Abstract
Context:
The clinical consequences of insulin resistance and hyperinsulinemia on bone remain largely unknown.
Objective:
The objective of the study was to evaluate the effect of insulin resistance on peripheral bone geometry, volumetric bone mineral density (vBMD), bone microarchitecture, and estimated bone strength.
Design, Setting, and Participants:
This cross-sectional study included 146 postmenopausal, nondiabetic Caucasian women (mean age 60.3 ± 2.7 y) who were participating in the Study of Women's Health Across the Nation.
Interventions:
There were no interventions.
Main Outcome Measures:
High-resolution peripheral quantitative computed tomography was used to assess bone density and microstructure at the distal radius and tibia. Fasting insulin and glucose were measured and insulin resistance was estimated using homeostasis model assessment of insulin resistance (HOMA-IR), with higher values indicating greater insulin resistance.
Results:
There was a negative association between HOMA-IR and bone size and a positive association between HOMA-IR and total vBMD, trabecular vBMD, trabecular thickness, and cortical thickness at the radius and tibia. These relationships remained, even after adjusting for body weight and other potential covariates (eg, time since menopause, cigarette smoking, physical activity, prior use of osteoporosis medications or glucocorticoids).
Conclusions:
In nondiabetic, postmenopausal women, insulin resistance was associated with smaller bone size, greater volumetric bone mineral density, and generally favorable bone microarchitecture at weight-bearing and nonweight-bearing skeletal sites. These associations were independent of body weight and other potential covariates, suggesting that hyperinsulinemia directly affects bone structure independent of obesity and may explain, in part, the higher trabecular bone density and favorable trabecular microarchitecture seen in individuals with type 2 diabetes mellitus.
The association between insulin resistance and bone microarchitecture was assessed in postmenopausal, non-diabetic women. Insulin resistance was associated with favorable bone microarchitecture at the distal radius and tibia.
The presence of the metabolic syndrome greatly increases an individual's risk of developing diabetes and is associated with a 2- to 3-fold increase in cardiovascular mortality (1). One of the hallmarks of the metabolic syndrome is peripheral insulin resistance within hepatic, skeletal muscle, and adipose tissues, resulting in hyperinsulinism. The skeletal consequences of increased circulating insulin levels are not entirely clear, but in recent years, studies have indicated that insulin has direct effects on bone cells. Animal models with osteoblast-specific deletions of the insulin receptor show reductions in osteoblastic differentiation and proliferation, leading to lower osteoblast numbers and reduced bone mass (2). These data, coupled with the identification of skeletal fragility in patients with insulin deficiency due to type 1 diabetes, have led to the hypothesis that insulin is osteoanabolic (3–5). This hypothesis is further supported by the finding of high bone mass in a variety of hyperinsulinemic conditions (6–9), a finding that also suggests that peripheral resistance to insulin's hypoglycemic actions (referred to as insulin resistance throughout this paper) is not necessarily accompanied by resistance to insulin at the level of the skeleton.
Nevertheless, owing to the strong association between obesity and insulin resistance, it is challenging to distinguish the independent effects of obesity and insulin resistance on the skeleton. Therefore, the association between insulin resistance and bone mass remains uncertain. Several studies point to the presence of a positive association between circulating insulin levels and bone mineral density (BMD) independent of body mass index (BMI) (10–13), suggesting that the effects of insulin resistance on bone may be mediated independently of BMI. In contrast, other studies showed a loss of the positive association between insulin levels and BMD after adjusting for BMI (14–16), implying that the insulin resistance may affect BMD through indirect effects, eg, body weight. Furthermore, some studies have shown a lack of association (17) or even an inverse association between insulin resistance and BMD (18). The discrepancies in these results may be due to the heterogeneity of the various study populations, particularly differences in age (14, 16), ethnicity (14, 16, 17), menopausal status (14, 16, 17), and the inclusion of diabetic patients on hypoglycemic medications (11, 16–18).
Another avenue to investigate the skeletal effects of insulin resistance is to use high-resolution three-dimensional imaging to explore effects on bone microarchitecture. Using this technique, whereas studies in patients with type 2 diabetes (T2D) have suggested that an increase in cortical porosity may help explain the increase in fracture risk among these patients (19–22), most of these studies have also suggested a generally well-preserved (21–23) to superior trabecular compartment (20). Whether the advantageous trabecular microarchitecture seen in these women is the result of insulin resistance or obesity is uncertain. Recent studies using high-resolution peripheral quantitative computed tomography (CT) have characterized the effects of obesity on bone microarchitecture (7, 24); however, there are currently no studies exploring the association between insulin resistance and bone microstructure in healthy nondiabetic individuals.
The aims of this study were to evaluate the effect of insulin resistance on compartment-specific bone geometry, volumetric bone mineral density, microarchitecture, and estimated strength in postmenopausal nondiabetic women. We also sought to determine whether the effect of insulin resistance on bone is independent of obesity. Based on previous literature, we hypothesized that bone mass and microarchitecture would be positively associated with insulin resistance independent of BMI.
Materials and Methods
We studied a subset of postmenopausal, nondiabetic Caucasian women (n = 146) who were participating in the Study of Women's Health Across the Nation (SWAN). The SWAN study is a multisite, multiethnic, longitudinal cohort study of community-based women. The initial eligibility criteria and recruitment of the cohort have been described in detail previously (25). Briefly, approximately 3300 premenopausal women were initially recruited between 1996 and 1998 and have been followed up prospectively every 1–2 years to characterize changes during and beyond the menopausal transition. The current study was focused on women (n = 273) who received high-resolution peripheral quantitative CT (HR-pQCT) scans at the Boston site during study SWAN visit 11 or 12 (September 2008 through April 2011). Women were excluded if they were not postmenopausal (n = 12) or had a diagnosis of diabetes (n = 35, based on a self-reported history of diabetes or use of diabetic medications or if they had a fasting blood glucose >126 mg/dL at any SWAN visit) or did not have fasting insulin or glucose values (n = 5). Of note, we detected statistically significant race interactions for relationships between homeostasis model assessment of insulin resistance (HOMA-IR) and bone microarchitectural parameters, but the relatively smaller numbers and the unequal distribution of HOMA-IR in nondiabetic African-American participants (n = 75, with fewer African-American women having low HOMA-IR) limited our power to visualize correlations across the spectrum of HOMA-IR in this group. Thus, the current study was confined to data pertaining to Caucasian women only (n = 146). The SWAN parent study and HR-pQCT substudy were approved by the Institutional Review Board at Massachusetts General Hospital, and all women provided written, informed consent.
Study protocol
Assessment of clinical data
Medical history including age (years), medical diagnosis, cigarette smoking (yes/no), alcohol intake (drinks per day; no ≤ one drink per day and one ≥ one drink per day), medication use, menopause stage, and physical activity (modified Baeke questionnaire) (26) were detailed at the concurrent visit using standardized interviews and self-administered questionnaires as described previously (21). Medications were self-reported, including any use of diabetes medications, glucocorticoids (defined by self-report of glucocorticoid use >3 mo at the baseline visit or report of use at three or more subsequent follow up visits), systemic hormonal replacement therapy (HRT), and/or osteoporosis medications (including all oral and iv bisphosphonates, selective estrogen receptor modulators, teriparatide, and calcitonin). Anthropometric data including body weight and height were measured to the nearest 0.1 kg on a digital scale and to the nearest 0.1 cm on a wall-mounted fixed stadiometer in all participants dressed in casual indoor clothing without shoes, and BMI was calculated as weight (kilograms) divided by the square of height (meters).
Glycemic indices
Blood samples from the participants were drawn after an overnight 12-hour fast, and serum insulin and glucose were measured using a RIA procedure (Coat-a-Count; Diagnostic Products Corp) and hexokinase-coupled reaction (Roche Molecular Biochemicals Diagnostics), respectively. The quality control program for serum insulin in SWAN has been previously described (27). Insulin resistance was estimated using HOMA-IR ([fasting serum glucose [milligrams per deciliter]] × [fasting serum insulin [microinternational units per milliliter]]/405) (28), with higher values indicating insulin resistance (29). Quartiles (Qs) for the cohort distribution for the HOMA-IR were as follows: Q1, 0.480–1.062; Q2, 1.108–1.560; Q3, 1.571–2.651; and Q4, 2.689–8.908.
Areal BMD (aBMD)
Areal BMD (aBMD) was measured using dual-energy X-ray absorptiometry (DXA) (QDR4500A; Hologic Inc) at the lumbar spine (L1-L4) and total hip region. Short-term in vivo DXA precision was calculated by scanning 30 patients twice, with repositioning between scans. Root mean square SD values were 0.007 g/cm2, 0.008 g/cm2, and 0.012 g/cm2 for the lumbar spine, total hip, and femoral neck, respectively. The standard quality program for DXA measurements has been described in detail previously (25).
Bone microarchitecture
HR-pQCT (Xtreme CT; Scanco Medical, AG) was used to assess bone geometry, volumetric BMD (vBMD), microarchitecture, and estimated bone strength at the nondominant distal radius and distal tibia (or the opposite limb in the presence of a previous fracture) on the same day as the DXA measurement. The manufacturer's default protocol for in vivo imaging, image acquisition, analysis, and validation of the method has been described in detail (30–33) as has the protocol used at our center (25). Briefly, all scans were first analyzed using the manufacturer's standard evaluation protocol (software version V6.0) allowing the direct measurement of total and trabecular vBMD (Total vBMD; Tb.vBMD [milligrams of hydroxyapatite [HA]] per cubic centimeter) and Tb.vBMD (milligrams of HA per cubic centimeter) and trabecular number (Tb.N; [millimeters−1]). Trabecular thickness (Tb.Th) was then automatically calculated from Tb.N and Tb.vBMD. To characterize cortical and trabecular density and microarchitecture in greater detail, HR-pQCT images were processed by a semiautomated cortical bone segmentation technique as previously described (34). The outcome variables obtained from the extended cortical analysis included the following: cortical bone area fraction (ratio of cortical to total bone area [CtAr/TtAr] [percentage]); periosteal and endosteal perimeters (millimeters); cortical vBMD (Ct.vBMD [milligrams of HA per cubic centimeter]); cortical thickness (Ct.Th; millimeters); and cortical porosity (Ct.Po; percentage).
A microfinite element analysis solver provided by the manufacturer (Finite Element analysis software, version 1.15; Scanco Medical) was used to estimate the estimated failure load (kilonewton) and stiffness (kilonewton per millimeter) of the distal radius and the distal tibia after the uniaxial compression, as described previously (35).
Statistical analysis
Statistical analysis was performed using SAS 9.3 software (SAS Institute Inc). We used three different strategies to test our hypothesis that insulin resistance is positively associated with bone parameters. First, we divided women into quartiles according to the HOMA-IR, with the highest quartile representing the group with the greatest insulin resistance. An ANOVA or Fisher's exact tests were used to compare general subject characteristics in the various quartiles. BMD and bone microarchitecture results were similar among the bottom two quartiles and among the top two quartiles. Thus, the results were pooled into high HOMA-IR (top two quartiles) and low HOMA-IR (bottom two quartiles), and differences between the high HOMA-IR and low HOMA-IR groups were compared using independent t tests and expressed as SD scores (calculated by dividing the mean difference between the high HOMA-IR group and the low HOMA-IR group by the SD of the low HOMA-IR group). Second, to evaluate the association between HOMA-IR and bone, we used a univariate regression analysis to test linear trends between bone outcome variables and HOMA-IR (expressed as a continuous variable) in the entire cohort. Because the distribution of HOMA-IR was nonnormal, a log transformation was applied prior to analysis. We also performed multivariate linear regression analyses, adjusting for weight alone, and additionally for covariates that might have a strong independent effect on skeletal outcomes (eg, time since menopause, cigarette smoking, alcohol intake, physical activity, prior use of osteoporosis medications, systemic HRT or glucocorticoids). Third, to reduce the potential impact of obesity even further, we assessed the association between HOMA-IR and bone parameters independent of obesity by repeating univariate and multivariate linear regression analyses after the exclusion of obese individuals (defined as BMI > 30 kg/m2) from the analysis. All statistical tests were two tailed and the values of P < .05 were considered statistically significant.
Results
Cohort characteristics
One hundred seventy-three Caucasian women underwent HR-pQCT imaging, of whom 146 women were nondiabetic and postmenopausal and had available laboratory data (Table 1). The average age of the women, height, time since final menstrual period, physical activity score, and history of glucocorticoid and osteoporosis medication therapy use were similar between women across the quartiles of HOMA-IR. However, women in the higher quartiles of HOMA-IR were more likely to be overweight or obese than women in the lower quartiles (P < .001). Additionally, a history of smoking and prior exposure to HRT were significantly different among the women across the quartiles (P = .007 and P = .042, respectively).
Table 1.
General Characteristics of the Study Population Stratified by Quartiles of HOMA-IR
| All Subjects | Q1 | Q2 | Q3 | Q4 | P Valuea | |
|---|---|---|---|---|---|---|
| n | 146 | 37 | 36 | 37 | 36 | |
| Age, y | 60.3 ± 2.7 | 60.4 ± 2.6 | 60.5 ± 2.8 | 60.4 ± 3.0 | 60.0 ± 2.5 | .891 |
| Weight, kg | 74.5 ± 14.9 | 65.4 ± 10.8 | 71.4 ± 13.8 | 77.8 ± 13.3 | 83.9 ± 15.3 | <.001 |
| Height, cm | 164.5 ± 6.0 | 165.2 ± 5.8 | 164.5 ± 6.4 | 164.5 ± 5.8 | 163.7 ± 5.9 | .772 |
| BMI, kg/m2 | 27.5 ± 5.1 | 24.0 ± 3.9 | 26.3 ± 4.4 | 28.7 ± 4.5 | 31.2 ± 4.8 | <.001 |
| Tobacco use, n, % | 13 (9) | 0 | 3 (8) | 2 (5) | 8 (22) | .007 |
| Alcohol, ≥1 drink per day, n, % | 21 (14)7 | 7 (19) | 3 (8) | 2 (5) | 9 (25) | .060 |
| Time since menopause, y | 8.2 ± 2.9 | 8.3 ± 3.0 | 8.7 ± 2.7 | 7.8 ± 2.9 | 7.9 ± 3.0 | .509 |
| Medications, n, % | 17 (12) | 7 (19) | 4 (11) | 3 (8) | 3 (8) | .482 |
| Osteoporosis medications | 15 (10) | 6 (16) | 5 (14) | 2 (5) | 2 (5) | .359 |
| Glucocorticoids | 57 (39) | 20 (54) | 8 (22) | 16 (43) | 13 (36) | .042 |
| HRT | ||||||
| Physical activity scoreb | 8.7 ± 1.8 | 9.0 ± 1.8 | 9.1 ± 1.8 | 8.7 ± 1.3 | 8.1 ± 1.9 | .071 |
| Glucose, mg/dL | 89.3 ± 9.6 | 83.2 ± 5.5 | 85.8 ± 5.9 | 89.0 ± 5.6 | 99.4 ± 11.3 | <.001 |
| Insulin, μIU/mL | 9.2 ± 5.8 | 4.2 ± 0.8 | 6.4 ± 0.8 | 9.3 ± 1.6 | 17.2 ± 5.7 | <.001 |
| HOMA-IR | 2.1 ± 1.5 | 0.9 ± 0.2 | 1.4 ± 0.2 | 2.0 ± 0.4 | 4.2 ± 1.6 | <.001 |
Data are presented as mean ± SD or in numbers (percentages) as appropriate. Significant P values are in bold.
P values represent the significance of the differences in mean values across all the quartiles as calculated by an ANOVA.
Higher scores indicate increased physical activity.
Comparison of bone parameters between high and low HOMA-IR groups
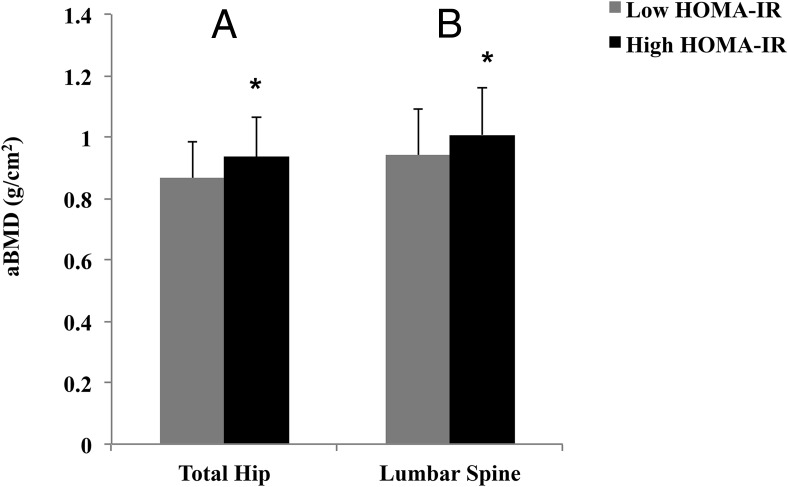
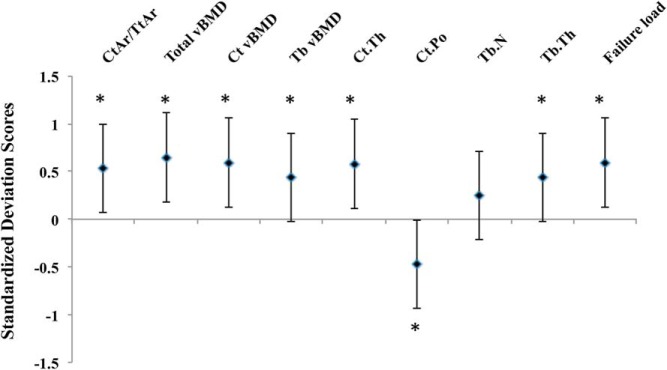
Women in the high HOMA-IR group (ie, HOMA-IR > 1.56) had greater aBMD at the total hip and lumbar spine (P = .001 and P = .011, respectively) compared with the women in the low HOMA-IR group (Figure 1). Women in the high HOMA-IR group had a higher CtAr/TtAr at the radius (P = .011) (Figure 2) and tibia (P = .002) (Figure 3) and higher total vBMD at the radius and the tibia (P ≤ .001 for both) in comparison with women in the low HOMA-IR group. The increase in total vBMD was driven by higher Tb.vBMD at both sites as well as higher Ct.vBMD at the tibia in those with higher levels of insulin resistance (P ≤ .01 for all). Cortical thickness was also greater in women in the high HOMA-IR group at both the radius and the tibia (P < .02 for all), whereas cortical porosity was lower at the tibia (P = .001). Furthermore, women in the high HOMA-IR group had higher Tb.N at the radius, and larger TbTh at the radius and the tibia (P < .05 for all). Finally, estimates of bone stiffness and failure load were greater at both sites (P ≤ .001 for all) in women in the high HOMA-IR group than in the low HOMA-IR group.
Figure 1.
Bar graphs of DXA measurements of the total hip (A) and lumbar spine (L1–L4) (B) for the low and high HOMA-IR groups. Asterisks represent significant differences (P < .05) between the groups.
Figure 2.
Standardized difference (in SD units ± 95% confidence interval) in bone microarchitecture variables at the distal radius for women in the top quartiles of HOMA-IR vs women in the lower quartiles. Zero line indicates the mean of the bottom two quartiles. Differences are expressed as the mean difference between the groups divided by the SD of the lower quartile group. The P value refers to the comparison between the top and bottom two quartiles (*, P < .05).
Figure 3.
Standardized difference (in SD units ± 95% confidence interval) in bone microarchitecture variables at the distal tibia for women in the top quartiles of HOMA-IR vs women in the lower quartiles. Zero line indicates the mean of the bottom two quartiles. Differences are expressed as the mean difference between the groups divided by the SD of the lower quartile group. The P value refers to the comparison between the top and bottom two quartiles (*, P < .05).
Associations between HOMA-IR and bone parameters
In univariate analyses, there was a positive association between HOMA-IR and total hip and lumbar spine aBMD (Table 2). However, these associations were reduced and/or eliminated in weight-adjusted models and multivariate models.
Table 2.
Univariate and Weight- and Multivariable-Adjusted Associations Between Log HOMA-IR and aBMD, Bone Size, and Bone Microarchitecture Parameters in Postmenopausal Women (n = 146)
| Unadjusted |
Weight Adjusted |
Multivariable Adjusteda |
||||
|---|---|---|---|---|---|---|
| β ± SEb | P Value | β ± SEb | P Value | β ± SEb | P Value | |
| DXA aBMD | ||||||
| Total hip | 0.337 ± 0.079 | <.001 | 0.166 ± 0.083 | .048 | 0.166 ± 0.089 | .060 |
| Lumbar spine | 0.190 ± 0.083 | .022 | 0.008 ± 0.086 | .920 | 0.009 ± 0.091 | .912 |
| Radius | ||||||
| Periosteal perimeter | −0.110 ± 0.083 | .189 | −0.256 ± 0.090 | .005 | −0.319 ± 0.094 | .001 |
| Endosteal perimeter | −0.179 ± 0.082 | .032 | −0.299 ± 0.090 | .001 | −0.369 ± 0.093 | <.001 |
| CtAr/TtAr | 0.226 ± 0.081 | .006 | 0.221 ± 0.092 | .018 | 0.288 ± 0.096 | .003 |
| Total vBMD | 0.270 ± 0.080 | .001 | 0.255 ± 0.091 | .006 | 0.301 ± 0.096 | .003 |
| Ct.vBMD | 0.143 ± 0.083 | .088 | 0.140 ± 0.094 | .137 | 0.166 ± 0.098 | .133 |
| Tb.vBMD | 0.223 ± 0.081 | .007 | 0.202 ± 0.092 | .030 | 0.199 ± 0.099 | .041 |
| Ct.Th | 0.219 ± 0.081 | .008 | 0.157 ± 0.091 | .088 | 0.218 ± 0.095 | .036 |
| Ct.Po | −0.105 ± 0.083 | .208 | −0.097 ± 0.094 | .302 | −0.084 ± 0.100 | .451 |
| Tb.N | 0.195 ± 0.082 | .018 | 0.107 ± 0.091 | .241 | 0.082 ± 0.098 | .370 |
| Tb.Th | 0.173 ± 0.082 | .037 | 0.224 ± 0.092 | .017 | 0.239 ± 0.100 | .016 |
| Stiffness | 0.223 ± 0.081 | .007 | 0.079 ± 0.088 | .374 | 0.070 ± 0.093 | .444 |
| Failure load | 0.217 ± 0.081 | .009 | 0.074 ± 0.088 | .401 | 0.059 ± 0.093 | .504 |
| Tibia | ||||||
| Periosteal perimeter | −0.145 ± 0.084 | .086 | −0.343 ± 0.089 | <.001 | −0.378 ± 0.096 | <.001 |
| Endosteal perimeter | −0.182 ± 0.083 | .030 | −0.401 ± 0.087 | <.001 | −0.466 ± 0.092 | <.001 |
| CtAr/TtAr | 0.325 ± 0.080 | <.001 | 0.400 ± 0.090 | <.001 | 0.457 ± 0.097 | <.001 |
| Total vBMD | 0.333 ± 0.080 | <.001 | 0.319 ± 0.091 | .001 | 0.362 ± 0.097 | <.001 |
| Ct.vBMD | 0.312 ± 0.081 | <.001 | 0.256 ± 0.091 | .006 | 0.268 ± 0.094 | .006 |
| Tb.vBMD | 0.198 ± 0.083 | .019 | 0.124 ± 0.094 | .188 | 0.141 ± 0.100 | .176 |
| Ct.Th | 0.341 ± 0.080 | <.001 | 0.351 ± 0.091 | .001 | 0.411 ± 0.096 | <.001 |
| Ct.Po | −0.218 ± 0.083 | .010 | −0.163 ± 0.094 | .084 | −0.179 ± 0.096 | .063 |
| Tb.N | 0.059 ± 0.085 | .483 | −0.123 ± 0.091 | .177 | −0.159 ± 0.097 | .104 |
| Tb.Th | 0.218 ± 0.083 | .009 | 0.316 ± 0.093 | .001 | 0.366 ± 0.098 | <.001 |
| Stiffness | 0.305 ± 0.081 | <.001 | 0.067 ± 0.082 | .414 | 0.089 ± 0.085 | .300 |
| Failure load | 0.291 ± 0.081 | <.001 | 0.033 ± 0.080 | .678 | 0.048 ± 0.083 | .563 |
Significant P values are in bold.
Adjusted for multiple covariates including weight, time since menopause, tobacco use, alcohol intake, physical activity, prior use of osteoporosis medications, systemic HRT, or glucocorticoids.
Standardized β-coefficients.
At the radius, in univariate analyses, HOMA-IR was positively associated with total vBMD, Tb.vBMD, Tb.N, Tb.Th, CtAr/TtAr, and Ct.Th as well as estimated compressive stiffness and failure load (Table 2). In weight-adjusted and multivariate-adjusted analyses, the strength of these associations was generally maintained, except for the loss of statistical significance for associations between HOMA-IR and Tb.N and estimates of bone strength. In comparison, adjustment for weight and other potential covariates revealed a significant inverse association between HOMA-IR and bone size (ie, periosteal and endosteal perimeters).
Associations between HOMA-IR and bone structure at the tibia were generally similar to those seen at the radius (Table 2). In particular, in univariate analyses, HOMA-IR was positively associated with total vBMD, Ct.vBMD, Tb.vBMD, Tb.Th, CtAr/TtAr, and Ct.Th as well as estimated stiffness and failure load. As at the radius, the strength of these associations were similar after weight and multivariable adjustments, except for loss of significance for associations between HOMA-IR and Tb.vBMD and estimates of bone strength. Similar to the radius, the negative association between HOMA-IR and bone size was apparent only in weight-adjusted and multivariable-adjusted models.
A prespecified sensitivity analysis in nonobese women (n = 102) demonstrated similar associations between HOMA-IR and bone parameters in univariate and multivariate-adjusted models (Supplemental Table 1).
Discussion
This is the first study to determine the association between insulin resistance and HR-pQCT-derived measures of bone geometry and microarchitecture as well as estimated compressive strength of the appendicular skeleton in healthy, nondiabetic, postmenopausal women. The results indicate that higher HOMA-IR is associated with greater vBMD and generally favorable bone microarchitecture at both weight-bearing and nonweight-bearing skeletal sites, independent of body weight. The associations between HOMA-IR and bone microarchitecture persisted after adjusting for multiple potential covariates including time since menopause and prior use of medications known to affect bone metabolism, suggesting that the presence of insulin resistance may protect, in part, against bone loss due to estrogen deficiency and/or aging in postmenopausal women and may contribute to higher aBMD and greater trabecular vBMD and microarchitecture consistently seen in individuals with T2D (9, 20, 36).
Consistent with our findings of a higher vBMD with increasing HOMA-IR, Abrahamsen et al (13) reported that insulin resistance assessed during an intravenous glucose tolerance test positively correlated with aBMD independent of BMI. Similarly, Reid et al (10), using quantitative CT, identified a positive association between vBMD and circulating insulin levels, independent of fat mass and body weight. Several other DXA-based studies have consistently shown a significant positive association between aBMD and circulating insulin levels (11, 12, 14) or HOMA-IR (15, 16), although these relationships were muted after accounting for body weight in some of the studies (14–16). In contrast, Shin et al (18) reported an inverse relationship between HOMA-IR and aBMD in a population-based study of young South Korean men (mean age 49.9 ± 0.6 y), suggesting that insulin resistance was a negative predictor of bone health. Our current study differs from the study by Shin et al (18) in several aspects: our subjects were older (mean age 60.3 ± 2.7 y) postmenopausal Caucasian women, and importantly, our subjects were nondiabetic. It is currently unknown whether the effects of insulin resistance or hyperinsulinemia on bone are age, gender, or race specific. However, the inclusion of patients with diabetes in the aforementioned study (18) may confound results due to the difficulty in interpretation and unreliability of HOMA-IR estimation in subjects on antidiabetic medications, as well as the potential effects of chronic hyperglycemia and/or antidiabetic medications on skeletal microarchitecture, making it difficult to make reliable comparisons with our study.
Our finding of a positive association between HOMA-IR and bone microarchitecture is consistent with recent studies comparing bone microarchitecture in obese and nonobese men and women (7, 24). Sornay-Rendu et al (24) reported higher total, cortical, and trabecular vBMD and superior trabecular and cortical microarchitecture at the distal radius and tibia in elderly obese women. Similar findings were also reported by Evans et al (7) in young and elderly adults. At first glance, it would appear that the positive effect of insulin resistance on bone microarchitecture is due to the concomitant obesity because in our study, women with higher HOMA-IR had higher body weight and BMI than women with lower HOMA-IR. It is possible that factors related to obesity other than insulin resistance, such as greater circulating estrogen in obesity (37) and the possible positive effects of leptin on the skeleton (38), might be responsible for the beneficial effects of obesity on the skeleton. However, we specifically addressed potential confounding by weight by showing that the positive associations between HOMA-IR and volumetric bone density and microarchitecture were independent of body weight and furthermore that the strength of these associations, particularly for the trabecular compartment, were similar at the nonweight-bearing distal radius and weight-bearing distal tibia. Moreover, a separate analysis in nonobese women in our study revealed similar results, further substantiating the notion that the effects of HOMA-IR on bone are mediated independently from body weight.
In line with the notion that insulin is osteoanabolic, the increased bone mass seen in patients with T2D (9) has been attributed partly to the insulin resistance seen in these patients (15). Our findings of a positive association between insulin resistance (a harbinger of future T2D) and superior total and trabecular bone density and microarchitecture are consistent with previous studies of bone microarchitecture by HR-pQCT in postmenopausal women with T2D that suggested a relatively well-preserved or slightly improved trabecular microarchitecture at the distal radius and tibia (20–23, 39). However, unlike some of these prior studies that reported an increase in cortical porosity in adults with T2D (20, 21), we found a tendency toward an inverse association between HOMA-IR and cortical porosity in our nondiabetic cohort. Bearing in mind the cross-sectional design of the studies, it could be speculated that during the early years prior to the development of diabetes, the presence of insulin resistance is associated with high bone mass and favorable bone microarchitecture. With the development and progression to full-blown diabetes, other negative factors, such as the relative decline in hyperinsulinemia, chronic hyperglycemia, accumulation of advanced glycation end products, oxidative stress, and the development of chronic complications of diabetes, gain ascendency, leading to the decline in bone mass and microstructure, reflected in particular, for reasons that are presently unclear, by an increase in cortical porosity.
In weight-adjusted and multivariate models, we found an inverse association between HOMA-IR and periosteal circumference at the distal radius and tibia, suggesting lower bone size in women with greater insulin resistance. This is a surprising finding if it is assumed that insulin has a direct anabolic effect on bone (2), which might be expected to lead to increased bone size via periosteal apposition (40). However, hyperinsulinism may have indirect effects on bone, such as increasing the free concentrations of androgens and estrogens (due to a negative impact on SHBG) (41). Indeed, our findings of decreased bone size and increased cortical thickness in women with greater insulin resistance are analogous to the estrogen-replete skeletal phenotype of decreased periosteal apposition and reduced endocortical bone resorption (42). The smaller bone size associated with higher HOMA-IR may explain why we failed to demonstrate a positive association between HOMA-IR and estimates of bone strength after weight adjustment. Because microfinite element analysis-derived estimates reflect loading in pure axial compression and resistance to compressive loading is directly proportional to the cross-sectional area, the negative effect of smaller bone size may have offset the advantages in bone density and microarchitecture associated with higher HOMA-IR, even after multivariate adjustment.
A limitation of the study is the cross-sectional design, allowing only suggestions of causal relationships, necessitating further prospective studies. In particular, the exact mechanisms underlying insulin's role in bone homeostasis are still unclear, as is the question of whether bone cells also develop resistance to insulin's actions similar to what is observed in muscle and adipose tissues. Moreover, this study focused on postmenopausal women of Caucasian ethnicity, precluding the generalizability of our results to a non-Caucasian population. Note that we detected interactions with race in the larger SWAN cohort, which suggests that postmenopausal African-American women may have a different relationship with HOMA-IR and bone microarchitecture, but we were unable to make further conclusions in this group due to the relatively small sample. Future studies including a large number of subjects across a wide age distribution in both genders, as well as in other ethnicities, are necessary to further address this issue. On the other hand, our relatively homogeneous study population (in terms of race, age, months since menopause, and physical activity levels) may have strengthened our findings because many of these factors are known to profoundly impact insulin resistance and bone morphology.
In summary, data from our study indicate that the presence of peripheral insulin resistance and hyperinsulinemia have a generally favorable outcome on bone density and microarchitecture independent of body size in healthy, nondiabetic, postmenopausal women. These results suggest the possibility that bone cells may remain sensitive to the actions of insulin in conditions of peripheral insulin resistance, although further experimental studies at the bone tissue and cellular levels are needed to confirm this notion.
Acknowledgments
We thank the Massachusetts General Hospital Bone Density Center and the Massachusetts General Hospital HR-pQCT Core Facility.
The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging, the National Institute of Nursing Research, the Office of Research on Women's Health, or the National Institutes of Health.
The Study of Women's Health Across the Nation has grant support from the National Institutes of Health (NIH), Department of Health and Human Services, through the National Institute on Aging, the National Institute of Nursing Research, and the NIH Office of Research on Women's Health Grants U01NR004061, U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, and U01AG012495. We are grateful to the Massachusetts General Hospital Bone Density Center and the Massachusetts General Hospital HR-pQCT Core Facility, which is supported by NIH Grant 1S10RR023405. E.W.Y. was supported by NIH Grant DK093713.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- aBMD
- areal BMD
- BMD
- bone mineral density
- BMI
- body mass index
- CT
- computed tomography
- CtAr/TtAr
- ratio of cortical to total bone area
- Ct.Th
- cortical thickness
- Ct.vBMD
- cortical vBMD
- DXA
- dual-energy X-ray absorptiometry
- HA
- hemagglutinin
- HOMA-IR
- homeostasis model assessment of insulin resistance
- HR-pQCT
- high-resolution peripheral quantitative CT
- HRT
- hormonal replacement therapy
- Q
- quartile
- SWAN
- Study of Women's Health Across the Nation
- Tb.N
- trabecular number
- TbTh
- trabecular thickness
- Tb.vBMD
- trabecular vBMD
- T2D
- type 2 diabetes
- vBMD
- volumetric BMD.
References
- 1. Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288(21):2709–2716. [DOI] [PubMed] [Google Scholar]
- 2. Fulzele K, Riddle RC, DiGirolamo DJ, et al. Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell. 2010;142(2):309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thrailkill KM, Lumpkin CK, Jr, Bunn RC, Kemp SF, Fowlkes JL. Is insulin an anabolic agent in bone? Dissecting the diabetic bone for clues. Am J Physiol Endocrinol Metab. 2005;289(5):E735–E745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maor G, Karnieli E. The insulin-sensitive glucose transporter (GLUT4) is involved in early bone growth in control and diabetic mice, but is regulated through the insulin-like growth factor I receptor. Endocrinology. 1999;140(4):1841–1851. [DOI] [PubMed] [Google Scholar]
- 5. Ogata N, Chikazu D, Kubota N, et al. Insulin receptor substrate-1 in osteoblast is indispensable for maintaining bone turnover. J Clin Invest. 2000;105(7):935–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dagogo-Jack S, al-Ali N, Qurttom M. Augmentation of bone mineral density in hirsute women. J Clin Endocrinol Metab. 1997;82(9):2821–2825. [DOI] [PubMed] [Google Scholar]
- 7. Evans AL, Paggiosi MA, Eastell R, Walsh JS. Bone density, microstructure and strength in obese and normal weight men and women in younger and older adulthood. J Bone Miner Res. 2015;30(5):920–928. [DOI] [PubMed] [Google Scholar]
- 8. Christensen JD, Lungu AO, Cochran E, et al. Bone mineral content in patients with congenital generalized lipodystrophy is unaffected by metreleptin replacement therapy. J Clin Endocrinol Metab. 2014;99(8):E1493–E1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ma L, Oei L, Jiang L, et al. Association between bone mineral density and type 2 diabetes mellitus: a meta-analysis of observational studies. Eur J Epidemiol. 2012;27(5):319–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reid IR, Evans MC, Cooper GJ, Ames RW, Stapleton J. Circulating insulin levels are related to bone density in normal postmenopausal women. Am J Physiol. 1993;265(4 Pt 1):E655–E659. [DOI] [PubMed] [Google Scholar]
- 11. Stolk RP, Van Daele PL, Pols HA, et al. Hyperinsulinemia and bone mineral density in an elderly population: the Rotterdam Study. Bone. 1996;18(6):545–549. [DOI] [PubMed] [Google Scholar]
- 12. Barrett-Connor E, Kritz-Silverstein D. Does hyperinsulinemia preserve bone? Diabetes Care. 1996;19(12):1388–1392. [DOI] [PubMed] [Google Scholar]
- 13. Abrahamsen B, Rohold A, Henriksen JE, Beck-Nielsen H. Correlations between insulin sensitivity and bone mineral density in non-diabetic men. Diabet Med. 2000;17(2):124–129. [DOI] [PubMed] [Google Scholar]
- 14. Haffner SM, Bauer RL. The association of obesity and glucose and insulin concentrations with bone density in premenopausal and postmenopausal women. Metabolism. 1993;42(6):735–738. [DOI] [PubMed] [Google Scholar]
- 15. Dennison EM, Syddall HE, Aihie Sayer A, Craighead S, Phillips DI, Cooper C. Type 2 diabetes mellitus is associated with increased axial bone density in men and women from the Hertfordshire Cohort Study: evidence for an indirect effect of insulin resistance? Diabetologia. 2004;47(11):1963–1968. [DOI] [PubMed] [Google Scholar]
- 16. Srikanthan P, Crandall CJ, Miller-Martinez D, et al. Insulin resistance and bone strength: findings from the study of midlife in the United States. J Bone Miner Res. 2014;29(4):796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ishii S, Cauley JA, Crandall CJ, et al. Diabetes and femoral neck strength: findings from the Hip Strength Across the Menopausal Transition Study. J Clin Endocrinol Metab. 2012;97(1):190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shin D, Kim S, Kim KH, Lee K, Park SM. Association between insulin resistance and bone mass in men. J Clin Endocrinol Metab. 2014;99(3):988–995. [DOI] [PubMed] [Google Scholar]
- 19. Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes—a meta-analysis. Osteoporos Int. 2007;18(4):427–444. [DOI] [PubMed] [Google Scholar]
- 20. Burghardt AJ, Issever AS, Schwartz AV, et al. High-resolution peripheral quantitative computed tomographic imaging of cortical and trabecular bone microarchitecture in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2010;95(11):5045–5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yu EW, Putman MS, Derrico N, Abrishamanian-Garcia G, Finkelstein JS, Bouxsein ML. Defects in cortical microarchitecture among African-American women with type 2 diabetes. Osteoporos Int. 2015;26(2):673–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Patsch JM, Burghardt AJ, Yap SP, et al. Increased cortical porosity in type 2 diabetic postmenopausal women with fragility fractures. J Bone Miner Res. 2013;28(2):313–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shu A, Yin MT, Stein E, et al. Bone structure and turnover in type 2 diabetes mellitus. Osteoporos Int. 2012;23(2):635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sornay-Rendu E, Boutroy S, Vilayphiou N, Claustrat B, Chapurlat RD. In obese postmenopausal women, bone microarchitecture and strength are not commensurate to greater body weight: the Os des Femmes de Lyon (OFELY) study. J Bone Miner Res. 2013;28(7):1679–1687. [DOI] [PubMed] [Google Scholar]
- 25. Putman MS, Yu EW, Lee H, et al. Differences in skeletal microarchitecture and strength in African-American and white women. J Bone Miner Res. 2013;28(10):2177–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36(5):936–942. [DOI] [PubMed] [Google Scholar]
- 27. Santoro N, Torrens J, Crawford S, et al. Correlates of circulating androgens in mid-life women: the Study of Women's Health Across the Nation. J Clin Endocrinol Metab. 2005;90(8):4836–4845. [DOI] [PubMed] [Google Scholar]
- 28. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487–1495. [DOI] [PubMed] [Google Scholar]
- 29. Bonora E, Targher G, Alberiche M, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23(1):57–63. [DOI] [PubMed] [Google Scholar]
- 30. Boutroy S, Bouxsein ML, Munoz F, Delmas PD. In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab. 2005;90(12):6508–6815. [DOI] [PubMed] [Google Scholar]
- 31. Laib A, Ruegsegger P. Calibration of trabecular bone structure measurements of in vivo three-dimensional peripheral quantitative computed tomography with 28-microm-resolution microcomputed tomography. Bone. 1999;24(1):35–39. [DOI] [PubMed] [Google Scholar]
- 32. Laib A, Hauselmann HJ, Ruegsegger P. In vivo high resolution 3D-QCT of the human forearm. Technol Health Care. 1998;6(5–6):329–337. [PubMed] [Google Scholar]
- 33. Laib A, Hildebrand T, Hauselmann HJ, Ruegsegger P. Ridge number density: a new parameter for in vivo bone structure analysis. Bone. 1997;21(6):541–546. [DOI] [PubMed] [Google Scholar]
- 34. Burghardt AJ, Buie HR, Laib A, Majumdar S, Boyd SK. Reproducibility of direct quantitative measures of cortical bone microarchitecture of the distal radius and tibia by HR-pQCT. Bone. 2010;47(3):519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Boutroy S, Van Rietbergen B, Sornay-Rendu E, Munoz F, Bouxsein ML, Delmas PD. Finite element analysis based on in vivo HR-pQCT images of the distal radius is associated with wrist fracture in postmenopausal women. J Bone Miner Res. 2008;23(3):392–399. [DOI] [PubMed] [Google Scholar]
- 36. Melton LJ, 3rd, Riggs BL, Leibson CL, et al. A bone structural basis for fracture risk in diabetes. J Clin Endocrinol Metab. 2008;93(12):4804–4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Callewaert F, Sinnesael M, Gielen E, Boonen S, Vanderschueren D. Skeletal sexual dimorphism: relative contribution of sex steroids, GH-IGF1, and mechanical loading. J Endocrinol. 2010;207(2):127–134. [DOI] [PubMed] [Google Scholar]
- 38. Blain H, Vuillemin A, Guillemin F, et al. Serum leptin level is a predictor of bone mineral density in postmenopausal women. J Clin Endocrinol Metab. 2002;87(3):1030–1035. [DOI] [PubMed] [Google Scholar]
- 39. Farr JN, Drake MT, Amin S, Melton LJ, 3rd, McCready LK, Khosla S. In vivo assessment of bone quality in postmenopausal women with type 2 diabetes. J Bone Miner Res. 2014;29(4):787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kawai M, Rosen CJ. Insulin-like growth factor-I and bone: lessons from mice and men. Pediatr Nephrol. 2009;24(7):1277–1285. [DOI] [PubMed] [Google Scholar]
- 41. Birkeland KI, Hanssen KF, Torjesen PA, Vaaler S. Level of sex hormone-binding globulin is positively correlated with insulin sensitivity in men with type 2 diabetes. J Clin Endocrinol Metab. 1993;76(2):275–278. [DOI] [PubMed] [Google Scholar]
- 42. Seeman E. Estrogen, androgen, and the pathogenesis of bone fragility in women and men. Curr Osteoporos Rep. 2004;2(3):90–96. [DOI] [PubMed] [Google Scholar]