 | ||||||
| Ophidiidae sp. | 1 | 1 | 1 | ||||||
| Macrouridae | |||||||||
| Coryphaenoides armatus | 2 | 2 | 2 | ||||||
| Coryphaenoides rupestris | 1 | 1 | 1 | ||||||
| Nezumia bairdii* | 57 (153) | 4 | 5 | 8 | 2 | 19 | |||
| Moridae | |||||||||
| Antimora rostrata* | 44 (97) | 4 | 28 | 32 | |||||
| Lepidion guentheri | 1 | 1 | 1 | ||||||
| Phycidae | |||||||||
| Phycis chesteri | 17 | 1 | 4 | 5 | |||||
| Urophycis tenuis | 1 | 1 | 1 | ||||||
| Trachichthyidae | |||||||||
| Hoplostethus mediterraneus | 3 | 1 | 1 | ||||||
| Oreosomatidae | |||||||||
| Neocyttus helgae | 7 | 4 | 4 | ||||||
| Sebastidae | |||||||||
| Helicolenus dactylopterus | 4 | 1 | 1 | ||||||
| Sebastes mentella | 4 | 1 | 1 | ||||||
| Psychrolutidae | |||||||||
| Cottunculus thomsonii | 12 | 1 | 1 | ||||||
| Pleuronectidae | |||||||||
| Reinhardtius hippoglossoides | 1 | 1 | 1 | ||||||
| Total Observations | 1429 (2122) | 4 | 12 | 6 | 1 | 7 | 66 | 29 | 125 |
Table 2.
Observations of ectoparasite-host interactions by depth. Number of dives (n) occurring within a particular depth range where ectoparasite-host interactions were observed is also listed.
| 500–800 m n = 8 |
800–1100 m n = 11 |
1100–1400 m n = 12 |
1400–1700 m n = 9 |
1700–2000 m n = 3 |
2000–2300 m n = 3 |
2900–3300 m n = 1 |
|
|---|---|---|---|---|---|---|---|
| Copepoda | |||||||
| Sphyriidae | 3 | 8 | 1 | ||||
| Lernaeopodidae | 2 | 2 | |||||
| Unknown | 1 | 4 | |||||
| Isopoda | |||||||
| Aegidae | 3 | 3 | |||||
| Cymothoidae | 1 | ||||||
| Gnathiidae | 8 | 11 | 18 | 18 | 5 | 4 | 2 |
| Unknown Ectoparasites | 4 | 10 | 11 | 3 | 1 | 1 | |
| Total No. Observations | 19 | 42 | 31 | 20 | 5 | 5 | 3 |
| Total No. Host Spp. Infected | 8 | 10 | 12 | 7 | 2 | 3 | 2 |
Two families of siphonostomatoid copepods were identified on three species of fishes (Fig. 2A–C). Copepods from the family Lernaeopodidae, sized at approximately 1–3 cm total length (TL), were observed on A. rostrata (Fig. 2A). Copepods were attached to anal fins, below second dorsal fins, and behind the eyes. At least two species (likely Lophoura spp.) from the family Sphyriidae infected Synaphobranchus spp. (Fig. 2B) and N. bairdii (Fig. 2C). The copepods (∼2–3 cm TL) infecting N. bairdii were attached directly behind the dorsal fin, whereas larger copepods (∼4–5 cm TL) infecting Synaphobranchus spp. were attached mid body; either laterally or ventrally. Unidentified siphonostomatoid copepods were observed (∼2 cm TL) on mesopelagic fishes, including two Diaphus sp. individuals (Fig. 2D) and four unidentified myctophids. Copepods were attached behind the dorsal fins of myctophids. In all cases, only one copepod was observed on a single fish. Hyperparasitism was observed on N. bairdii, with each sphyriid copepod infected by at least three to eight leeches (Fig. 2C).
Fig. 2.
Example images of ectoparasites infecting various host species. A) Antimora rostrata with a lernaeopodid copepod (1059 m, Alvin Canyon); B) Synaphobranchus sp. with a sphyriid copepod (820 m, open slope); C) Nezumia bairdii with a sphyriid copepod parasitized by eight leeches (1035 m, Phoenix Canyon; D), Black fish, Diaphus sp. with an unknown siphonostomatid copepod (1130 m, cold seep) attached behind dorsal fin; E) N. bairdii with aegid isopod (780 m, open slope); F) Hoplostethus mediterraneus with a cymothoid isopod (744 m, Nygren Canyon); G) Amblyraja radiata with aegid isopod (1010 m, Alvin Canyon); and H) Cottunculus thomsonii with gnathiids (1210 m, Oceanographer Canyon).
Of the three families of isopods that infected demersal fishes, gnathiids were the most common, with 1 to 45 individuals infecting at least one side of each individual fish. Gnathiids were translucent, attached to all fins, heads, and sides of bodies (Fig. 2H), and ranged in size from 1 to 3 mm TL. Distinct species of aegids infected A. radiata and N. bairdii. One large (∼2.5 cm TL) aegid was attached mid-way on the body of A. radiata, at the juncture of the left pectoral wing and the central disk (Fig. 2G). The other fish individual had at least 15 smaller (1–2 mm TL) aegids attached to both the wings and the central disk. Five N. bairdii were observed each with an aegid isopod. Each aegid (∼2–3 cm TL) was attached behind the dorsal fin (Fig. 2E). One cymothoid isopod (∼4 cm TL) was observed attached on the side of the body below the dorsal fin of H. mediterraneus (Fig. 2F).
3.2. General patterns across sites and depths
Ectoparasite-host interactions were documented during 36 dives across the entire study region at depths ranging from 494 to 3262 m (temperature 5.6 to 2.6 °C, dissolved oxygen 3.6–5.6 ml L−1) (Fig. 1). Observations of host-ectoparasite interactions were more frequent in canyons (66%, n = 23 dives) than in open slope (23% n = 7 dives), cold seep (7%, n = 3 dives), and seamount (4%, n = 3 dives) habitats (Fig. 1). Of the six seamounts where fishes were observed (<20 individuals per dive), ectoparasites (gnathiids) were observed on five individuals, one individual each on Kelvin and Retriever seamounts and three individuals from Mytilus Seamount, at depths ranging from 2035 to 3260 m (Fig. 1, Table 2). The number of species infected with parasites was similar among open slope (6 spp), seamount (4 spp.), and cold seep (4 spp.) habitats, but higher in canyon habitats (21 spp.) Overall, ectoparasite-host interactions peaked at mid-slope depths. Frequencies of ectoparasite-host interactions ranged from 2 to 34%, with the most frequent observations in 800–1100 m and 1100–1400 m (Table 2). Few (n = 8) ectoparasite-host interactions were observed in the deeper areas (>1700 m) (Table 2). The number of host species infected with parasites was highest at depths of 800–1100 m (n = 12 species), followed by 1100–1400 m (n = 11 species), and then declined with increasing depth (Table 2).
Species richness of ectoparasites was similar among habitats, but declined with deeper depths (Table 2). Siphonostomatoid copepods were observed in canyon, cold seep, and open slope habitats at depths down to 1400 m. Aegids were observed in open slope and canyon habitats at depths down to 1100 m. One cymothoid isopod was observed in a canyon habitat at a depth of 739 m. Gnathiids were observed in all habitats and at the deepest depths surveyed (down to 3300 m).
3.3. Ectoparasitism on three common species
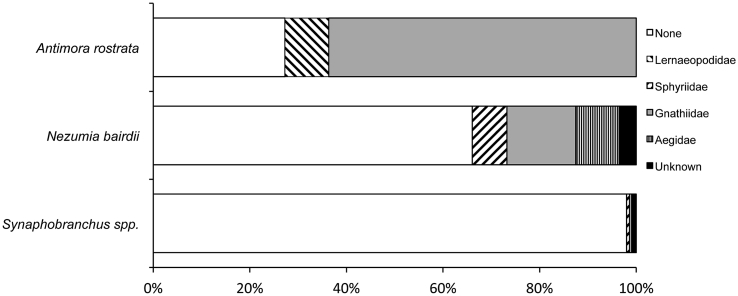
Out of the three common species enumerated on video, Antimora rostrata (n = 97 adults, n = 29 dives) was observed with parasites most frequently. We positively identified ectoparasites on 33% of all observed A. rostrata (25–40 cm TL). Of the individuals confirmed with parasites, 88% were infected with gnathiids and 12% were infected with lernaeopodid copepods (Fig. 3, Table 1). Only 12% of A. rostrata individuals did not have ectoparasites (Fig. 3). For the remaining 55% (n = 53) of individuals, it could not be determined whether or not individuals had ectoparasites because individuals in the video were too far from the camera to confirm whether or not ectoparasites were present. The average number of parasites infecting a single side of an individual was 7.72 ± 1.89 SE parasites (n = 26 individuals, 1 to 45 ectoparasites per individual). The most intense infections (9.05 ± 2.39 SE ectoparasites per side) were observed on individuals in canyon habitats, particularly at depths ranging from 1100 to 2000 m (Table 3). Although prevalence of infections did not differ (K-W, x2 = 0.14, p = 0.93) among the three dominant species, the infection intensity was significantly higher (K-W, x2 = 14.78, p = 0.0006) in A. rostrata than in the other species.
Fig. 3.
Percentage of ectoparasites infecting three common demersal fishes. Unconfirmed observations are not included.
Table 3.
Mean ± SE (and range) of infection intensity for each dominant species across four general habitat types (seamount, canyon, cold seep, and open slope/intercanyon). Intensity was calculated as the number of ectoparasites observed per one side of the individual. n = number of individuals used in calculations.
| Species | Cold seep | Seamount | Open slope/Intercanyon | Canyon |
|---|---|---|---|---|
| Antimora rostrata | 6.50 ± 2.00 (n = 2) | 6 (n = 1) | 2.00 ± 1.00 (1–4, n = 3) | 9.05 ± 2.39 (6–45, n = 8) |
| Nezumia bairdii | 2.67 ± 0.92 (1–7, n = 6) | 1.71 ± 0.57 (1–5, n = 7) | ||
| Synaphobranchus spp. | 1.00 ± 0.00 (1, n = 2) | 1.00 ± 0.00 (1, n = 5) | 2.00 ± 0.225 (2–3, n = 4) |
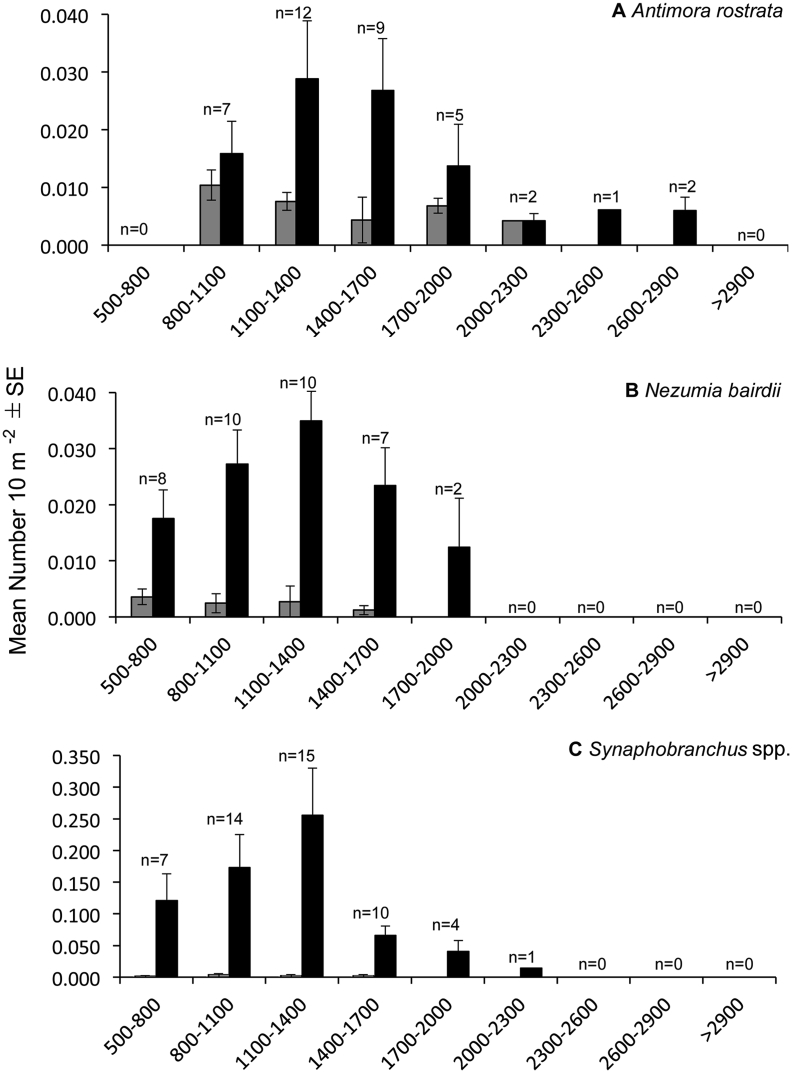
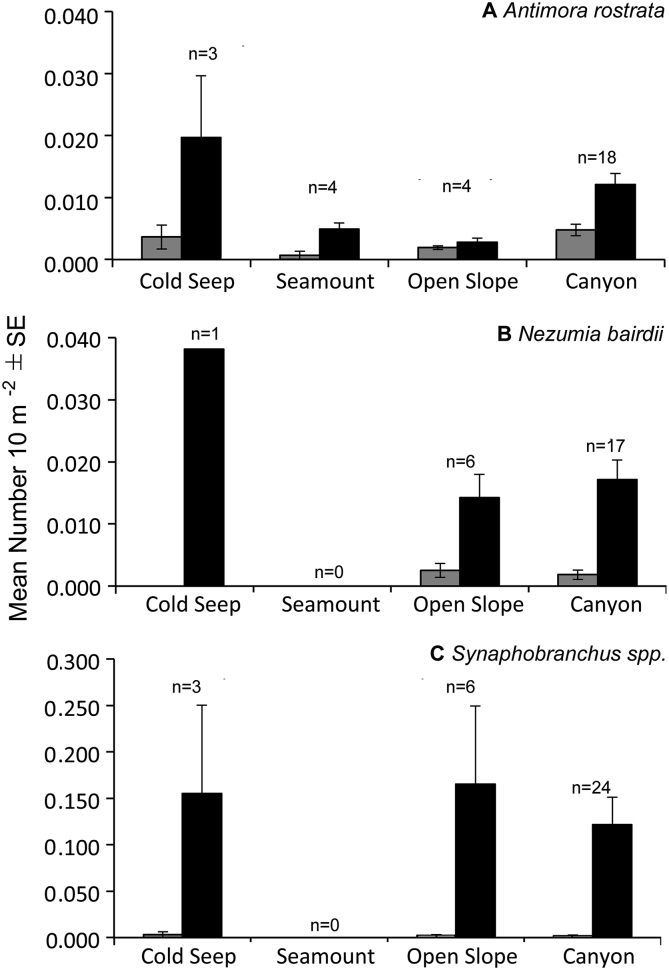
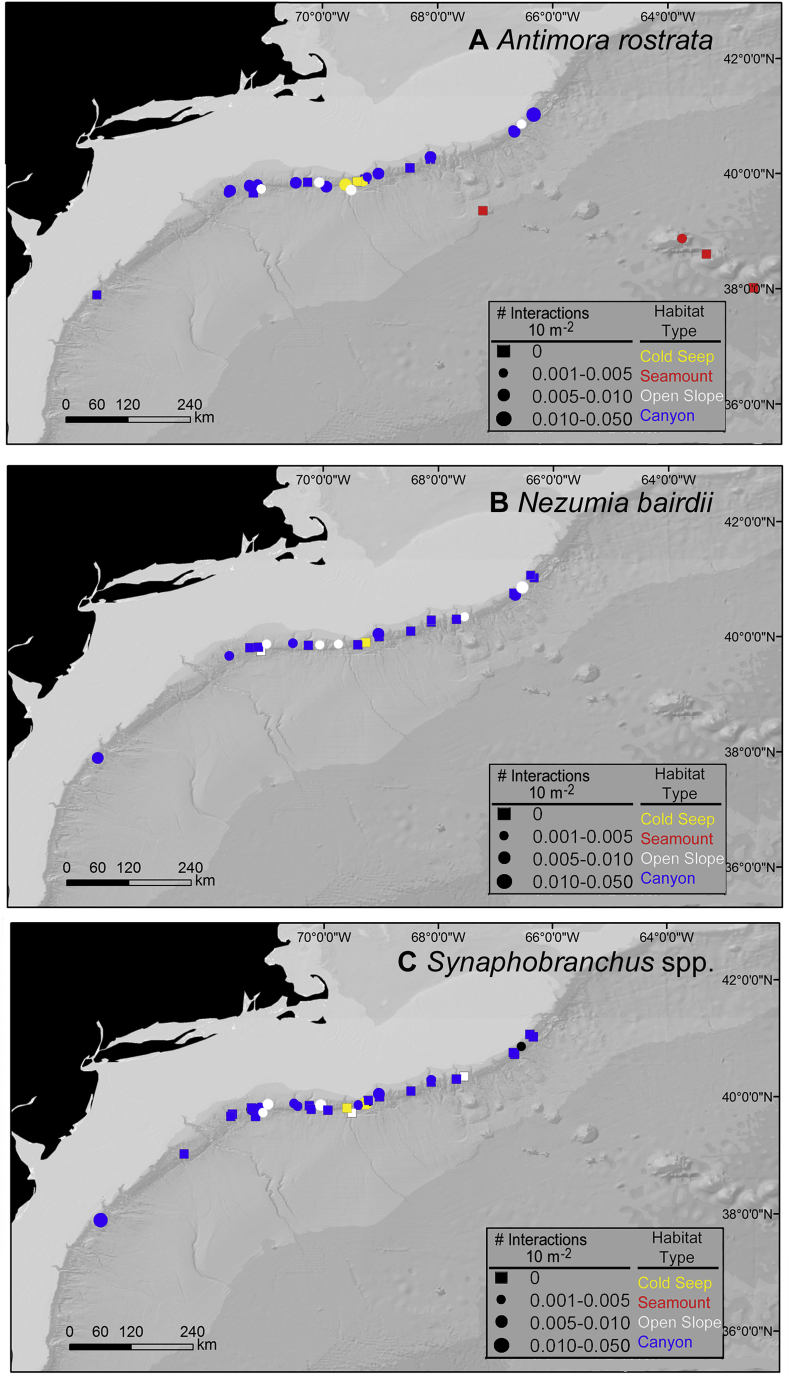
Antimora rostrata was observed at depths ranging from 810 to 2718 m. This species was most abundant at depths of 1100 to 1400 m (0.029 ± 0.010 SE individuals 10 m−2) followed by 1400 to 1700 m (0.027 ± 0.009 SE individuals 10 m−2) (Fig. 4A). Although ectoparasite-host interactions (0.010 ± 0.006 SE interactions 10 m−2) were slightly higher at 800 to 1100 m, there were no significant differences in ectoparasite-host interactions among depth zones (K-W, x2 = 4.28, p = 0.63, Fig. 4A). Only two individuals were infected with gnathiid parasites depths >2000 m. Among all habitats, A. rostrata was most abundant in cold seeps (0.020 ± 0.009 SE individuals 10 m−2, Fig. 5, Fig. 6A). A. rostrata was most abundant during a single dive at a cold seep site (0.37 individuals 10 m−2, 1412–1474 m depth). Here, only 0.004 ectoparasite-host interactions 10 m−2 was estimated. The greatest number (0.005 ± 0.001 SE interactions 10 m−2) of ectoparasite-host interactions was in submarine canyons, but interactions were not significantly different among habitats (K-W, x2 = 5.56, p = 0.13, Fig. 5A). The highest abundance (0.13 interactions 10 m−2) of ectoparasite-host interactions during a single dive was from Heezen Canyon (1694–1722 m), where the abundance of A. rostrata was 0.018 individuals 10 m−2.
Fig. 4.
Mean abundance (±SE) of A) Antimora rostrata, B) Nezumia bairdii, and C) Synaphobranchus spp. per depth zone (black bars). Mean abundance (±SE) of ectoparasite-host interactions (grey bars) also included. n = number of dives during which the species was present.
Fig. 5.
Mean abundance (±SE) of A) Antimora rostrata, B) Nezumia bairdii, and C) Synaphobranchus spp. per habitat (black bars). Mean abundance (±SE) of ectoparasite-host interactions (grey bars) is also included. n = number of dives during which the species was present.
Fig. 6.
Ectoparasite-host interactions for A) Antimora rostrata, B) Nezumia bairdii, and C) Synaphobranchus spp. across the region for which the presence or absence of ectoparasites could be confirmed. Circle Size = Number of confirmed ectoparasite-host interactions 10 m−2. Square = No ectoparasites observed. Colors denote habitats.
For N. bairdii (n = 153 individuals, n = 24 dives), we positively identified ectoparasites on 12% of all individuals (15–25 cm TL), while 25% of N. bairdii individuals had no ectoparasites. Of the individuals confirmed with parasites, 42% were infected with gnathiids, 26% were infected with aegid isopods, 21% were infected with sphyriid copepods, and 11% had unidentified parasites (Table 1, Fig. 3). For the remaining 63% of individuals, it could not be determined whether or not individuals hosted parasites. The average number of parasites infecting a single side of an individual was 2.15 ± 0.52 SE parasites per side (n = 13 individuals, 1 to 7 ectoparasites per individual). The highest intensity of infections (2.67 ± 0.92 SE ectoparasites per side) on N. bairdii occurred in open slope/intercanyon habitats (Table 3).
While N. bairdii was observed at depths of 500 to 1860 m, this species was most abundant (0.035 ± 0.007 SE individuals 10 m−2) between 1100 and 1400 m. However, ectoparasite-host interactions were not significantly different among depth zones (K-W, x2 = 7.57, p-value = 0.11) (Fig. 4B). Ectoparasite-host interactions also did not differ among habitats (K-W, x2 = 1.92, p = 0.38, Fig. 5, Fig. 6). Mean abundances ranged from 0.014 ± 0.004 SE individuals 10 m−2 (n = 6 dives, open slope) to 0.038 individuals 10 m−2 (n = 1 dive, cold seep). During a single dive, N. bairdii was most abundant (0.054 individuals 10 m−2) in Okeanos Canyon at depths ranging from 1360 to 1500 m; yet no individuals had ectoparasites at this site. The greatest number of individuals with ectoparasites (0.009 interactions 10 m−2) was during a single dive in Phoenix Canyon at depths ranging from 1035 to 1172 m. Here, abundance of N. bairdii was 0.041 individuals 10 m−2.
The cutthroat eel, Synaphobranchus spp. was the most abundant species observed (n = 1785 individuals, 33 dives) across the study area, but had the fewest ectoparasites. Ectoparasites were observed on only 1.4% of all Synaphobranchus spp. (25–50 cm TL), whereas 68.2% of Synaphobranchus individuals had no ectoparasites. Of the individuals confirmed with parasites, 32% were infected with sphyriid copepods, 2% were infected with gnathiids, and 48% had unidentified parasites (Fig. 3). For the remaining 30.4% of individuals, it could not be determined whether or not individuals hosted ectoparasites. The average number of parasites infecting a single side of an individual was 1.45 ± 0.21 SE parasites per side (1–3 ectoparasites per individual). The most intense infections (2.00 ± 0.25 SE ectoparasites per side) were in canyon habitats (Table 3).
Synaphobranchus spp. was observed at depths ranging from 500 to 2025 m and was most abundant (0.256 ± 0.074 SE individuals 10 m−2) at depths of 1100 to 1400 m. Ectoparasitism, however, was slightly more abundant at 800 to 1100 m, but not significantly higher (K-W, x2 = 4.20, p = 0.52) than other depth ranges (Fig. 4C). None of the Synaphobranchus spp. observed >2000 m were infected by ectoparasites. Although Synaphobranchus spp. were abundant in open slope habitats (0.165 ± 0.083 SE individuals 10 m−2), ectoparasite-host interactions did not differ among habitats (K-W, x2 = 0.79, p = 0.67, Fig. 5, Fig. 6C). Ectoparasite-host interactions ranged from 0.002 ± 0.001 to 0.003 ± 0.003 SE individuals 10 m−2 in open slope, canyon, and cold-seep habitats. During a single dive, both the highest abundances of Synaphobranchus spp. (0.68 individuals 10 m−2) and ectoparasite-host interactions (0.014 individuals 10 m−2) were observed in Phoenix Canyon at depths ranging from 1000 to 1170 m.
3.4. Behavioral observations
No notable differences in behavior were observed for the majority of fishes infected with ectoparasites, particularly those infected with gnathiids. Most of the individuals appeared to be either resting on the bottom [e.g., A. radiata, Bathysaurus ferox, Cottunculus thomsonii] or swimming normally (e.g., sharks, chimaeras, ophidiids, morids, macrourids, synaphobranchids) either close to or just a few meters above the seafloor. Only a few of the fishes that had large ectoparasites appeared to be behaving abnormally. One N. bairdii individual with a sphyriid copepod hyperparasitized by eight leeches (Fig. 2C) appeared to be underweight than other individuals of similar total lengths (∼15 cm TL). This individual was observed swimming in circles and appeared to be leaning towards one side (Suppl. Video). One Hoplostethus mediterraneus with a large cymothoid isopod on its left side was observed making short, erratic movements using its pectoral fins. Finally, one Synaphobranchus individual with a large (∼5 cm TL) sphyriid copepod was swimming so close to the seafloor that both host and copepod were in contact with the sediment, perhaps increasing the chance for parasite removal. Although ROV lights and noise can alter individual fish behavior (Stoner et al., 2008), abnormal swimming behaviors were likely not an effect of the ROV because these behaviors were not observed in numerous uninfected individuals.
Supplementary video related to this article can be found at http://dx.doi.org/10.1016/j.ijppaw.2016.07.004.
The following is the supplementary data related to this article:
Nezumia bairdii with a sphyriid copepod hyperparasitized by eight leeches (Phoenix Canyon, 800–1100 m depth). Video recorded by ROV D2 during the NOAA Okeanos Explorer Program, 2014 Atlantic Canyons and Seamounts Expedition. Video narration by S. France (Univ. Louisiana Lafayette).
4. Discussion
ROV video provided remarkable observations of ectoparasite infections on deep-sea fishes. These observations enabled us to determine that ectoparasitism occurs across a variety of depths (500–3300 m), habitats (seamounts, canyons, cold seeps, open slope), and host species (25 species) along the northeastern U.S. (NEUS) continental margin and New England Seamount Chain. We found that the abundance of ectoparasite-host interactions and intensity of infections peaked within particular depths and habitats depending upon the host species examined, but that submarine canyons may enhance ectoparasitism. We also found that species richness of ectoparasites declined with depth; only gnathiids were observed at the deepest depths surveyed. Thus, our results strengthen the notion that as temperature decreases (Poulin and Rohde, 1997) and the number of host species decline (Campbell et al., 1980) with increasing depth, the diversity of host-ectoparasite interactions decreases as well. We also note that, at least at the family level, ectoparasites infecting demersal fishes appear to be both generalist (gnathiids, infecting 19 host species) and specialist (copepods, aegids, and cymothoids, each infecting 1–2 host species) species, likely due to differences in parasite life cycles. Although our estimates of ectoparasite diversity are conservative, as species cannot be identified without collections, our study demonstrates the utility of using an ROV to observe and count ectoparasite-host interactions across a variety of depths, habitats, and host species, while providing the opportunity to examine in situ the impact of ectoparasite infections on fish behavior.
4.1. Ectoparasite-host interactions
Ectoparasitism was widespread across fish species, with 18 families of teleosts and chondrichthyans observed with ectoparasite infections. Demersal species were more frequently infected than mesopelagic species. Many ectoparasites have benthic life stages (e.g., Smit and Davies, 2004) and thus would more likely encounter a demersal fish host than a mesopelagic host. The majority of fish species harboring ectoparasites were both relatively abundant in the region and/or were habitat generalists (Auster et al., 1995, Ross et al., 2015, Quattrini et al., 2015). Thus, the number of ectoparasite-host interactions could be a consequence of the host population size or the host adopting a generalist strategy by utilizing a wide range of niches, including food resources and habitats. Other host behaviors, such as spawning (e.g., scyliorhinids) or feeding on the benthos (e.g., N. bairdii, Campbell et al., 1980) or aggregating (A. rostrata, Iwamoto, 1975), may also increase ectoparasite infections (Boxshall, 1998).
Our study also revealed that demersal lifestyle alone, of hosts, was not sufficient to explain ectoparasite infections. The number of demersal host species (24 species) with ectoparasites constituted approximately one third of all demersal species (69 species) observed across the same depth range in the region (Table S1, Quattrini et al., 2015). Although ectoparasite-host interactions may be underestimated, our results are comparable to those documented from deep-sea fishes (to 1000 m) collected off Australia using surface-deployed gear (e.g., traps, trawls) (Rohde et al., 1995). The absence of ectoparasites on several species might provide evidence of unoccupied niches for the ectoparasites (Rohde et al., 1995). Alternatively, the absence of ectoparasites could be a result of a fish's resistance to infection (e.g. mucous production, skin/scale resiliency, Coile and Sikkel, 2013), rarity of the fish host (Boxshall, 1998) or fish behavior (Boxshall, 1998).
The intensity of infections and the abundance of ectoparasite-host interactions was not a function of host abundance. Ectoparasitism was not most abundant where both N. bairdii and A. rostrata were locally most abundant. Furthermore, Synaphobranchus spp. was the most abundant species observed; yet ectoparasitism was relatively low in this species compared to the others. Additionally, among the three dominant species, the infection intensity was highest in A. rostrata, yet all three species were common across depths and habitats and all are generalist feeders. A. rostrata and Synaphobranchus spp. scavenge (Collins et al., 1999, Jamieson et al., 2011) or feed on benthopelagic species and N. bairdii feeds mainly on benthic invertebrates (Campbell et al., 1980, Houston and Haedrich, 1986). Perhaps the higher intensity of infections on A. rostrata relate to movement and/or aggregation of individuals (Boxshall, 1998) for reproduction (Iwamoto, 1975, Wenner and Musick, 1977). Aggregating at a single, dominant spawning site or undergoing periodic re-distribution during reproduction (White et al., 2011) may increase transmission rates of ectoparasites. Higher infection intensity in A. rostrata may also be due to reduced resistance to infection. Gnathiids could perhaps more easily penetrate A. rostrata, as this species has relatively large, overlapping cycloid scales.
In addition to the ecology and biology of the host species, the ecology and life history traits of the ectoparasites also influence prevalence, specificity, and intensity of infections. Compared to the other ectoparasite families, gnathiids infected a variety of host species (19 spp.) across the entire depth range. A single species of gnathiid is known to infect numerous host species in shallow waters (e.g., Coile and Sikkel, 2013). Life history characteristics of gnathiids likely increase their ability to infect a variety of species and more than one host species in their lifetime (Lafferty and Kuris, 2002, Jones et al., 2007, Grutter et al., 2008). Although the three larval stages of gnathiids are obligate fish parasites, between each stage, larval gnathiids return to the benthos (e.g., sponges, corals, serpulid tubeworms, tunicates, sediments, among rocks, wood) to molt until infecting another species or until the final, non-feeding adult stage (Mouchet, 1928, Stoll, 1962, Upton, 1987, Jacoby and Greenwood, 1988, Klitgaard, 1991, Smit et al., 1999, Smit et al., 2003, Smit and Davies, 2004). Thus, gnathiids may have been so successful at colonizing, with high intensity, a diversity of host fishes from shallow waters to the deep sea because of attributes of their life cycle.
In contrast to gnathiids, siphonostomatoids, aegids, and cymothoids isopods are known to be highly host specific (Wilson, 1919, Ho, 1985, Boxshall, 1998, Bunkley-Williams and Williams, 1998, Ross et al., 2001). In our study, siphonostomatoid copepods and aegid and cymothoid isopods infected four, two, and one species, respectively. For cymothoids and copepods, free-living juvenile stages attach to hosts and remain on the host for life until reproduction (Boxshall, 1998, Bunkley-Williams and Williams, 1998). Although these ectoparasites have reproductive strategies that would help them complete their life cycle in the deep sea [e.g., males parasitizing females (copepods, Boxshall, 1998) and hermaphroditism (cymothoids, Bunkley-Williams and Williams, 1998)], specializing on only a few host species may help increase encounter rates of male and females during periods of sexual reproduction. In contrast, aegids are temporary parasites, changing hosts during their lifetimes by settling on the benthos until infecting another species (Bunkley-Williams and Williams, 1998). This behavior may result in higher infection rates of demersal fishes that feed on the benthos, such as N. bairdii and A. radiata (e.g., Campbell et al., 1980).
Based on previous research, ectoparasites from the host-specific families observed in this study were most likely different species. Lophoura spp. are known to exhibit high host specificity. In the NEUS region, Leptodactylus gracilis has been reported from S. kaupii (Wilson, 1919) whereas Leptodactylus pentaloba and Leptodactylus bouvieri have been reported from N. bairdii (Wilson, 1919, Ho, 1985). Parabrachiella pinguis is the only lernaeopodid that has been reported from A. rostrata in the NEUS region (Wilson, 1915, Ho, 1985). Sarcotretes scopeli (family Pennellidae) is the only copepod recorded from myctophids in the Atlantic (Gartner and Zwerner, 1989, Boxshall, 1998). As for the isopods, the aegid Syscenus infelix has been reported from N. bairdii along the NEUS slope (Ross et al., 2001). Aega psora is the only aegid recorded from Antimora radiata, documented only once in the Bay of Fundy (Wallace and Huntsman, 1919). One cymothoid was observed in this study, and to our knowledge constitutes the first record of ectoparasitism on H. mediterraneus. Few cymothoids are known to inhabit deep waters (Brusca, 1981), particularly below 800 m (Poore and Bruce, 2012).
4.2. Patterns across depth and habitat
Ectoparasite-host interactions were observed in all habitats, but our data suggest that submarine canyons may increase abundance of ectoparasite-host interactions, the number of host species infected, and the intensity of infections, at least for some species. Canyons (Alvin, Nygren, Hydrographer, Phoenix) with the highest ectoparasite-host interactions observed contained relatively high numbers of fish species observed (14–20 species per dive) than other sites in the region (see Quattrini et al., 2015). Additionally, for each dominant species, ectoparasite-host interactions were most abundant during a single dive in a canyon habitat. For A. rostrata, the mean abundance of ectoparasite-host interactions and the intensity of ectoparasite infections were also higher in submarine canyons than other habitats. Campbell et al. (1980) found a higher endoparasite load in both A. rostrata and N. bairdii occupying canyon habitats in the same region. Higher intensities of infections in canyon environments may in part be related to increased habitat heterogeneity, including higher abundances of both corals and sponges (Huvenne et al., 2011, Quattrini et al., 2015). Corals and sponges have been noted to house resting stages of gnathiid larvae (Klitgaard, 1991). In fact, all resting larval and adult stages have been previously collected from a single sponge in deep waters (150–487 m, Klitgaard, 1991), suggesting some site fidelity for parasites. Submarine canyons also channel organic matter (Canals et al., 2006, Oliveira et al., 2007), and have been documented with higher abundances of fauna compared to the surrounding slopes (Vetter and Dayton, 1999). It is possible that deep-sea fishes, including A. rostrata, are more actively feeding in submarine canyons, and thus these behaviors may increase infection rates of ectoparasites.
Depth was an important factor influencing ectoparasitism. Peaks in both ectoparasite diversity and ectoparasite-host interactions were observed at mid-slope depths. Ectoparasite-host interactions were most abundant at depths of 800 to 1100 m for A. rostrata and Synaphobranchus spp. and at 500 to 800 m for N. bairdii. Ectoparasitism diversity was highest at depths of 500 to 1400 m, and then declined with increasing depth. Siphonostomatoid copepods, aegids, and cymothoids were absent at depths >1400 m; only gnathiids were observed at the deepest depths surveyed (up to 3260 m). Peaks at mid-slope depths appear to correspond to higher species richness of fishes. ROV dives from the 2013–2014 expeditions documented fewer numbers of species (5–12 species per dive) at deeper depths (>1400 m) than in shallower (500–1400 m) depths (9–20 species per dive, see Quattrini et al., 2015). The absence of the host-specific ectoparasites at deeper depths is due to decreased diversity and depth range limits of host species, similar to the endoparasite fauna sampled from fishes in the same region (Campbell et al., 1980). But in contrast to patterns in the endoparasite fauna, the number of ectoparasite-host interactions did not decrease linearly with depth in this region; similar abundances of ectoparasite-host interactions were observed at depths >1100 m (Campbell et al., 1980). In addition to host distribution, environmental conditions, such as temperature, could also limit the distribution of ectoparasites. Temperature has a significant effect on the species richness of ectoparasite communities (Rohde et al., 1995). In the NEUS region, temperature changes from 4-5 °C to 3–4 °C at a depth boundary of approximately 1300 m, corresponding to a change in deep water masses (Pickart, 1992).
4.3. Sampling considerations
This study was part of a larger expedition that was not focused solely on documenting ectoparasites on deep-sea fishes. Thus, we note a few methodological limitations and suggest modifications for future use of ROVs to fully document ectoparasitism in the deep sea. Due to inadequate camera angles, we were unable to determine whether ectoparasites were present on a portion of the dominant species. Further, we could not quantify ectoparasitism on all fishes observed in this study. Targeted ROV surveys that incorporate frequent zooms and discrete collections, perhaps in combination with museum collections, would be best to elucidate fine-scale patterns of ectoparasitism in the deep sea. We acknowledge that further sampling across similar depths and habitats is necessary to resolve confounding effects of habitat and depth on the distribution of ectoparasites and ectoparasite-host interactions; most effort was conducted at depths of 500 to 1100 m in canyon habitats. Further quantification is necessary to determine whether submarine canyons significantly influence the prevalence, abundance, and infection intensity of ectoparasitism in deep-sea fishes.
4.4. Further considerations
Metazoan parasites are an important, yet overlooked, component of deep-sea communities. Similar to shallow-water communities, an estimated 1.5 metazoan parasite species occur per fish species; thus parasites likely have significant impacts on ecosystem functioning in the deep sea (Klimpel et al., 2001). In the present study, the widespread occurrence of ectoparasitism across a variety of host species, depths, habitats, and locations indicate that ectoparasites are a significant component of deep-sea biodiversity. Because ectoparasitism is widespread and many fishes also have wide-ranging distributions (e.g., Moore et al., 2003), ectoparasites could alter behavior and population dynamics of hosts, while increasing trophic connections (Amundsen et al., 2009, Demopoulos and Sikkel, 2015) in communities throughout the deep sea. Understanding parasite ecology may thus serve as a proxy for determining healthy ecosystems (Hudson et al., 2006). For example, recent studies in shallow water ecosystems have demonstrated the important connections between fishes, ectoparasites, and cleaner species (Johnson et al., 2010); disruptions to these connections can cause community changes (e.g., Lafferty et al., 2008, Sun et al., 2015). Focused parasitology studies are sorely needed to further our understanding of the roles of parasites in both community and trophic ecology in the deep sea (Poulin et al., 2016). By demonstrating the widespread occurrence of ectoparasitism in the deep sea using visual based surveys, we hope that this study can serve as a basis for testing further hypotheses regarding the role of parasitism throughout the deep sea.
Author contribution
AWJD conceived the study. AMQ analyzed the video, conducted analyses, and wrote the text with significant contributions from AWJD.
Conflicting interests
We have no competing interests.
Acknowledgements
NOAA’s Office of Ocean Exploration and Research funded and supported the 2013 and 2014 NEUS Canyons and Seamounts Expeditions. Funding to Demopoulos was provided by the USGS Environments Program. We thank the crews of the NOAA Ship Okeanos Explorer and the ROV Deep Discoverer. Julianne Passarelli provided copepod identifications. Nico Smit provided isopod identifications. Jennie McClain-Counts identified the myctophids. Thanks to the three anonymous reviewers for providing helpful suggestions for improvement. We particularly thank Tom Munroe for thoughtful discussions. Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Contributor Information
Andrea M. Quattrini, Email: aquattrini@usgs.gov.
Amanda W.J. Demopoulos, Email: ademopoulos@usgs.gov.
References
- Adlard R.D., Lester R.G.J. The life cycle and biology of Anilocra pomacentri (Isopoda: Cymothoidae), an ectoparasitic isopod of the coral reef fish, Chromis nitida (Perciformes: pomacentridae) Aust. J. Zool. 1995;43:271–281. [Google Scholar]
- Amundsen P.A., Lafferty K.D., Knudsen R., Primicerio R., Klemetsen A., Kuris A.M. Food web topology and parasites in the pelagic zone of a subarctic lake. J. Anim. Ecol. 2009;78(3):563–572. doi: 10.1111/j.1365-2656.2008.01518.x. [DOI] [PubMed] [Google Scholar]
- Arias-González J.E., Morand S. Trophic functioning with parasites: a new insight for ecosystem analysis. Mar. Ecol. Prog. Ser. 2006;320:43–53. [Google Scholar]
- Artim J.M., Sellers J.C., Sikkel P.C. Micropredation by gnathiid isopods on settlement-stage reef fish in the eastern Caribbean Sea. Bul. Mar. Sci. 2015;91(4):479–487. [Google Scholar]
- Auster P.J., Malatesta R.J., LaRosa S.C. Patterns of microhabitat utilization by mobile megafauna on the southern New England continental shelf and slope. Mar. Ecol. Prog. Ser. 1995;127:77–85. [Google Scholar]
- Barber I., Hoare D., Krause J. Effects of parasites on fish behaviour: a review and evolutionary perspective. Rev. Fish Biol. Fish. 2000;10:131–165. [Google Scholar]
- Boxshall G.A. Host specificity in copepod parasites of deep-sea fishes. J. Mar. Syst. 1998;15(1):215–223. [Google Scholar]
- Brusca R.C. A monograph on the isopoda Cymothoidae (Crustacea) of the eastern pacific. Zoo. J. Linn. Soc. 1981;73(2):117–199. [Google Scholar]
- Bunkley-Williams L., Williams E.H. Isopods associated with fishes: a synopsis and corrections. J. Parasitol. 1998;84:893–896. [PubMed] [Google Scholar]
- Campbell R.A., Haedrich R.L., Munroe T.A. Parasitism and ecological relationships among deep-sea benthic fishes. Mar. Biol. 1980;57(4):301–313. [Google Scholar]
- Canals M., Puig P., de Madron X.D., Heussner S., Palanques A., Fabres J. Flushing submarine canyons. Nature. 2006;444(7117):354–357. doi: 10.1038/nature05271. [DOI] [PubMed] [Google Scholar]
- Coile A.M., Sikkel P.C. An experimental field test of susceptibility to ectoparasitic gnathiid isopods among Caribbean reef fishes. Parasitology. 2013;140:888–896. doi: 10.1017/S0031182013000097. [DOI] [PubMed] [Google Scholar]
- Collins M.A., Priede I.G., Bagley P.M. In situ comparison of activity in two deep-sea scavenging fishes occupying different depth zones. Proc. R. Soc. B. 1999;266(1432):2011–2016. [Google Scholar]
- Davies A.J., Smit N.J. The life cycle of Haemogregarina bigemina (adeleina: haemogregarinidae) in south african hosts. Folia Parasitol. 2001;48(3):169–177. doi: 10.14411/fp.2001.029. [DOI] [PubMed] [Google Scholar]
- Davies A.J., Wisshak M., Orr J.C., Roberts J.M. Predicting suitable habitat for the cold-water coral Lophelia pertusa (Scleractinia) Deep Sea Res. 2008;I 55(8):1048–1062. [Google Scholar]
- Demopoulos A.W., Sikkel P.C. Enhanced understanding of ectoparasite–host trophic linkages on coral reefs through stable isotope analysis. Int. J. Parasitol. 2015;4:125–134. doi: 10.1016/j.ijppaw.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson A.P., Hudson P.J. Parasites, disease and the structure of ecological communities. Trends Ecol. Evol. 1986;1(1):11–15. doi: 10.1016/0169-5347(86)90060-1. [DOI] [PubMed] [Google Scholar]
- Doughty C.L., Quattrini A.M., Cordes E.E. Insights into the population dynamics of the deep-sea coral genus Paramuricea in the Gulf of Mexico. Deep Sea Res. II. 2014;99:71–82. [Google Scholar]
- Gartner J.V., Zwerner D.E. The parasite faunas of meso- and bathypelagic fishes of Norfolk Submarine Canyon, western North Atlantic. J. Fish. Bio. 1989;34:79–95. [Google Scholar]
- Grutter A.S., Pickering J.L., McCallum H., McCormick M.I. Impact of micropredatory gnathiid isopods on young coral reef fishes. Coral Reefs. 2008;27:655–661. [Google Scholar]
- Ho J.S. Copepod parasites of deep-sea benthic fishes from the western North Atlantic. Parasitol. 1985;90(03):485–497. [Google Scholar]
- Houston K.A., Haedrich R.L. Food habits and intestinal parasites of deep demersal fishes from the upper continental slope east of Newfoundland, northwest Atlantic Ocean. Mar. Biol. 1986;92(4):563–574. [Google Scholar]
- Hudson P.J., Dobson A.P., Lafferty K.D. Is a healthy ecosystem one that is rich in parasites? Trends. Ecol. Evol. 2006;21(7):381–385. doi: 10.1016/j.tree.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Huvenne V.A., Tyler P.A., Masson D.G., Fisher E.H., Hauton C., Hühnerbach V. A picture on the wall: innovative mapping reveals cold-water coral refuge in submarine canyon. PloS One. 2011;6(12):e28755. doi: 10.1371/journal.pone.0028755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto T. The abyssal fish Antimora rostrata (Günther) Comp. Biochem. Physiology Part B Comp. Biochem. 1975;52:7–11. doi: 10.1016/0305-0491(75)90108-x. [DOI] [PubMed] [Google Scholar]
- Jacoby C.A., Greenwood J.G. Spatial, temporal, and behavioral patterns in emergence of zooplankton in the lagoon of Heron Reef, Great Barrier Reef, Australia. Mar. Biol. 1988;97:309–328. [Google Scholar]
- Jamieson A.J., Kilgallen N.M., Rowden A.A., Fujii T., Horton T., Lörz A.N., Priede I.G. Bait-attending fauna of the Kermadec Trench, SW Pacific Ocean: evidence for an ecotone across the abyssal–hadal transition zone. Deep Sea Res. I. 2011;58(1):49–62. [Google Scholar]
- Johnson P.T., Dobson A., Lafferty K.D., Marcogliese D.J., Memmott J., Orlofske S.A. When parasites become prey: ecological and epidemiological significance of eating parasites. Trends Ecol. Evol. 2010;25(6):362–371. doi: 10.1016/j.tree.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Jones C.M., Nagel L., Hughes G.L., Grutter A.S. Host specificity of two species of Gnathia (Isopoda) determined by DNA sequencing blood meals. Int. J. Parasitol. 2007;37:927–935. doi: 10.1016/j.ijpara.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Klimpel S., Seehagen A., Palm H.W., Rosenthal H. Logos Verlag Berlin; 2001. Deep-water Metazoan Fish Parasites of the World. [Google Scholar]
- Klimpel S., Palm H.W., Busch M.W., Kellermanns E., Rückert S. Fish parasites in the Arctic deep-sea: poor diversity in pelagic fish species vs. heavy parasite load in a demersal fish. Deep Sea Res. I. 2006;53(7):1167–1181. [Google Scholar]
- Klitgaard A.B. Gnathia abyssorum (g.o. sars, 1872) (Crustacea, Isopoda) associated with sponges. Sarsia. 1991;76(1–2):33–39. [Google Scholar]
- Lafferty K.D., Kuris A. Trophic strategies, animal diversity and body size. Trends Ecol. Evol. 2002;17:507–513. [Google Scholar]
- Lafferty K.D., Allesina S., Arim M., Briggs C.J., De Leo G., Dobson A.P., Dunne J.A., Johnson P.T., Kuris A.M., Marcogliese D.J., Martinez N.D. Parasites in food webs: the ultimate missing links. Ecol. Lett. 2008;11:533–546. doi: 10.1111/j.1461-0248.2008.01174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler A., Howse H.D., Cook D.W. Silver perch, Bairdiella chrysura: new host for Lymphocystis. Copeia. 1974:266–269. [Google Scholar]
- Lester R.J.G., Roubal F.R., Woo P.T.K. Phylum arthropoda. In: Woo P.T.K., editor. vol. 1. CABI Publishing; Wallingford, UK: 1995. pp. 475–598. (Fish Diseases and Disorders, Protozoan and Metazoan Infections). [Google Scholar]
- Manter H.W. Some digenetic trematodes from deep-water fish of Tortugas. Fla. Pubis Carnegie Instn. 1934;28:257–345. [Google Scholar]
- Marcogliese D.J. Food webs and the transmission of parasites to marine fish. Parasitol. 2002;124(07):83–99. doi: 10.1017/s003118200200149x. [DOI] [PubMed] [Google Scholar]
- Moore J.A., Hartel K.E., Craddock J.E., Galbraith J.K. An annotated list of deepwater fishes from off the New England region, with new area records. NE Nat. 2003;10(2):159–248. [Google Scholar]
- Mouchet S. Note sur Ie cycleevolutif des Gnathiidae. Bull. Soc. Zool. 1928;53:392–400. [Google Scholar]
- Noble E.R. Parasites and fishes in a deep-sea environment. Adv. Mar. Biol. 1973;11:121–195. [Google Scholar]
- Oliveira A., Santos A.I., Rodrigues A., Vitorino J. Sedimentary particle distribution and dynamics on the Nazaré canyon system and adjacent shelf (Portugal) Mar. Geol. 2007;246(2):105–122. [Google Scholar]
- Palm H.W., Klimpel S. Metazoan fish parasites of Macrourus berglax Lacepède, 1801 and other macrourids of the North Atlantic: invasion of the deep sea from the continental shelf. Deep Sea Res. I. 2008;55(1):236–242. [Google Scholar]
- Pickart R.S. Water mass components of the North Atlantic deep western boundary current. Deep-Sea Res. I. 1992;39:1553–1572. [Google Scholar]
- Poore G.C., Bruce N.L. Global diversity of marine isopods (except Asellota and crustacean symbionts) PLoS One. 2012;7(8):e43529. doi: 10.1371/journal.pone.0043529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin R. The functional importance of parasites in animal communities: many roles at many levels? Int. J. Parasitol. 1999;29(6):903–914. doi: 10.1016/s0020-7519(99)00045-4. [DOI] [PubMed] [Google Scholar]
- Poulin R., Rohde K. Comparing the richness of metazoan ectoparasite communities of marine fishes: controlling for host phylogeny. Oecologia. 1997;110(2):278–283. doi: 10.1007/s004420050160. [DOI] [PubMed] [Google Scholar]
- Poulin R., Blasco-Costa I., Randhawa H.S. Integrating parasitology and marine ecology: seven challenges towards greater synergy. J. Sea Res. 2016;113:3–10. [Google Scholar]
- Quattrini A.M., Nizinski M.S., Chaytor J.D., Demopoulos A.W.J., Roark E.B., France S.C. Exploration of the canyons-incised margin off the northeastern United States reveals dynamic habitats and diverse communities. PlosOne. 2015 doi: 10.1371/journal.pone.0139904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2015. R: a language and environment for statistical computing.http://www.R-project.org/ [Google Scholar]
- Rohde K., Hayward C., Heap M. Aspects of the ecology of metazoan ectoparasites of marine fishes. Int. J. Parasitol. 1995;25(8):945–970. doi: 10.1016/0020-7519(95)00015-t. [DOI] [PubMed] [Google Scholar]
- Ross S.W., Sulak K.J., Munroe T.A. Association of Syscenus infelix (Crustacea: isopoda: Aegidae) with benthopelagic rattail fishes, Nezumia spp. (Macrouridae), along the western North Atlantic continental slope. Mar. Biol. 2001;138(3):595–601. [Google Scholar]
- Ross S.W., Rhode M., Quattrini A.M. Demersal fish distribution and habitat use within and near Baltimore and Norfolk Canyons, U.S. middle Atlantic slope. Deep-Sea Res. I. 2015;103:137–154. [Google Scholar]
- Sikkel P.C., Cheney K.L., Côté I.M. In situ evidence for ectoparasites as a proximate cause of cleaning interactions in reef fish. Anim. Behav. 2004;68:241–247. [Google Scholar]
- Smit N.J., Davies A.J. The curious life-style of the parasitic stages of gnathiid isopods. Adv. Parasitol. 2004;58:289–391. doi: 10.1016/S0065-308X(04)58005-3. [DOI] [PubMed] [Google Scholar]
- Smit N.J., Van As J.G., Basson L. A redescription of the adult male and larvae of Gnathia africana Barnard, 1914 (Gnathiidae: Crustacea: isopoda) from southern Africa. Folia Parasitol. 1999;46:229–240. [Google Scholar]
- Smit N.J., Basson L., Van As J.G. Life cycle of the temporary fish parasite, Gnathia africana (Crustacea: isopoda: Gnathiidae) Folia Parasitol. 2003;50:135–142. doi: 10.14411/fp.2003.024. [DOI] [PubMed] [Google Scholar]
- Stoll C. Cycle evolutif de Paragnathia formica (Hesse) (Isopodes – Gnathiidae) Cah. Biol. Mar. 1962;3:401–416. [Google Scholar]
- Stoner A.W., Ryer C.H., Parker S.J., Auster P.J., Wakefield W.W. Evaluating the role of fish behavior in surveys conducted with underwater vehicles. Can. J. Fish. Aquat. Sci. 2008;65:1230–1243. [Google Scholar]
- Sun D., Cheney K.L., Werminghausen J., Meekan M.G., McCormick M.I., Cribb T.H., Grutter A.S. Presence of cleaner wrasse increases the recruitment of damselfishes to coral reefs. Biol. Lett. 2015;11(8):20150456. doi: 10.1098/rsbl.2015.0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upton N.P.D. Asynchronous male and female life cycles in the sexually dimorphic; harem-forming isopod Paragnathia formica (Crustacea:lsopoda) J. Zool. 1987;212:677–690. [Google Scholar]
- Vetter E.W., Dayton P.K. Organic enrichment by macrophyte detritus, and abundance patterns of megafaunal populations in submarine canyons. Mar. Ecol. Prog. Ser. 1999;186:137–148. [Google Scholar]
- Wallace N.A., Huntsman A.G. University Library; Toronto: 1919. The Isopoda of the Bay of Fundy. [Google Scholar]
- Welicky R.L., Sikkel P.C. Variation in occurrence of the fish-parasitic cymothoid isopod, Anilocra haemuli, infecting French grunt (Haemulon flavolineatum) in the north-eastern Caribbean. Mar. Fresh. Res. 2014;65:1018–1026. [Google Scholar]
- Wenner C.A., Musick J.A. Biology of the morid fish Antimora rostrata in the western North Atlantic. J. Fish. Res. Board Can. 1977;34(12):2362–2368. [Google Scholar]
- White T.A., Fotherby H.A., Stephens P.A., Hoelzel A.R. Genetic panmixia and demographic dependence across the North Atlantic in the deep-sea fish, blue hake (Antimora rostrata) Heredity. 2011;106(4):690–699. doi: 10.1038/hdy.2010.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C.B. North American parasitic copepods belonging to the Lernaeopodidae with a revision of the entire family. Proc. U. S. Natl. Mus. 1915;47:565–729. [Google Scholar]
- Wilson C.B. North American parasitic copepods belonging to the new family Sphyriidae. Proc. U. S. Natl. Mus. 1919;55:605–649. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Nezumia bairdii with a sphyriid copepod hyperparasitized by eight leeches (Phoenix Canyon, 800–1100 m depth). Video recorded by ROV D2 during the NOAA Okeanos Explorer Program, 2014 Atlantic Canyons and Seamounts Expedition. Video narration by S. France (Univ. Louisiana Lafayette).