Abstract
Context:
Denosumab and zoledronic acid (ZOL) are parenteral treatments for patients with osteoporosis.
Objective:
The objective of the study was to compare the effect of transitioning from oral bisphosphonates to denosumab or ZOL on bone mineral density (BMD) and bone turnover.
Design and Setting:
This was an international, multicenter, randomized, double-blind trial.
Participants:
A total of 643 postmenopausal women with osteoporosis previously treated with oral bisphosphonates participated in the study.
Interventions:
Subjects were randomized 1:1 to sc denosumab 60 mg every 6 months plus iv placebo once or ZOL 5 mg iv once plus sc placebo every 6 months for 12 months.
Main Outcome Measures:
Changes in BMD and bone turnover markers were measured.
Results:
BMD change from baseline at month 12 was significantly greater with denosumab compared with ZOL at the lumbar spine (primary end point; 3.2% vs 1.1%; P < .0001), total hip (1.9% vs 0.6%; P < .0001), femoral neck (1.2% vs −0.1%; P < .0001), and one-third radius (0.6% vs 0.0%; P < .05). The median decrease from baseline was greater with denosumab than ZOL for serum C-telopeptide of type 1 collagen at all time points after day 10 and for serum procollagen type 1 N-terminal propeptide at month 1 and at all time points after month 3 (all P < .05). Median percentage changes from baseline in serum intact PTH were significantly greater at months 3 and 9 with denosumab compared with ZOL (all P < .05). Adverse events were similar between groups. Three events consistent with the definition of atypical femoral fracture were observed (two denosumab and one ZOL).
Conclusions:
In postmenopausal women with osteoporosis previously treated with oral bisphosphonates, denosumab was associated with greater BMD increases at all measured skeletal sites and greater inhibition of bone remodeling compared with ZOL.
Postmenopausal women with osteoporosis transitioned from oral bisphosphonates had greater BMD increases and greater inhibition of bone turnover with denosumab compared with zoledronic acid.
Osteoporosis is a chronic, progressive condition that generally requires long-term management. Oral bisphosphonates are a commonly prescribed treatment for osteoporosis (1), but inconvenient dosing regimens and side effects can lead to low adherence (2, 3). Suboptimal adherence to osteoporosis medication can reduce antifracture efficacy (4–7) and increase health care use and costs (8, 9). Although more extended dosing intervals can improve adherence (2, 10, 11), efficacy remains an influential determinant of patient preference for and adherence with osteoporosis medications (12, 13).
Once-yearly iv bisphosphonate therapy with zoledronic acid (ZOL) has been shown to reduce the risk of hip, vertebral, and nonvertebral fractures (14). Although parenteral bisphosphonates, such as ZOL, have become a treatment option for osteoporosis, there is no evidence that cycling through bisphosphonate agents offers therapeutic benefit to patients with osteoporosis, whether assessed by bone mineral density (BMD) or bone turnover markers (BTMs). Although patients in one clinical trial expressed a preference for once-yearly ZOL over a weekly bisphosphonate regimen, switching from oral bisphosphonates to ZOL did not further increase BMD (15).
Denosumab (Prolia; Amgen Inc) is a fully human monoclonal antibody against RANKL administered sc every 6 months. In a 3-year, placebo-controlled, pivotal osteoporosis trial, denosumab significantly reduced BTMs, increased BMD, and reduced the risk of hip, vertebral, and nonvertebral fractures (16). Three studies have shown that individuals who received prior bisphosphonate therapy and transitioned to denosumab had greater BMD gains at all measured skeletal sites compared with continuing alendronate or initiating ibandronate or risedronate (17–19).
This study assessed whether transitioning from an oral bisphosphonate to a parenteral therapy in the same treatment class (iv bisphosphonate [ZOL]) or an antiresorptive therapy with a different mode of action (sc RANKL inhibitor [denosumab]) was associated with greater efficacy and comparable safety profile in postmenopausal women with osteoporosis.
Materials and Methods
Study subjects
Ambulatory postmenopausal women aged 55 years or older who received oral bisphosphonate therapy for 2 years or longer immediately before screening were eligible if they had a T-score of −2.5 or less at the lumbar spine, total hip, or femoral neck, two or more lumbar vertebrae, and one hip evaluable by dual-energy x-ray absorptiometry (DXA) and baseline serum C-telopeptide of type 1 collagen (CTX) of 500 pg/mL or less. Subjects were excluded if they had received denosumab or ZOL at any time; fluoride, strontium ranelate, or iv bisphosphonate other than ZOL within the previous 5 years; PTH or PTH derivatives within the year before enrollment; or other bone-active drugs in the 3 months before screening. All subjects provided written informed consent prior to enrollment in the study.
Study design
This was a 12-month international, multicenter, randomized, double-blind, double-dummy, active-controlled, parallel-group study (clinicaltrials.gov; number NCT01732770) conducted at 37 study centers in Belgium, Denmark, Poland, Spain, Canada, the United States, and Australia. Subjects were randomized 1:1 to one of two treatment arms. Subjects in the denosumab arm received sc denosumab 60 mg and iv saline (ZOL placebo) on day 1 and sc denosumab 60 mg at the month 6 visit. Subjects in the ZOL arm received iv ZOL 5 mg and denosumab placebo sc on day 1 and denosumab placebo sc at month 6. Subjects were required to take 1000 mg or greater elemental calcium and 800 IU or greater vitamin D daily. Of 643 subjects enrolled, 117 subjects from 11 centers consented to the increased frequency of sample draws and enrolled in a substudy assessing serum BTMs, intact PTH (iPTH), and albumin-adjusted calcium.
The study protocol was approved by an institutional review board or ethics committee for each site. The study was conducted in accordance with the International Conference on Harmonisation Good Clinical Practice guidelines. All authors had access to the data, participated in drafting or revising the manuscript, and approved the final version for submission; N.P. and C.W. take responsibility for the integrity of the data analysis.
Study assessments
DXA scans were performed in duplicate at baseline and month 12 or early termination visit for the lumbar spine (L1–L4); left proximal femur (for total hip and femoral neck), unless the left was unsuitable for analysis, in which case the right was used; and nondominant forearm (one-third radius) using GE Lunar or Hologic scanners. The same side was used for the proximal femur or forearm scan at baseline and month 12 or early termination visit. DXA scan data were submitted to a central imaging vendor (BioClinica) for blinded analyses. BMD values obtained at early termination were carried forward as the month 12 value. The analysis was based on the mean BMD values from duplicate DXA scans.
In subjects enrolled in the substudy, serum CTX and procollagen type 1 N-terminal propeptide (P1NP) were assessed at day 1, day 10, and months 1, 3, 6, 6 + 10 days, months 7, 9, and 12 using electrochemiluminescence immunoassays. Albumin-adjusted calcium was assessed at the same time points on a Roche Modular clinical chemistry analyzer. Serum iPTH was assessed at months 1, 3, 6, 7, 9, and 12 using an IMMULITE iPTH chemiluminescence assay (Siemens Healthcare). Blood samples were collected after an overnight fast and prior to study drug administration and analyzed centrally by Quintiles Laboratories.
Evaluation of antidenosumab antibodies in subjects receiving denosumab was performed on day 1 and month 12 or end-of-study visit by the study sponsor. Adverse events (AEs) were recorded at each study visit.
End points
The primary end point was mean percentage change from baseline in lumbar spine BMD at month 12. The secondary end point was the mean percentage change from baseline in total hip BMD at month 12. Additional end points included the mean percentage change from baseline in the femoral neck and one-third radius BMD at month 12 in the overall population and the median percentage changes from baseline in serum BTMs, iPTH, and albumin-adjusted calcium in subjects participating in the BTM substudy. Safety end points included AEs.
Statistical analyses
The primary hypothesis was that treatment with denosumab was not inferior to ZOL for the mean percentage change from baseline in lumbar spine BMD at month 12 based on a margin of −0.46%. Secondary hypotheses included the following: 1) noninferiority in total hip BMD with denosumab vs ZOL based on a margin of −0.51%, 2) superiority of denosumab for the mean percentage change from baseline in lumbar spine BMD at month 12, and 3) superiority of denosumab for the mean percentage change from baseline in total hip BMD at month 12. A stepdown sequential testing procedure was used to maintain the overall type I error rate at 5% among BMD end points. Specifically, only if the primary noninferiority hypothesis was demonstrated was the individual secondary hypothesis tested in the prespecified sequence.
To derive the noninferiority margins, the effect of ZOL treatment at 12 months on the lumbar spine and total hip BMD relative to placebo in postmenopausal women previously treated with oral bisphosphonates was needed. Due to the paucity of relevant data, the ZOL and placebo effects in similar pretreated populations were estimated separately (Supplemental Material). A 50% preservation of the net ZOL treatment effect was deemed clinically meaningful, which led to noninferiority margins of −0.46% for the lumbar spine and −0.51% for the total hip end points. A sample size of 310 subjects per group, assuming a 10% dropout rate through month 12, was estimated to provide 99% power for the primary analysis.
The primary analysis population included all randomized subjects who had a baseline BMD measurement and one or more postbaseline measurements. An analysis of covariance model assessed the treatment difference in the primary efficacy analysis and included treatment, screening serum CTX category (<300 pg/mL or 300–500 pg/mL), baseline BMD, DXA machine type, and baseline BMD value-by-machine type interaction. Data are least squares means of the treatment difference and two-sided 95% confidence intervals (CIs). The lower bound of the two-sided 95% CI was compared with the noninferiority margin for assessing noninferiority.
Precision errors based on duplicate DXA scans from individual subjects were pooled to derive an estimate for the least significant change (LSC) in BMD measurements at each skeletal site assessed (20). The proportions of women with a BMD response less than the LSC or equal to or greater than the LSC at each skeletal site were evaluated using a logistic model with treatment as the explanatory variable. The estimated odds ratio (OR) of achieving a BMD gain of equal to or greater than the LSC for denosumab vs ZOL was also provided along with the 95% CI and the corresponding P value.
The BTM analysis subset included all randomized subjects enrolled in the substudy and had a baseline measurement and one or more postbaseline measurements. Descriptive statistics were calculated and expressed as a median and interquartile range. The significance of treatment difference at each visit was assessed using a Wilcoxon rank sum test.
The safety analysis population included all subjects who received one or more doses of the study drug. Subject incidence of treatment-emergent AEs and events of interest were summarized. MedDRA version 17.1 was used to code AEs. All potential cases of osteonecrosis of the jaw (ONJ) and atypical femoral fracture (AFF) were reviewed by separate independent blinded external adjudication committees. Available x-ray images of femoral fractures were reviewed by a panel at the central radiographic vendor (BioClinica). The major criteria established by the American Society for Bone and Mineral Research and reported in the second report of the Atypical Femoral Fractures Task Force were used for adjudication (21). A finding of indeterminate was not permitted.
Results
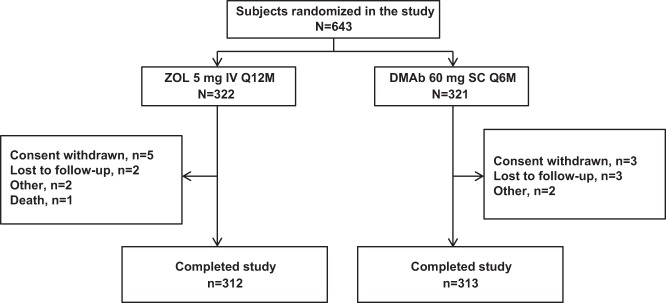
Of 1297 women screened, 643 were enrolled in the study, and 625 (97.2%) completed 12 months of follow-up. Eighteen subjects (2.8%) discontinued the study, with rates of discontinuation similar between the denosumab and ZOL groups (Figure 1). A total of 117 subjects (18.2%) were enrolled in the BTM substudy (denosumab, n = 61; ZOL, n = 56); 110 substudy subjects (94.0%) completed 12 months of follow-up.
Figure 1.
Subject disposition. DMAb, denosumab; Q6M, every 6 months; Q12M, every 12 months.
Baseline demographics and clinical characteristics were similar between the two treatment groups (Table 1) and between the overall and substudy populations. Mean (SD) age at baseline was 68.5 (7.1) and 69.5 (7.7) years, and mean (SD) duration of prior oral bisphosphonate therapy was 6.2 (3.8) and 6.4 (3.7) years in the denosumab and ZOL groups, respectively. Median baseline serum concentrations of CTX, P1NP, and iPTH were similar in the two treatment groups (Table 1).
Table 1.
Baseline Demographics and Characteristics
| Denosumab (n = 321) | Zoledronic Acid (n = 322) | |
|---|---|---|
| Age, y, mean (SD) | 68.5 (7.1) | 69.5 (7.7) |
| Race/ethnic group, n, % | ||
| White or Caucasian | 309 (96.3) | 314 (97.5) |
| Asian | 5 (1.6) | 4 (1.2) |
| Black or African American | 1 (0.3) | 0 |
| Othera | 6 (1.8) | 4 (1.2) |
| BMI, kg/m2, mean (SD) | 24.3 (4.0) | 24.3 (4.2) |
| Years since menopause, mean (SD) | 19.9 (8.2) | 20.8 (8.9) |
| History of fracture, n, % | ||
| Any | 169 (52.6) | 159 (49.4) |
| Osteoporotic | 120 (37.4) | 121 (37.6) |
| Nonvertebral | 109 (34.0) | 106 (32.9) |
| Vertebral | 24 (7.5) | 28 (8.7) |
| Lumbar spine BMD T-score | ||
| Mean (SD) | −2.74 (0.83) | −2.64 (0.86) |
| ≤−2.5, n, % | 230 (71.7) | 229 (71.1) |
| >−2.5, n, % | 91 (28.3) | 91 (28.3) |
| Total hip BMD T-score | ||
| Mean (SD) | −1.93 (0.74) | −1.93 (0.80) |
| ≤−2.5, n, % | 74 (23.1) | 75 (23.3) |
| >−2.5, n, % | 246 (76.6) | 243 (75.5) |
| Prior oral bisphosphonate treatment duration, y, mean (SD) | 6.2 (3.8) | 6.4 (3.7) |
| Serum CTX, pg/mL, median (Q1, Q3) | 209 (146, 303) | 212 (151, 297) |
| Serum CTX,b pg/mL, median (Q1, Q3) | 211 (134, 303) | 194 (133, 292) |
| Serum P1NP,b ng/mL, median (Q1, Q3) | 26 (16, 34) | 23 (19, 32) |
| Serum iPTH,b ng/mL, median (Q1, Q3) | 39 (29, 49) | 36 (29, 51) |
Abbreviations: Q1, quartile 1; Q3, quartile 3.
Includes subjects who self-identified as Native, Native Hawaiian, or other Pacific Islander, multiple, or other.
Data represent subjects enrolled in the bone turnover marker substudy.
Bone mineral density
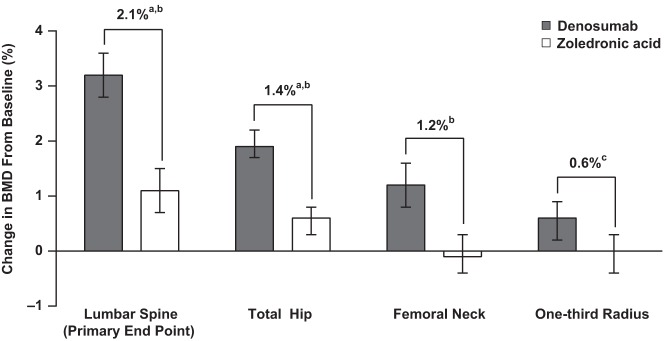
BMD at the lumbar spine increased by 3.2% (95% CI 2.8%–3.6%) from baseline in the denosumab group compared with 1.1% (95% CI 0.7%–1.5%) in the ZOL group (Figure 2). This treatment difference of 2.1% (95% CI 1.6%–2.6%) at month 12 excluded the predefined noninferiority margin of −0.46% and also achieved superiority (P < .0001). BMD at the total hip increased by 1.9% (95% CI 1.7%–2.2%) from baseline in the denosumab group compared with 0.6% (95% CI 0.3%–0.8%) in the ZOL group. This difference of 1.4% (95% CI 1.0%–1.7%) at month 12 excluded the noninferiority margin of −0.51% and also achieved superiority (P < .0001) (Figure 2). Femoral neck BMD increased by 1.2% (95% CI 0.8%–1.6%) from baseline in the denosumab group compared with a change of −0.1% (95% CI −0.4% to 0.3%) in the ZOL group, for a significant difference of 1.2% (95% CI 0.7%–1.7%; P < .0001) (Figure 2). BMD at the one-third radius increased by 0.6% (95% CI 0.2%–0.9%) from baseline in the denosumab group compared with 0% (95% CI −0.4% to 0.3%) in the ZOL group, resulting in a significant difference of 0.6% (95% CI 0.1%–1.0%; P = .018).
Figure 2.
Mean percentage change from baseline in areal BMD at month 12. Data represent least squares means and 95% CIs based on an analysis of covariance model adjusting for treatment, serum CTX stratification variable (<300 pg/mL vs 300–500 pg/mL), baseline BMD, DXA machine type, and baseline value-by–machine type interaction. a, P < .0001 for noninferiority; b, P < .0001 for superiority; c, P = .018 for superiority.
BMD effects were also assessed by calculating the LSC at month 12. The calculated LSC was 2.07% at the lumbar spine, 2.26% at the total hip, 3.42% at the femoral neck, and 3.66% at the one-third radius. More subjects in the denosumab group than in the ZOL group had BMD gains of the LSC or greater at the lumbar spine (66% vs 33%; OR 3.98 [95% CI 2.86–5.55], P < .0001), total hip (39% vs 21%; OR 2.34 [95% CI 1.64–3.34], P < .0001), and femoral neck (17% vs 10%; OR 1.88 [95% CI 1.17–3.02], P < .01). For the one-third radius, small percentages of subjects in the denosumab (11%) and ZOL (9%) groups achieved a BMD increase of the LSC or greater at 12 months, with no significant difference between groups (OR 1.28 [95% CI 0.75–2.18], P = .360).
Bone turnover markers, calcium, and intact parathyroid hormone
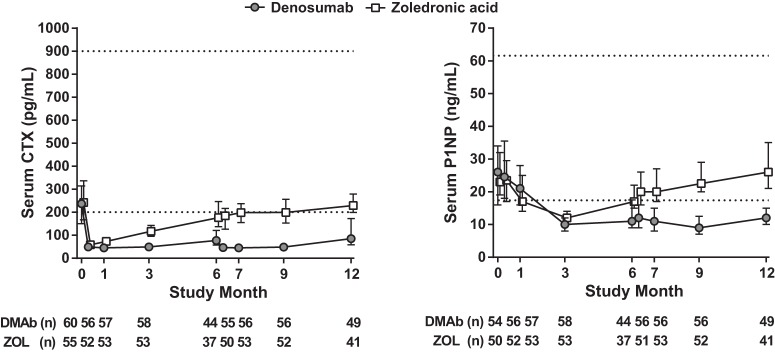
Absolute concentrations of serum CTX and P1NP decreased from baseline in both treatment groups at most time points for subjects enrolled in the BTM substudy (Figure 3). The median percentage decrease from baseline in serum CTX was significantly greater with denosumab compared with ZOL at all time points after day 10 (all P < .01). The median percentage decrease from baseline in serum P1NP was significantly greater with denosumab compared with ZOL at month 1 (P < .05) and at all time points after month 3 (all P < .01).
Figure 3.
Serum CTX and P1NP concentrations. Values represent median and interquartile (Q1, Q3) range. Lower limit of quantification is 40 pg/mL for CTX and 5 ng/mL for P1NP. Dashed horizontal lines indicate premenopausal reference ranges for CTX (200–900 pg/mL) and P1NP (17.4–61.6 ng/mL) (28). DMAb, denosumab.
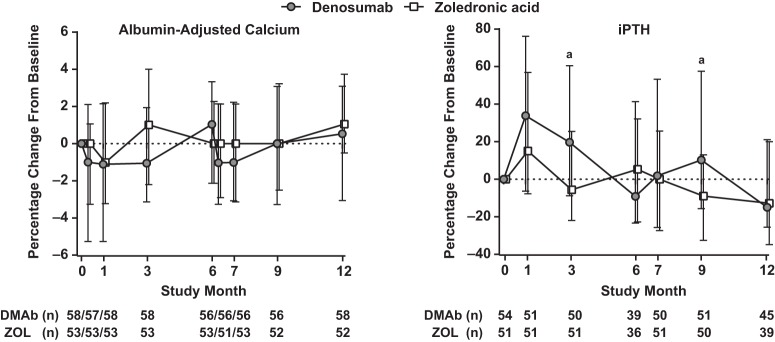
There were no clinically meaningful changes in albumin-adjusted calcium with denosumab or ZOL (Figure 4). Transient increases in serum iPTH concentrations from baseline occurred in both groups after dosing. When paired baseline and on-treatment values were compared, significant increases from baseline in serum iPTH were observed at months 1 (33.9%), 3 (19.7%), and 9 (10.3%) with denosumab and at month 1 with ZOL (15.1%) (all P < .05; Figure 4). The percentage increases from baseline observed with denosumab were significantly greater compared with ZOL at months 3 and 9 (P < .05).
Figure 4.
Median (Q1, Q3) percentage change in albumin-adjusted calcium and iPTH concentrations from baseline to month 12. Values represent median and interquartile (Q1, Q3) range. a, P < .05 compared with ZOL. DMAb, denosumab.
Safety
Overall, a similar number of subjects in each treatment group reported AEs during the study (62.2% in each; Table 2). Serious AEs were reported in 25 subjects (7.8%) in the denosumab group and 29 subjects (9.1%) in the ZOL group. There was one death during the study. A 79-year-old woman in the ZOL group died due to sepsis, anemia, and multiple organ failure after a fall, complicated by Clostridium difficile diarrhea. This death was not considered related to treatment by the investigator. AEs observed with ZOL were generally similar to those observed in previous trials of ZOL.
Table 2.
Adverse Events
| Denosumab (N = 320) n, % | Zoledronic Acid (N = 320) n, % | |
|---|---|---|
| Any AE | 199 (62.2) | 199 (62.2) |
| Serious AEs | 25 (7.8) | 29 (9.1) |
| AEs leading to discontinuation of study drug | 4 (1.3) | 9 (2.8) |
| Fatal AEs | 0 (0.0) | 1 (0.3) |
| Selected AEs of interest | ||
| Atypical femoral fracture | 2 (0.6) | 1 (0.3) |
| AEs potentially related to hypersensitivity | 12 (3.8) | 6 (1.9) |
| Serious infection | 5 (1.6) | 6 (1.9) |
| Malignancy | 5 (1.6) | 8 (2.5) |
| Cardiac disorders | 11 (3.4) | 4 (1.3) |
| Vascular disorders | 13 (4.1) | 16 (5.0) |
| Eczemaa | 5 (1.6) | 1 (0.3) |
| Musculoskeletal pain | 43 (13.4) | 63 (19.7) |
Abbreviations: N, number of subjects who received one or more doses of study drug; n, number of subjects reporting one or more events.
Events included eczema, dermatitis, and allergic dermatitis.
Three femoral fractures were adjudicated as consistent with the definition of AFF: two in denosumab subjects and one in a ZOL subject (Table 2). All three subjects had baseline serum CTX concentration at or below the lower limit of the reference range for healthy premenopausal women, implying compliance with previous oral bisphosphonate therapy. One AFF occurred in a woman previously treated with monthly oral ibandronate for approximately 2.5 years. Prodromal pain was reported at the fracture site. Approximately 2 months after her first denosumab dose, the subject fell from a standing height or less and sustained a left femoral shaft fracture. The subject discontinued denosumab and the fracture was reported as healed by the investigator within 10 months of the event. The second case of AFF in the denosumab group involved a woman previously treated with an unspecified oral bisphosphonate followed by risedronate for a total of approximately 5–6 years. No prodromal pain was reported at the fracture site. Approximately 5 months after her first denosumab dose, the subject lost her balance and tripped and fell on slippery ground while wearing a walking boot foot brace and using crutches due to a previous metatarsal fracture, sustaining a subtrochanteric fracture of the left femur. The subject discontinued denosumab and the fracture was reported as healed; although the investigator did not specify the duration of time to heal, the report of healed was received within 15 months of the event. The AFF in the ZOL group involved a woman previously treated with risedronate followed by alendronate for a total of approximately 6 years. The subject had prodromal pain over the fracture site at rest. Approximately 9.3 months after receiving ZOL, the subject stumbled and sustained a right distal femur fracture. The subject underwent surgery and after an x-ray, the fracture was reported as healed by the investigator within 3 months of the event.
Among other selected AEs of interest, there were no cases of hypocalcemia, fracture healing complications, acute pancreatitis, or events adjudicated as consistent with the definition of ONJ in either treatment group. The incidences of AEs of infection (20.9% and 16.9%), cardiac disorders (3.4% and 1.3%), and eczema (including dermatitis and dermatitis allergic; 1.6% and 0.3%) were numerically higher in the denosumab group compared with the ZOL group. Musculoskeletal pain was significantly lower in the denosumab group compared with the ZOL group (13.4% and 19.7%; P < .05) (Table 2). Cardiac AEs were reported by 11 subjects (14 events) in the denosumab group and four subjects (six events) in the ZOL group, with no consistent trend in events and a small number of individual events (two or fewer per group), coded with MedDRA version 17.1 based on AE terms reported by the investigator: heart valve incompetence (no denosumab subjects, one ZOL subject), aortic valve disease (one, none), palpitations (one, one), tachycardia (one, none), arrhythmia (two, none), supraventricular arrhythmia (one, none), extrasystoles (one, none), supraventricular extrasystoles (none, one), sick sinus syndrome (one, none), atrial tachycardia (none, one), atrial flutter (one, none), atrial fibrillation (two, one), coronary artery disease (one, none), acute myocardial infarction (none, one), myocardial infarction (one, none), and chronic cardiac failure (one, none). No serious AEs were reported for eczema, and incidences of serious infections and serious cardiovascular disorders (cardiac plus vascular) were similar in the two treatment groups. No women developed binding antibodies to denosumab.
This study was not designed with adequate statistical power to evaluate differences between treatment groups in fracture incidence. Fractures were recorded as AEs and were not adjudicated (other than potential cases of AFF). Osteoporosis-related fractures (definition available in Supplemental Material) were reported in seven denosumab subjects and 15 ZOL subjects. Fractures included radius (three denosumab, three ZOL), rib (one denosumab, three ZOL), foot (one denosumab, two ZOL), hip (one denosumab, two ZOL), humerus (one denosumab, one ZOL), tibia (one denosumab), femur (one denosumab), spine (four ZOL), hand (one ZOL), and pelvis (one ZOL) fractures.
Discussion
In this randomized trial of postmenopausal women with osteoporosis after more than 6 years of oral bisphosphonate therapy, treatment with denosumab resulted in significantly greater BMD increases at all skeletal sites evaluated as well as greater reductions in BTMs, compared with ZOL. Previous head-to-head studies of women who had received oral bisphosphonate therapy for several years showed that those who were transitioned to denosumab also experienced greater increases in BMD at the lumbar spine, hip, and one-third radius compared with those who remained on oral bisphosphonates, including alendronate, risedronate, or ibandronate (17–19). In the current study, BMD changes with denosumab and ZOL were of a lesser magnitude than in studies of subjects naïve to previous osteoporosis therapy (14, 16, 22), consistent with reduced remodeling space from prior bisphosphonate therapy at the time of initiating the parenteral osteoporosis treatments. The impact of prior bisphosphonate therapy also seemed evident by low baseline BTM concentrations. Despite prolonged bisphosphonate therapy, baseline BMD in this population was low, and the early decrease in BTMs after transitioning to denosumab or ZOL suggests a potential for an additional increase in bone mass by further control of remodeling activity.
The early reduction in BTMs upon transition to denosumab persisted throughout the 6-month denosumab dosing interval, whereas BTMs began to increase, after the initial decline, within 3 months of ZOL administration. The transience of BTM reductions after transition from oral bisphosphonate to ZOL may explain the relatively minor BMD gains observed 12 months after transitioning to ZOL. These findings are generally consistent with those from a study in postmenopausal women previously treated with oral alendronate, which showed that transition to ZOL was associated with an early reduction in BTMs followed by progressive increases with minimal BMD gains over the 12 months after ZOL administration (15). In the current study, BTMs were persistently reduced upon transition from oral bisphosphonates to denosumab, and BMD was significantly increased at each measured site. Significantly more subjects in the denosumab group achieved BMD gains exceeding the LSC threshold at the lumbar spine, total hip, and femoral neck, compared with the ZOL group. These data suggest that denosumab may be a more potent antiresorptive than ZOL in patients transitioning from long-term oral bisphosphonate therapy.
Consistent with greater reductions in BTMs, the denosumab group also exhibited significantly greater increases in endogenous PTH at months 3 and 9 compared with ZOL. A similar observation has been noted in a comparative study of denosumab-, alendronate-, and placebo-treated subjects, in which subjects treated with denosumab showed increases in PTH and decreases in cortical porosity, which were not observed with alendronate (23). The prolonged yet oscillatory pattern of endogenous PTH exposure induced by the 6-month denosumab regimen has uncertain but potentially positive implications for BMD. Studies in rodents and cynomolgus monkeys showed that prolonged oscillatory exposure to exogenous PTH, which persisted for several days per treatment interval, increased cortical and cancellous BMD (24, 25). A recent bone histomorphometry study, however, that explored the anabolic potential of the endogenous PTH response to denosumab, did not find absolute increases in bone formation (26), consistent with the lack of absolute increases in the formation marker serum P1NP in the current study's denosumab group. The PTH hypothesis as an explanation for the efficacy response with denosumab remains under evaluation.
AEs and serious AEs were similar in the two treatment groups. No events were adjudicated as consistent with ONJ. Two fractures were adjudicated as consistent with AFF with denosumab and one with ZOL. With the short time to onset of the AFF in the study subjects, the AFF is likely a result of prior long-term bisphosphonate use (from 3 to 6 y on average) and not related to the initiation of denosumab or ZOL because four other studies did not report any AFF with transition to denosumab after prior oral bisphosphonate use of varying duration or with suboptimal adherence (17–19, 27).
Strengths of the study include a robust sample size, a low dropout rate, and a reflection of the clinical practice setting wherein many patients switch osteoporosis therapies. Limitations of the study include the relatively short-term follow-up.
In summary, in postmenopausal women with osteoporosis who were previously taking oral bisphosphonates, transitioning to denosumab was well tolerated and more effective at increasing BMD at all sites measured than transitioning to ZOL. Greater BMD gains with denosumab may relate to persistent inhibition of bone turnover throughout the 6-month dosing interval. This study completes a suite of head-to-head clinical trials indicating greater clinical benefits, in terms of greater BMD increases at all sites measured and larger decreases in BTMs, of transitioning from one osteoporosis therapeutic class to another (oral bisphosphonates to denosumab), compared with cycling through the same therapeutic class (from one bisphosphonate to another).
Acknowledgments
We thank Paul Kostenuik (Phylon Pharma Services) and Mandy Suggitt (Amgen Inc), who provided medical writing support.
The study had a trial registration number of NCT01732770 (clinicaltrials.gov).
This work was supported by Amgen Inc.
Disclosure Summary: P.D.M. has received research grants from Alexion, Lilly, Amgen, Novartis, NBHA, Pfizer, the University of Alabama, Boehringer Ingelheim, Merck, Merck Serono, and Radius; is a consultant for Grünenthal, Shionogi, Radius, Amgen, and Lilly; and is a speakers' bureau member for Radius, Alexion, and Amgen. N.P., C.W., and R.B.W. are employed by Amgen and may have Amgen stock/stock options. J.P.B. has received research grants from Amgen, Eli Lilly, and Novartis and is a consultant and speakers' bureau member for Amgen and Eli Lilly. E.C. has received research grants and lecture fees from Amgen. B.S.N. is a principal investigator for several Amgen studies. M.A.B. has received research grants from Pfizer, Amgen, Sanofi, and Lilly and is a consultant and speakers' bureau member for Amgen. J.M. is a speakers' bureau member for Amgen, Lilly, Grünenthal, and Mundipharma and has received other financial support from AbbVie. H.G.B. has received research grants from Amgen and Merck; is a consultant for Amgen, Merck, and Grünenthal; and is a speakers' bureau member for Amgen and Shire. J.-Y.R. has received research grants from Bristol-Myers Squibb, Merck Sharp and Dohme, Rottapharm, Teva, Roche, Amgen, Lilly, Novartis, GlaxoSmithKline, Servier, Pfizer, Theramex, Danone, Organon, Therabel, Boehringer Ingelheim, Chiltern, and Galapagos; is a consultant or adviser for Servier, Novartis, Negma, Lilly, Wyeth, Amgen, GlaxoSmithKline, Roche, Merckle, Nycomed-Takeda, NPS, IBSA-Genévrier, Theramex, UCB, Asahi Kasei, and Endocyte; and has received lecture fees from Merck Sharp and Dohme, Lilly, Rottapharm, IBSA-Genévrier, Novartis, Servier, Roche, GlaxoSmithKline, Merckle, Teijin, Teva, Analis, Theramex, Nycomed, Novo Nordisk, Ebewe Pharma, Zodiac, Danone, Will-Pharma, and Amgen. A.S. has received research grants from Amgen; is a consultant for Amgen, Actavis, and Eli Lilly; and is a speakers' bureau member for Amgen and Actavis. S.R.C. is a consultant for Amgen.
Footnotes
- AE
- adverse event
- AFF
- atypical femoral fracture
- BMD
- bone mineral density
- BTM
- bone turnover marker
- CI
- confidence interval
- CTX
- C-telopeptide of type 1 collagen
- DXA
- dual-energy x-ray absorptiometry
- iPTH
- intact PTH
- LSC
- least significant change
- ONJ
- osteonecrosis of the jaw
- OR
- odds ratio
- P1NP
- procollagen type 1 N-terminal propeptide
- ZOL
- zoledronic acid.
References
- 1. Sambrook P, Cooper C. Osteoporosis. Lancet. 2006;367:2010–2018. [DOI] [PubMed] [Google Scholar]
- 2. Recker RR, Gallagher R, MacCosbe PE. Effect of dosing frequency on bisphosphonate medication adherence in a large longitudinal cohort of women. Mayo Clin Proc. 2005;80:856–861. [DOI] [PubMed] [Google Scholar]
- 3. Silverman SL, Schousboe JT, Gold DT. Oral bisphosphonate compliance and persistence: a matter of choice? Osteoporos Int. 2011;22:21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gallagher AM, Rietbrock S, Olson M, van Staa TP. Fracture outcomes related to persistence and compliance with oral bisphosphonates. J Bone Miner Res. 2008;23:1569–1575. [DOI] [PubMed] [Google Scholar]
- 5. Gold DT, Martin BC, Frytak JR, Amonkar MM, Cosman F. A claims database analysis of persistence with alendronate therapy and fracture risk in post-menopausal women with osteoporosis. Curr Med Res Opin. 2007;23:585–594. [DOI] [PubMed] [Google Scholar]
- 6. Kanis JA, Cooper C, Hiligsmann M, Rabenda V, Reginster JY, Rizzoli R. Partial adherence: a new perspective on health economic assessment in osteoporosis. Osteoporos Int. 2011;22:2565–2573. [DOI] [PubMed] [Google Scholar]
- 7. Penning-van Beest FJ, Erkens JA, Olson M, Herings RM. Loss of treatment benefit due to low compliance with bisphosphonate therapy. Osteoporos Int. 2008;19:511–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Halpern R, Becker L, Iqbal SU, Kazis LE, Macarios D, Badamgarav E. The association of adherence to osteoporosis therapies with fracture, all-cause medical costs, and all-cause hospitalizations: a retrospective claims analysis of female health plan enrollees with osteoporosis. J Manag Care Pharm. 2011;17:25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Olsen KR, Hansen C, Abrahamsen B. Association between refill compliance to oral bisphosphonate treatment, incident fractures, and health care costs—an analysis using national health databases. Osteoporos Int. 2013;24:2639–2647. [DOI] [PubMed] [Google Scholar]
- 10. Cramer JA, Gold DT, Silverman SL, Lewiecki EM. A systematic review of persistence and compliance with bisphosphonates for osteoporosis. Osteoporos Int. 2007;18:1023–1031. [DOI] [PubMed] [Google Scholar]
- 11. Cotte FE, Fardellone P, Mercier F, Gaudin AF, Roux C. Adherence to monthly and weekly oral bisphosphonates in women with osteoporosis. Osteoporos Int. 2010;21:145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gold DT, Safi W, Trinh H. Patient preference and adherence: comparative US studies between two bisphosphonates, weekly risedronate and monthly ibandronate. Curr Med Res Opin. 2006;22:2383–2391. [DOI] [PubMed] [Google Scholar]
- 13. Lee S, Glendenning P, Inderjeeth CA. Efficacy, side effects and route of administration are more important than frequency of dosing of anti-osteoporosis treatments in determining patient adherence: a critical review of published articles from 1970 to 2009. Osteoporos Int. 2011;22:741–753. [DOI] [PubMed] [Google Scholar]
- 14. Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. The Horizon Pivotal Fracture Trial. N Engl J Med. 2007;356:1809–1822. [DOI] [PubMed] [Google Scholar]
- 15. McClung M, Recker R, Miller P, et al. Intravenous zoledronic acid 5 mg in the treatment of postmenopausal women with low bone density previously treated with alendronate. Bone. 2007;41:122–128. [DOI] [PubMed] [Google Scholar]
- 16. Cummings S, San Martin J, McClung M, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. The FREEDOM Trial. N Engl J Med. 2009;361:756–765. [DOI] [PubMed] [Google Scholar]
- 17. Kendler D, Roux C, Benhamou C, et al. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women transitioning from alendronate therapy. J Bone Miner Res. 2010;25:72–81. [DOI] [PubMed] [Google Scholar]
- 18. Recknor C, Czerwinski E, Bone HG, et al. Denosumab compared with ibandronate in postmenopausal women previously treated with bisphosphonate therapy: a randomized open-label trial. Obstet Gynecol. 2013;121:1291–1299. [DOI] [PubMed] [Google Scholar]
- 19. Roux C, Hofbauer LC, Ho PR, et al. Denosumab compared with risedronate in postmenopausal women suboptimally adherent to alendronate therapy: efficacy and safety results from a randomized open-label study. Bone. 2014;58:48–54. [DOI] [PubMed] [Google Scholar]
- 20. Bonnick SL, Johnston CC, Jr, Kleerekoper M, et al. Importance of precision in bone density measurements. J Clin Densitom. 2001;4:105–110. [DOI] [PubMed] [Google Scholar]
- 21. Shane E, Burr D, Abrahamsen B, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2014;29:1–23. [DOI] [PubMed] [Google Scholar]
- 22. Brown JP, Prince RL, Deal C, et al. Comparison of the effect of denosumab and alendronate on BMD and biochemical markers of bone turnover in postmenopausal women with low bone mass: a randomized, blinded, phase 3 trial. J Bone Miner Res. 2009;24:153–161. [DOI] [PubMed] [Google Scholar]
- 23. Seeman E, Libanati C, Austin M, et al. The transitory increase in PTH following denosumab administration is associated with reduced intracortical porosity: a distinctive attribute of denosumab therapy. J Bone Miner Res. 2011;26(suppl 1):S22. [Google Scholar]
- 24. Kostenuik PJ, Ferrari S, Pierroz D, et al. Infrequent delivery of a long-acting PTH-Fc fusion protein has potent anabolic effects on cortical and cancellous bone. J Bone Miner Res. 2007;22:1534–1547. [DOI] [PubMed] [Google Scholar]
- 25. Atkinson JE, Kostenuik PJ, Smith SY, et al. Four weekly treatments with sustained-duration PTH-Fc increases bone mineral density in cynomolgus monkeys. J Bone Miner Res. 2003;18(suppl 1):S385. [Google Scholar]
- 26. Dempster DW, Zhou H, Recker RR, et al. Differential effects of teriparatide and denosumab on intact PTH and bone formation indices: AVA Osteoporosis Study. J Clin Endocrinol Metab. 2016;101:1353–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Freemantle N, Satram-Hoang S, Tang ET, et al. Final results of the DAPS (Denosumab Adherence Preference Satisfaction) study: a 24-month, randomized, crossover comparison with alendronate in postmenopausal women. Osteoporos Int. 2012;23:317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eastell R, Christiansen C, Grauer A, et al. Effects of denosumab on bone turnover markers in postmenopausal osteoporosis. J Bone Miner Res. 2011;26:530–537. [DOI] [PubMed] [Google Scholar]