Abstract
Context:
Polycystic ovary syndrome (PCOS) is associated with reduced health-related quality of life (HRQOL) and increased prevalence of depressive and anxiety disorders. The impact of PCOS-specific treatments on these co-morbidities is unclear.
Objective:
To assess the impact of weight loss and decreasing hyperandrogenism on HRQOL and mood and anxiety disorders in women with PCOS.
Design/Setting/Participants:
A secondary analysis of a randomized controlled trial (OWL-PCOS) of preconception treatment conducted at two academic centers in women (age, 18–40 years; body mass index, 27–42 kg/m2) with PCOS defined by Rotterdam criteria.
Intervention:
Continuous oral contraceptive pill (OCP) or intensive lifestyle intervention or the combination (Combined) for 16 weeks.
Main Outcome Measure(s):
Changes in HRQOL assessed by PCOSQ and SF-36 and prevalence of depression and anxiety disorder assessed by PRIME-MD PHQ.
Results:
The lowest scores were noted on the general health domain of the SF-36 and the weight and infertility domains on the PCOSQ. All three interventions resulted in significant improvement in the general health score on the SF-36. Both the OCP and Combined groups showed improvements in all domains of the PCOSQ (P < .01) compared to baseline scores. The Combined group had significant improvements in the weight, body hair, and infertility domains compared to a single treatment group (P < .05). In a linear regression model, change in weight correlated with improvements in the weight domain (P < .001) and physical well-being (P < .02), change in T correlated with improvements in the hair domain (P < .001), and change in both weight and T correlated with the infertility (P < .001) and menstrual domains (P < .05).
Conclusions:
Both weight loss and OCP use result in significant improvements in several physical and mental domains related to quality of life, depressive symptoms, and anxiety disorders, and combined therapies offer further benefits in overweight/obese women with PCOS.
Weight loss and normalization of hyperandrogenism results in improvement in health related quality of life parameters in overweight/obese women with polycystic ovary syndrome.
Polycystic ovary syndrome (PCOS) is a common endocrine disorder occurring in 5–15% of reproductive-age women with significant health-related comorbidities and lifetime physical and financial burden (1). There is also increasing evidence to suggest untoward changes in psychosocial functioning in this population. Health-related quality of life (HRQOL) assessment is multidimensional and encompasses physical, emotional, and social aspects associated with a specific disease or its treatment. Several systematic reviews have confirmed that PCOS has an overall negative impact on HRQOL (2–4). In addition, cross-sectional studies report increased symptoms of depression and anxiety associated with PCOS (5–7). In a longitudinal study, we demonstrated that women with PCOS had a high conversion risk for depression over a 2-year period (8). New-onset anxiety and depressive disorders were also significantly increased over a 5-year period in a large cohort of Taiwanese women with PCOS compared to age-matched controls (9).
Despite the evidence for increased prevalence of emotional distress and mood disorders in PCOS, the exact etiology for this association is unclear. In the general population, obesity, particularly among women, has been independently associated with an increased risk of depression (10), with some studies suggesting a bidirectional link between obesity and depression (11). Obesity is also associated with decreased psychosocial functioning, both in general HRQOL as well as disease-specific areas of quality of life (12). Although weight concerns are common and are associated with low HRQOL scores in women with PCOS (13), a meta-analysis showed only a modest impact of weight on depression and anxiety scores (7). Diabetes and insulin resistance have also been implicated as risk factors for depression in the general population (14, 15). However, studies examining this association in PCOS show mixed results (16, 17). Similarly, the precise association between increased androgen levels, as seen in PCOS, and depressive and anxiety symptoms or HRQOL is unclear (3, 7, 16, 17).
There is a paucity of data on the impact of PCOS-specific treatments on HRQOL and depressive and anxiety symptoms. In the general population, weight loss is typically associated with improvements in quality of life as well as depressive symptoms (18, 19). Only a few studies have examined the impact of weight loss interventions on HRQOL scores in women with PCOS (20–22) and reported improvements in the weight domain. Some studies indicate that hirsutism and menstruation issues are significantly associated with lower quality of life with PCOS (3), suggesting that therapeutic interventions aimed at improving these symptoms may also improve overall well-being. Hormonal contraceptive use for 6 months improved HRQOL scores in lean women with PCOS (23), but use of antiandrogen medication did not significantly affect HRQOL or depressive scores (13). HRQOL is a required outcome measure for drug development and may be an important outcome for efficacy of treatment of PCOS, given the complexity of the phenotype and the wide range of treatments that affect some, but not all, of the abnormal parameters and symptoms of PCOS.
We recently conducted a three-arm randomized controlled trial (OWL-PCOS) to examine the impact of prepregnancy interventions that included lifestyle (LS) change to induce weight loss, hormonal contraception to suppress androgens, and the combination on live birth rates in overweight/obese women with PCOS (24). These treatments are also first-line therapies for the chronic treatment of women with PCOS not seeking pregnancy (25). In this secondary analysis, we examined changes in HRQOL, depression, and anxiety symptoms in the three intervention groups after 16 weeks of treatment (phase 1); and we hypothesized that both weight loss and normalization of androgens would be associated with improvements in HRQOL scores and that combining these treatments would provide additional benefit.
Patients and Methods
Trial design
The trial was a randomized, open-label, two-site study of three treatment groups: oral contraceptive pills (OCPs; 20 μg ethinyl estradiol/1 mg norethindrone acetate every day), a LS modification program designed to produce a 10% weight loss, and combined OCP and LS modification (Combined) (24). The protocol was approved by the Investigational Review Boards at the Penn State College of Medicine, Hershey, Pennsylvania, and the University of Pennsylvania, Philadelphia, Pennsylvania. We have published the main outcomes for the OWL PCOS Study (24), and the protocol and case report forms can be accessed at http://ctsi.psu.edu/owl-pcos/. The trial was registered at Clinicaltrials.gov (OWL PCOS: NCT00704912).
Patients
We randomized 149 overweight or obese (body mass index [BMI], 27–42 kg/m2) women between the ages of 18 and 40 years with PCOS as previously described in detail (24). We used modified Rotterdam criteria (26) to diagnose PCOS; all women had ovulatory dysfunction with either hyperandrogenism assessed by a Ferriman-Gallwey (FG) score >8 or an elevated T level or a polycystic ovary on transvaginal ultrasound. Participants were allowed to continue the use of antianxiety and antidepressant medications.
LS intervention
The LS program was multifocal and included recommendations for caloric restriction, increased physical activity, and counseling in behavioral modification strategies. Caloric restriction occurred through the use of meal replacement products, and participants were also instructed to consume two servings of fruit, three servings of vegetables, and two servings of skim milk per day. This diet was designed to create a 500-calorie deficit based on initial weight, consistent with the LS modification protocol of the Look AHEAD study and POWER-UP Trial (27, 28). We followed the Diabetes Prevention Program recommendations for increasing physical activity (principally brisk walking or similar aerobic activity) 5 days per week (29). Activity goals began at 10 minutes on each of those 5 days and gradually increased over 16 weeks to 30–35 minutes, for a total activity goal of 150 minutes per week (24). To promote additional weight loss, we used a weight loss medication in those participants who were medically appropriate for usage. We began sibutramine at a dose of 5 mg/d and titrated up to a maximum dose of 15 mg/d if tolerated. When sibutramine was removed from the market by the Food and Drug Administration secondary to health concerns, we substituted over-the-counter orlistat 60 mg with breakfast, lunch, and dinner. Compliance with interventions was tracked in all groups with diet records and activity logs.
Screening tools
Participants from the OWL-PCOS who completed questionnaires at baseline and at the end of phase 1 of the study (132 of 149) were included in this analysis. The questionnaires were scored as directed by each form's standard instructions.
PCOS quality of life survey
The PCOS Health-Related Quality of Life (PCOSQ) survey is a self-administered questionnaire validated for women with PCOS and includes 26 questions in five domains: emotional, body hair, infertility, weight, and menstrual problems (30). Each domain score is graded on a scale of 1 (poorest function) to 7 (optimal function) with a change of 0.5 approximating the minimal important difference, the smallest change in score that women feel is important in their daily lives. The mean score of all items in a domain provides the domain score, with lower scores indicating a lower negative impact. We previously reported the domain scores from this survey in our primary report of this trial (24).
Short form health survey
The 36-item Short Form (SF-36) Health Survey was used to assess HRQOL not specific for PCOS. Eight subscales were used to assess separate domains of health and related functioning. Items on the physical functioning, physical role functioning, pain, and general health subscales were used to calculate a physical health summary score. Items on the vitality, social functioning, emotional role functioning, and mental health subscales were used to calculate a mental health summary score. Higher scores indicate a more positive HRQOL.
PRIME-MD patient health questionnaire (31)
Participants were diagnosed with major depressive disorder if they had depressed mood and loss of interest or pleasure in daily activities for a period of at least 2 weeks. In addition, they experienced at least five of the following symptoms nearly every day for at least 2 consecutive weeks: changes in appetite, psychomotor retardation or agitation, feelings of worthlessness or guilt, thoughts of death or suicidal ideation, changes in sleep, decreased energy, and difficulty thinking or concentrating. Depressive episode with insufficient symptoms classified under other specified depressive disorder is similar to major depressive disorder in that depressive symptoms must be present for at least 2 consecutive weeks and interfere with daily activities. However, at least one symptom, but fewer than five, is required to meet criteria for this category.
Serum assays
Assays for total T and SHBG were performed at the Core Ligand Laboratory at the University of Virginia. These assays had interassay coefficients of variation <10%, including the T assay, which was optimized by increasing the sample volume to reproducibly measure T levels in the female range.
Statistical analysis
The Pearson correlation coefficient assessed the association between HRQOL parameters and PCOS patient characteristics (eg, weight, FG score) at baseline, as well as the association between the changes in HRQOL parameters and the changes in weight, T, and homeostasis model of assessment for insulin resistance (HOMA-IR). Fisher's Z transformation was used to derive 95% confidence intervals (CIs) for the Pearson correlation coefficient. Multiple linear regression assessed the strength of the relationship between the changes in HRQOL parameters and the changes in weight, T, and insulin sensitivity index (ISI). For all continuous outcomes, an ANOVA model estimated the change from baseline within each treatment group and compared the changes between the groups. Logistic regression via generalized estimating equations with a logit link, to account for the within-subject correlation due to two visits per subject, compared the rates of depression from baseline to the end of study. Effect sizes from logistic regression models are reported as odds ratio (OR) and 95% CI.
Results
Patient characteristics
Table 1 displays the baseline demographics of the three study groups. A total of 132 women between the ages of 21 and 40 years who completed both baseline and 16-week visits were included. Overall, the majority (88.37%) of the subjects had phenotype A, 9.3% had phenotype B, and 2.3% had phenotype C based on the Rotterdam criteria. The groups were similar with respect to age, race, education, and marital status. Table 2 shows clinical and biochemical parameters related to PCOS. There were no significant baseline differences in weight, BMI, or clinical and biochemical hyperandrogenism between those assigned to the different intervention groups.
Table 1.
Baseline Demographic Characteristics of Women With PCOS Randomized to the Three Treatment Groups
| LS | OCP | Combined | |
|---|---|---|---|
| N | 44 | 45 | 43 |
| Age, ya | 28.7 (3.4) | 29.9 (3.6) | 28.8 (4.3) |
| Hispanic | 4 (9.1) | 5 (11.1) | 5 (11.6) |
| Race | |||
| Caucasian | 31 (70.5) | 36 (80.0) | 27 (62.8) |
| Black/African American | 5 (11.4) | 7 (15.6) | 12 (27.9) |
| Other/multiracial | 8 (18.2) | 2 (4.4) | 4 (9.3) |
| Married | 31 (70.5) | 40 (88.9) | 31 (72.1) |
| High school graduate | 43 (97.7) | 42 (93.3) | 41 (95.3) |
| Self-reported history of depression and/or anxiety | 12 (27.3) | 10 (22.7) | 10 (23.3) |
| Depression only | 10 (22.7) | 8 (18.2) | 8 (18.6) |
| Anxiety only | 6 (13.6) | 3 (6.8) | 5 (11.6) |
| On medication for depression | 3 (6.8) | 1 (2.2) | 2 (4.7) |
| On medication for anxiety | 2 (4.5) | 1 (2.2) | 0 (0.0) |
Data are expressed as number (percentage), unless otherwise described.
Mean (SD).
Table 2.
Baseline Data on PCOS Characteristics in Study Participants
| LS | OCP | Combined | |
|---|---|---|---|
| N | 44 | 45 | 43 |
| Weight, kg | 97.0 (15.5) | 95.1 (14.6) | 94.6 (15.0) |
| BMI, kg/m2 | 35.4 (4.6) | 35.3 (4.2) | 35.3 (4.4) |
| No. of menstrual cycles per year | 3.5 (2.5) | 3.6 (2.7) | 4.5 (3.0) |
| FG hirsutism score | 19.3 (8.7) | 16.9 (7.9) | 17.2 (9.1) |
| Total T, ng/dL | 57.2 (29.2) | 56.4 (23.0) | 60.8 (29.8) |
| SHBG, nmol/L | 26.5 (21.5, 37.8) | 27.1 (20.2, 34.3) | 28.0 (18.6, 45.0) |
| Free androgen index | 6.4 (4.0, 8.8) | 6.2 (4.7, 12.2) | 6.3 (4.2, 9.6) |
| HOMA-IR | 3.6 (1.9) | 3.1 (1.3) | 3.2 (2.1) |
| Acne lesion count | 1.0 (0.0, 4.0) | 1.0 (0.0, 3.0) | 1.0 (0.0, 4.0) |
Values are expressed as mean (SD) or median (25th percentile, 75th percentile).
Baseline psychosocial functioning
Table 3 shows the baseline scores for HRQOL in all three groups. The lowest scores were noted on the general health domain of the SF-36 and the weight and infertility domains on the PCOSQ. When considering the PCOSQ scores for all participants at baseline, a high FG score correlated with a lower body hair domain score (r = −0.64; 95% CI, −0.73, −0.53; P < .001), and a higher BMI correlated with a lower weight domain score (r = −0.21; 95% CI, −0.37, −0.04; P = .02).
Table 3.
Baseline Scores for Assessment of HRQOL Parameters, Mood, and Anxiety Disorders in Women With PCOS
| LS | OCP | Combined | |
|---|---|---|---|
| N | 44 | 45 | 43 |
| PCOSQ | |||
| Emotion domain | 4.5 (1.2) | 4.8 (1.3) | 4.5 (1.2) |
| Body hair domain | 4.0 (1.9) | 4.3 (1.8) | 4.1 (1.6) |
| Weight domain | 2.9 (1.5) | 3.0 (1.5) | 2.8 (1.7) |
| Infertility domain | 3.0 (1.4) | 3.3 (1.4) | 2.6 (1.5) |
| Menstrual problems domain | 3.9 (1.1) | 4.1 (1.2) | 4.3 (1.0) |
| Overall physical well-being | 4.1 (1.3) | 4.0 (1.4) | 3.8 (1.6) |
| Overall emotional well-being | 4.3 (1.2) | 4.4 (1.2) | 4.3 (1.5) |
| Overall general well-being | 4.5 (1.2) | 4.6 (1.0) | 4.5 (1.2) |
| SF-36 | |||
| Physical functioning | 51.6 (7.0) | 51.8 (6.8) | 54.0 (4.0) |
| Role physical | 54.3 (4.9) | 54.5 (4.3) | 53.7 (6.8) |
| Bodily pain | 56.0 (7.9) | 55.6 (7.4) | 57.7 (5.3) |
| General health | 46.5 (8.2) | 45.9 (8.1) | 46.1 (7.6) |
| Vitality | 48.1 (9.9) | 50.4 (9.4) | 50.8 (8.6) |
| Social functioning | 49.7 (8.3) | 50.5 (8.9) | 52.5 (7.4) |
| Role emotional | 50.8 (10.3) | 49.0 (10.6) | 52.6 (6.5) |
| Mental health | 49.3 (8.1) | 49.7 (9.2) | 50.5 (8.1) |
| Physical component summary | 53.2 (6.5) | 53.4 (5.8) | 53.8 (5.1) |
| Mental component summary | 48.1 (8.6) | 48.2 (11.1) | 50.2 (7.2) |
| Depression (PRIME-MD), n (%) | |||
| Major depressive syndrome | 4 (9.1) | 4 (8.9) | 1 (2.3) |
| Other depressive syndrome | 3 (6.8) | 1 (2.2) | 2 (4.7) |
| Anxiety (PRIME-MD), n (%) | |||
| Panic disorder | 2 (4.5) | 0 (0.0) | 0 (0.0) |
| Other anxiety disorder | 4 (9.1) | 2 (4.4) | 1 (2.3) |
Data are expressed as mean (SD) unless specified otherwise.
Changes in HRQOL scores with treatment
On the SF-36 survey, all three groups showed significant improvement in their general health score after 16 weeks of treatment (Table 4). In addition, the OCP group showed significant improvement in their vitality, emotional role, bodily pain, and mental component summary scores (P < .05), and the LS group showed significant improvement in their vitality score (P < .01) compared to baseline. There was significant improvement in emotional role in the OCP group compared to the Combined group (P < .05) only.
Table 4.
Mean Difference (95% CI) in SF-36 Scores After 16-Week Intervention Compared to Baseline
| LS | OCP | Combined | |
|---|---|---|---|
| Physical function | 1.70 (−0.31, 3.71) | 1.25 (−0.76, 3.26) | 0.44 (−1.59, 2.47) |
| Role physical | −0.16 (−2.15, 1.83) | 0.48 (−1.51, 2.48) | −0.38 (−2.40, 1.63) |
| Bodily pain | −0.37 (−2.57, 1.84) | 2.24 (0.06, 4.41)a | −0.75 (−2.97, 1.48) |
| General health | 2.11 (0.36, 3.87)a | 3.14 (1.38, 4.90)b | 2.34 (0.56, 4.12)a |
| Vitality | 5.20 (2.86, 7.54)b | 2.42 (0.10, 4.73)a | 1.89 (−0.48, 4.26) |
| Social functioning | 0.25 (−2.60, 3.09) | 1.57 (−1.24, 4.38) | 0.38 (−2.50, 3.26) |
| Role emotional | 1.32 (−1.32, 3.95) | 3.59 (0.96, 6.23)b | −0.98 (−3.65, 1.69) |
| Mental health | 0.84 (−1.33, 3.02) | 0.98 (−1.18, 3.13) | 0.46 (−1.74, 2.67) |
| Physical component summary | 0.79 (−0.89, 2.46) | 1.20 (−0.48, 2.87) | 0.54 (−1.15, 2.23) |
| Mental component summary | 1.90 (−0.49, 4.29) | 2.40 (0.004, 4.79)a | 0.33 (−2.09, 2.75) |
P < .05.
P < .01.
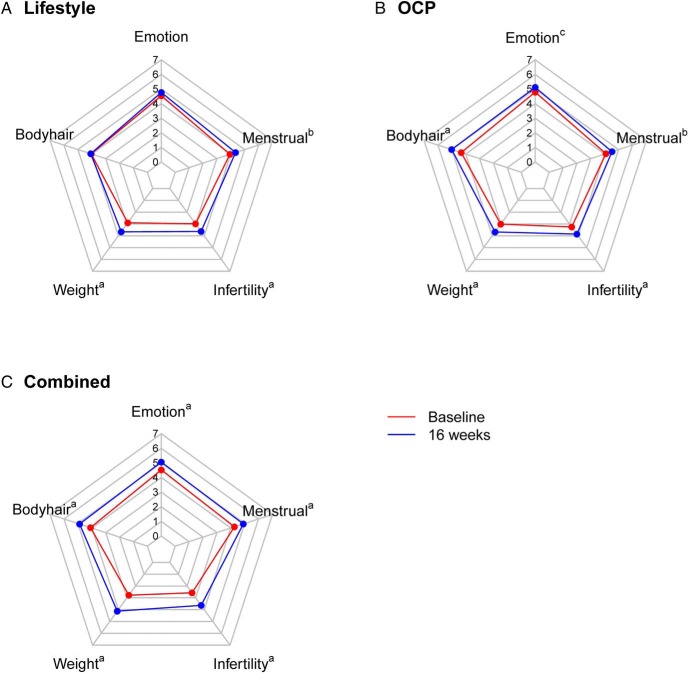
Subjects showed significant improvements in most domains of the PCOSQ after 16 weeks of treatment in all three groups (Figure 1). The OCP group and the Combined group showed significant improvements in all five domains, whereas the LS group did not show improvements in the hair and emotional domains compared to baseline scores. Both the OCP and Combined groups showed significant improvement in the body hair domain compared to the LS group (P < .001). There was also significant improvement in the weight domain in the Combined group compared to the LS and OCP groups (P < .05). The Combined group also had significant improvement in the infertility domain compared to OCP alone (P < .05). As a sensitivity analysis, we adjusted for race, education, and marital status, and the differences between the groups for both the SF-36 and PCOSQ remained consistent with the unadjusted results (data not shown).
Figure 1.
Polar chart for PCOSQ domain scores at baseline (red) and after 16-week intervention (blue) in the LS (A), OCP (B) and Combined groups (C). The scores on the PCOSQ range from 0 (poorest function) to 7 (optimum function). Changes from baseline are indicated as follows: a P < .001, b P < .01, c P < .05.
Impact of change in weight and androgen levels on HRQOL
The percentage weight loss after 16 weeks of intervention was significantly higher in the LS group (6.4 ± 4.3 kg) and the Combined group (6.4 ± 4.2 kg) compared to the OCP group (1.2 ± 3.0 kg) (P < .001). Weight loss across all three groups correlated with a significant improvement in the weight domain score (r = −0.40; 95% CI, −0.53, −0.25; P < .001) and infertility domain score (r = −0.29; 95% CI, −0.44, −0.12; P < .001). Also, there was a significant decrease in mean T levels in the OCP (30.5 ± 20.9 ng/dL) and Combined (32.7 ± 22.8 ng/dL) groups compared to the LS alone group (3.1 ± 20.0 ng/dL; P < .001). The overall decrease in T levels correlated with improvement in body hair domain scores (r = −0.29; 95% CI, −0.44, −0.12; P = .001). The OCP and Combined groups had a significantly greater improvement in body hair scores (P < .01) as compared to the LS group. The change in FG scores also correlated with a change in body hair domain scores (r = −0.24; 95% CI, −0.40, −0.08; P < .01). There was no significant correlation between a change in HOMA-IR, ISI, glucose, and insulin area under the curve and a change in PCOSQ domains. In a linear regression model including weight, T, and ISI, a change in weight had a significant correlation with the weight domain (P < .001), change in T had a significant correlation with the hair domain (P < .001), and change in both weight and T correlated with the infertility domain (P < .001) and the menstrual domain (P < .05). Furthermore, a change in weight correlated with improvement in physical well-being on the PCOSQ (P < .02).
Depressive and anxiety symptoms
At baseline, overall 24.4% of subjects reported a history of depression or anxiety on the medical questionnaire, and 6.8% were taking medications to treat one or both conditions (Table 1). There were no significant differences between the treatment groups for anxiety, depression, or medication use at baseline. Over the study period, the prevalence of depression, as assessed by positive screens on the Prime-MD and/or use of medications, decreased from 13.3 to 4.4% in the OCP group (OR, 0.30; 95% CI, 0.09, 0.99; P < .05) and from 22.7 to 15.9% in the LS group (OR, 0.64; 95% CI, 0.34, 1.22; P = .17). There was no change in the prevalence of depression in the Combined group (11.6 vs 11.9%). The prevalence of anxiety, as assessed by positive screens on the Prime-MD and/or use of medications, decreased from 15.9 to 4.7% in the LS group (OR, 0.30; 95% CI, 0.10, 0.85; P = .02) and from 6.7 to 2.2% in the OCP group (OR, 0.32; 95% CI, 0.06, 1.64; P = .17). In the Combined group, only one subject had anxiety at baseline, and there were no cases of anxiety at follow-up.
Discussion
Our study is the largest to assess the impact of decreasing weight and serum androgens in overweight/obese women with PCOS on psychosocial status and mood after three randomized interventions, including common treatments such as OPCs and LS modification. We report that low-dose hormonal contraceptives and LS interventions are both associated with significant improvements in HRQOL measures, using a validated questionnaire specifically developed to capture the range of health-related problems identified in PCOS (32). In fact, we noted an improvement in all domains of the PCOSQ after 16 weeks of therapy in both the OCP and Combined groups. Furthermore, the combined treatment showed the most significant improvements in the body hair, weight, infertility, and overall physical well-being domains compared to a single intervention. Changes in serum T levels, FG scores, and body weight had the most impact on improvements in their respective PCOSQ domains. On the other hand, changes in insulin sensitivity did not correlate with changes in HRQOL scores. Our results indicate that in addition to recognized benefits such as improved menstrual cyclicity and decreased hirsutism, low-dose hormonal contraceptive treatment combined with LS changes also leads to improved psychosocial functioning in overweight/obese women PCOS.
In a recent meta-analysis, hirsutism and menstruation were the most affected PCOSQ domains, suggesting that targeting these complaints might improve HRQOL (4). Hirsutism has been associated with decreased HRQOL scores in women with and without PCOS (4, 33, 34). In our study, high baseline FG scores correlated with low body hair domain scores on the PCOSQ as reported previously (35). Similarly, FG scores have been shown to correlate with emotional distress (36) and the sum physical component of SF-36 domains (37). We hypothesized that a decrease in androgens with OCP use will result in improvement in HRQOL scores. In our study, improvement in the body hair domain scores after 16 weeks of treatment with a low-dose (20 μg ethinyl estradiol) OCP significantly correlated with both a decrease in serum T levels and improvement in FG scores. In lean women with PCOS, the use of OCPs was also associated with improvement in hirsutism and emotion domain and a decrease in total T (23). Improvement in psychological domain scores after laser treatment for hirsutism without change in androgens has been described in women with PCOS (38).
Hormonal contraceptives are the first-line treatment for regulating menses in women with PCOS. In a survey of over 1000 women in the general population, the use of OCPs was associated with significant improvement in the mental component but not in the physical component of HRQOL (39). In our study, the use of OCPs alone in overweight/obese women for 4 months was associated with improvements in all domains of PCOSQ scores. Moreover, the scores were further improved when LS interventions were combined with OCP treatment. Although the use of continuous OCPs resulted in complaints of breakthrough bleeding (8.2–12%), it did not adversely impact the menstrual domain scores. In the adolescent population, one study showed improvement in all domains of the PCOSQ using OCPs combined with a LS management program for 24 weeks (40). These studies indicate an overall beneficial effect of the use of OCPs combined with LS changes on HRQOL assessments in the obese PCOS population.
On the PCOSQ survey, the lowest score among the five domains is typically in the weight domain (2, 13). We confirmed these findings and also found an inverse correlation between the weight domain scores and BMI as reported by other groups (37, 35). In our study, LS changes resulted in independent improvements in weight and menstrual and physical well-being scores. The change in weight domain scores significantly correlated with weight loss in both the LS and Combined groups. Moran et al (21) also reported significant improvement in weight and menstrual PCOSQ scores in women participating in an 8-week meal replacement program resulting in 6% weight loss. In the maintenance phase of the same study (6 months), no further changes in the PCOSQ domains were noted, indicating that the initial improvement in PCOSQ scores corresponded to the active weight loss phase. In a randomized controlled trial comparing diet only, diet and aerobic exercise, and diet and aerobic/resistance exercise, all groups showed significant improvement in emotion, weight, and menstrual domain scores after 20 weeks of intervention (20). Another study comparing weight loss associated with two dietary interventions reported significantly higher scores in all domains of PCOSQ after 12 months of a low glycemic index diet compared to a conventional healthy diet (22). Our study supports the use of hormonal contraception with LS changes because we noted an improvement in all domains of PCOS-specific quality of life parameters.
In a recent review on hormonal contraception and depression, it was concluded that there was likely no worsening of mood symptoms with the use of combination hormonal contraception (41). Although OCP use is not contraindicated in women with depression or anxiety, few studies have examined their impact on mood and anxiety disorders in women with PCOS. We have previously reported that women with PCOS are at a 4-fold increased risk for depressive and anxiety symptoms (5, 6). This increased risk is independent of obesity (5, 7); however, higher depression scores were observed with higher mean BMI (3). In our current study, we report a significant decrease in the prevalence of depressive symptoms in the OCP group and anxiety symptoms in the LS group after 16 weeks of treatment. Of note, there was improvement in both depressive and anxiety symptoms with both interventions, although we did not have the power to detect significant differences. These are important findings in a population at increased risk of both mood and anxiety disorders (5, 6). However, given the small numbers of subjects with depressive and anxiety symptoms, we were unable to determine the interaction with changes in serum androgens, FG score, or weight. One randomized study of laser treatment for hirsutism showed an improvement in both depression and anxiety scores after 6 months of intervention (38), and another weight loss study showed improvement in depression scores (20). Collectively, these studies suggest that PCOS-specific treatments may potentially improve depressive symptoms.
We acknowledge certain limitations to our study. We did not have a “no-treatment” group, so it is difficult to assess whether improvement in scores was due to study participation and social support rather than specific interventions. Because there are effective treatments for the symptoms of PCOS, a no-treatment arm may not provide equipoise for patient participants. Our results may not be generalizable because all our participants were overweight or obese, used a low-dose continuous OCPs, and were seeking pregnancy after phase 1 of this study. The latter may explain why we observed infertility domain scores to be one of the lowest at baseline. Although we saw significant improvements with short-duration interventions, we are unable to confirm whether these improvements will sustain over longer intervention durations. Our study has several strengths including a randomized trial of three treatment interventions in well-characterized women with PCOS and the use of tools validated in both the general population and women with PCOS for assessment of HRQOL.
Our study shows that common treatments for PCOS, including hormonal contraception and weight loss interventions, are both associated with significant improvements in HRQOL. Consistent with findings in the general obesity literature, in our study, overweight and obese women with PCOS treated with an intensive weight loss intervention reported significant improvements in most domains of disease-specific quality of life as well as general HRQOL parameters. Perhaps more novel and noteworthy, we noted improvements in both physical and some mental domains of the PCOSQ and significant improvement in depressive symptoms with the use of a low-dose OCP, a first-line treatment for PCOS. Finally and most importantly, the combination of the two treatments, aimed to decrease hyperandrogenism and body weight, resulted in significant improvements in most domains of PCOSQ, suggesting further benefits of the combined therapy.
Acknowledgments
This project was supported by the Eunice Kennedy Shriver National Institutes of Child Health and Human Development (NICHD), National Center for Research Resources, and the National Center for Advancing Translational Sciences at the National Institutes of Health, through Grants R01 HD056510 (RSL), UL1 TR000127 (Penn State Clinical and Translational Institute) and U54 HD29834 (UVA Core Ligand Assay Core of the Specialized Cooperative Centers Program in Reproduction of the NICHD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH).
Clinical Trial Registration No. OWL PCOS: NCT00704912.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- body mass index
- CI
- confidence interval
- FG
- Ferriman-Gallwey
- HOMA-IR
- homeostasis model of assessment for insulin resistance
- HRQOL
- health-related quality of life
- ISI
- insulin sensitivity index
- LS
- lifestyle
- OCP
- oral contraceptive pill
- OR
- odds ratio
- PCOS
- polycystic ovary syndrome.
References
- 1. Azziz R, Marin C, Hoq L, Badamgarav E, Song P. Health care-related economic burden of the polycystic ovary syndrome during the reproductive life span. J Clin Endocrinol Metab. 2005;90(8):4650–4658. [DOI] [PubMed] [Google Scholar]
- 2. Jones GL, Hall JM, Balen AH, Ledger WL. Health-related quality of life measurement in women with polycystic ovary syndrome: a systematic review. Hum Reprod Update. 2008;14(1):15–25. [DOI] [PubMed] [Google Scholar]
- 3. Veltman-Verhulst SM, Boivin J, Eijkemans MJ, Fauser BJ. Emotional distress is a common risk in women with polycystic ovary syndrome: a systematic review and meta-analysis of 28 studies. Hum Reprod Update. 2012;18(6):638–651. [DOI] [PubMed] [Google Scholar]
- 4. Bazarganipour F, Taghavi SA, Montazeri A, Ahmadi F, Chaman R, Khosravi A. The impact of polycystic ovary syndrome on the health-related quality of life: a systematic review and meta-analysis. Iran J Reprod Med. 2015;13(2):61–70. [PMC free article] [PubMed] [Google Scholar]
- 5. Dokras A, Clifton S, Futterweit W, Wild R. Increased risk for abnormal depression scores in women with polycystic ovary syndrome: a systematic review and meta-analysis. Obstet Gynecol. 2011;117(1):145–152. [DOI] [PubMed] [Google Scholar]
- 6. Dokras A, Clifton S, Futterweit W, Wild R. Increased prevalence of anxiety symptoms in women with polycystic ovary syndrome: systematic review and meta-analysis. Fertil Steril. 2012;97(1):225–230.e2. [DOI] [PubMed] [Google Scholar]
- 7. Barry JA, Kuczmierczyk AR, Hardiman PJ. Anxiety and depression in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2011;26(9):2442–2451. [DOI] [PubMed] [Google Scholar]
- 8. Kerchner A, Lester W, Stuart SP, Dokras A. Risk of depression and other mental health disorders in women with polycystic ovary syndrome: a longitudinal study. Fertil Steril. 2009;91(1):207–212. [DOI] [PubMed] [Google Scholar]
- 9. Hung JH, Hu LY, Tsai SJ, et al. Risk of psychiatric disorders following polycystic ovary syndrome: a nationwide population-based cohort study. PLoS One. 2014;9(5):e970419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stunkard AJ, Faith MS, Allison KC. Depression and obesity. Biol Psychiatry. 2003;54(3):330–337. [DOI] [PubMed] [Google Scholar]
- 11. Luppino FS, de Wit LM, Bouvy PF, et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry. 2010;67(3):220–229. [DOI] [PubMed] [Google Scholar]
- 12. Sarwer DB, Lavery M, Spitzer JC. A review of the relationships between extreme obesity, quality of life, and sexual function. Obes Surg. 2012;22(4):668–676. [DOI] [PubMed] [Google Scholar]
- 13. Barnard L, Ferriday D, Guenther N, Strauss B, Balen AH, Dye L. Quality of life and psychological well being in polycystic ovary syndrome. Hum Reprod. 2007;22(8):2279–2286. [DOI] [PubMed] [Google Scholar]
- 14. Wiltink J, Michal M, Wild PS, et al. Associations between depression and diabetes in the community: do symptom dimensions matter? Results from the Gutenberg Health Study. PLoS One. 2014;9(8):e105499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kan C, Silva N, Golden SH, et al. A systematic review and meta-analysis of the association between depression and insulin resistance. Diabetes Care. 2013;36(2):480–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jedel E, Gustafson D, Waern M, et al. Sex steroids, insulin sensitivity and sympathetic nerve activity in relation to affective symptoms in women with polycystic ovary syndrome. Psychoneuroendocrinology. 2011;36(10):1470–1479. [DOI] [PubMed] [Google Scholar]
- 17. Greenwood EA, Pasch LA, Shinkai K, Cedars MI, Huddleston HG. Putative role for insulin resistance in depression risk in polycystic ovary syndrome. Fertil Steril. 2015;104(3):707–714.e1. [DOI] [PubMed] [Google Scholar]
- 18. Sarwer DB, Moore RH, Diewald LK, et al. The impact of a primary care-based weight loss intervention on the quality of life. Int J Obes (Lond). 2013;37(suppl 1):S25–S30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Strain GW, Kolotkin RL, Dakin GF, et al. The effects of weight loss after bariatric surgery on health-related quality of life and depression. Nutr Diabetes. 2014;4:e132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thomson RL, Buckley JD, Lim SS, et al. Lifestyle management improves quality of life and depression in overweight and obese women with polycystic ovary syndrome. Fertil Steril. 2010;94(5):1812–1816. [DOI] [PubMed] [Google Scholar]
- 21. Moran LJ, Noakes M, Clifton PM, Wittert GA, Williams G, Norman RJ. Short-term meal replacements followed by dietary macronutrient restriction enhance weight loss in polycystic ovary syndrome. Am J Clin Nutr. 2006;84(1):77–87. [DOI] [PubMed] [Google Scholar]
- 22. Marsh KA, Steinbeck KS, Atkinson FS, Petocz P, Brand-Miller JC. Effect of a low glycemic index compared with a conventional healthy diet on polycystic ovary syndrome. Am J Clin Nutr. 2010;92(1):83–92. [DOI] [PubMed] [Google Scholar]
- 23. Cinar N, Harmanci A, Demir B, Yildiz BO. Effect of an oral contraceptive on emotional distress, anxiety and depression of women with polycystic ovary syndrome: a prospective study. Hum Reprod. 2012;27(6):1840–1845. [DOI] [PubMed] [Google Scholar]
- 24. Legro RS, Dodson WC, Kris-Etherton PM, et al. Randomized controlled trial of preconception interventions in infertile women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2015;100(11):4048–4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Legro RS, Arslanian SA, Ehrmann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2013;98(12):4565–4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. [DOI] [PubMed] [Google Scholar]
- 27. Look AHEAD Research Group, Wadden TA, West DS, Delahanty L, et al. The Look AHEAD study: A description of the lifestyle intervention and the evidence supporting it. Obesity. 2006;14:737–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wadden TA, Volger S, Sarwer DB, et al. A two-year randomized trial of obesity treatment in primary care practice. N Engl J Med. 2011;365(21):1969–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Coffey S, Bano G, Mason HD. Health-related quality of life in women with polycystic ovary syndrome: a comparison with the general population using the Polycystic Ovary Syndrome Questionnaire (PCOSQ) and the Short Form-36 (SF-36). Gynecol Endocrinol. 2006;22(2):80–86. [DOI] [PubMed] [Google Scholar]
- 31. Spitzer RL, Williams JB, Kroenke K, et al. Utility of a new procedure for diagnosing mental disorders in primary care. The PRIME-MD 1000 study. JAMA. 1994;272(22):1749–1756. [PubMed] [Google Scholar]
- 32. Cronin L, Guyatt G, Griffith L, et al. Development of a health-related quality-of-life questionnaire (PCOSQ) for women with polycystic ovary syndrome (PCOS). J Clin Endocrinol Metab. 1998;83(6):1976–1987. [DOI] [PubMed] [Google Scholar]
- 33. Drosdzol A, Skrzypulec V, Plinta R. Quality of life, mental health and self-esteem in hirsute adolescent females. J Psychosom Obstet Gynaecol. 2010;31(3):168–175. [DOI] [PubMed] [Google Scholar]
- 34. Ekbäck MP, Lindberg M, Benzein E, Årestedt K. Health-related quality of life, depression and anxiety correlate with the degree of hirsutism. Dermatology. 2013;227(3):278–284. [DOI] [PubMed] [Google Scholar]
- 35. McCook JG, Reame NE, Thatcher SS. Health-related quality of life issues in women with polycystic ovary syndrome. J Obstet Gynecol Neonatal Nurs. 2005;34(1):12–20. [DOI] [PubMed] [Google Scholar]
- 36. Kumarapeli V, Seneviratne Rde A, Wijeyaratne C. Health-related quality of life and psychological distress in polycystic ovary syndrome: a hidden facet in South Asian women. BJOG. 2011;118(3):319–328. [DOI] [PubMed] [Google Scholar]
- 37. Hahn S, Janssen OE, Tan S, et al. Clinical and psychological correlates of quality-of-life in polycystic ovary syndrome. Eur J Endocrinol. 2005;153(6):853–860. [DOI] [PubMed] [Google Scholar]
- 38. Clayton WJ, Lipton M, Elford J, Rustin M, Sherr L. A randomized controlled trial of laser treatment among hirsute women with polycystic ovary syndrome. Br J Dermatol. 2005;152(5):986–992. [DOI] [PubMed] [Google Scholar]
- 39. Borenstein J, Yu HT, Wade S, Chiou CF, Rapkin A. Effect of an oral contraceptive containing ethinyl estradiol and drospirenone on premenstrual symptomatology and health-related quality of life. J Reprod Med. 2003;48(2):79–85. [PubMed] [Google Scholar]
- 40. Harris-Glocker M, Davidson K, Kochman L, Guzick D, Hoeger K. Improvement in quality-of-life questionnaire measures in obese adolescent females with polycystic ovary syndrome treated with lifestyle changes and oral contraceptives, with or without metformin. Fertil Steril. 2010;93(3):1016–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Böttcher B, Radenbach K, Wildt L, Hinney B. Hormonal contraception and depression: a survey of the present state of knowledge. Arch Gynecol Obstet. 2012;286(1):231–236. [DOI] [PubMed] [Google Scholar]