Abstract
Context:
Controversy persists over: 1) how best to restore low serum 25-hydroxyvitamin D (25D) levels (vitamin D2 [D2] vs vitamin D3 [D3]); 2) how best to define vitamin D status (total [protein-bound + free] vs free 25D); and 3) how best to assess the bioactivity of free 25D.
Objective:
To assess: 1) the effects of D2 vs D3 on serum total and free 25D; and 2) whether change in intact PTH (iPTH) is more strongly associated with change in total vs free 25D.
Design:
Participants previously enrolled in a D2 vs D3 trial were matched for age, body mass index, and race/ethnicity. Participants received 50 000 IU of D2 or D3 twice weekly for 5 weeks, followed by a 5-week equilibration period. Biochemical assessment was performed at baseline and at 10 weeks.
Setting and Participants:
Thirty-eight adults (19 D2 and 19 D3) ≥18 years of age with baseline 25D levels <30 ng/mL were recruited from an academic ambulatory osteoporosis clinic.
Outcome Measures:
Serum measures were total 25D, free 25D (directly measured), 1,25-dihydroxyvitamin D, calcium, and iPTH. Urine measure was fasting calcium:creatinine ratio.
Results:
Baseline total (22.2 ± 3.3 vs 23.3 ± 7.2 ng/mL; P = .5) and free (5.4 ± 0.8 vs 5.3 ± 1.7 pg/mL; P = .8) 25D levels were similar between D2 and D3 groups. Increases in total (+27.6 vs +12.2 ng/mL; P = .001) and free (+3.6 vs +6.2 pg/mL; P = .02) 25D levels were greater with D3 vs D2. Percentage change in iPTH was significantly associated with change in free (but not total) 25D, without and with adjustment for supplementation regimen, change in 1,25-dihydroxyvitamin D, and change in calcium.
Conclusions:
D3 increased total and free 25D levels to a greater extent than D2. Free 25D may be superior to total 25D as a marker of vitamin D bioactivity.
500,000 IU of vitamin D2 or D3 was given over 10 weeks. Total and free 25-hydroxyvitamin D (25D) increased more robustly with D3. Change in iPTH was associated with change in free, but not total, 25D.
Low serum 25-hydroxyvitamin D (25D) is associated with adverse skeletal health outcomes. In particular, low 25D leads to decreased intestinal calcium absorption, increased PTH secretion, and increased bone resorption (1, 2). When serum 25D levels are low (<30 ng/mL), clinicians frequently recommend supplementation with either ergocalciferol (D2) or cholecalciferol (D3). Based on early studies demonstrating that both D2 and D3 reverse rickets in infants (3), current clinical practice guidelines consider D2 and D3 to be therapeutically equivalent (2). Over the last two decades, however, multiple studies have assessed the equivalence of equimolar dosing regimens of D2 vs D3 by comparing each supplement's ability to raise and maintain serum total 25D levels (4–11). Although most completed studies have found orally administered D3 to raise total serum 25D more robustly than D2 (4, 6–11), others have found them to be equivalent (5).
In serum, 25D is primarily bound to serum vitamin D binding protein (DBP) and albumin, with less than 1% of total 25D circulating in its free (unbound) form (12). In the renal epithelial cell, filtered DBP-bound 25D is internalized via a megalin-mediated mechanism and subsequently converted to the active form of vitamin D (1,25-dihydroxyvitamin D [1,25D]) via the 1-α-hydroxylase (CYP27B1) (13). On the other hand, many extrarenal tissues express CYP27B1 and the vitamin D receptor; thus, intracrine regulation could occur through intracellular conversion of 25D to 1,25D (14, 15). In these tissues, it has been postulated that only free 25D (unbound to serum proteins) is available for uptake and subsequent intracrine conversion to 1,25D. Under these conditions, the free fraction of 25D represents a more superior marker of vitamin D substrate bioavailability than total 25D.
Because the human serum DBP is reported to bind 25-hydroxylated D2 (25D2) metabolites less avidly than 25-hydroxylated D3 (25D3) metabolites (16–18), we theorized that relatively more of the 25D2 metabolite would be free and bioavailable to target cells. Indeed, among vitamin D-deficient mice that are placed on diets containing equal amounts of D2 or D3, those fed D3 had lower free 25D levels vs those provided D2 (19). Only one prior study comparing the equivalence of D2 vs D3 has reported their relative effects in terms of both total and “free” 25D levels in humans. Instead of directly measuring free 25D concentrations, this study calculated free 25D levels from serum levels of total 25D, DBP, and albumin using previously published equations (11, 20). Importantly, recent studies demonstrated that calculated free 25D levels could overestimate directly measured free concentrations (21). Owing to discrepancies in the methodology for measuring DBP concentrations in human serum and the varying avidities with which different DBP isoforms bind 25D (21–23), our study was designed to address the following questions: 1) what are the comparative effects of D2 vs D3 replacement on total and directly measured serum 25D levels; and 2) does free 25D represent a superior in vivo marker of vitamin D-mediated bioactivity above and beyond total 25D?
Subjects and Methods
Subjects
We evaluated 38 paired serum samples (before and after vitamin D supplementation) from a parent trial (24) in which patients were randomized in a 1:2 ratio to receive a total dose of 500 000 IU D2 or 500 000 IU D3 (ClinicalTrials.gov Identifier: NCT01848236). Patients from the original parent trial were recruited from an ambulatory osteoporosis clinic at the University of California, Los Angeles (UCLA). Inclusion criteria were age ≥18 years and baseline 25D level <30 ng/mL. Exclusion criteria included evidence of hypercalcemia, hypercalciuria, nephrolithiasis, primary hyperparathyroidism, intestinal malabsorption, or dysregulated vitamin D metabolism (from underlying comorbidity or medication). Sera from 38 control participants (screening 25D level ≥30 ng/mL), matched for age, body mass index (BMI) and race/ethnicity, were available. All participants gave informed consent, and the study was approved by the UCLA Institutional Review Board.
Intervention
Participants were those with a serum total 25D concentration <30 ng/mL at baseline (24–26). Participants received 50 000 IU of either D2 or D3 twice weekly for 5 weeks, followed by a 5-week period during which we allowed serum 25D concentrations to equilibrate (24–26). The total vitamin D dose of 500 000 IU was chosen because it has been reported to successfully raise serum total 25D levels to >30 ng/mL and reduce fracture risk (26, 27). D2 and D3 were obtained from Swanson Health Products and Schwartz Pharma, respectively, at a concentration of 1000 IU (in vegetable oil) per drop. These were compounded into capsules for distribution to study participants by the UCLA Investigational Pharmacy. Equivalent D2 and D3 content was confirmed by liquid chromatography-mass spectrometry (LC/MS/MS) (Heartland Assays). Adherence to supplementation was assessed by patient interview at week 10, and only those who reported ≥80% adherence (on a yes/no basis) were included in this analysis.
Measurements
Biochemical assessment was performed at baseline before initiation of D2 or D3 supplementation and repeated 10 weeks after initiation of the supplementation regimen. Serum measurements included total 25D, free 25D, total 1,25D, calcium, and intact PTH (iPTH). Urinary measurement included a calcium:creatinine excretion ratio on an early morning fasting sample. Total 25D was measured by chemiluminescence immunoassay (Diasorin Liaison) in the UCLA Department of Pathology and Laboratory Medicine; this laboratory participates in the College of American Pathologists Accuracy-Based Vitamin D Survey. The assay is reported by the manufacturer to demonstrate equimolar cross-reactivity with 25D2 (104%) and 25D3 (100%) and minimal cross-reactivity with 3-epi-25D3 (<1.0%). The equimolar cross-reactivity of 25D2 and 25D3 with the detecting antibody has been separately demonstrated (28). This assay has also been shown to have acceptable performance for 25D2 cross-reactivity when compared to HPLC and LC/MS/MS (29, 30). Intra- and interassay coefficients of variation (CVs) were 2.1–2.2 and 4.0–4.5%, respectively. In a subset of participants in whom additional serum was available, total 25D was also measured by LC/MS/MS (Esoterix, Inc.). Interassay CV was 6%. Free 25D was measured using an antibody-based assay from Future Diagnostics as previously described (31, 32). In this assay, an antibody that reacts with free 25D (25D2 and 25D3) is coated on wells of a microtiter plate. Serum is added to a well, and free 25D is captured by the anti-25D antibody. After washing, a fixed amount of biotinylated 25D3 is added. After washing to remove unbound biotinylated 25D3, a streptavidin peroxidase conjugate is added, followed by a tetramethyl benzidine chromogenic substrate. The reaction is then stopped, and the absorbance (A450 nm) of the sample is measured using a plate spectrophotometer. The free 25D (25D2 and 25D3) concentration is inversely proportional to the absorbance in the sample well. The assay limit of detection is 1.9 pg/mL. In the range of concentrations measured, the CV was ≤7%. Total 1,25D was measured by chemiluminescence immunoassay (Diasorin Liaison). Intra- and interassay CVs were 2.4–3.9 and 4.5–7.8%, respectively. iPTH was measured by electrochemiluminescence immunoassay (Roche Cobas; Roche Diagnostics). Intra- and interassay CVs were 0.8–1.5 and 1.5–1.8%, respectively.
Statistical analyses
Descriptive statistics of relevant continuous clinical covariates and biochemical measurements were generated and assessed for normality. Differences in baseline characteristics between D2 and D3 groups were assessed by the independent samples t test (continuous variables) or χ2 test (categorical variables). Changes in biochemical measurements within D2 and D3 treatment groups were examined by the paired t test. Differences in biochemical measurements at week 10 between D2 and D3 groups were assessed by the independent samples t test. Associations between change in iPTH (outcome) and change in total or free 25D (primary predictor) were examined using: 1) Lowess plots (for visual inspection of unadjusted relationship); 2) simple linear regression (unadjusted); and 3) multiple linear regression (adjusted). In adjusted models, we included supplementation regimen, change in 1,25D, and change in calcium as covariates. These covariates were chosen because they have been previously reported to influence PTH secretion.
Results
Patient characteristics
Each supplementation group included 19 individuals matched for age, BMI, and race/ethnicity. Age, BMI, and race/ethnicity were not significantly different between D2 and D3 groups (Table 1). Baseline total 25D (22.2 ± 3.3 vs 23.3 ± 7.2; P = .5), free 25D (5.4 ± 0.8 vs 5.3 ± 1.7; P = .8), calcium, and iPTH were similar in D2 and D3 groups. The proportion of participants with baseline total 25D levels <20 ng/mL (26.3 vs 21.1%; P = .7) and between 20 and 30 ng/mL (73.7 vs 78.9%, P = .7) was similar between treatment groups. Baseline characteristics between supplemented participants who received D2 or D3 vs controls (screening total 25D ≥30 ng/mL) were similar, except that controls had higher total 25D (40.2 ± 10.2 vs 22.8 ± 4.8; P < .0001) and lower iPTH (41.3 ± 11.4 vs 50.6 ± 18.2; P = .03) levels (Table 2).
Table 1.
Descriptive Statisticsa for Baseline Clinical Characteristics and Biochemical Measurement
| D2 | D3 | P Valueb | |
|---|---|---|---|
| n | 19 | 19 | |
| Age, y | 50.2 (18.8) | 56.4 (19.6) | .3 |
| BMI, kg/m2 | 26.2 (5.6) | 24.9 (4.8) | .4 |
| Ethnicity | |||
| Caucasian | 11 (57.9) | 11 (57.9) | .9 |
| African American | 1 (5.3) | 1 (5.3) | .9 |
| Asian American | 3 (15.8) | 3 (15.8) | .9 |
| Hispanic/Latino | 4 (21.1) | 4 (21.1) | .9 |
| 25D, ng/mL | 22.2 (3.3) | 23.3 (7.2) | .5 |
| Free 25D, pg/mL | 5.4 (0.8) | 5.3 (1.7) | .8 |
| 1,25D, pg/mL | 60.0 (25.0) | 61.2 (30.8) | .9 |
| Serum calcium, mg/dL | 9.3 (0.3) | 9.5 (0.4) | .2 |
| Serum PTH, pg/mL | 50.3 (19.6) | 50.8 (17.2) | .6 |
Count (percentage) for categorical variables; mean (standard deviation) for continuous variables.
Difference between groups in count (percentage) for categorical variables; mean (standard deviation) for continuous variables.
Table 2.
Descriptive Statisticsa for Baseline Clinical Characteristics and Biochemical Measurements Between Vitamin D (D2 or D3) Supplemented and Control Participants
| Supplemented | Control | P Valueb | |
|---|---|---|---|
| n | 38 | 38 | |
| Age, y | 53.3 (19.2) | 61.6 (18.2) | .09 |
| BMI, kg/m2 | 25.6 (5.2) | 25.0 (4.0) | .6 |
| Ethnicity | |||
| Caucasian | 22 (57.9) | 22 (57.9) | .9 |
| African American | 2 (5.3) | 2 (5.3) | .9 |
| Asian American | 6 (15.8) | 6 (15.8) | .9 |
| Hispanic/Latino | 8 (21.1) | 8 (21.1) | .9 |
| 25D, ng/mL | 22.8 (4.8) | 40.2 (10.2) | <.0001 |
| Free 25D, pg/mL | 5.3 (1.3) | N/A | N/A |
| 1,25D, pg/mL | 60.6 (27.6) | 62.6 (27.9) | .8 |
| Serum calcium, mg/dL | 9.4 (0.3) | 9.6 (0.4) | .2 |
| Serum PTH, pg/mL | 50.6 (18.2) | 41.3 (11.4) | .03 |
Abbreviation: N/A, not available.
Count (percentage) for categorical variables; mean (standard deviation) for continuous variables.
Difference between groups in count (percentage) for categorical variables; mean (standard deviation) for continuous variables.
Effects of D2 vs D3 on vitamin D metabolites
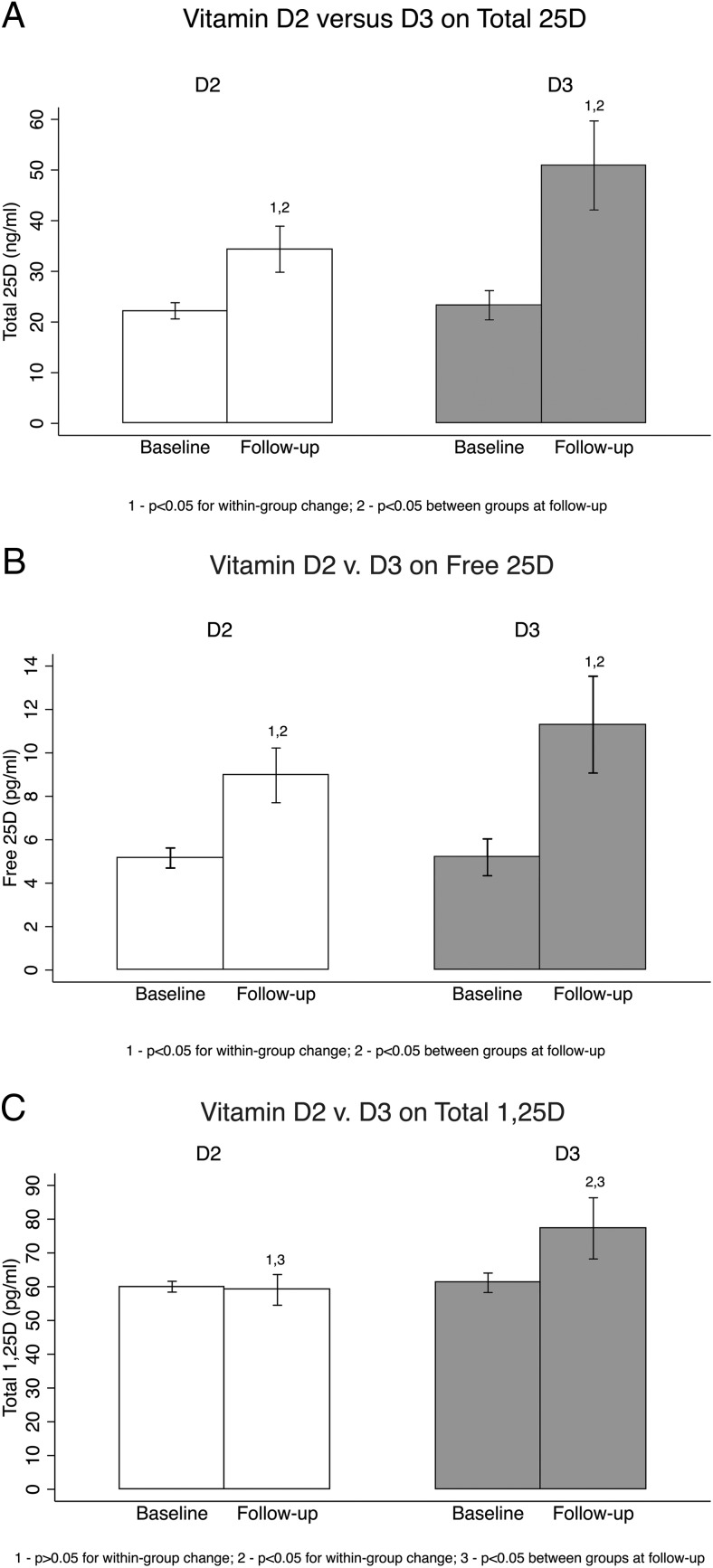
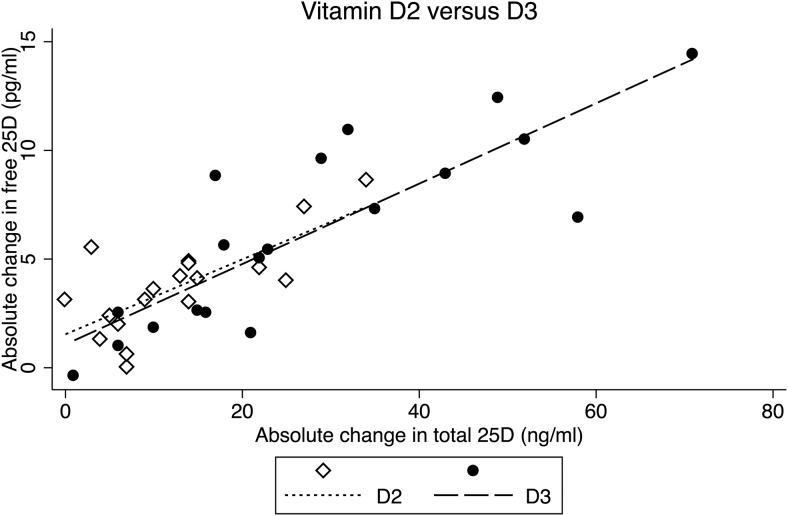
Total 25D increased to a greater extent with D3 (+27.6 ng/mL) vs D2 (+12.2 ng/mL) (P = .001) (Figure 1). Final total 25D was 34.3 ± 9.4 ng/mL with D2, compared to 50.9 ng/mL ± 18.2 ng/mL with D3 (P = .001). Among those who received D2, the 25D2:25D3 ratio was 0.31 at baseline and 1.12 at follow-up. Among those receiving D3, the 25D2:25D3 ratio was 0.07 at baseline and 0.03 at follow-up. Serum free 25D increased to a greater extent with D3 (+6.2 pg/mL) than with D2 (+3.7 pg/mL) (P = .02) (Figure 1). Final free 25D was 9.0 ± 2.3 pg/mL with D2 compared to 11.5 ± 4.4 pg/mL with D3 (P = .03). Serum free 25D at 10-week follow-up remained significantly higher with D3 than D2 supplementation, even after adjusting for baseline total or free 25D levels (P = .03 for both) (Figure 1). The increase in free 25D per ng/mL increment in total 25D was similar between groups (+0.2 pg/mL for D2 and D3; P = .8) (Figure 2).
Figure 1.
Effects of D2 vs D3 supplementation on serum vitamin D metabolites. A) D2 vs D3 on total 25D. B) D2 vs D3 on free 25D. C) D2 vs D3 on total 1,25D.
Figure 2.
Associations between changes in free and total 25D after D2 vs D3 supplementation.
Total 1,25D increased to a greater extent with D3 (+15.1 pg/mL) than with D2 (−0.9 pg/mL) (P = .02) (Figure 1). Serum total 1,25D at 10-week follow-up remained significantly higher with D3 than with D2 supplementation, even after adjusting for baseline total 1,25D level (P = .01). The percentage change in 1,25D was significantly associated with a change in total 25D (P = .02; R2 = 0.28), but not a change in free 25D (P = .07; R2 = 0.21).
Effects of D2 vs D3 on markers of calcium homeostasis
Changes in serum calcium (+0.03 vs +0.1 mg/dl; P = .5) and fasting urinary calcium:creatinine (−0.02 vs −0.02; P = .9) were not significantly different with D2 vs D3 supplementation. The change in iPTH was also similar (−5.8 vs −6.0 pg/mL; P = .5) with D2 vs D3 supplementation.
Association between percentage change in iPTH and total vs free 25D
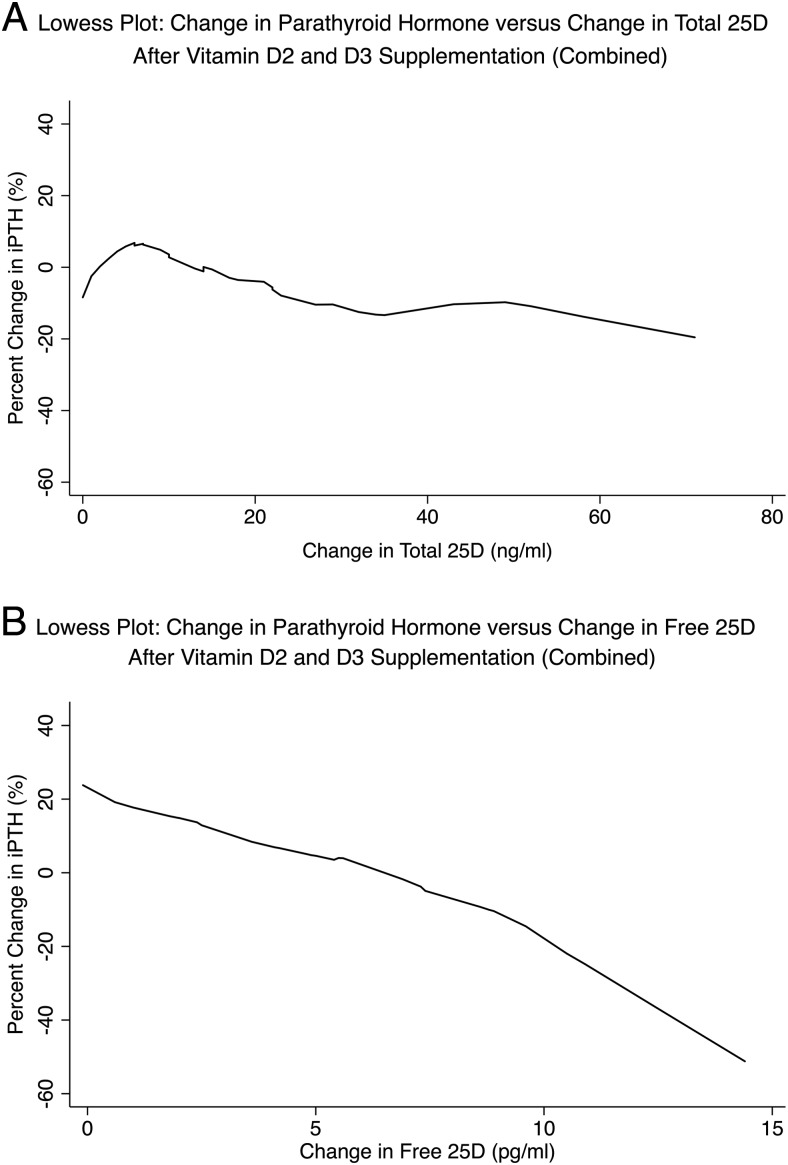
The associations between percentage change in iPTH and change in total vs free 25D were examined via Lowess plots, as well as unadjusted and adjusted linear regression models (Figure 3 and Table 3). Percentage change in iPTH was not significantly associated with change in total 25D alone or after adjustment for supplementation regimen, change in total 1,25D level, and change in calcium. In contrast, the percentage change in iPTH was significantly associated with a change in free 25D alone and after adjustment for the same covariates. Change in iPTH was not significantly associated with a change in 1,25D or calcium.
Figure 3.
Lowess plots of change in iPTH as a function of change in total 25D (A) vs free (B) 25D after D2 and D3 supplementation. The slope of the relationship between change in iPTH is greater (indicating a stronger association) with change in free compared to total 25D.
Table 3.
Associations Between Percentage Change in iPTH and Change in Total vs Free 25D
| Percentage Change in iPTH (95% CI) per Unit Increment Change in Serum Total vs Free 25D |
||
|---|---|---|
| β Coefficienta (95% CI) | P Value | |
| Total 25D | ||
| Unadjusted | −0.507 (−1.124, 0.110) | .2 |
| Adjusted for D2 vs D3 | −0.516 (−1.230, 0.197) | .2 |
| Adjusted for D2 vs D3, change in 1,25D and calciumb | −0.498 (−1.269, 0.273) | .2 |
| Free 25D | ||
| Unadjusted | −3.383 (−6.247, −0.519) | .02 |
| Adjusted for D2 vs D3 | −3.366 (−6.463, −0.268) | .03 |
| Adjusted for D2 vs D3, change in 1,25D and calciumb | −3.384 (−6.594, −0.175) | .03 |
The β coefficient should be interpreted as follows: for each ng/mL increase in total 25D or pg/mL increase in free 25D, PTH changes by “β” percent.
Adjusted for covariates that have previously been reported to influence PTH secretion.
Discussion
The aims of this study were to characterize the effects of D2 vs D3 supplementation on changes in total and free 25D and to assess whether a change in PTH with supplementation is more strongly associated with a change in total vs free 25D. We found that D3 increased total and free 25D levels to a greater extent than D2. We also report that change in serum iPTH is more strongly associated with change in free vs total 25D, independent of changes in serum 1,25D and calcium, suggesting that free 25D is indeed a useful marker of vitamin D-mediated bioactivity in vivo.
Our first key finding is that follow-up 25D levels were higher with 50 000 IU D3 than with D2 twice weekly. Prior studies have examined the effects of D2 vs D3 on total serum 25D using various supplementation regimens. With the exception of one study that found 1000 IU/d of D2 and D3 to be comparable in raising total 25D levels (5), D3 has been shown to increase serum total 25D levels to a greater extent than D2 when administered at 50 000 IU/mo, 50 000 IU/wk, 2000 IU/d, and 4000 IU/d (4, 6–11). In fact, most studies comparing D2 with D3 report a greater incremental increase in total 25D levels with D3, even if the regimen was not always successful in achieving a final total 25D level that was in the “normal” range (>30 ng/mL) (11). One factor that accounts for the greater incremental increase in total 25D levels with D3 is the relatively longer half-life of 25D3 in the serum. On average, the 25D3 half-life is 8% longer than 25D2, owing to the greater avidity of DBP for 25D3 than 25D2 (33). Another factor that may also account for the greater incremental increase in 25D levels with D3 compared to D2 is that studies differed in attempts to minimize endogenous synthesis of D3 (and 25D3) during the course of the trial (5, 8, 34).
A second key finding in this study is that measured free 25D levels increased to a greater extent with D3 than with D2. This is consistent with emerging evidence to suggest that free and total 25D levels are highly correlated, such that the rise of free 25D is proportional to the rise in total 25D (31, 32, 34). This is in contrast to our understanding, however, that 25D2 is bound less avidly by DBP than is 25D3 (16–18). Based on the above, one would speculate that for a given total 25D level, the free fraction would be greater if a greater percentage of the total 25D pool is composed of 25D2. Indeed, we have shown that mice receiving a D2-containing diet had higher free 25D (all 25D2) concentrations compared to those receiving a D3-containing diet (all 25D3) (19). In humans, one prior study comparing 1000 IU/d of D2 and D3 found that despite achieving higher total 25D levels with D3, calculated free 25D levels were similar (11). One possible explanation for this observed discrepancy is related to our supplementation regimen. We gave patients 50 000 IU of D2 or D3 twice weekly for 5 weeks, followed by a 5-week period during which we allowed serum vitamin D metabolite levels to equilibrate (24–26). Given that the serum half-life of 25D2 is shorter than 25D3 (33), it is possible that the total 25D2 fraction of the total 25D pool diminished over the equilibration period such that any advantage in serum free 25D that may have existed at week 5 was lost by week 10. In addition, total serum 25D3 concentrations remained constant and represented 50% of all total 25D at follow-up in the D2 group. This is in contrast to our prior mouse study in which D2- or D3-fed mice had exclusively 25D2 or 25D3 in the circulation. This is also in contrast to prior human studies in which D2 supplementation decreased circulating 25D3 concentrations, possibly owing to competition by D2 for 25-hydroxylation, the presence of multiple 25-hydroxylases with varying avidity for D2 and D3, or increased metabolic degradation of 25D3 (4, 8, 9, 35). However, whereas the above studies were conducted in more northern latitudes (4, 8, 9) and asked participants to wear sunscreen when outside for more than 15 minutes (8), we conducted our study in Southern California where sun exposure and therefore endogenous production of D3 and 25D3 remained relevant. As such, any advantage in serum free 25D level that could be obtained from D2 supplementation would be attenuated. Taken within the context of the available literature, it seems that whereas D2 may have theoretical advantages in human and nonhuman species (17–19), an equivalent dose of D3 is more likely to increase free 25D levels to a greater extent than D2 in “real-world” community-dwelling individuals. Because 1,25D2 and 1,25D3 have similar biological activity at the vitamin D receptor (36), the supplement that most efficiently raises total and free 25D levels (D3 in this case) without causing toxicity would seem preferable. Nonetheless, it would be informative to replicate this clinical experiment at a more northern latitude during winter, while ensuring minimal sunlight exposure to participants.
The third key (and perhaps most novel) finding in this study is that dynamic change in serum iPTH was significantly associated with change in free, but not total, 25D even after adjusting for supplementation regimen, change in 1,25D, and change in serum calcium. Interestingly, change in iPTH was not significantly associated with change in 1,25D, suggesting that PTH suppression may have been more strongly driven by free 25D. It is classically understood that 1,25D suppresses PTH secretion, but this was demonstrated by studies that reported PTH response to intravenous 1,25D administration (37). This represents a different physiological context than the present study. To our knowledge, this is the first study to date to show this, and it argues that free 25D is indeed a useful biomarker of vitamin D-mediated bioactivity in vivo above and beyond total serum 25D. In serum, nearly 90% of 25D is bound to DBP, and approximately 10% of 25D is bound to albumin. As is evidenced in our studies (Table 1 and Figure 1), less than 0.1% circulates free, unbound to serum proteins (12, 19). The “free-hormone” hypothesis postulates that free 25D represents a superior marker of vitamin D substrate bioavailability than total 25D (12, 20, 38, 39). The cross-sectional association between total and free 25D and markers of skeletal health, such as serum iPTH, bone turnover markers, and bone mineral density, has been assessed in multiple studies (20, 21, 31, 32, 34, 38–42). Although some studies have reported a stronger correlation with free 25D levels (20, 34, 38, 39), others have not (21, 31, 32, 40–42). These observed inconsistencies may be due to differences in study populations and differences in methodologies for determining bioavailable and/or free 25D levels; whereas some studies directly measured free 25D concentrations by assay technology similar to that used here (21, 31, 32, 34, 41), others calculated bioavailable 25D indirectly with algorithms based on DBP quantity and circulating isoforms harboring varying affinities for 25D (20, 38–40, 42). Importantly, recent studies have called into question the accuracy of a commonly used monoclonal antibody-based assay for measuring DBP levels, as well as the frequent practice of using a single DBP affinity constant when calculating free 25D levels. One study reported an approximately 1.5-fold overestimation of calculated compared to directly measured free 25D level (21). Given their cross-sectional nature and heterogeneity in methodology for assessing free 25D levels, as well as the questionable accuracy of calculating free 25D levels, it is difficult to draw cause-and-effect outcome conclusions from the above studies. In particular, it is challenging to infer the mechanism by which 25D is internalized by a target cell (DBP-bound or free).
This question is better assessed by examining the associations between dynamic changes in markers of vitamin D bioactivity and change in total vs free 25D after a provocation to the human system (ie, vitamin D supplementation wherein each subject serves as his/her own control). Here, we had two outcome measures of note: 1) percentage change in serum 1,25D; and 2) percentage change in serum iPTH. Change in 1,25D is mediated by DBP-bound 25D entry into the renal tubular epithelial cell (13), whereas change in iPTH may be mediated by free 25D entry into the parathyroid cell (43). In the human renal tubular epithelial cells that house most human CYP27B1, entry of substrate 25D into the target cell is dependent on megalin-mediated endocytosis of DBP-bound 25D from the glomerular filtrate (13). Megalin is also expressed in several extrarenal tissues, including the placenta, mammary gland, and parathyroid gland, suggesting the possibility of a DBP-megalin interaction at these sites (12). However, the functional significance of this is unclear. Megalin-mediated entry of DBP-bound 25D into renal epithelial cells likely explains why supplementation with D3, which is bound more avidly by DBP than is D2, was associated with a significant increase in total serum 1,25D, whereas treatment with D2 was not (Figure 1C). Along these lines, percentage change in 1,25D was significantly associated with change in total, but not free 25D after adjustment for supplementation regimen. The source of increased 1,25D was likely renal because the vast majority of CYP27B1 is expressed in the kidney under normal physiological conditions (44–47). This is in contrast to pathological conditions (eg, lymphoma) in which dysregulated CYP27B1 expression in cells outside of the kidney leads to substantial extrarenal 1,25D synthesis (48). At extrarenal tissue sites (in this study, the parathyroid gland), the preferred mechanism of 25D (and 1,25D) entry may be diffusion of free unbound metabolite into the target cell, followed by subsequent CYP27B1-mediated conversion to 1,25D in an intracrine fashion (12, 14, 43). Indeed, the parathyroid cell has previously been shown to express CYP27B1 and therefore possesses the requisite machinery to convert internalized free 25D to 1,25D, which in turn can direct suppression of PTH expression (49–51). Of note, megalin is known to be expressed in parathyroid gland cells (52); however, the extent to which 25D enters the cell bound to DBP (via a megalin-mediated mechanism) vs free remains an open question. A prior animal study showed that DBP−/− mice placed on a vitamin D-containing diet had serum iPTH levels similar to that of DBP+/+ mice. In contrast, when the DBP−/− mice were placed on a vitamin D-deficient diet, their serum iPTH levels doubled (53). These findings suggest to us that, at least in mice, entry of free 25D into the parathyroid cell occurs in vivo. Consistent with this report in mice, we found that the percentage change in iPTH after supplementation was significantly associated with a change in free, but not total, 25D. This remained significant even after adjusting for supplementation regimen and factors known to influence iPTH secretion, namely change in serum 1,25D and calcium. Based on our findings, we posit that: 1) movement of free 25D into the parathyroid cell represents a physiologically relevant mechanism of target cell entry; and 2) free 25D can serve as a useful in vivo biomarker of vitamin D-mediated bioactivity above and beyond total 25D. Future studies should aim to clarify: 1) whether entry of free 25D into target cells occurs via simple diffusion vs other mechanisms; and 2) whether the association between a broader range of free 25D levels and different markers of vitamin D bioactivity is different.
This study has several weaknesses that warrant mention. First, the sample size was relatively small. This would, however, bias our results toward null. Therefore, the significant association seen between change in iPTH and change in free 25D would likely only be strengthened with increased sample size. Second, our study participants were not severely vitamin D deficient. Nonetheless, we found that supplemented subjects (baseline total 25D <30 ng/mL) had significantly higher iPTH levels than their external controls (baseline total 25D ≥30 ng/mL). This suggests that whereas individuals with lower total 25D levels did not have frankly elevated iPTH levels, they had a “relative” secondary hyperparathyroidism compared to those with 25D levels >30 ng/mL. Perhaps if our study had included patients with baseline total 25D levels <20 ng/mL and frankly elevated serum iPTH (ie, secondary hyperparathyroidism) exclusively, a more pronounced biomarker benefit would have been observed with supplementation. Third, our study was only 10 weeks in duration. This may explain why iPTH levels at follow-up were not significantly lower in D3- vs D2-treated subjects, despite achieving higher total and free 25D levels. It has previously been shown that among patients with vitamin D deficiency and secondary hyperparathyroidism, iPTH levels may remain persistently elevated even up to 17 months after initiation of vitamin D supplementation (54). If our study duration had been longer, perhaps an advantage in iPTH suppression would have become evident with D3 vs D2.
Despite these limitations, we are the first to: 1) compare the effects of D2 vs D3 on serum total and directly measured free 25D; and 2) evaluate whether changes in iPTH, a recognized biomarker of the host's calcium homeostasis, with vitamin D supplementation is significantly more strongly associated with a change in free vs total 25D. We conclude that D3 increased both total and free 25D levels more robustly than D2 and that change in iPTH was more strongly associated with change in free 25D, independent of changes in serum 1,25D and calcium.
Acknowledgments
Research described in this manuscript was supported by: 1) National Institutes of Health/National Center for Advancing Translational Science UCLA CTSI Grant UL1TR000124; 2) National Institute of Arthritis and Musculoskeletal and Skin Diseases award 5R01AR063810; and 3) the UCLA Specialty Training and Advanced Research Program. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ClinicalTrials.gov Identifier: NCT01848236.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- body mass index
- CV
- coefficient of variation
- 1,25D
- 1,25-dihydroxyvitamin D
- D2
- vitamin D2
- D3
- vitamin D3
- 25D
- 25-hydroxyvitamin D
- 25D2
- 25-hydroxyvitamin D2
- 25D3
- 25-hydroxyvitamin D3
- DBP
- vitamin D binding protein
- LC/MS/MS
- liquid chromatography-mass spectrometry
- iPTH
- intact PTH.
References
- 1. Holick M. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. [DOI] [PubMed] [Google Scholar]
- 2. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2011;96:1911–1930. [DOI] [PubMed] [Google Scholar]
- 3. Park EA. The therapy of rickets. JAMA. 1940;115:370–379. [Google Scholar]
- 4. Trang HM, Cole DE, Rubin LA, Pierratos A, Siu S, Vieth R. Evidence that vitamin D3 increases serum 25-hydroxyvitamin D more efficiently than does vitamin D2. Am J Clin Nutr. 1998;68:854–858. [DOI] [PubMed] [Google Scholar]
- 5. Holick MF, Biancuzzo RM, Chen TC, et al. Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. J Clin Endocrinol Metab. 2008;93:677–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Romagnoli E, Mascia ML, Cipriani C, et al. Short and long-term variations in serum calciotropic hormones after a single very large dose of ergocalciferol (vitamin D2) or cholecalciferol (vitamin D3) in the elderly. J Clin Endocrinol Metab. 2008;93:3015–3020. [DOI] [PubMed] [Google Scholar]
- 7. Thacher TD, Obadofin MO, O'Brien KO, Abrams SA. The effect of vitamin D2 and vitamin D3 on intestinal calcium absorption in Nigerian children with rickets. J Clin Endocrinol Metab. 2009;94:3314–3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Binkley N, Gemar D, Engelke J, et al. Evaluation of ergocalciferol or cholecalciferol dosing, 1,600 IU daily or 50,000 IU monthly in older adults. J Clin Endocrinol Metab. 2011;96:981–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Heaney RP, Recker RR, Grote J, Horst RL, Armas LA. Vitamin D(3) is more potent than vitamin D(2) in humans. J Clin Endocrinol Metab. 2011;96:E447–E452. [DOI] [PubMed] [Google Scholar]
- 10. Lehmann U, Hirche F, Stangl GI, Hinz K, Westphal S, Dierkes J. Bioavailability of vitamin D(2) and D(3) in healthy volunteers, a randomized placebo-controlled trial. J Clin Endocrinol Metab. 2013;98:4339–4345. [DOI] [PubMed] [Google Scholar]
- 11. Glendenning P, Chew GT, Inderjeeth CA, Taranto M, Fraser WD. Calculated free and bioavailable vitamin D metabolite concentrations in vitamin D-deficient hip fracture patients after supplementation with cholecalciferol and ergocalciferol. Bone. 2013;56:271–275. [DOI] [PubMed] [Google Scholar]
- 12. Chun RF, Peercy BE, Orwoll ES, Nielson CM, Adams JS, Hewison M. Vitamin D and DBP: the free hormone hypothesis revisited. J Steroid Biochem Mol Biol. 2014;144PA:132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Christensen EI, Birn H. Megalin and cubilin: synergistic endocytic receptors in renal proximal tubule. Am J Physiol Renal Physiol. 2001;280:F562–F573. [DOI] [PubMed] [Google Scholar]
- 14. Rosen CJ, Adams JS, Bikle DD, et al. The nonskeletal effects of vitamin D: an Endocrine Society Scientific Statement. Endocr Rev. 2012;33:456–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chun RF, Lauridsen AL, Suon L, et al. Vitamin D-binding protein directs monocyte responses to 25-hydroxy- and 1,25-dihydroxyvitamin D. J Clin Endocrinol Metab. 2010;95:3368–3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Belsey R, Clark MB, Bernat M, et al. The physiologic significance of plasma transport of vitamin D and metabolites. Am J Med. 1974;57:50–56. [DOI] [PubMed] [Google Scholar]
- 17. Jones G, Byrnes B, Palma F, Segev D, Mazur Y. Displacement potency of vitamin D2 analogs in competitive protein-binding assays for 25-hydroxyvitamin D3, 24,25-dihydroxyvitamin D3, and 1,25-dihydroxyvitamin D3. J Clin Endocrinol Metab. 1980;50:773–775. [DOI] [PubMed] [Google Scholar]
- 18. Hollis B. Comparison of equilibrium and disequilibrium assay conditions for ergocalciferol, cholecalciferol and their major metabolites. J Steroid Biochem. 1984;21:81–86. [DOI] [PubMed] [Google Scholar]
- 19. Chun RF, Pereira R, Huijs T, et al. Differential effects of vitamin D2 vs. vitamin D3 on bone are associated with variations in free 25-hydroxyvitamin D. J Bone Miner Res. 2015;30 Available at http://www.asbmr.org/education/AbstractDetail?aid=4880c4877e-4200a-4884cb4889-ac4886e-4881e658604f658671 Accessed February 658628, 652016. [Google Scholar]
- 20. Powe CE, Evans MK, Wenger J, et al. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N Engl J Med. 2013;369:1991–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schwartz JB, Lai J, Lizaola B, et al. A comparison of measured and calculated free 25(OH) vitamin D levels in clinical populations. J Clin Endocrinol Metab. 2014;99:1631–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nielson CM, Jones KS, Bouillon R, et al. Role of assay type in determining free 25-hydroxyvitamin D levels in diverse populations. N Engl J Med. 2016;374:1695–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nielson CM, Jones KS, Chun RF, et al. Free 25-hydroxyvitamin D: impact of vitamin D binding protein assays on racial-genotypic associations. J Clin Endocrinol Metab. 2016;101:2226–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Adams JS, Hewison M. Update in vitamin D. J Clin Endocrinol Metab. 2010;95:471–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Adams JS, Kantorovich V, Wu C, Javanbakht M, Hollis BW. Resolution of vitamin D insufficiency in osteopenic patients results in rapid recovery of bone mineral density. J Clin Endocrinol Metab. 1999;84:2729–2730. [DOI] [PubMed] [Google Scholar]
- 26. Pepper KJ, Judd SE, Nanes MS, Tangpricha V. Evaluation of vitamin D repletion regimens to correct vitamin D status in adults. Endocr Pract. 2009;15:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chapuy MC, Arlot ME, Duboeuf F, et al. Vitamin D3 and calcium to prevent hip fractures in the elderly women. N Engl J Med. 1992;327:1637–1642. [DOI] [PubMed] [Google Scholar]
- 28. Hsu SA, Soldo J, Gupta M. Evaluation of two automated immunoassays for 25-OH vitamin D: comparison against LC-MS/MS. J Steroid Biochem Mol Biol. 2013;136:139–145. [DOI] [PubMed] [Google Scholar]
- 29. Horst R. Exogenous versus endogenous recovery of 25-hydroxyvitamins D2 and D3 in human samples using high-performance liquid chromatography and the DiaSorin LIAISON Total-D assay. J Steroid Biochem Mol Biol. 2010;121:180–182. [DOI] [PubMed] [Google Scholar]
- 30. Freeman J, Wilson K, Spears R, Shalhoub V, Sibley P. Performance evaluation of four 25-hydroxyvitamin D assays to measure 25-hydroxyvitamin D2. Clin Biochem. 2015;48:1097–1104. [DOI] [PubMed] [Google Scholar]
- 31. Aloia J, Mikhail M, Dhaliwal R, et al. Free 25(OH)D and the vitamin D paradox in African Americans. J Clin Endocrinol Metab. 2015;100:3356–3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Aloia J, Dhaliwal R, Mikhail M, et al. Free 25(OH)D and calcium absorption, PTH, and markers of bone turnover. J Clin Endocrinol Metab. 2015;100:4140–4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jones KS, Assar S, Harnpanich D, et al. 25(OH)D2 half-life is shorter than 25(OH)D3 half-life and is influenced by DBP concentration and genotype. J Clin Endocrinol Metab. 2014;99:3373–3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schwartz JB, Kane L, Bikle D. Response of vitamin D concentration to vitamin D3 administration in older adults without sun exposure: a randomized double-blind trial. J Am Geriatr Soc. 2016;64:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Swanson CM, Nielson CM, Shrestha S, et al. Higher 25(OH)D2 is associated with lower 25(OH)D3 and 1,25(OH)2D3. J Clin Endocrinol Metab. 2014;99:2736–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tsugawa N, Nakagawa K, Kawamoto Y, et al. Biological activity profiles of 1α,25-dihydroxyvitamin D2, D3, D4, D7, and 24-epi-1α,25-dihydroxyvitamin D2. Biol Pharm Bull. 1999;22:371–377. [DOI] [PubMed] [Google Scholar]
- 37. Delmez JA, Tindira C, Grooms P, Dusso A, Windus DW, Slatopolsky E. Parathyroid hormone suppression by intravenous 1,25-dihydroxyvitamin D. A role for increased sensitivity to calcium. J Clin Invest. 1989;83:1349–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Powe CE, Ricciardi C, Berg AH, et al. Vitamin D-binding protein modifies the vitamin D-bone mineral density relationship. J Bone Miner Res. 2011;26:1609–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bhan I, Powe CE, Berg AH, et al. Bioavailable vitamin D is more tightly linked to mineral metabolism than total vitamin D in incident hemodialysis patients. Kidney Int. 2012;82:84–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jemielita TO, Leonard MB, Baker J, et al. Association of 25-hydroxyvitamin D with areal and volumetric measures of bone mineral density and parathyroid hormone: impact of vitamin D-binding protein and its assays. Osteoporos Int. 2016;27:617–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sollid ST, Hutchinson MY, Berg V, et al. Effects of vitamin D binding protein phenotypes and vitamin D supplementation on serum total 25(OH)D and directly measured free 25(OH)D. Eur J Endocrinol. 2016;174:445–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Goswami R, Saha S, Sreenivas V, Singh N, Lakshmy R. Vitamin D-binding protein, vitamin D status and serum bioavailable 25(OH)D of young Asian Indian males working in outdoor and indoor environments [published online January 30, 2016]. J Bone Miner Metab. doi: 10.1007/s00774-016-0739-x. [DOI] [PubMed] [Google Scholar]
- 43. Bienaimé F, Prié D, Friedlander G, Souberbielle J. Vitamin D metabolism and activity in the parathyroid gland. Mol Cell Endocrinol. 2011;347:30–41. [DOI] [PubMed] [Google Scholar]
- 44. Zehnder D, Bland R, Williams MC, et al. Extrarenal expression of 25-hydroxyvitamin d(3)-1 α-hydroxylase. J Clin Endocrinol Metab. 2001;86:888–894. [DOI] [PubMed] [Google Scholar]
- 45. Zehnder D, Bland R, Chana RS, et al. Synthesis of 1,25-dihydroxyvitamin D(3) by human endothelial cells is regulated by inflammatory cytokines: a novel autocrine determinant of vascular cell adhesion. J Am Soc Nephrol. 2002;13:621–629. [DOI] [PubMed] [Google Scholar]
- 46. Hewison M, Burke F, Evans KN, et al. Extra-renal 25-hydroxyvitamin D3-1α-hydroxylase in human health and disease. J Steroid Biochem Mol Biol. 2007;103:316–321. [DOI] [PubMed] [Google Scholar]
- 47. Vanhooke JL, Prahl JM, Kimmel-Jehan C, et al. CYP27B1 null mice with LacZreporter gene display no 25-hydroxyvitamin D3-1α-hydroxylase promoter activity in the skin. Proc Natl Acad Sci USA. 2006;103:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hewison M, Kantorovich V, Liker HR, et al. Vitamin D-mediated hypercalcemia in lymphoma: evidence for hormone production by tumor-adjacent macrophages. J Bone Miner Res. 2003;18:579–582. [DOI] [PubMed] [Google Scholar]
- 49. Segersten U, Correa P, Hewison M, et al. 25-Hydroxyvitamin D(3)-1α-hydroxylase expression in normal and pathological parathyroid glands. J Clin Endocrinol Metab. 2002;87:2967–2972. [DOI] [PubMed] [Google Scholar]
- 50. Silver J, Russell J, Sherwood L. Regulation by vitamin D metabolites of messenger ribonucleic acid for preproparathyroid hormone in isolated bovine parathyroid cells. Proc Natl Acad Sci USA. 1985;82:4270–4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Canterbury JM, Lerman S, Claflin AJ, Henry H, Norman A, Reiss E. Inhibition of parathyroid hormone secretion by 25-hydroxycholecalciferol and 24,25-dihydroxycholecalciferol in the dog. J Clin Invest. 1978;61:1375–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lundgren S, Carling T, Hjälm G, et al. Tissue distribution of human gp330/megalin, a putative Ca(2+)-sensing protein. J Histochem Cytochem. 1997;45:383–392. [DOI] [PubMed] [Google Scholar]
- 53. Safadi FF, Thornton P, Magiera H, et al. Osteopathy and resistance to vitamin D toxicity in mice null for vitamin D binding protein. J Clin Invest. 1999;103:239–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kantorovich V, Gacad MA, Seeger LL, Adams JS. Bone mineral density increases with vitamin D repletion in patients with coexistent vitamin D insufficiency and primary hyperparathyroidism. J Clin Endocrinol Metab. 2000;85:3541–3543. [DOI] [PubMed] [Google Scholar]