Abstract
Electrospinning is the process by which a scaffold containing micrometer and nanometer diameter fibers are drawn from a polymer solution or melt using a large voltage gradient between a polymer emitting source and a grounded collector. Ramakrishna and colleagues first investigated electrospun fibers for neural applications in 2004. After this initial study, electrospun fibers are increasingly investigated for neural tissue engineering applications. Electrospun fibers robustly support axonal regeneration within in vivo rodent models of spinal cord injury. These findings suggest the possibility of their eventual use within patients. Indeed, both spinal cord and peripheral nervous system regeneration research over the last several years shows that physical guidance cues induce recovery of limb, respiration, or bladder control in rodent models. Electrospun fibers may be an alternative to the peripheral nerve graft (PNG), because PNG autografts injure the patient and are limited in supply, and allografts risk host rejection. In addition, electrospun fibers can be engineered easily to confront new therapeutic challenges. Fibers can be modified to release therapies locally or can be physically modified to direct neural stem cell differentiation. This review summarizes the major findings and trends in the last decade of research, with a particular focus on spinal cord injury. This review also demonstrates how electrospun fibers can be used to study the central nervous system in vitro.
Key words: : axonal regeneration, in vitro studies, in vivo studies, spinal cord injury
Spinal Cord Injury and Guidance Strategies for Regeneration
Methylprednisolone is currently the only drug approved by the Food and Drug Administration (FDA) for treatment of patients with spinal cord injury (SCI). There is skepticism among some clinicians about its efficacy, however.1 This steroidal treatment protects neurons by reducing inflammation after SCI, but it is only marginally effective at preventing further damage after SCI.2 While approaches that provide neuroprotection have increased the life expectancy of persons living with SCI,3 there remains no method of neuroregeneration to restore lost function.
With an estimated 12,000 new cases of SCI added each year and at an individual cost of more than $1.5 million over the lifetime of a patient,4 there is incentive to find a treatment option that restores function and reduces chronic injury. New methods provide regenerating neurons with both factors and guidance cues to overcome the barriers to regeneration.
Many cell- and drug-based therapies have been introduced for the purpose of restoring lost function. There are three primary approaches to SCI: (1) transplanting cells to repopulate the region, (2) reducing the physical and chemical barriers for regenerating cells, or (3) promoting growth through a series of factors. Transplantation of neural stem cells has received the most attention within the decade, and in the United States, there is one completed clinical trial and four ongoing clinical trials that use neural stem cells to repair SCI.
A recent alternative to stem cell-based therapies, however, uses a peripheral nerve graft (PNG), and this approach has produced evidence of functional recovery equivalent to pre-injury levels in animal models of SCI. PNGs use sections of nerve from the peripheral nervous system and can either be autografts or allografts. The first study that used a PNG in a SCI model showed partial recovery of limb function.5 Studies from Houle and associates6 and Alilain and colleagues7 present an effective synergistic approach where a PNG is used with the glycosaminoglycan degrading enzyme chondroitinase ABC (ChABC). The synergistic approach partially restored limb function6 and completely restored respiratory function.7
Recently, an intercostal nerve graft in combination with ChABC and acidic fibroblast growth factor (aFGF) restored the complex set of systems involved in bladder control.8 Strategies that promote functional recovery using a nerve graft to guide regenerating axons are an important divergence from past approaches that focused on the application of cells or biologically active agents alone.
The application of a biologically active agent alone has failed to restore lost function to the same extent as when a nerve graft is used, and this important concept was validated by Alilain and colleagues7 in a rat model of SCI. ChABC (in the study by Alilain and colleagues7) use within rodent injury models over the last decade was recently reviewed.9 The appeal of ChABC stems from the ability of the enzyme to degrade glycosaminoglycans attached to the protein core of chondroitin sulfate proteoglycans (CSPGs). Glycosaminoglycans inhibit axonal extension through protein tyrosine phosphatase sigma (PTPσ) signaling.10
Importantly, the study by Alilain and colleagues7 demonstrated that the use of ChABC alone did not restore respiratory function to the same extent as when ChABC was combined with a PNG.7 Thus, the inclusion of a guidance strategy is imperative when the experimental approach is focused on restoring lost function.
Electrospinning (presented in several recent reviews11–13 and briefly described later in this review) is a technique used to generate fibers with nano to micro scale diameters. Electrospun fibers mimic the scale and high surface area to volume ratio found in the extracellular matrix and are currently studied as synthetic nerve guides. Synthetic guides may be a desirable alternative to natural nerve guides because allografts may induce an inflammatory response and autografts create loss of function or sensation at the harvest site. Further, electrospinning of nano or microfibers can be performed using many types of FDA-approved polymers such as polylactic acid (PLA), polyglycolic acid (PGA), and polycaprolactone (PCL).
Pioneering work from the group at the National University of Singapore introduced electrospun fibers as a promising scaffold for neural regeneration a decade ago.14,15 Since then, many studies have attempted to engineer electrospun fibers to better guide the extension of axons.
More recently, electrospun fibers have been used to create in vitro culture systems that recapitulate the topographical features observed in the native spinal cord. These new topographical tools are used to understand cell behavior on topography known to exist in the white matter tracts. For example, new in vitro culture systems have been created using patterned electrospun fibers and smooth films to study how astrocytes respond to local changes in surface topography16,17 that may be similar to topographical changes after SCI. In subsequent sections of this review, electrospun fiber research over the past decade in the context of SCI is presented, both as a material to promote spinal cord regeneration and as tools to better understand how topography affects the response of cells known to exist in the central nervous system (CNS).
Fabrication of Fibers Using Electrospinning
Creation of polymer fibers from electrospinning is accomplished by applying a high voltage to a small bead of polymer solution residing on the tip of a needle. The high voltage propels a solvent/polymer jet toward a grounded collector. Over several milliseconds, the jet elongates to around 10,000 times its original length and solidifies (by solvent evaporation) as it whips unstably through the air.18 Rapid elongation of the jet and the high charge density in the polymer cause the jet to bend into a series of coils.19,20 As a consequence, fibers fall randomly onto the collection surface without any specific orientation. These fibers can be aligned, however, if collected on an oscillating collection plate,21 a rotating disc,22 or between two grounded plates.23 By tuning these collection parameters, fiber orientation can range from random to highly aligned.22,24–26
The versatility of the electrospinning process allows researchers to observe the effects of both fiber alignment and fiber diameter on neural cell models. Electrospun fibers can be generated with diameters ranging from tens of microns to tens of nanometers, with the smallest diameter fibers containing fewer than 10 elongated polymer chains.18 The simplest method of altering fiber diameter is to change the concentration of polymer in the electrospinning solution, where a higher concentration of polymer leads to a larger fiber diameter27 because of an increased solution viscosity.28,29 Other factors, however, influence fiber diameter such as the dielectric constant of the electrospinning solvent,30 the distance between the electrospinning tip and the collector,31 and vapor pressure of the solvent.32 These methods of controlling fiber diameter make electrospinning a versatile tool for scientific research.
The following sections present research that has investigated how neurite extension was affected by fiber geometry (fiber alignment and diameter), the material from which the fiber was made (including surface chemistry modification), and drug and protein delivery from the fibers. Fiber geometry is presented first because fiber geometry is easily modified and generally results in a drastic change in neurite extension. Material and chemical composition of the fibers is presented next because attempts to improve neurite guidance by changing materials or chemical modification tend to produce marginal differences in neurite extension compared with traditional materials alone (polycaprolactone or polylactic acid). Finally, drug and protein delivery are presented last because of the complexity of drug release and other considerations such as protein denaturation.
The importance of electrospun fiber geometry in directing the extension of neurites
Electrospun fiber alignment, neurite extension, and axonal guidance
The first study to combine electrospun fibers with nerve cells was conducted in 2004. Neural stem cells (multipotent neural cell line, C17.233) were placed on randomly oriented poly-l-lactic acid (PLLA) fibers.14 C17.2 cells cultured on fibers adhered more strongly when compared with cells cultured on a polymer film control. A subsequent study from the same group using aligned PLLA fibers revealed the capability of aligned fibers to guide neurite extension from C17.2 cells along the length of the fibers.34 The ability of electrospun fibers to direct the extension of neurites is similar to other engineered approaches where finely grooved surfaces35 and hydrogels with partially aligned fibrous networks (to a limited extent) were capable of directing neurite guidance.36
The ability of aligned electrospun fibers to direct the extension of neural processes is now routinely reported, using both primary neurons and neural cell lines. Dorsal root ganglia (DRG) cultures are useful in vitro models for the study of a biomaterial's ability to direct neurite extension.37 DRG extend longer neurites than neurites extending from cell line cultures. Thus, if the electrospun fiber approach is meant to induce longer neurite extension, then a DRG model may be more appropriate than a cell line.
DRG placement onto electrospun fibers routinely occurs within studies demonstrating the ability of aligned electrospun fibers to direct neurite extension. The correlation between fiber alignment and neurite extension from embryonic rat DRG was demonstrated in pioneering experiments by Corey and coworkers38 using aligned PLLA electrospun fibers. In general, electrospun fiber scaffolds with greater fiber alignment result in a more elongated DRG body, with longer neurites that align to the orientation of fibers compared with results where DRG were cultured on randomly organized fibers. The study by Corey and coworkers38 also included the use of neuroblastoma cell lines (neuroblastoma cell lines SH-EP39 and SH-SY5Y40), and these cells extended neurites along the electrospun fibers.
In an article published by Chow and colleagues,41 DRG from embryonic day 16 rats were cultured on both aligned and randomly oriented polydioxanone (PDS) fibers. This study was unique because the DRG were cultured on top of electrospun fibers with adherent astrocytes. The presence of astrocytes significantly increased the length of neurites from DRG cultured on the electrospun fibers.
Schnell and associates42 cultured embryonic day 10 DRG from chicken on aligned electrospun fibers composed of PCL or a blend of collagen type I and PCL (mixed 1:3 [w/w]) to study the ability of collagen to promote long extension of neurites. Interestingly, Schnell and colleagues42 collected fibers on a surface coated with star-poly(ethylene glycol), which was used to adhere fibers to the collection surface, but also prevented cell adhesion to the collection surface. There was no comparison of neurite lengths when DRG were cultured on aligned or randomly oriented fibers, but neurites from whole and dissociated DRG traveled along the length of both PCL and PCL/blended fibers. The DRG experiments from these initial studies confirm the ability of electrospun fibers to guide the extension of neurites, with aligned fibers inducing the longest neurite extension.
From these initial studies, one can easily conclude that neurites extend parallel to fiber orientation and extend longer processes when the electrospun fibers are aligned. Since these initial studies, several publications document the ability of aligned, electrospun fibers to induce long neurite extension from DRG. Several subsequent studies document similar results stating that different cells from different animal sources all extend neurites along aligned fibers. DRG from postnatal day 1,43 day 4, and day 1648 rat pups,44,45 DRG from day 8 chicken,46,47 PC12 cells (rat adrenal pheochromocytoma cells49),50,51 and C17.2 cells52,53 all increased neurite extension along aligned, electrospun fibers compared with neurite extension observed on randomly oriented fiber controls.
Also, different types of neurons respond to aligned fibers in unique ways. In a study comparing DRG neurite outgrowth and hippocampal neurite outgrowth, DRG neurites extended along the length of aligned fibers while hippocampal neurites extended both parallel and perpendicular to the aligned fibers.54 Recently, the need for fibers to be aligned to direct neurite extension was challenged in a study revealing neurite extension can occur perpendicular to the direction of fiber alignment when the fiber density was high (3000 fibers/mm).55 The study, however, did not quantify fiber alignment, so it is difficult to determine whether fiber alignment played a role in their results.
From the above studies, neurites extend to a greater extent on aligned fibers compared with neurite extension on randomly oriented fibers. The literature presents two explanations for why aligned fibers more efficiently direct neurite extension. The first explanation is supported by observations in cell lines. C17.2 and PC12 derived neurons are more viable when cultured on aligned fibers compared with randomly oriented fibers,24,56–58 and increased viability was also confirmed when primary neural progenitor cells from rats were cultured on fibers.59
Most of these studies assess cell viability with either a metabolic assay (without normalizing to cell count) or by a simple cell count, meaning an increase in cellular viability could merely represent an increase in the rate of cellular proliferation. If fibers enable neural cells to attach and cells in culture possess greater viability, then it is reasonable to assume that cells on fibers will extend longer processes than cells having difficulty attaching to the substrate.
The second explanation revolves on the premise that aligned fibers do not have as many barriers to extension as do randomly organized fibers. Studies analyzing the effect of fiber crossing inhibiting neurite extension reveal the ability of crossed fibers to stop neurite extension. As stated previously, a study from Corey and coworkers38 demonstrated that increased neurite extension correlated with increased fiber alignment. In addition, a study by Wang and colleagues25 developed a method for generating highly aligned electrospun fibers. This study also introduced fiber scaffolds with two fiber layers of highly aligned PLLA fibers oriented at 45-degree angles to each other. Interestingly, when DRG from day 9 chickens were cultured on these scaffolds, neurites extended along the top layer of fibers until they came into contact with the underlying fibers oriented at a 45-degree angle, at which point the neurites did not extend. The results from these two studies demonstrate that aligned fibers improve total neurite extension and guide neurites along the length of the fibers, while randomly oriented fibers restrict neurite extension.
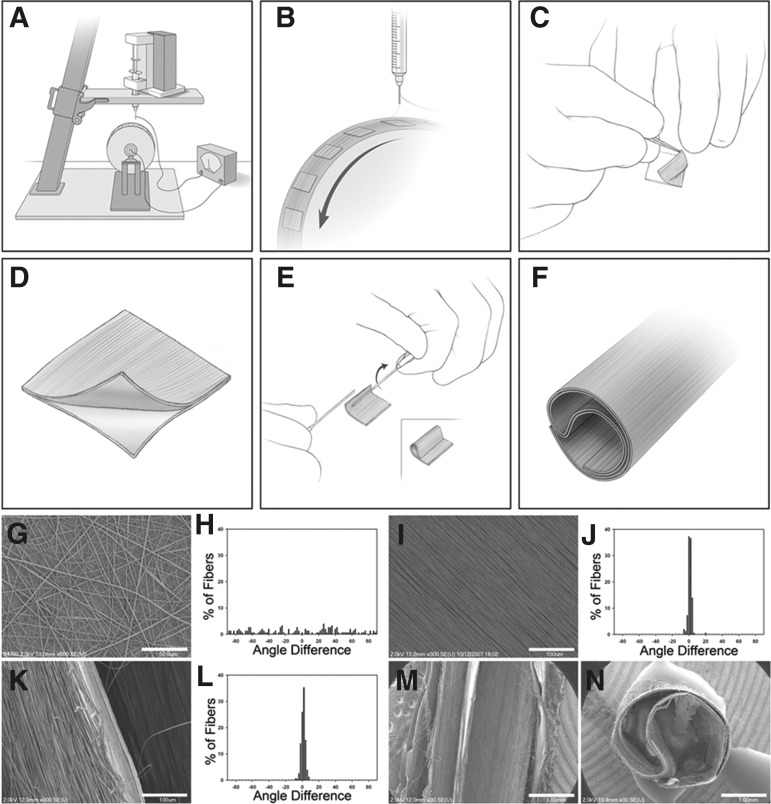
The importance of fiber alignment within in vivo models of SCI was also demonstrated by Hurtado and associates.45 To apply the fibers within a three-dimensional in vivo SCI environment, an innovative approach was used to place fibers within a conduit construct (Fig. 1). Specifically, in this study, Hurtado and associates45 placed aligned or randomly oriented fibers in such a conduit structure within a complete transection rat model (T9–T10, 3 mm gap) of acute SCI.45 In this study, Hurtado and associates45 verified that aligned PLLA microfibers permitted the greatest extension of pioneering axons (2055 μm) 4 weeks after implantation. The result was statistically different from axonal extension in rats with randomly oriented PLLA fibers (1162 μm) or a PLLA film (413 μm).
FIG. 1.
The panel A–F provides a schematic representation of conduit fabrication. PLLA fibers are electrospun onto coverslips coated with PLLA films on a rotating mandrel (A,B). The film and fibers are removed from the coverslip (C) and placed back-to-back, so the films meet, and are rolled into a conduit (D,E). The final conduit shape is shown as a schematic (F) and the lumen (M) and coronal view (N) are shown under Scanning electron microscopy (SEM.) Random fibers were produced by electrospinning onto a stationary target (G) while aligned fibers were produced using the rotating mandrel (I). Aligned fibers in the conduit maintained their alignment through the processing in steps C–E (K,L). The alignment for each group was determined by SEM imaging. Alignment was reported as a histogram of the angle differences from the median fiber orientation for 150 fibers per condition (H,J,L). Scale Bars: (G) 50 μm; (I, K) 100 μm; (M,N) 1 mm. This figure was reproduced from Hurtado and associates.45
In contrast to the study from Hurtado and associates,45 Liu and coworkers60 found that rats with a complete spinal cord transection (C3, 2.5 mm gap) had little neurite sprouting into the lesion regardless of scaffold alignment. The differences between these two studies could be the result of differences in fiber diameter, because Hurtado and associates45 used microscale fiber diameters while Liu and coworkers60 used nanoscale fiber diameters (nearly an order of magnitude difference). Material selection may have also been a factor, because the study from Hurtado and associates45 used PLLA, while the study from Liu and coworkers60 used collagen.
Because of the discrepancy between the results of these two studies, it may be beneficial to compare with trends seen in the peripheral nervous system. In a rat tibial nerve model, aligned poly(acrylonitrile-co-methylacrylate) fibers improved axonal extension compared with extension on random electrospun fibers.43 Using the same model, aligned PCL fibers yielded increased axonal density compared with randomly aligned PCL scaffolds.61 Therefore, it may be possible to attribute results of the study by Liu and coworkers60 in the spinal cord to the use of collagen and small diameter fibers (<400 nm), two factors that could potentially reduce axonal guidance and extension (described later in this review).
Few in vivo studies, however, have been performed using electrospun scaffolds in the spinal cord. While evidence supports electrospun fiber alignment as a strong factor in improving axonal guidance, further work is required to understand how fiber alignment aids in axonal guidance and extension in vivo.
Neurite response to electrospun fiber scaffolds of varying diameter
Although there was significant focus on developing fibers with nanoscale diameters when electrospun fibers were first being investigated as neuron regeneration scaffolds, studies focusing on the effects of fiber diameter suggest a unique response of neurons and neural stem cells to fibers on the nanoscale versus the microscale. In 2005, Yang and colleagues34 placed C17.2 cells on aligned and randomly oriented electrospun PLLA fibers with a mean diameter of 300 nm (nanofibers) and on fibers with a mean diameter of 1.5 μm (microfibers). From this study, aligned nanofibers induced longer neurite outgrowth than fibers with diameters on the microscale.
In addition to this finding, a large portion of the C17.2 cells preferentially differentiated into neurons on nanofibers (∼80%) compared with microfibers (∼40%) regardless of fiber orientation.34 This initial study examining the role of fiber diameter in inducing neurite extension also suggests fiber diameter is as important as alignment in directing the extension of neural processes.
The results from C17.2 cells cultured on fibers of varying diameter initially proposed the idea of nanoscale diameter fibers being superior to microscale diameters for neural regeneration applications. Nanoscale fiber diameters, however, do not always provide optimum results when directing the extension of neurites. He and associates52 created unique electrospun PLLA scaffolds (both aligned and randomly oriented) with diameters ranging from 300 nm to 900 nm in 200 nm increments. C17.2 cells cultured on scaffolds with a mean fiber diameter of 500 nm induced the longest neurite extension and the greatest amount of neuronal differentiation.52
Unlike their previous study,34 C17.2 differentiation was highly dependent on fiber alignment as more cells differentiated into a neuronal lineage on aligned fibers regardless of fiber diameter. This result led the authors to conclude that fibers with a 500 nm diameter were optimal for neurite guidance. The authors did acknowledge, however, that their observation might be a result of the high degree of neuronal differentiation of the C17.2 cells because neurite extension was longest on scaffolds with the greatest amount of neuronal differentiation.
Similarly, electrospun silk fibers with diameters on the nanoscale (400 nm) improved neurite extension in a coculture of neurons and astrocytes from postnatal day 1 rat pups versus neurons and astrocytes cultured on microscale diameter fibers (1200 nm).62 Yao and colleagues51 performed a similar study using PC12 cells cultured on a range of larger diameter PCL fibers (800 nm to 8800 nm). In this study, PC12 cells generated the longest neurites on aligned fibers with mean diameters of 3.7 μm and 5.9 μm. A similar result was observed when ND7/23 sensory neuronal cells (neuroblastoma cell line63) were cultured onto PLLA fibers, where neuronal cells cultured on fibers with mean diameter of 750 nm had shorter neurites than neuronal cells cultured on fibers with diameters of 5–6 μm.64
Daud and coworkers66 cultured NG108-15 cells (neuroblastoma cell line65) or adult rat DRG onto PCL fibers with diameters similar to those reported by Yao and colleagues51 (1 μm to 8 μm). The neuroblastoma cells cultured on 8 μm fibers had longer neurites than neuroblastoma cell lines cultured on fibers with smaller diameters. When neuroblastoma cells were cocultured with Schwann cells, however, neurite extension was the same on every scaffold regardless of fiber diameter, and neurite extension was similar to the neuroblastoma cells cultured without Schwann cells on the 8 μm fibers. Interestingly, neurite extension from rat DRG was greatest when cultured on fibers with a 1 μm diameter. These studies suggest a micron scale fiber diameter is more suitable for neurite extension (>750 nm).
It is difficult to compare the studies from Yang and associates34 and He and colleagues,52 however, with the studies from Daud and coworkers66 and Yao and colleagues51 because there was no overlap in the diameter of the fibers used between the studies. Wang and coworkers67 used a range of fiber diameters that sufficiently overlap the other studies (diameters ranging from 293 nm to 1325 nm); they cultured embryonic day 9 chicken DRG on PLLA fibers with diameters that were large (1325 nm), intermediate (759 nm), or small (293 nm). DRG cultured on electrospun fibers with large and intermediate diameters had significantly longer neurites than DRG cultured on small diameter fibers.
The study by Wang and coworkers67 helps to bridge the gap of many studies that propose a fiber diameter between 750 nm and 5 μm may be most suitable for instigating the greatest neurite extension. It is possible that discrepancies are merely a result of cell source (cell line vs. primary cells), and this is supported by the study from Daud and coworkers.66 Unfortunately, there is no study that investigates how fiber diameter alters neurite extension in vivo in the central or peripheral nervous system. Regardless, a consistent theme throughout all of the in vitro studies is that fiber diameter significantly alters neurite extension, but more research must be performed to examine the significance of fiber diameter in animal models of SCI.
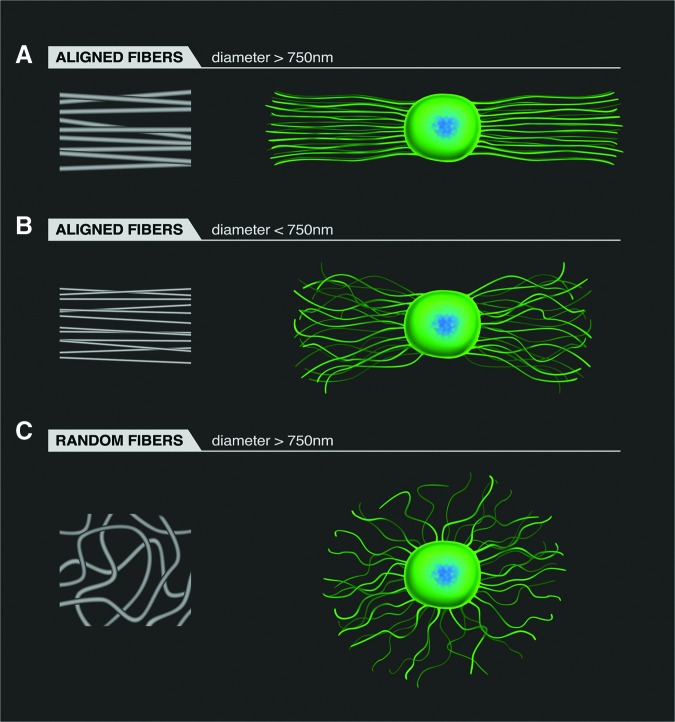
An illustration summarizing the general results of several independent studies presented above reveals the ability of fibers with specific fiber diameters and fiber alignment to direct the extension of neuritis from DRG (Fig. 2). Specifically, highly aligned fibers (with diameters larger than 750 nm) strongly direct neurite extension (Fig. 2A), while aligned fibers with fiber diameters less than 750 nm direct neurite elongation to a lesser degree (Fig. 2B). Randomly organized fibers (with diameters greater than 750 nm) effectively direct neurite outgrowth, but the outgrowth is randomly distributed from the neural explant (Fig. 2C).
FIG. 2.
An illustration of how fiber geometry affects neurite extension from dorsal root ganglia (DRG) on electrospun fibers. The blue/green objects in the illustrations on the right side of the figure indicate the DRG body. When electrospun fibers are aligned and the fiber diameter is above 750 nm, neurites extend along the fibers (A). When electrospun fibers are aligned but the fiber diameter is below 750 nm, neurites will still follow the fibers, but the neurites tend to be shorter and appear to wander in less of a straight line along the fibers (B). When electrospun fibers are randomly aligned, neurites tend to grow in all directions (C). Color image is available online at www.liebertpub.com/neu
Polymer material selection/surface modification
In addition to the geometric properties of fiber orientation and diameter, material selection may also influence neurite extension. Synthetic, degradable polymers have been used to electrospin fibers for neural applications including PLLA, poly (l–lactic-co-glycolic acid) (PLGA), and PCL. Several different natural polymers were also examined, including cellulose, chitosan, collagen, gelatin, and silk.68 Combining polymers or surface modification to alter the surface chemistry seeks to create fibers with unique surface characteristics to improve neural response and regeneration potential.
Biodegradable, synthetic polyesters can be mixed with proteins (such as laminin, fibronectin, or L1) or peptide sequences (such as YIGSR, RGD, or IKVAV) before electrospinning to incorporate these biological molecules within the fibers. The above molecules can also be placed onto the surface of fibers modified through covalent coupling of proteins or coupling of integrin binding peptides to support the extension of neurites.69–71
Synthetic polymers can be mixed with proteins in the electrospinning solution and subsequently electrospun, but there is some evidence that proteins such as collagen denature during the process of electrospinning.72 Further, addition of a protein to the electrospinning solution can significantly alter fiber morphology (e.g., fiber alignment and diameter) because proteins are charge carriers.73 In addition, there is no guarantee that the added protein or peptide will remain functional or in a high enough concentration at the surface to significantly improve extension of neurites.
An alternative method of adding proteins to fibers is through covalent linkage or physical adsorption. To promote protein adsorption to hydrophobic polymers, surface treatments are frequently made to the fibers before exposure to proteins or peptides. Surface treatment affects the hydrophobicity and surface free energy, which alters the type of protein adsorbing to the fibers.74 Decreasing the hydrophobicity of the surface tends to increase protein adsorption,75 but intermediate values of surface hydrophobicity are optimal for cell adhesion.76
A comparison of blending, covalent linkage, and physical adsorption of laminin on electrospun fibers was performed by Koh and associates77 to determine which method improved neurite extension, and electrospun fibers blended with laminin were most likely to induce long neurite extension. Thus, specific concentrations of proteins or peptides are necessary to improve the rate of neurite extension compared with unmodified fiber controls.78
A protein commonly incorporated into electrospun fibers is collagen. Polymer and collagen blends or collagen alone improves neuron adhesion/viability. Generally, collagen inclusion within polymers reduces neurite extension when neurite extension is compared between polymer fiber only controls and collagen containing fibers. A PLLA-PCL blend electrospun with collagen I and III created fibers with diameters ranging from 196–288 nm. The inclusion of collagen improved C17.2 neural stem cell viability, but it was unclear how collagen blends of PCL affected neurite extension.79 Schnell and colleagues42 observed neurites from DRG explants were shorter on collagen/PCL scaffolds when compared with neurite outgrowth on PCL alone.42
The reasons why collagen inclusion does not promote long neurite extension are unknown. Collagen is denatured by electrospinning, however.72 Thus, it is possible that denatured collagen may be incapable of promoting the extension of neurites. A study by Liu and coworkers60 placed collagen I electrospun fibers within an in vivo rat model of SCI. Unexpectedly, the fibrous scaffolds did not induce axonal extension into or onto the electrospun collagen scaffolds, which is a different result to the significant amount of axonal extension observed when electrospun PLLA fibers were placed into the rat spinal cord.45 While it is difficult to directly compare these studies because of the major differences in fiber diameter in addition to the different materials, the lack of axonal extension into the scaffold may be because of the selection of collagen.80
Another protein incorporated into electrospun fibers is the basement membrane glycoprotein laminin. As mentioned previously, Koh and colleagues77 analyzed neurite extension on fibers where laminin was adsorbed to fibers, electrospun into, or covalently bound to the fiber surface. The study by Koh and colleagues77 proved laminin inclusion in the electrospinning solution induced the longest extension of neurites.
In another study,81 investigators electrospun pure laminin into fibers and cultured neurons on them. The length of neurites on these fibers, however, was not compared with neurite extension on other types of fibers. In a follow-up study,61 blends of PCL and laminin (10% weight laminin) had the same effect on maximum neurite extension from mouse DRG cultured on electrospun fibers with a higher percentage of laminin (range 10–100% laminin).61
Another method to modify the surface chemistry of fibers is through plasma treatment. Oxygen plasma treatment adds oxygen-containing molecules to the fiber surface, which decreases hydrophobicity. Corey and coworkers82 sought to improve adhesion of primary rat sensory DRG or motor neurons through plasma treatment of aligned 2 μm diameter PLLA fibers. Interestingly, plasma treatment did not improve motor neuron survival when compared with motor neurons cultured onto a nonfibrous control. Further, motor neuron survival was statistically higher on untreated electrospun fibers. From these findings, Corey and colleagues82 concluded that plasma treatment may restrict neuronal adhesion possibly because of electrostatic repulsion between the cell membrane and the negatively charged surface imparted by plasma treatment.
Similar findings were recently reported from our laboratory when chick DRG extended shorter neurites when cultured on oxygen plasma treated electrospun PLLA fibers.83 In our study, different surface chemistries were covalently linked to the surface of the electrospun fibers to determine how different chemistries changed the hydrophilicity and ability to guide neurite extension. Although we found that all surface modifications resulted in a similar degree of hydrophilic character of the scaffolds, all of the treatments resulted in a decrease in neurite extension except the group that was modified with RGD, the only group with a biologically functional component. In these studies, neurons were cultured in media free of significant amounts of serum. Thus, the reduced neurite extension observed on charged surfaces is likely because of the presence of surface chemistry and not from differential adhesion of protein.
While plasma treatment may not directly improve neural adhesion or neurite extension by itself, the presence of oxygen species can provide a binding site for proteins. PLLA fibers with diameters ranging from 100 to 500 nm were plasma treated and functionalized with laminin and bFGF using a di-amino-poly(ethylene glycol) (di-NH2-PEG) linker covalently attached to carboxyl groups on the surface of the fibers using EDC-NHS chemistry. The tethered growth factors significantly improved the length and density of neurites extending from rat DRG.84
Tethering the neural adhesion ligand L1 to the fiber surface enhanced both functionality in primary neuronal cells and differentiation of human embryonic stem cell derived neural progenitor cells (hESC-NPC) into GABAergic neural lineages.85 L1 presents an RGD sequence that binds preferentially to neurons instead of astrocytes and oligodendrocytes. The selection of the L1 molecule is appropriate for SCI applications because L1 binds to α5β1 and αvβ3 integrins on the neural growth cone and improves functional recovery and axonal regeneration after SCI. Cherry and associates85 electrospun a 1.25 μm tyrosine derived polycarbonate polymer and functionalized the fibers with L1. L1 ligand orientation on the fiber surface was improved using a tether that was comprised of the Fc receptor and protein A, and this combined method improved neurite extension.
One material aspect that has not received a significant amount of attention is how different surface modifications of electrospun fibers might degrade over time. This is an especially important topic considering the work from the group at Case Western Reserve with PNGs, where months of recovery time are required before functional recovery is observed.7,8
Recent work from our laboratory suggests that some types of surface modifications may be very transient, lasting less than a few days.83 The only electrospun fiber group modified with a peptide (RGD), however, appeared to be stable with no noticeable signs of degradation, so peptides and proteins that are linked to the surface may not have as transient a presence at the surface of electrospun fibers. Still, further work must be performed to better understand how surfaces can be modified to improve neurite extension, and an analysis of the stability of the surface modification should be included considering the potential length of time required to grow neurites across a scaffold in vivo.
Drug and protein delivery
While the previous sections have detailed the importance of fiber geometry (alignment and diameter) or surface chemistry with regard to neurite guidance, electrospun fibers are also capable of delivering therapeutic agents to stimulate neural regeneration or reduce the secondary injury response. The simplest method of adding a therapeutic agent to electrospun fibers is by direct addition of the agent to a solution before electrospinning. The small fiber diameters generate a high surface area to volume ratio, allowing for a sustained release profile via diffusion in slowly degrading polymers such as high molecular weight PLLA.86–88 The ability to release therapies from electrospun fibers locally enables the sustained release of therapies that otherwise might have difficulty reaching the injury site if the therapy were delivered systemically.
The first article to incorporate a therapeutic agent into electrospun fibers for nerve regeneration was a study presented by Chew and colleagues73 in 2005. Chew and colleagues73 loaded human β-nerve growth factor (NGF) into electrospun fibers. NGF was incorporated into an electrospinning solution containing a copolymer of PCL and poly(ethyl ethylene phosphate) (PCLEEP), and the release rate of NGF was sustained over a 90-day period. Chew tested the bioactivity of the NGF released from PCLEEP fibers by adding the released NGF to PC12 cells. The NGF released from the fibers lost some of its ability to induce neurite extension from PC12 cells. To improve NGF bioactivity, polymer and NGF were mixed with a surfactant. The inclusion of the surfactant improved NGF bioactivity in the presence of PC12 qualitatively, but the neurite extension results were not quantified.89
A later study by Valmikinathan and colleagues90 included bovine serum albumin (BSA) in an electrospinning solution containing NGF and PCL. BSA inclusion increased the total amount of NGF released from fibers and improved the bioactivity of the released NGF as shown when released NGF was cultured with PC12 cells inducing them to form neurites. Another article by Chew and coworkers91 demonstrated the ability to release glial derived neurotrophic factor (GDNF) from PCLEEP. Although no bioactivity experiment was conducted, fibers loaded with GDNF induced recovery in animals with peripheral nerve injury compared with the controls with no GDNF. These studies demonstrate the ability of electrospun fibers to release proteins, but these studies also highlight the importance of protecting proteins from the large electrical fields required for the electrospinning process.
Other studies present strategies for delivery of proteins from electrospun fibers. Proteins (such as collagen) are denatured by the electrospinning process,72 potentially because the organic solvents used for electrospinning can cause the protein to denature. In addition, fibers undergo intense stretching because of violent motion of the electrospinning jet, and the intense stretching may denature proteins.
One potential solution to maintain protein activity may be to covalently bind proteins to the fiber surface. Chew and colleagues92 used microbial transglutaminase to covalently couple either ChABC or neurotrophin-3 (NT3) to the surface of electrospun collagen fibers. Collagen in the supernatant linked to NT3 was less capable than soluble NT3 at promoting neurite extension from embryonic day 15 rat DRG. When DRG were placed on fibers with covalently attached NT3, however, there were no differences in the length of neurite extension when comparing neurite outgrowth from DRG placed on plain collagen scaffolds with the addition of soluble NT3. In addition, collagen fibers covalently coupled to heparin or ChABC were more apt to maintain ChABC's ability to digest decorin over a 32-day period compared with soluble heparin.
Thus, post-fabrication surface inclusion of protein may maintain a protein's function better than mixing the protein in with the polymer and solvent before electrospinning. Very few studies have investigated the release of proteins from fibers for nerve regeneration applications, so more work is needed to develop new approaches to protect protein and better understand the mechanisms by which proteins diffuse out of the fibers.
Rather than delivering large proteins, some groups have delivered small organic molecules to improve neurite extension. Delivery of small organic molecules has a number of advantages over the release of protein. There is less of a concern that organic molecules will lose their bioactivity as a result of the electrospinning process. Further, the size of small organic molecules permits them to diffuse out of slowly degrading polymers at a much higher rate than proteins. This attribute is important, because slowly degrading polymers are of greater interest in nerve guidance because of the long periods required for functional regeneration in animal models of SCI (3–6 months).6–8
One of the first small organic molecules released from electrospun fibers for spinal cord repair was the antimetabolite 6-aminonicotinamide (6AN). 6AN is known to inhibit astrocyte metabolism at low levels while having a lesser effect on neurons.93–95 Schaub and Gilbert96 released 6AN from electrospun PLLA, and the released 6AN was found to inhibit astrocyte viability without interfering with neurite extension at a concentration of 10% of the weight of the polymer. The intention was to try to inhibit astrocyte viability to reduce the negative effects from astrocyte reactivity after SCI, but no in vivo work was performed.
A more in-depth study was performed using rolipram by Downing and associates,97 where rolipram was released from electrospun PLLA and placed in a C5 hemisection rat model of SCI. The study by Downing and associates97 found that animals that received electrospun fibers that released a low dose of rolipram (∼3 μg/cm2 over 12 days) had significantly improved functional recovery after injury over a control group with injury alone. Interestingly, the treatment group that received electrospun fibers that released a low dose of rolipram had significantly improved motor function over another group that included electrospun fibers that released a large amount of rolipram (∼60 μg/cm2 over 12 days). This study demonstrates the ability to easily tune the amount of drug released from electrospun fibers and the ability to release therapeutic levels of drug.
In addition to the concern of maintaining protein bioactivity, if the protein or organic molecule is incorporated within the electrospun fiber, it is also important to note that protein incorporation or inclusion of small molecular weight therapeutic agents alters fiber geometry. Because fiber diameter is known to alter cellular function, fiber diameter must be maintained when performing cell culture experiments on fibers that release therapeutic agents. In general, inclusion of protein in an electrospinning solution results in electrospun fibers with drastically smaller fiber diameters compared with electrospinning solutions without protein.73,89,98 The decreased fiber diameter may be attributed to the large charge character of the protein.
Schaub and Gilbert,97 however, found a decreased fiber diameter even when using 6AN, a small organic molecule. To compensate, Schaub and Gilbert96 reduced the concentration of PLLA fibers with no 6AN to obtain fibers with similar fiber diameter. The change in fiber diameter when protein or therapeutic agent is added to an electrospinning solution can interfere with a proper interpretation of the results if the fiber diameter is not compensated for, because either a small fiber diameter or the release of a therapeutic agent may be the source of any observed changes in neurite extension.
Electrospun Fibers as a Tool to Understand the CNS and SCI
Although the focus of this review thus far has detailed the use of electrospun fibers to guide axons after SCI, a new area of biological research is emerging that uses electrospun fibers to understand the behavior of CNS cells in the presence of topography. As stated in the introduction, dissociated DRG placed in the corpus callosum extended neurites along the length of white matter tracts.99
Neurite extension, however, could be attributed to any number of factors, including trophic or structural cues. Electrospun fibers provide the opportunity to reduce the complexity of the system by generation of synthetic structures of similar dimension to axons because aligned fibers can be fabricated with diameters similar to those of axons found in the CNS (myelinated axons range in diameter from 0.2 μm to more than 16 μm).100
In the absence of serum, which contains a complex assortment of proteins, neurites extend along the length of electrospun PLLA fibers.82 Neurite guidance by electrospun fibers in the absence of other cells or protein gradients is a powerful indication that the physical, isotropic organization of white matter tracts plays a significant role in guiding axonal extension. In addition, simple experiments on electrospun fibers can help elucidate how biological factors, such as fibronectin, contribute to axonal guidance on these three dimensional structures.101
A very recent discovery from the laboratories of the University of California, San Francisco, and the University of Michigan demonstrated that oligodendrocytes myelinate electrospun fibers in a diameter dependent manner, and that oligodendrocyte progenitors begin electrospun fiber ensheathment before differentiation.102,103 This discovery effectively decoupled the molecular characteristics from the physical characteristics of an axon to demonstrate that the physical dimension of an axon is a critical component to myelination.
Myelination is a critical component in the action potential conduction velocity of neurons and is an important factor in restoring full functional recovery. In addition to being a useful tool for understanding oligodendrocyte myelination, it also gives further credence to a previous study that found primary neural stem cells differentiate into oligodendrocytes or neurons in response to electrospun fiber diameter.104 These discoveries demonstrate how electrospun fibers can be used to better understand basic biological processes involved in the CNS, but also may prove useful for SCI repair because neural stem cells are currently being pursued as a treatment option.105
In addition to oligodendrocytes, astrocytes have also been found to respond uniquely to electrospun fibers versus a flat surface. Zuidema and coworkers106 demonstrated how astrocytes cultured on electrospun fibers produce more GLT-1 (a glutamate transport protein) when cultured on aligned or randomly oriented electrospun PLLA fibers compared with astrocytes cultured on a flat surface of PLLA. The significance of this finding is that GLT-1 is found to be down regulated after SCI107 and contributes to neuronal excitotoxicity.108
The results of the study from Zuidema and associates106 suggest that the microscale, axon-like structure of the fibers contributes to the expression of GLT-1. Taking the results of the astrocyte study and the oligodendrocyte data from the preceding paragraph, the axon-like structure of electrospun fibers may be a more suitable surface for in vitro culture of glial cells because these cell types appear to behave more like glial cells in native tissue. Therefore, electrospun fibers may not simply be an approach to repairing SCI, but also a tool to better study glial cells in vitro.
In addition to better understanding the biology of neurons and other cell types in the CNS, electrospun fibers may also prove useful in understanding the mechanisms responsible for failure of axons to regenerate into a SCI lesion. Although the glial scar is believed to be a chemical and physical barrier to axonal extension,109–111 the extent of glial scarring in humans is marginal compared with rodents,112 and some researchers suggest the mesenchymal scar plays a larger role in the failure of regeneration after SCI.80,113
One study hypothesized that the orientation of collagen fibrils in the mesenchymal scar may contribute to the scar's inhibitory nature,114 while other studies have demonstrated how certain collagens inhibit axonal growth.80,115 Electrospun fibers have supported these ideas by demonstrating that neurites extend along electrospun collagen fibers,57,116,117 but fibers containing collagen type I tend to have shorter neurites than neurites from neurons grown on biologically benign synthetic polymers.42 These results suggest that collagen fibril organization within the fibrous mesenchymal scar may act as a barrier to nerve growth across the site of injury because the collagen fibrils are oriented parallel to the edge of the scar, which is perpendicular to the direction needed for nerve regeneration. Thus, electrospun fibers may aid in understanding whether components in the injured region are biologically or structurally inhibitory.
Conclusions
Although electrospun fibers have only been applied to neural applications for a decade, they have contributed to the understanding of how geometric structure may guide axonal extension. The initial in vitro and in vivo studies show that optimization of electrospun fibers can improve axonal extension and demonstrate the promise that electrospun fibers can be used as synthetic nerve guidance scaffolds. In addition to the fibers acting as a physical guide, further utility is found in the ability to use a wide range of materials and inclusion of therapeutic agents that can be released over time.
There is a great need for additional work to optimize fiber diameter, material properties, and release characteristics specifically for SCI, however. In addition to using electrospun fibers as a repair strategy, electrospun fibers may also prove a useful tool in understanding biological processes in the healthy and injured CNS.
Acknowledgments
The authors acknowledge grant support to RJG provided by the National Science Foundation (CAREER grant 11050125), National Institutes of Health (R01 NS092754), and the New York Spinal Cord Injury Research Trust (Contract # C030239). Nicholas Schaub was supported by the Ajit Prabhu '98 Fellowship.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Silver J.R. (2012). Response to “The administration of high-dose methylprednisolone for 24 h reduced muscle size and increased atrophy-related gene expression in spinal cord-injured rats.” Spinal Cord 50, 479. [DOI] [PubMed] [Google Scholar]
- 2.Bracken M.B. (1996). Steroids for acute spinal cord injury., in: Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd: Chichester, UK [Google Scholar]
- 3.Varma A.K., Das A., Wallace G., IV, Barry J., Vertegel A.A., Ray S.K., and Banik N.L. (2013). Spinal cord injury: a review of current therapy, future treatments, and basic science frontiers. Neurochem. Res. 38, 895–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.No authors (2012). Spinal cord injury facts and figures at a glance. J. Spinal Cord Med. 35, 197–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng H., Cao Y., and Olson L. (1996). Spinal cord repair in adult paraplegic rats: partial restoration of hind limb function. Science 273, 510–513 [DOI] [PubMed] [Google Scholar]
- 6.Houle J.D., Tom V.J., Mayes D., Wagoner G., Phillips N., and Silver J. (2006). Combining an autologous peripheral nervous system “bridge” and matrix modification by chondroitinase allows robust, functional regeneration beyond a hemisection lesion of the adult rat spinal cord. J. Neurosci. 26, 7405–7415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alilain W.J., Horn K.P., Hu H., Dick T.E., and Silver J. (2011). Functional regeneration of respiratory pathways after spinal cord injury. Nature 475, 196–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee Y.S., Lin C.Y., Jiang H.H., DePaul M., Lin V.W., and Silver J. (2013). Nerve regeneration restores supraspinal control of bladder function after complete spinal cord injury. J. Neurosci. 33, 10591–10606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bradbury E.J., and Carter L.M. (2011). Manipulating the glial scar: Chondroitinase ABC as a therapy for spinal cord injury. Brain Res. Bull. 84, 306–316 [DOI] [PubMed] [Google Scholar]
- 10.Shen Y., Tenney A.P., Busch S.A., Horn K.P., Cuascut F.X., Liu K., He Z., Silver J., and Flanagan J.G. (2009). PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science 326, 592–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garg K., and Bowlin G.L. (2011). Electrospinning jets and nanofibrous structures. Biomicrofluidics 5, 13403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sill T.J., and von Recum H.A. (2008). Electrospinning: applications in drug delivery and tissue engineering. Biomaterials 29, 1989–2006 [DOI] [PubMed] [Google Scholar]
- 13.Lee Y.S., and Livingston Arinzeh T. (2011). Electrospun nanofibrous materials for neural tissue engineering. Polymers 3, 413–426 [Google Scholar]
- 14.Yang F., Xu C.Y., Kotaki M., Wang S., and Ramakrishna S. (2004). Characterization of neural stem cells on electrospun poly(L-lactic acid) nanofibrous scaffold. J. Biomater. Sci. Polym. Ed. 15, 1483–1497 [DOI] [PubMed] [Google Scholar]
- 15.Bini T.B., Gao S., Tan T.C., Wang S., Lim A., Hai L.B., and Ramakrishna S. (2004). Electrospun poly(L-lactide-co-glycolide) biodegradable polymer nanofibre tubes for peripheral nerve regeneration. Nanotechnology 15, 1459 [Google Scholar]
- 16.Zuidema J.M., Desmond G.P., Rivet C.J., Kearns K.R., Thompson D.M., and Gilbert R.J. (2015). Nebulized solvent ablation of aligned PLLA fibers for the study of neurite response to anisotropic-to-isotropic fiber/film transition (AFFT) boundaries in astrocyte–neuron co-cultures. Biomaterials 46, 82–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weightman A.P., Pickard M.R., Yang Y., and Chari D.M. (2014). An in vitro spinal cord injury model to screen neuroregenerative materials. Biomaterials 35, 3756–3765 [DOI] [PubMed] [Google Scholar]
- 18.Reneker D.H., and Yarin A.L. (2008). Electrospinning jets and polymer nanofibers. Polymer 49, 2387–2425 [Google Scholar]
- 19.Reneker D.H., Yarin A.L., Fong H., and Koombhongse S. (2000). Bending instability of electrically charged liquid jets of polymer solutions in electrospinning. J. Appl. Phys. 87, 4531–4547 [Google Scholar]
- 20.Yarin A.L., Koombhongse S., and Reneker D.H. (2001). Bending instability in electrospinning of nanofibers. J. Appl. Phys. 89, 3018–3026 [Google Scholar]
- 21.Fong H., Liu W., Wang C.-S., and Vaia R.A. (2002). Generation of electrospun fibers of nylon 6 and nylon 6-montmorillonite nanocomposite. Polymer 43, 775–780 [Google Scholar]
- 22.Fennessey S.F., and Farris R.J. (2004). Fabrication of aligned and molecularly oriented electrospun polyacrylonitrile nanofibers and the mechanical behavior of their twisted yarns. Polymer 45, 4217–4225 [Google Scholar]
- 23.Kakade M.V., Givens S., Gardner K., Lee K.H., Chase D.B., and Rabolt J.F. (2007). Electric field induced orientation of polymer chains in macroscopically aligned electrospun polymer nanofibers. J. Am. Chem. Soc. 129, 2777–2782 [DOI] [PubMed] [Google Scholar]
- 24.Subramanian A., Krishnan U.M., and Sethuraman S. (2011). Fabrication of uniaxially aligned 3D electrospun scaffolds for neural regeneration. Biomed. Mater. 6, 025004. [DOI] [PubMed] [Google Scholar]
- 25.Wang H.B., Mullins M.E., Cregg J.M., Hurtado A., Oudega M., Trombley M.T., and Gilbert R.J. (2009). Creation of highly aligned electrospun poly-L-lactic acid fibers for nerve regeneration applications. J. Neural Eng. 6, 016001. [DOI] [PubMed] [Google Scholar]
- 26.Wang W., Itoh S., Konno K., Kikkawa T., Ichinose S., Sakai K., Ohkuma T., and Watabe K. (2009). Effects of Schwann cell alignment along the oriented electrospun chitosan nanofibers on nerve regeneration. J. Biomed. Mater. Res. A 91, 994–1005 [DOI] [PubMed] [Google Scholar]
- 27.Deitzel J., Kleinmeyer J., Harris D., and Beck Tan N. (2001). The effect of processing variables on the morphology of electrospun nanofibers and textiles. Polymer 42, 261–272 [Google Scholar]
- 28.Kim K.W., Lee K.H., Khil M.S., Ho Y.S., and Kim H.Y. (2004). The effect of molecular weight and the linear velocity of drum surface on the properties of electrospun poly(ethylene terephthalate) nonwovens. Fibers Polym. 5, 122–127 [Google Scholar]
- 29.Gupta P., Elkins C., Long T.E., and Wilkes G.L. (2005). Electrospinning of linear homopolymers of poly(methyl methacrylate): exploring relationships between fiber formation, viscosity, molecular weight and concentration in a good solvent. Polymer 46, 4799–4810 [Google Scholar]
- 30.Guarino V., Cirillo V., Taddei P., Alvarez-Perez M.A., and Ambrosio L. (2011). Tuning size scale and crystallinity of pcl electrospun fibres via solvent permittivity to address hMSC response. Macromol. Biosci. 11, 1694–1705 [DOI] [PubMed] [Google Scholar]
- 31.Lee K.H., Kim H.Y., La Y.M., Lee D.R., and Sung N.H. (2002). Influence of a mixing solvent with tetrahydrofuran and N,N-dimethylformamide on electrospun poly(vinyl chloride) nonwoven mats. J. Polym. Sci. Part B Polym. Phys. 40, 2259–2268 [Google Scholar]
- 32.Asran A.S., Salama M., Popescu C., and Michler G. h. (2010). Solvent influences the morphology and mechanical properties of electrospun poly(l-lactic acid) scaffold for tissue engineering applications. Macromol. Symp. 294, 153–161 [Google Scholar]
- 33.Snyder E.Y., Deitcher D.L., Walsh C., Arnold-Aldea S., Hartwieg E.A., and Cepko C.L. (1992). Multipotent neural cell lines can engraft and participate in development of mouse cerebellum. Cell 68, 33–51 [DOI] [PubMed] [Google Scholar]
- 34.Yang F., Murugan R., Wang S., and Ramakrishna S. (2005). Electrospinning of nano/micro scale poly(L-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials 26, 2603–2610 [DOI] [PubMed] [Google Scholar]
- 35.Rajnicek A., Britland S., and McCaig C. (1997). Contact guidance of CNS neurites on grooved quartz: influence of groove dimensions, neuronal age and cell type. J. Cell Sci. 110, 2905–2913 [DOI] [PubMed] [Google Scholar]
- 36.Dubey N., Letourneau P.C., and Tranquillo R.T. (2001). Neuronal contact guidance in magnetically aligned fibrin gels: effect of variation in gel mechano-structural properties. Biomaterials 22, 1065–1075 [DOI] [PubMed] [Google Scholar]
- 37.Smith G.M., Falone A.E., and Frank E. (2012). Sensory axon regeneration: rebuilding functional connections in the spinal cord. Trends Neurosci. 35, 156–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corey J.M., Lin D.Y., Mycek K.B., Chen Q., Samuel S., Feldman E.L., and Martin D.C. (2007). Aligned electrospun nanofibers specify the direction of dorsal root ganglia neurite growth. J. Biomed. Mater. Res. A 83, 636–645 [DOI] [PubMed] [Google Scholar]
- 39.Reddy U.R., Venkatakrishnan G., Roy A.K., Chen J., Hardy M., Mavilio F., Rovera G., Pleasure D., and Ross A.H. (1991). Characterization of two neuroblastoma cell lines expressing recombinant nerve growth factor receptors. J. Neurochem. 56, 67–74 [DOI] [PubMed] [Google Scholar]
- 40.Biedler J.L., Roffler-Tarlov S., Schachner M., and Freedman L.S. (1978). Multiple neurotransmitter synthesis by human neuroblastoma cell lines and clones. Cancer Res. 38, 3751–3757 [PubMed] [Google Scholar]
- 41.Chow W.N., Simpson D.G., Bigbee J.W., and Colello R.J. (2007). Evaluating neuronal and glial growth on electrospun polarized matrices: bridging the gap in percussive spinal cord injuries. Neuron Glia Biol. 3, 119–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schnell E., Klinkhammer K., Balzer S., Brook G., Klee D., Dalton P., and Mey J. (2007). Guidance of glial cell migration and axonal growth on electrospun nanofibers of poly-ɛ-caprolactone and a collagen/poly-ɛ-caprolactone blend. Biomaterials 28, 3012–3025 [DOI] [PubMed] [Google Scholar]
- 43.Kim Y., Haftel V.K., Kumar S., and Bellamkonda R.V. (2008). The role of aligned polymer fiber-based constructs in the bridging of long peripheral nerve gaps. Biomaterials 29, 3117–3127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Griffin J., Delgado-Rivera R., Meiners S., and Uhrich K.E. (2011). Salicylic acid‐derived poly(anhydride‐ester) electrospun fibers designed for regenerating the peripheral nervous system. J. Biomed. Mater. Res. A 97, 230–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hurtado A., Cregg J.M., Wang H.B., Wendell D.F., Oudega M., Gilbert R.J., and McDonald J.W. (2011). Robust CNS regeneration after complete spinal cord transection using aligned poly-L-lactic acid microfibers. Biomaterials 32, 6068–6079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xie J., MacEwan M.R., Willerth S.M., Li X., Moran D.W., Sakiyama-Elbert S.E., and Xia Y. (2009). Conductive core-sheath nanofibers and their potential application in neural tissue engineering. Adv. Funct. Mater. 19, 2312–2318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xie J., MacEwan M.R., Li X., Sakiyama-Elbert S.E., and Xia Y. (2009). Neurite outgrowth on nanofiber scaffolds with different orders, structures, and surface properties. ACS Nano 3, 1151–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee Y.S., Collins G., and Arinzeh T.L. (2011). Neurite extension of primary neurons on electrospun piezoelectric scaffolds. Acta Biomater. 7, 3877–3886 [DOI] [PubMed] [Google Scholar]
- 49.Greene L.A., and Tischler A.S. (1976). Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc. Natl. Acad. Sci. U. S. A. 73, 2424–2428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee J.Y., Bashur C.A., Goldstein A.S., and Schmidt C.E. (2009). Polypyrrole-coated electrospun PLGA nanofibers for neural tissue applications. Biomaterials 30, 4325–4335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yao L., O'Brien N., Windebank A., and Pandit A. (2009). Orienting neurite growth in electrospun fibrous neural conduits. J. Biomed. Mater. Res. B Appl. Biomater. 90, 483–491 [DOI] [PubMed] [Google Scholar]
- 52.He L., Liao S., Quan D., Ma K., Chan C., Ramakrishna S., and Lu J. (2010). Synergistic effects of electrospun PLLA fiber dimension and pattern on neonatal mouse cerebellum C17.2 stem cells. Acta Biomater. 6, 2960–2969 [DOI] [PubMed] [Google Scholar]
- 53.Prabhakaran M.P., Venugopal J.R., Chyan T.T., Hai L.B., Chan C.K., Lim A.Y., and Ramakrishna S. (2008). Electrospun biocomposite nanofibrous scaffolds for neural tissue engineering. Tissue Eng. Part A 14, 1787–1797 [DOI] [PubMed] [Google Scholar]
- 54.Bourke J.L., Coleman H.A., Pham V., Forsythe J.S., and Parkington H.C. (2013). Neuronal electrophysiological function and control of neurite outgrowth on electrospun polymer nanofibers are cell type dependent. Tissue Eng. Part A 20, 1089–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xie J., Liu W., MacEwan M.R., Bridgman P.C., and Xia Y. (2014). Neurite outgrowth on electrospun nanofibers with uniaxial alignment: the effects of fiber density, surface coating, and supporting substrate. ACS Nano 8, 1878–1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ghasemi-Mobarakeh L., Prabhakaran M.P., Morshed M., Nasr-Esfahani M.H., and Ramakrishna S. (2008). Electrospun poly([epsilon]-caprolactone)/gelatin nanofibrous scaffolds for nerve tissue engineering. Biomaterials 29, 4532–4539 [DOI] [PubMed] [Google Scholar]
- 57.Kijeńska E., Prabhakaran M.P., Swieszkowski W., Kurzydlowski K.J., and Ramakrishna S. (2012). Electrospun bio-composite P(LLA-CL)/collagen I/collagen III scaffolds for nerve tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 100, 1093–1102 [DOI] [PubMed] [Google Scholar]
- 58.Subramanian A., Krishnan U.M., and Sethuraman S. (2012). Axially aligned electrically conducting biodegradable nanofibers for neural regeneration. J. Mater. Sci. Mater. Med. 23, 1797–1809 [DOI] [PubMed] [Google Scholar]
- 59.Wang Y., Yao M., Zhou J., Zheng W., Zhou C., Dong D., Liu Y., Teng Z., Jiang Y., Wei G., and Cui X. (2011). The promotion of neural progenitor cells proliferation by aligned and randomly oriented collagen nanofibers through β1 integrin/MAPK signaling pathway. Biomaterials 32, 6737–6744 [DOI] [PubMed] [Google Scholar]
- 60.Liu T., Houle J.D., Xu J., Chan B.P., and Chew S.Y. (2012). Nanofibrous collagen nerve conduits for spinal cord repair. Tissue Eng. Part A 18, 1057–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Neal R.A., Tholpady S.S., Foley P.L., Swami N., Ogle R.C., and Botchwey E.A. (2012). Alignment and composition of laminin–polycaprolactone nanofiber blends enhance peripheral nerve regeneration. J. Biomed. Mater. Res. A 100, 406–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qu J., Wang D., Wang H., Dong Y., Zhang F., Zuo B., and Zhang H. (2013). Electrospun silk fibroin nanofibers in different diameters support neurite outgrowth and promote astrocyte migration. J. Biomed. Mater. Res. A 101, 2667–2678 [DOI] [PubMed] [Google Scholar]
- 63.Wood J.N., Bevan S.J., Coote P.R., Dunn P.M., Harmar A., Hogan P., Latchman D.S., Morrison C., Rougon G., Theveniau M., and Wheatley S. (1990). Novel cell lines display properties of nociceptive sensory neurons. Proc. Biol. Sci. 241, 187–194 [DOI] [PubMed] [Google Scholar]
- 64.Binder C., Milleret V., Hall H., Eberli D., and Lühmann T. (2013). Influence of micro and submicro poly(lactic-glycolic acid) fibers on sensory neural cell locomotion and neurite growth. J. Biomed. Mater. Res. B Appl. Biomater. 101, 1200–1208 [DOI] [PubMed] [Google Scholar]
- 65.Fishman M.C., and Spector I. (1981). Potassium current suppression by quinidine reveals additional calcium currents in neuroblastoma cells. Proc. Natl. Acad. Sci. U. S. A. 78, 5245–5249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Daud M.F., Pawar K.C., Claeyssens F., Ryan A.J., and Haycock J.W. (2012). An aligned 3D neuronal-glial co-culture model for peripheral nerve studies. Biomaterials 33, 5901–5913 [DOI] [PubMed] [Google Scholar]
- 67.Wang H.B., Mullins M.E., Cregg J.M., McCarthy C.W., and Gilbert R.J. (2010). Varying the diameter of aligned electrospun fibers alters neurite outgrowth and Schwann cell migration. Acta Biomater. 6, 2970–2978 [DOI] [PubMed] [Google Scholar]
- 68.Schiffman J.D., and Schauer C.L. (2008). A review: electrospinning of biopolymer nanofibers and their applications. Polym. Rev. 48, 317–352 [Google Scholar]
- 69.Xie J., MacEwan M.R., Schwartz A.G., and Xia Y. (2010). Electrospun nanofibers for neural tissue engineering. Nanoscale 2, 35–44 [DOI] [PubMed] [Google Scholar]
- 70.Bockelmann J., Klinkhammer K., von Holst A., Seiler N., Faissner A., Brook G.A., Klee D., and Mey J. (2011). Functionalization of electrospun poly(ɛ-caprolactone) fibers with the extracellular matrix-derived peptide GRGDS improves guidance of Schwann cell migration and axonal growth. Tissue Eng. Part A 17, 475–486 [DOI] [PubMed] [Google Scholar]
- 71.Kim T.G., and Park T.G. (2006). Biomimicking extracellular matrix: cell adhesive RGD peptide modified electrospun poly(D,L-lactic-co-glycolic acid) nanofiber mesh. Tissue Eng. 12, 221–233 [DOI] [PubMed] [Google Scholar]
- 72.Zeugolis D.I., Khew S.T., Yew E.S., Ekaputra A.K., Tong Y.W., Yung L.Y., Hutmacher D.W., Sheppard C., and Raghunath M. (2008). Electro-spinning of pure collagen nano-fibres—just an expensive way to make gelatin? Biomaterials 29, 2293–2305 [DOI] [PubMed] [Google Scholar]
- 73.Chew S.Y., Wen J., Yim E.K., and Leong K.W. (2005). Sustained release of proteins from electrospun biodegradable fibers. Biomacromolecules 6, 2017–2024 [DOI] [PubMed] [Google Scholar]
- 74.Alves C.M., Yang Y., Marton D., Carnes D.L., Ong J.L., Sylvia V.L., Dean D.D., Reis R.L., and Agrawal C.M. (2008). Plasma surface modification of poly(D,L-lactic acid) as a tool to enhance protein adsorption and the attachment of different cell types. J. Biomed. Mater. Res. B Appl. Biomater. 87, 59–66 [DOI] [PubMed] [Google Scholar]
- 75.Gessner A., Waicz R., Lieske A., Paulke B.R., Mäder K., and Müller R.H. (2000). Nanoparticles with decreasing surface hydrophobicities: influence on plasma protein adsorption. Int. J. Pharm. 196, 245–249 [DOI] [PubMed] [Google Scholar]
- 76.Ikada Y. (1994). Surface modification of polymers for medical applications. Biomaterials 15, 725–736 [DOI] [PubMed] [Google Scholar]
- 77.Koh H.S., Yong T., Chan C.K., and Ramakrishna S. (2008). Enhancement of neurite outgrowth using nano-structured scaffolds coupled with laminin. Biomaterials 29, 3574–3582 [DOI] [PubMed] [Google Scholar]
- 78.Zander N.E., Orlicki J.A., Rawlett A.M., and Beebe T.P. (2012). Quantification of protein incorporated into electrospun polycaprolactone tissue engineering scaffolds. ACS Appl. Mater. Interfaces 4, 2074–2081 [DOI] [PubMed] [Google Scholar]
- 79.Kijeńska E., Prabhakaran M.P., Swieszkowski W., Kurzydlowski K.J., and Ramakrishna S. (2012). Electrospun bio-composite P(LLA-CL)/collagen I/collagen III scaffolds for nerve tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 100, 1093–1102 [DOI] [PubMed] [Google Scholar]
- 80.Klapka N., and Müller H.W. (2006). Collagen matrix in spinal cord injury. J. Neurotrauma 23, 422–435 [DOI] [PubMed] [Google Scholar]
- 81.Neal R.A., McClugage S.G., Link M.C., Sefcik L.S., Ogle R.C., and Botchwey E.A. (2009). Laminin nanofiber meshes that mimic morphological properties and bioactivity of basement membranes. Tissue Eng. Part C Methods 15, 11–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Corey J.M., Gertz C.C., Wang B.S., Birrell L.K., Johnson S.L., Martin D.C., and Feldman E.L. (2008). The design of electrospun PLLA nanofiber scaffolds compatible with serum-free growth of primary motor and sensory neurons. Acta Biomater. 4, 863–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schaub N.J., Le Beux C., Miao J., Linhardt R.J., Alauzun J.G., Laurencin D., and Gilbert R.J. (2015). The effect of surface modification of aligned poly-l-lactic acid electrospun fibers on fiber degradation and neurite extension. PLoS One 10, e0136780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Patel S., Kurpinski K., Quigley R., Gao H., Hsiao B.S., Poo M.M., and Li S. (2007). Bioactive nanofibers: synergistic effects of nanotopography and chemical signaling on cell guidance. Nano Lett. 7, 2122–2128 [DOI] [PubMed] [Google Scholar]
- 85.Cherry J.F., Carlson A.L., Benarba F.L., Sommerfeld S.D., Verma D., Loers G., Kohn J., Schachner M., and Moghe P.V. (2012). Oriented, multimeric biointerfaces of the L1 cell adhesion molecule: an approach to enhance neuronal and neural stem cell functions on 2-D and 3-D polymer substrates. Biointerphases 7, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zong X., Kim K., Fang D., Ran S., Hsiao B.S., and Chu B. (2002). Structure and process relationship of electrospun bioabsorbable nanofiber membranes. Polymer 43, 4403–4412 [Google Scholar]
- 87.Luu Y.K., Kim K., Hsiao B.S., Chu B., and Hadjiargyrou M. (2003). Development of a nanostructured DNA delivery scaffold via electrospinning of PLGA and PLA–PEG block copolymers. J. Control. Release 89, 341–353 [DOI] [PubMed] [Google Scholar]
- 88.Kenawy E.R., Bowlin G.L., Mansfield K., Layman J., Simpson D.G., Sanders E.H., and Wnek G.E. (2002). Release of tetracycline hydrochloride from electrospun poly(ethylene-co-vinylacetate), poly(lactic acid), and a blend. J. Control. Release 81, 57–64 [DOI] [PubMed] [Google Scholar]
- 89.Li X., Su Y., Liu S., Tan L., Mo X., and Ramakrishna S. (2010). Encapsulation of proteins in poly(l-lactide-co-caprolactone) fibers by emulsion electrospinning. Colloids Surf. B Biointerfaces 75, 418–424 [DOI] [PubMed] [Google Scholar]
- 90.Valmikinathan C.M., Defroda S., and Yu X. (2009). Polycaprolactone and bovine serum albumin based nanofibers for controlled release of nerve growth factor. Biomacromolecules 10, 1084–1089 [DOI] [PubMed] [Google Scholar]
- 91.Chew S.Y., Mi R., Hoke A., and Leong K.W. (2007). Aligned protein-polymer composite fibers enhance nerve regeneration: a potential tissue-engineering platform. Adv. Funct. Mater. 17, 1288–1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu T., Xu J., Chan B.P., and Chew S.Y. (2012). Sustained release of neurotrophin-3 and chondroitinase ABC from electrospun collagen nanofiber scaffold for spinal cord injury repair. J. Biomed. Mater. Res. A 100, 236–242 [DOI] [PubMed] [Google Scholar]
- 93.Sotelo C. (1967). Cerebellar neuroglia: Morphological and histochemical aspects., in: Progress in Brain Research. Fox C.A. and Snider R.S. (eds). Elsevier: Amsterdam, pps. 226–250 [DOI] [PubMed] [Google Scholar]
- 94.Haghighat N., and McCandless D.W. (1997). Effect of 6-aminonicotinamide on metabolism of astrocytes and C6-glioma cells. Metab. Brain Dis. 12, 29–45 [DOI] [PubMed] [Google Scholar]
- 95.Hunting D., Gowans B., and Henderson J.F. (1985). Effects of 6-aminonicotinamide on cell growth, poly(ADP-ribose) synthesis and nucleotide metabolism. Biochem. Pharmacol. 34, 3999–4003 [DOI] [PubMed] [Google Scholar]
- 96.Schaub N.J., and Gilbert R.J. (2011). Controlled release of 6-aminonicotinamide from aligned, electrospun fibers alters astrocyte metabolism and dorsal root ganglia neurite outgrowth. J. Neural Eng. 8, 046026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Downing T.L., Wang A., Yan Z.Q., Nout Y., Lee A.L., Beattie M.S., Bresnahan J.C., Farmer D.L., and Li S. (2012). Drug-eluting microfibrous patches for the local delivery of rolipram in spinal cord repair. J. Control. Release 161, 910–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zeng J., Xu X., Chen X., Liang Q., Bian X., Yang L., and Jing X. (2003). Biodegradable electrospun fibers for drug delivery. J. Control. Release 92, 227–231 [DOI] [PubMed] [Google Scholar]
- 99.Davies S.J., Fitch M.T., Memberg S.P., Hall A.K., Raisman G., and Silver J. (1997). Regeneration of adult axons in white matter tracts of the central nervous system. Nature 390, 680–683 [DOI] [PubMed] [Google Scholar]
- 100.Ritchie J.M. (1982). On the relation between fibre diameter and conduction velocity in myelinated nerve fibres. Proc. R. Soc. Lond. B Biol. Sci. 217, 29–35 [DOI] [PubMed] [Google Scholar]
- 101.Mukhatyar V.J., Salmerón-Sánchez M., Rudra S., Mukhopadaya S., Barker T.H., García A.J., and Bellamkonda R.V. (2011). Role of fibronectin in topographical guidance of neurite extension on electrospun fibers. Biomaterials 32, 3958–3968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee S., Leach M.K., Redmond S.A., Chong S.Y., Mellon S.H., Tuck S.J., Feng Z.Q., Corey J.M., and Chan J.R. (2012). A culture system to study oligodendrocyte myelination processes using engineered nanofibers. Nat. Methods 9, 917–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee S., Chong S.Y., Tuck S.J., Corey J.M., and Chan J.R. (2013). A rapid and reproducible assay for modeling myelination by oligodendrocytes using engineered nanofibers. Nat. Protoc. 8, 771–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Christopherson G.T., Song H., and Mao H.Q. (2009). The influence of fiber diameter of electrospun substrates on neural stem cell differentiation and proliferation. Biomaterials 30, 556–564 [DOI] [PubMed] [Google Scholar]
- 105.Abematsu M., Tsujimura K., Yamano M., Saito M., Kohno K., Kohyama J., Namihira M., Komiya S., and Nakashima K. (2010). Neurons derived from transplanted neural stem cells restore disrupted neuronal circuitry in a mouse model of spinal cord injury. J. Clin. Invest. 120, 3255–3266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zuidema J.M., Hyzinski-García M.C., Van Vlasselaer K., Zaccor N.W., Plopper G.E., Mongin A.A., and Gilbert R.J. (2014). Enhanced GLT-1 mediated glutamate uptake and migration of primary astrocytes directed by fibronectin-coated electrospun poly-l-lactic acid fibers. Biomaterials 35, 1439–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lepore A.C., O'Donnell J., Kim A.S., Yang E.J., Tuteja A., Haidet-Phillips A., O'Banion C.P., and Maragakis N.J. (2011). Reduction in expression of the astrocyte glutamate transporter, GLT1, worsens functional and histological outcomes following traumatic spinal cord injury. Glia 59, 1996–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rothstein J.D., Dykes-Hoberg M., Pardo C.A., Bristol L.A., Jin L., Kuncl R.W., Kanai Y., Hediger M.A., Wang Y., Schielke J.P., and Welty D.F. (1996). Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron 16, 675–686 [DOI] [PubMed] [Google Scholar]
- 109.Silver J., and Miller J.H. (2004). Regeneration beyond the glial scar. Nat. Rev. Neurosci. 5, 146–156 [DOI] [PubMed] [Google Scholar]
- 110.Sofroniew M.V. (2009). Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 32, 638–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sofroniew M.V., and Vinters H.V. (2010). Astrocytes: biology and pathology. Acta Neuropathol. 119, 7–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Norenberg M.D., Smith J., and Marcillo A. (2004). The pathology of human spinal cord injury: defining the problems. J. Neurotrauma 21, 429–440 [DOI] [PubMed] [Google Scholar]
- 113.Stichel C.C., and Müller H.W. (1998). The CNS lesion scar: new vistas on an old regeneration barrier. Cell Tissue Res. 294, 1–9 [DOI] [PubMed] [Google Scholar]
- 114.Stichel C.C., Hermanns S., Luhmann H.J., Lausberg F., Niermann H., D'Urso D., Servos G., Hartwig H.G., and Müller H.W. (1999). Inhibition of collagen IV deposition promotes regeneration of injured CNS axons. Eur. J. Neurosci. 11, 632–646 [DOI] [PubMed] [Google Scholar]
- 115.Liesi P., and Kauppila T. (2002). Induction of type IV collagen and other basement-membrane-associated proteins after spinal cord injury of the adult rat may participate in formation of the glial scar. Exp. Neurol. 173, 31–45 [DOI] [PubMed] [Google Scholar]
- 116.Yu W., Zhao W., Zhu C., Zhang X., Ye D., Zhang W., Zhou Y., Jiang X., and Zhang Z. (2011). Sciatic nerve regeneration in rats by a promising electrospun collagen/poly(epsilon-caprolactone) nerve conduit with tailored degradation rate. BMC Neurosci. 12, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Panseri S., Cunha C., Lowery J., Del Carro U., Taraballi F., Amadio S., Vescovi A., and Gelain F. (2008). Electrospun micro- and nanofiber tubes for functional nervous regeneration in sciatic nerve transections. BMC Biotechnol. 8, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]