Abstract
The central nervous system (CNS) is an important target of HIV, and cerebrospinal fluid (CSF) can provide a window into host–virus interactions within the CNS. The goal of this study was to determine whether HIV-specific CD8+ T cells are present in CSF of HIV controllers (HC), who maintain low to undetectable plasma viremia without antiretroviral therapy (ART). CSF and blood were sampled from 11 HC, defined based on plasma viral load (VL) consistently below 2,000 copies/ml without ART. These included nine elite controllers (EC, plasma VL <40 copies/ml) and two viremic controllers (VC, VL 40–2,000 copies/ml). All controllers had CSF VL <40 copies/ml. Three comparison groups were also sampled: six HIV noncontrollers (NC, VL >10,000 copies/ml, no ART); seven individuals with viremia suppressed due to ART (Tx, VL <40 copies/ml); and nine HIV-negative controls. CD4+ and CD8+ T cells in CSF and blood were analyzed by flow cytometry to assess expression of CCR5, activation markers CD38 and HLA-DR, and memory/effector markers CD45RA and CCR7. HIV-specific CD8+ T cells were quantified by major histocompatibility complex class I multimer staining. HIV-specific CD8+ T cells were detected ex vivo at similar frequencies in CSF of HC and noncontrollers; the highest frequencies were in individuals with CD4 counts below 500 cells/μl. The majority of HIV-specific CD8+ T cells in CSF were effector memory cells expressing CCR5. Detection of these cells in CSF suggests active surveillance of the CNS compartment by HIV-specific T cells, including in individuals with long-term control of HIV infection in the absence of therapy.
Introduction
The central nervous system (CNS) is an important target of HIV-1 infection. Neurological manifestations of HIV-1 include HIV encephalitis and its clinical manifestation, HIV-associated dementia.1,2 The brain and CNS are generally considered as immune privileged; nevertheless, the presence of HIV-specific T cells in brain and cerebrospinal fluid (CSF) has been documented, both in HIV-infected humans3–6 and in rhesus macaques infected with simian immunodeficiency virus (SIVmac).7,8 Sampling of CSF from HIV-infected subjects can provide a window into host–virus interactions in the CNS.9 The CSF reflects events occurring in the CNS since the brain interstitial fluid is in contact with the CSF produced in the choroid plexus and circulating through the subarachnoid space.10,11 The CSF compartment is accessible by lumbar puncture and multiple sampling is possible.
The CNS is thought of as immune privileged due to the presence of the blood–brain barrier (BBB) and the blood–cerebrospinal fluid barrier (BCSFB), but immune surveillance of the CNS does take place,12–15 as highlighted by recent evidence of CNS lymphatic vessels.16 The BBB is established by endothelial cells of brain parenchyma microvessels, and the BCSFB is formed by epithelial cells of the choroid plexus.17,18 Under physiological conditions, both barriers contain tight junctions,18 limiting leukocyte entry to small numbers of activated lymphocytes and monocytes. However, under inflammatory conditions, an increased number of activated leukocytes can enter the CNS.17,19 While it is known that virus-specific CD8+ T cells can infiltrate the CNS,12,20,21 few studies have addressed the role of these cells in fighting infection. In HIV infection, CD8+ T cells may mediate viral clearance from the CNS by killing infected CD4+ T cells and monocytes/macrophages; however, they may also contribute to inflammation by releasing proinflammatory cytokines and chemokines.22,23 The purpose of this study was to explore the potential role of T cells in CNS immune surveillance, focusing on HIV controllers (HC).
HC are defined as individuals with chronic HIV infection whose plasma viral load (VL) remains consistently below 2,000 viral RNA copies per milliliter without antiretroviral therapy (ART).24 This group includes “elite” controllers, who maintain VLs below 40 copies/ml, and “viremic” controllers, whose VLs are above 40 but below 2,000 copies/ml.24 The search for genetic factors that predispose to control of HIV infection revealed two major histocompatibility complex (MHC) class I alleles: HLA-B27 and HLA-B5725–29; however, although common among controllers, these alleles are neither necessary nor sufficient to confer protection from disease progression.30 Nevertheless, MHC class I-restricted, HIV-specific CD8+ T cells are believed to contribute to HIV control,31–33 and earlier studies from our group revealed polyfunctional HIV-specific CD8+ T cells in the gastrointestinal mucosa and blood of HC.34,35 HC generally lack detectable CNS infection and intrathecal inflammation.36,37 However, some individuals who meet the definition of HC nevertheless experience significant T-cell loss, demonstrate increased immune activation, and experience non-AIDS defining morbidities.38–41 This study provided an opportunity to determine the phenotype and frequency of HIV-specific CD8+ T cells in CSF of HIV-infected individuals, including controllers, noncontrollers (NC), and subjects on ART. In particular, in undertaking this study, we speculated that the detection of HIV-specific T cells in CSF of HC might suggest an ongoing antigen presence in the CSF or brain compartment despite apparent control of HIV replication as indicated by CSF VLs below detectable limits.
Materials and Methods
Participant characteristics
HC were defined based on plasma VL measurements consistently below 2,000 copies/ml in the absence of ART. Study participants included nine elite controllers (EC, plasma VL consistently <40 copies/ml, no ART); two viremic controllers (VC, VL consistently >40 but <2,000 copies/ml, no ART); six HIV NC (VL 70,000–650,000 copies/ml, no ART); seven ART-suppressed individuals (Tx, VL <40 copies/ml); and nine negative controls (Table 1).
Table 1.
Patient Characteristics
| Patient ID | Gender | Ethnicity | Years since diagnosis | Plasma viral load, copies/ml | CSF viral load, copies/ml | CD4 count, cells/μl | CSF WBC, count/mm3 | HIV-specific response studied |
|---|---|---|---|---|---|---|---|---|
| HIV controllers | ||||||||
| EC 7164 | M | C | 20 | <40 | <40 | 740 | 2 | B*5701 Gag KF11 |
| EC 7176 | F | H | 10 | <40 | <40 | 582 | 1 | B*5703 Gag TW10 |
| EC 7178 | F | AA | 18 | <40 | <40 | 1,738 | 7 | n/a |
| EC 7179 | M | C | 22 | <40 | <40 | 535 | 3 | n/a |
| EC 7180 | M | AA | 25 | <40 | <40 | 588 | 6 | n/a |
| EC 7183 | M | C | 25 | <40 | <40 | 731 | 7 | A*0301 Gag RK9 |
| EC 7187 | F | C | 5 | <40 | <40 | 725 | 2 | n/a |
| EC 7191 | M | C | 25 | <40 | <40 | 1,090 | 3 | n/a |
| EC 9038 | M | C | 2 | <40 | <40 | 1,251 | 6 | B*2705 Gag KK10 |
| VC 7190 | M | C | 20 | 231 | <40 | 468 | 1 | B*2705 Gag KK10 |
| VC 7109 | M | AA | 23 | 221 | <40 | 485 | 3 | B*5703 Gag KF11 |
| Noncontrollers | ||||||||
| NC 9012 | M | C | 1.2 | 71,700 | 296 | 544 | 8 | n/a |
| NC 9016 | M | C | 1.1 | 116,000 | 2,380 | 344 | 0 | A*0301 Gag RK9 |
| NC 9036 | M | C | 1.2 | 10,164 | 792 | 590 | 5 | A*0201 Gag SL9 |
| NC 9040 | M | C | 1.75 | 219,377 | 4,415 | 494 | 2 | n/a |
| NC 4141 | M | C | 19 | 62,285 | 63 | 94 | 2 | B*5701 Gag KF11 |
| NC 7193 | M | C | 27 | 15,483 | 11,496 | 396 | 7 | B*2705 Gag KK10 |
| HAART suppressed, n = 7 | 100% M | 5C, 2H | (2–26) | <40 | <40 | (410–917) | (0–4) | n/a |
| Negative controls, n = 9 | 100% M | 7C, 1As, 2AA | n/a | n/a | n/a | (534–1,070) | (0–5) | n/a |
Numbers between parentheses indicate ranges for HAART suppressed and negative controls.
AA, African American; As, Asian; C, Caucasian; CSF, cerebrospinal fluid; EC, elite controllers; H, Hispanic/Latino; n/a, not applicable; NC, noncontrollers; VC, viremic controllers.
Sample collection
Blood and CSF were obtained at San Francisco General Hospital (San Francisco, CA). Written informed consent for phlebotomy and lumbar puncture was obtained from all subjects under protocols approved by the Committee on Human Research, University of California, San Francisco, and the Institutional Review Board, University of California, Davis. Exclusion criteria included pregnancy, presence of a brain mass lesion at risk of herniation, bleeding diathesis, or any other contraindication to lumbar puncture. Approximately 20 ml of blood and 20 ml of CSF were obtained from each subject. Fresh samples were transported to the laboratory at the University of California, Davis, for immediate processing of fresh cells. CSF samples contained ∼2–16 × 103 WBC per milliliter (Table 1).
Sample processing
One milliliter of blood was set aside for antibody staining before processing. Blood was layered onto Ficoll–Hypaque (Pfizer, New York, NY) and centrifuged at 400 g for 20 min to isolate peripheral blood mononuclear cells (PBMC). CSF was centrifuged at 400 g for 10 min to pellet mononuclear cells. PBMC were maintained in RPMI 1640 (Gibco, Grand Island, NY) supplemented with 15% fetal calf serum, penicillin (100 U/ml), streptomycin (100 μg/ml), and l-glutamine (2 nM), referred to as R15. To generate sufficient CD8+ T cells for enzyme-linked ImmunoSpot (ELISPOT) assays, PBMC were polyclonally expanded in R15 with 50 U/ml human recombinant interleukin-2 (R&D Systems, Minneapolis, MN) and stimulated with 0.62 μg/ml anti-CD3/4 bispecific antibody for CD8+ T-cell enrichment.35
ELISPOT assays
To map HIV-specific CD8+ T-cell responses, PBMC were tested for responsiveness to HIV peptides in ELISPOT assays. Polyclonally expanded CD8+ T lymphocytes from PBMC were plated at 1 × 105 to 2 × 105 cells per well; pooled or individual peptides (10 μg/ml; NIH Reagent Program, Germantown, MD) were added to duplicate wells. Staphylococcal enterotoxin B was used as positive control; the R15 medium was used for negative controls. ELISPOT assays were performed as previously described.35 Plates were read with an ELISPOT reader (Autoimmun Diagnostika GMBH, Strasberg, Germany). Responses were quantified as spot-forming cells per 106 cells (SFC/million) after subtracting negative control values, and values of >50 SFC/million were considered positive.35 The goal of these assays was not to perform comprehensive epitope mapping, but instead to identify immunodominant responses for each study participant (data not shown) and select MHC class I tetramer and pentamer reagents for subsequent flow cytometry experiments using CSF.
HLA class I typing
Genomic DNA was isolated from 5 to 6 million PBMC using a QIAamp DNA Blood Mini Kit (Qiagen, Valencia, CA) and quantified by a spectrophotometer. Low-resolution MHC class I typing was performed using polymerase chain reaction (PCR) and an ABC SSP (sequence-specific primer) UniTray kit (Invitrogen, Carlsbad, CA). High-resolution typing was performed using HLA-A or HLA-B SSP UniTray kits (Invitrogen). PCR products were resolved by electrophoresis on a 2% agarose gel, photographed, and analyzed using UniMatch Plus software (Invitrogen). (Table 1 and Supplementary Table S1).
Antibodies, staining, and flow cytometry
Fluorochrome-labeled monoclonal antibodies to CD3 (Clone UCHT-1), CD4, CD14, and CD19 were purchased from BD Biosciences (San Jose, CA). Fluorochrome-labeled antibody to CD8 (Clone 3B5) was purchased from Invitrogen; antibodies to CCR7 and CD45RA were purchased from BD and Beckman Coulter (Brea, CA), respectively. Antibodies to CD38 (ADP-ribosyl cyclase), HLA-DR (MHC class II), and CCR5 were purchased from BD Biosciences. Samples were stained with amine binding Aqua dye (Invitrogen) to label dead cells. MHC class I peptide tetramers were purchased from Beckman Coulter; MHC class I peptide pentamers were purchased from ProImmune (Oxford, United Kingdom); HLA-B5703-restricted KF11 and TW10 tetramers were generated at the Weatherall Institute of Molecular Medicine (Oxford, United Kingdom).
Antibody staining of CSF and whole blood was performed on fresh samples without prior T-cell expansion. CSF cells were washed once in phosphate-buffered saline and stained with Aqua amine dye (Invitrogen) at a 1:10 dilution for 30 min and then quenched with fetal bovine serum. For whole blood staining, 100 μl of blood was dispensed directly into staining tubes. MHC class I peptide multimers were added to respective tubes and stained for 20 min; other surface stains were then added and incubated an additional 20 min. Blood samples were treated with 3 ml of cold ACK lysing buffer for 10 min. All samples were washed with the FACS buffer and fixed in 1% paraformaldehyde for at least 1 h at 4°C, before acquisition on an LSRII (BD Biosciences).
Data analysis
Flow cytometry data were analyzed with FlowJo software (TreeStar, Ashland, OR). Bivariate plots were constructed using “fluorescence minus one” controls to confirm placement of individual gates.42 Statistical analyses were performed using GraphPad Prism software (GraphPad software, San Diego, CA). Nonparametric tests were used for all analyses and paired tests were used where applicable. Differences in variables between subject groups were analyzed using the Mann–Whitney U test or the Wilcoxon test.
Results
HIV-specific CD8+ T cells are present in CSF of HC and NC
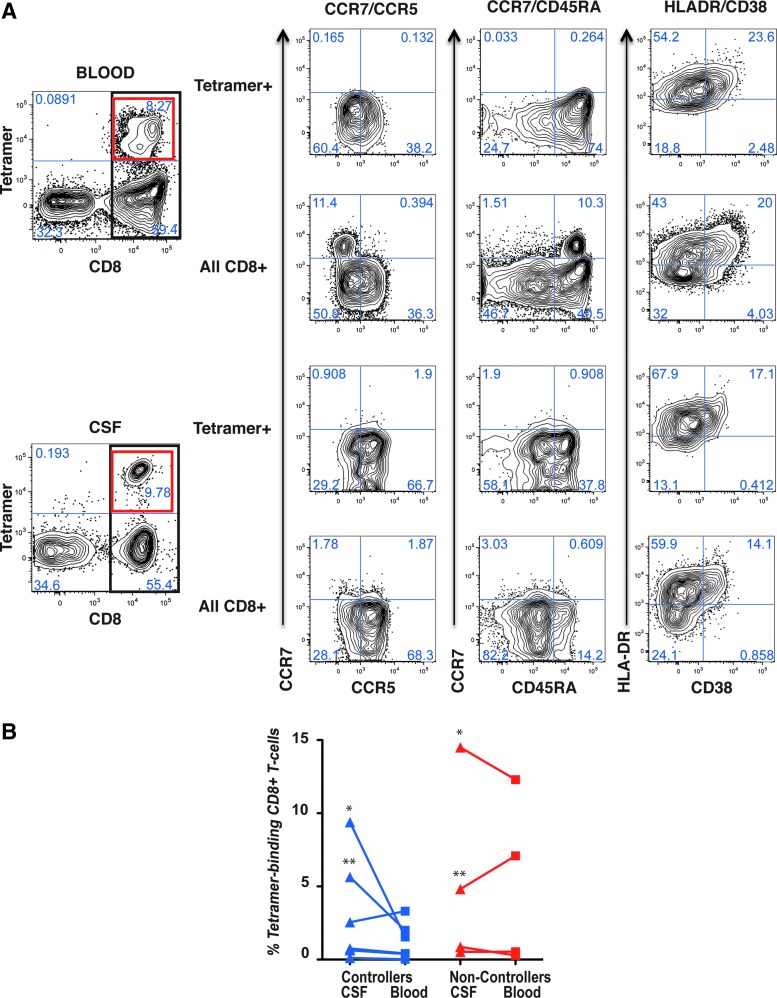
Participant characteristics are summarized in Table 1. Due to the low number of leukocytes isolated from CSF (Table 1), epitope mapping was performed on CD8+ T cells from a preliminary blood sample obtained before lumbar puncture. MHC class I tetramers or pentamers corresponding to appropriate MHC class I/peptide combinations were available for 6 of the 11 HC and 4 of the 6 chronically infected NC, none of whom was on therapy at the time of sampling. Using the appropriate MHC-matched reagents, HIV-specific CD8+ T cells were detected in blood and CSF as shown in the flow cytometry plots in Figure 1A. Two controllers, 7190 and 7109 (Fig. 1B), showed substantially higher frequencies of MHC multimer-binding cells in CSF compared to blood. Interestingly, these two subjects had “protective” MHC Class I alleles but were classified as VC based on their VLs, which exceeded the threshold for EC.24 These two participants also had the lowest CD4 T-cell counts of any in the controller group. HIV-specific CD8+ T cells were also detected in CSF and blood of NC. Intriguingly, the noncontroller group included two participants, 7193 and 4141, with “protective” MHC class I alleles, whose plasma VLs exceeded 10,000 copies/ml (Fig. 1B); these two subjects had the highest percentages of HIV-specific CD8+ T cells in blood and CSF among the noncontroller group, and also had CD4 T-cell counts below 500 cells/μl. Thus, somewhat unexpectedly, HC and NC had similar frequencies of HIV-specific CD8+ T cells in CSF, and in both groups, the participants with the highest frequencies had blood CD4+ T-cell counts below 500 cells/μl.
FIG. 1.
MHC class I multimer staining and multicolor phenotyping. (A) Shows flow cytometry bivariate contour plots for whole blood (top two rows) and CSF (bottom two rows). At the far left, MHC class I multimer staining (y-axis) is plotted versus CD8 (x-axis). The heavy black rectangle indicates cells staining positive for CD8, which are further delineated in the bivariate plots labeled “All CD8+” at right. The heavy red square in the upper right quadrant indicates CD8+ T cells stained with MHC class I multimer, which are further delineated in the bivariate plots labeled “Tetramer+” at right. Three additional surface staining combinations are shown: from left to right, CCR7 (y-axis) versus CCR5 (x-axis); CCR7 (y-axis) versus CD45RA (x-axis); and HLA-DR (y-axis) versus CD38 (x-axis). Numbers in each quadrant indicate the percentages of cells in that quadrant. All data in (A) were from an HIV noncontroller, subject number 7193. (B) Shows the percentage of CD8+ T cells that bound MHC class I multimers in HIV controllers (blue shapes) or noncontrollers (red shapes). Values for CSF are indicated by triangles; values for blood are indicated by squares. Each subject corresponds to a single line connecting blood and CSF values. For one subject, noncontroller 9016, PBMC were stained instead of whole blood (0.544%). Asterisks indicate samples from subjects specifically discussed in the text, as follows: left side, shown in blue: *HIV controller 7190; **HIV controller 7109; right side, shown in red: *HIV noncontroller 7193; **HIV noncontroller 4141. CSF, cerebrospinal fluid; MHC, major histocompatibility complex; PBMC, peripheral blood mononuclear cells.
Memory T cells dominate in CSF of all subject groups
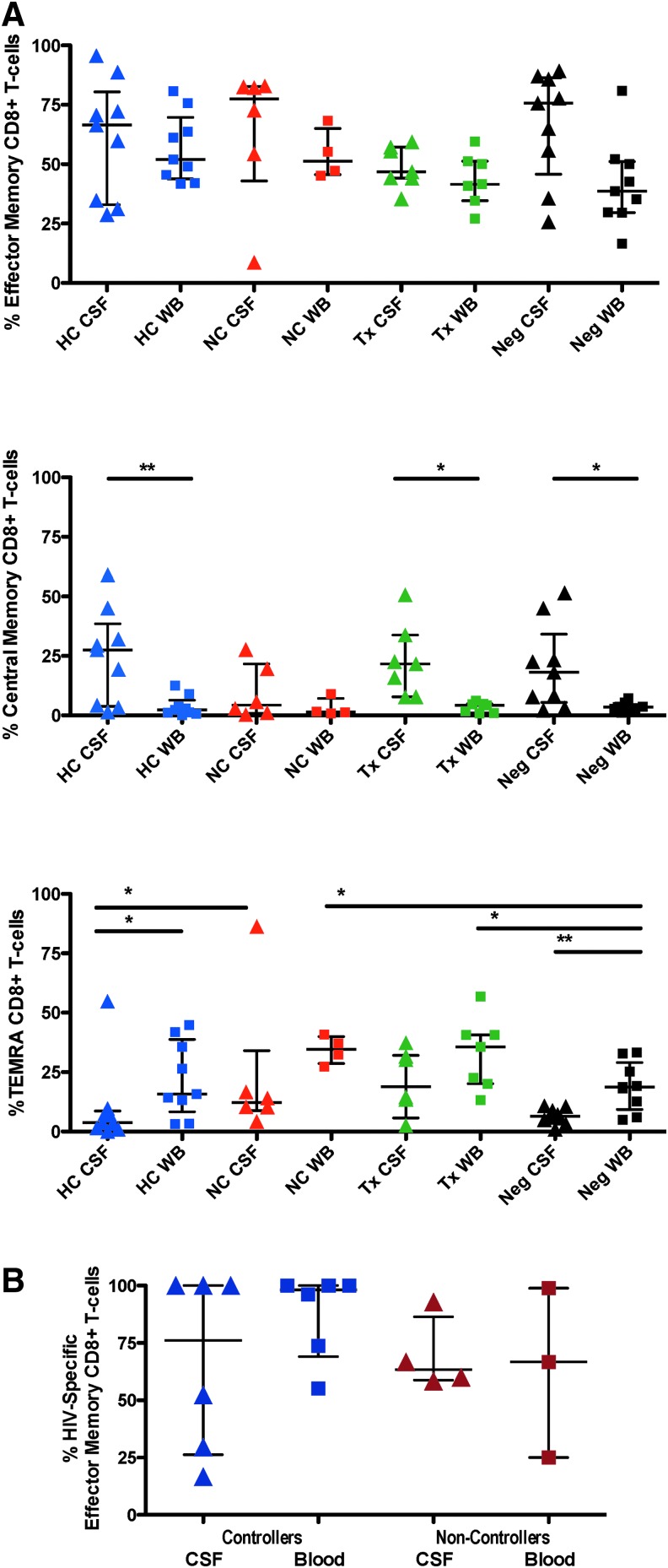
We next addressed the differentiation phenotype of all CSF T lymphocytes, regardless of antigen specificity. Coexpression patterns of CD45RA and CCR7 were used to delineate memory/effector differentiation status of CD8+ T cells in CSF and blood (Fig. 2A). CD45RA+CCR7+ cells were defined as naive, CD45RA−CCR7+ as central memory (CM), CD45RA−CCR7− as effector memory (EM), and CD45RA+CCR7− as terminally differentiated effector cells.43,44 As expected, naive T cells were rarely detected in CSF (not shown). Memory CD8+ T cells (both EM and CM) were more abundant in CSF than in blood regardless of subject group, consistent with previous reports.4,6 EM CD8+ T cells were the most abundant subset in CSF, followed by CM cells (Fig. 2A). CM CD8+ T cells were significantly more frequent in CSF than blood in all subject groups except NC. Terminally differentiated effector cells were consistently more abundant in blood than CSF.
FIG. 2.
(A) Shows the breakdown of CD8+ T cells in whole blood and CSF that had a central memory, effector memory, or “TEMRA” (terminally differentiated, RA expressing) phenotype. HC, HIV controllers (blue shapes); NC, noncontrollers (red shapes); Tx, subjects on ART with VL <50 (green shapes); Neg, HIV-negative controls (black shapes). Lines and asterisks above the graphs indicate p values for statistically significant differences between groups (*p < .05; **p < .01). (B) Summarizes the percentages of HIV-specific CD8+ T cells (i.e., binding MHC class I multimers) that were effector memory cells. ART, antiretroviral therapy; VL, viral load.
CSF HIV-specific CD8+ T cells are mainly “effector memory” cells
In most subjects for whom HIV-specific CD8+ T-cell responses could be quantified using MHC class I multimers, greater than 50% of the HIV-specific, CD8+ T cells in CSF were EM T cells (Fig. 2B). The two exceptions to this trend were HC 9038 and 7183, in whom greater than 50% of multimer-binding CSF CD8+ T cells had a CM phenotype.
CSF T cells in all groups express CCR5
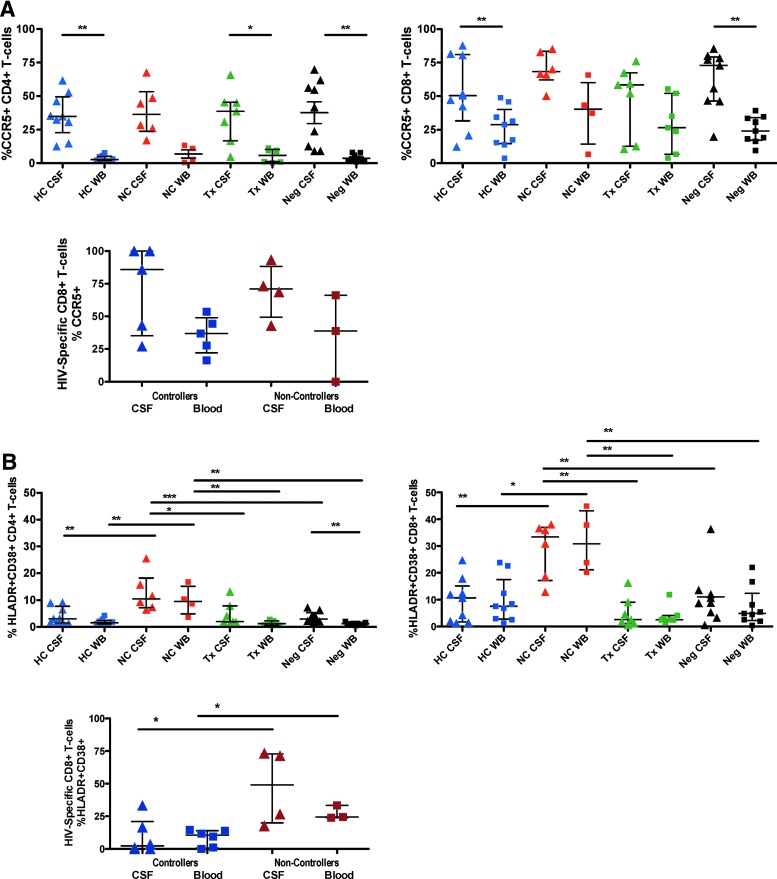
In all subject groups, CCR5, the receptor for beta chemokines CCL-3, CCL-4, and CCL-5, was expressed on a higher percentage of CD4+ and CD8+ T cells in CSF compared to blood (Fig. 3A). The difference was statistically significant for CD4+ T cells in all groups except HIV NC (Fig. 3A, top left panel) and for CD8+ T cells in both HC and healthy controls (right panel). Most HIV-specific, MHC class I multimer-binding CD8+ T cells in CSF expressed CCR5. Previous reports have suggested a role for CCR5 and its ligands in recruitment of antigen-specific T cells to the CNS.6 However, CCR5 is also highly expressed on activated T cells, and expression levels are modulated by factors affecting T-cell activation.45
FIG. 3.
(A) The top row shows the percentage of all CD4+ T cells (left) or all CD8+ T cells (right) in blood or CSF that were positive for CCR5. Second row: the graph shows the percentages of HIV-specific CD8+ T cells (i.e., binding MHC class I multimers) that were positive for CCR5 in blood or CSF. HC, HIV controllers (blue shapes); NC, noncontrollers (red shapes); Tx, subjects on ART with VL <50 (green shapes); Neg, HIV-negative controls (black shapes). Lines and asterisks above the graphs indicate p values for statistically significant differences between groups (*p < .05; **p < .01; ***p < 0.001). (B) Shows the percentage of all CD4+ T cells (left) or all CD8+ T cells (right) in blood or CSF that coexpressed the activation markers HLA-DR and CD38. The third panel shows the percentages of HIV-specific CD8+ T cells (i.e., binding MHC class I multimers) that were positive for both HLA-DR and CD38 in blood or CSF. Lines and asterisks above the graphs indicate p values for statistically significant differences between groups (*p < .05).
Increased CNS immune activation in HIV NC
CD38 (ADP-ribosyl cyclase) and HLA-DR (MHC class II) are considered markers of T-cell activation and their coexpression in HIV infection strongly correlates with clinical parameters such as plasma VL and CD4+ T-cell count.46,47 HIV NC had significantly higher percentages of CD38+/HLADR+ T cells in CSF and blood compared to all other subject groups, as shown in Figure 3B. Percentages of CD38+/HLA-DR+ CD8+ T cells in HC were similar to healthy controls, but slightly elevated compared to subjects on ART. The percentage of HIV-specific CD8+ T cells coexpressing CD38 and HLA-DR was higher in NC than controllers (Fig. 3B, lower panel); however, the comparison involved relatively few data points and should be interpreted with caution.
Plasma neopterin levels reveal immune activation in HC
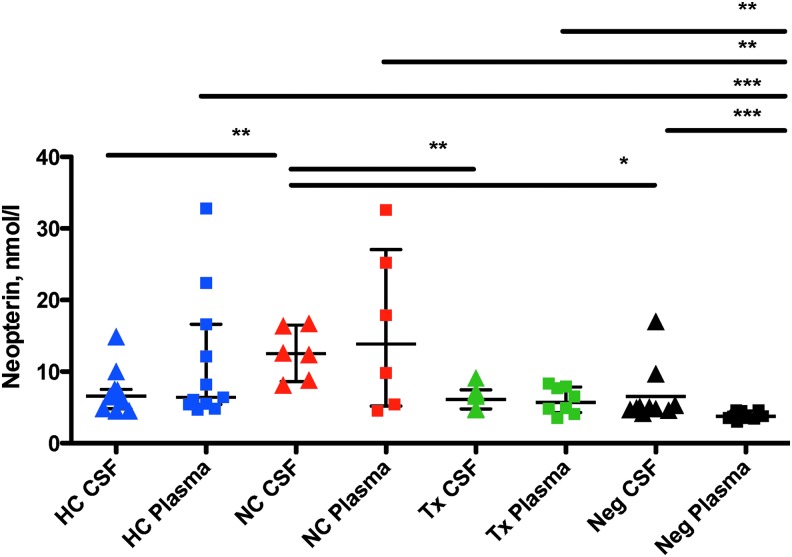
Another biomarker of CNS immune activation is neopterin, a soluble protein found in plasma and CSF that indicates macrophage activation.48 In a recent study of HC, neopterin levels in plasma and CSF did not differ significantly between controllers, negative controls, and subjects on ART.37 A similar trend was observed in the present study: CSF neopterin concentrations for HC were similar to those in ART subjects and negative controls (p > .05 for all pairwise comparisons). In contrast, NC had elevated CSF neopterin relative to all other groups (Fig. 4). However, plasma neopterin levels in the controller group were significantly elevated compared to HIV-negative controls.
FIG. 4.
Neopterin. The graph shows levels of neopterin (nM) measured in CSF (triangles) and plasma (squares) obtained from participants in each group. HC, HIV controllers (blue shapes); NC, noncontrollers (red shapes); Tx, subjects on ART with VL <50 (green shapes); Neg, HIV-negative controls (black shapes). Lines and asterisks above the graphs indicate p values for statistically significant differences between groups (*p < .05; **p < .01; ***p < .001).
Discussion
These studies revealed that HIV-specific CD8+ T cells are present in the CSF of HC and NC. Earlier studies of HC indicated that HIV-specific CD8+ T cells are abundant in blood49,50 and the gastrointestinal tract,34,51 raising the possibility that widespread dissemination of these cells throughout the body could be a correlate of nonprogression. However, in this study, we did not find significant differences in CSF T-cell frequency or phenotype between HC and NC. Earlier studies have also suggested that HIV-specific CD8+ T cells in HC are more highly polyfunctional or more effective at eliminating HIV-infected cells than their counterparts in NC.35,49 Unfortunately, such studies require direct ex vivo analysis of >1 × 105 cells, and the relatively low number of leukocytes isolated from CSF made it impossible to explore T-cell functionality in this study. The widespread dissemination of HIV-specific CD8+ T cells throughout the body may also be a result of normal T-cell trafficking processes, which are enhanced by the presence of antigen and/or inflammation in certain anatomical reservoirs. Mathematical modeling of the relationship between antigen localization and T-cell trafficking will require highly sensitive methods of T-cell and antigen tracking, such as those developed for use in animal model systems.52,53
The factors contributing to CSF T-cell trafficking are complex and incompletely understood. Migration of T cells across the BCSFB is a multistep process involving interactions between T cells and choroid plexus epithelial cells.19 Systemic viral infection increases generalized inflammation and expression of adhesion molecules that regulate T-cell trafficking. In addition, the presence of virus-infected cells within the CNS leads to local inflammation and expression of chemokines.6,54 The presence of HIV-specific CD8+ T cells in CSF of controllers may be a response to low-level CNS infection that is below the detection threshold of standard assays.55 However, we observed comparable frequencies of HIV-specific T cells in CSF of controllers (with undetectable CSF VL) and NC (including several with CSF VL >103 copies/ml), arguing against a straightforward relationship between these cells and viremia. Furthermore, the four individuals with the highest frequencies of HIV-specific T cells in CSF in this study had CD4 counts below 500 cells/μl, suggesting an association between high CSF HIV-specific T-cell frequencies and disease progression.
CNS T-cell trafficking may also be enhanced by persistent immune activation, which has been reported in EC.38,56,57 In the present study, however, CSF biomarkers of immune activation (T-cell activation marker expression and CSF neopterin levels) were highest in HIV NC and were similar among controllers, treated individuals, and HIV-negative controls. Similarly, in a recent study of a panel of CSF biomarkers in 143 subjects from 8 HIV-infected groups, we found that 7 of 8 HC had CSF biomarkers in the normal range; an exception was elevated CSF neurofilament light chain marker in 1 subject after age correction.58 Nevertheless, several HC in the present study had elevated plasma neopterin compared to negative controls and ART-treated subjects, indicating persistent systemic immune activation in these controllers despite control of HIV replication.
Chemokines and their receptors clearly play a major role in leukocyte trafficking in health and disease. CCR5 serves as a major coreceptor for HIV,59–62 as well as a receptor for chemokines CCL-3, CCL-4, and CCL-5, which mediate leukocyte migration to tissues.6,63,64 CCR5 is also highly expressed on activated T cells, and expression levels are modulated by factors affecting T-cell activation.45 In this study, higher percentages of CCR5-expressing CD4+ and CD8+ T cells were present in CSF compared to blood in all subject groups. CNS viral infection or inflammation may induce local secretion of β-chemokines, which would chemoattract CCR5+ leukocytes to the CNS.6 Earlier studies have implicated a role for CCR5 and its ligands in leukocyte trafficking to the CNS in multiple sclerosis,21–23 other inflammatory conditions,65,66 as well as HIV and other viral infections.6,67
Not surprisingly, EM CD8+ cells were the predominant subset in CSF of all subject groups; EM T cells also predominate at other nonlymphoid immune effector sites throughout the body.68 Interestingly, in this study, CM cells were present at higher frequency in CSF than blood of all subject groups. CM cells, long-lived cells generally harbored in lymph nodes, are noteworthy for high proliferative capacity and relative resistance to apoptosis.69 The precise lineage relationship between CM and EM cells remains controversial70; nevertheless, the abundance of CM cells in CSF, including in healthy individuals, supports the concept that these cells play an active role in CNS immunosurveillance, returning to deep cervical lymph nodes after circulating through CSF.71–73 This is an evolving concept in neuroimmunology, supported by recent studies in animal models of both infectious and neurodegenerative diseases.14,63
Intriguingly, in this study, the two HIV NC with the highest frequencies of HIV-specific CD8+ T cells in CSF had “protective” MHC class I alleles, HLA-B27, and B57. Viral escape from epitopes restricted by these alleles has been documented: controllers positive for HLA-B27 show a robust response to a single CD8+ T-cell epitope, and viral escape from this response results in disease progression.74,75 In contrast, CD8+ T cells of controllers positive for HLA-B57 may respond to one or more of several HLA-B57-restricted epitopes, and viral escape from one of these epitopes can often be compensated by responses to other epitopes.27,76,77 Unfortunately, we were not able to test for the presence of viral escape mutants in plasma or CSF of these two subjects. Longitudinal studies of individuals with these alleles, to track the emergence of CTL-resistant viral variants, may improve our understanding of the relationship between immune escape and viral dissemination to the CNS.
In summary, this study demonstrated that individuals meeting a stringent definition of HC, as well as more typical HIV NC, have HIV-specific CD8+ T cells in CSF. Additional studies of these cohorts may shed further light on how immunological control relates to CNS involvement in HIV disease and may inform approaches to HIV remission/cure strategies, where the goal is prevention of viral replication and suppression of abnormal immune activation.
Supplementary Material
Acknowledgments
We thank the study volunteers for their participation in this project. We also thank the staff of the UCSF-CTSI Clinical Research Center at San Francisco General Hospital, for their assistance, and Rebecca Hoh, San Francisco General Hospital, for assistance with the subject cohort. This work was supported by the National Institutes of Health research grant R21 NS069219, the UCSF/Gladstone Institute of Virology & Immunology CFAR (P30 AI027763), the UCSF Clinical and Translational Research Institute (UL1 TR000004 and UL1 RR024131), the Center for AIDS Prevention Studies (P30 MH62246), and NIAID (K24 AI069994). This investigation was conducted in a facility constructed with support from the NIH Research Facilities Improvement Program (grant C06 RR012088). The LSR-II violet laser was upgraded with support from the James B. Pendleton Charitable Trust. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the sponsors.
Authors' Contributions
This work was performed by A.G. in partial fulfillment of the requirements for the degree of Master of Science (MS) in Genetics at UC Davis. B.L.S., S.S.S., and R.W.P. planned the study with consultation from S.G.D. and P.W.H.; A.G. and D.L. performed T-cell experiments and analyzed data; D.F. performed neopterin assays and analyzed data; S.G.D., P.W.H., R.W.P., S.S., E.L., and J.P. oversaw the clinical cohorts and collected samples; G.M.G. provided MHC class I tetramers; B.E.M. assisted with flow cytometry; A.L.F. helped characterize the cohort; A.G. and B.L.S. wrote the manuscript; all authors read and edited the manuscript.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Fuller GN, Guiloff RJ, Gazzard B, Harcourt-Webster JN, Scarvilli F: Neurological presentations of AIDS—when to test for HIV. J R Soc Med 1989;82:717–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levy JA, Shimabukuro J, Hollander H, Mills J, Kaminsky L: Isolation of AIDS-associated retroviruses from cerebrospinal fluid and brain of patients with neurological symptoms. Lancet 1985;2:586–588 [PubMed] [Google Scholar]
- 3.Jassoy C, Johnson RP, Navia BA, Worth J, Walker BD: Detection of a vigorous HIV-1-specific cytotoxic T lymphocyte response in cerebrospinal fluid from infected persons with AIDS dementia complex. J Immunol 1992;149:3113–3119 [PubMed] [Google Scholar]
- 4.Sadagopal S, Lorey SL, Barnett L, et al. : Enhancement of human immunodeficiency virus (HIV)-specific CD8+ T cells in cerebrospinal fluid compared to those in blood among antiretroviral therapy-naive HIV-positive subjects. J Virol 2008;82:10418–10428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sethi KK, Naher H, Stroehmann I: Phenotypic heterogeneity of cerebrospinal fluid-derived HIV-specific and HLA-restricted cytotoxic T-cell clones. Nature 1988;335:178–181 [DOI] [PubMed] [Google Scholar]
- 6.Shacklett BL, Cox CA, Wilkens DT, et al. : Increased adhesion molecule and chemokine receptor expression on CD8+ T cells trafficking to cerebrospinal fluid in HIV-1 infection. J Infect Dis 2004;189:2202–2212 [DOI] [PubMed] [Google Scholar]
- 7.Marcondes MC, Burdo TH, Sopper S, et al. : Enrichment and persistence of virus-specific CTL in the brain of simian immunodeficiency virus-infected monkeys is associated with a unique cytokine environment. J Immunol 2007;178:5812–5819 [DOI] [PubMed] [Google Scholar]
- 8.Marcondes MC, Burudi EM, Huitron-Resendiz S, et al. : Highly activated CD8(+) T cells in the brain correlate with early central nervous system dysfunction in simian immunodeficiency virus infection. J Immunol 2001;167:5429–5438 [DOI] [PubMed] [Google Scholar]
- 9.Price RW: The two faces of HIV infection of cerebrospinal fluid. Trends Microbiol 2000;8:387–391 [DOI] [PubMed] [Google Scholar]
- 10.Cserr HF, Depasquale M, Patlak CS, Pullen RG: Convection of cerebral interstitial fluid and its role in brain volume regulation. Ann N Y Acad Sci 1986;481:123–134 [DOI] [PubMed] [Google Scholar]
- 11.Weller RO, Kida S, Zhang ET: Pathways of fluid drainage from the brain—morphological aspects and immunological significance in rat and man. Brain Pathol 1992;2:277–284 [DOI] [PubMed] [Google Scholar]
- 12.Hickey WF, Hsu BL, Kimura H: T-lymphocyte entry into the central nervous system. J Neurosci Res 1991;28:254–260 [DOI] [PubMed] [Google Scholar]
- 13.Hickey WF: Basic principles of immunological surveillance of the normal central nervous system. Glia 2001;36:118–124 [DOI] [PubMed] [Google Scholar]
- 14.Ousman SS, Kubes P: Immune surveillance in the central nervous system. Nat Neurosci 2012;15:1096–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ransohoff RM, Engelhardt B: The anatomical and cellular basis of immune surveillance in the central nervous system. Nat Rev Immunol 2012;12:623–635 [DOI] [PubMed] [Google Scholar]
- 16.Louveau A, Smirnov I, Keyes TJ, et al. : Structural and functional features of central nervous system lymphatic vessels. Nature 2015;523:337–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Couraud PO, Nassif X, Bourdoulous S: Mechanisms of infiltration of immune cells, bacteria and viruses through brain endothelium. Adv Mol Cel Biol 2003;31:255–268 [Google Scholar]
- 18.Coisne C, Engelhardt B: Tight junctions in brain barriers during central nervous system inflammation. Antioxid Redox Signal 2011;15:1285–1303 [DOI] [PubMed] [Google Scholar]
- 19.Engelhardt B, Coisne C: Fluids and barriers of the CNS establish immune privilege by confining immune surveillance to a two-walled castle moat surrounding the CNS castle. Fluids Barriers CNS 2011;8:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raine CS: The Dale E. McFarlin Memorial Lecture: The immunology of the multiple sclerosis lesion. Ann Neurol 1994;36 Suppl:S61–S72 [DOI] [PubMed] [Google Scholar]
- 21.Kivisakk P, Trebst C, Liu Z, et al. : T-cells in the cerebrospinal fluid express a similar repertoire of inflammatory chemokine receptors in the absence or presence of CNS inflammation: implications for CNS trafficking. Clin Exp Immunol 2002;129:510–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balashov KE, Rottman JB, Weiner HL, Hancock WW: CCR5(+) and CXCR3(+) T cells are increased in multiple sclerosis and their ligands MIP-1alpha and IP-10 are expressed in demyelinating brain lesions. Proc Natl Acad Sci U S A 1999;96:6873–6878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sorensen TL, Tani M, Jensen J, et al. : Expression of specific chemokines and chemokine receptors in the central nervous system of multiple sclerosis patients. J Clin Invest 1999;103:807–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deeks SG, Walker BD: Human immunodeficiency virus controllers: Mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity 2007;27:406–416 [DOI] [PubMed] [Google Scholar]
- 25.Kiepiela P, Leslie AJ, Honeyborne I, et al. : Dominant influence of HLA-B in mediating the potential co-evolution of HIV and HLA. Nature 2004;432:769–775 [DOI] [PubMed] [Google Scholar]
- 26.Kosmrlj A, Read EL, Qi Y, et al. : Effects of thymic selection of the T-cell repertoire on HLA class I-associated control of HIV infection. Nature 2010;465:350–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Migueles SA, Sabbaghian MS, Shupert WL, et al. : HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc Natl Acad Sci U S A 2000;97:2709–2714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao X, O'Brien TR, Welzel TM, et al. : HLA-B alleles associate consistently with HIV heterosexual transmission, viral load, and progression to AIDS, but not susceptibility to infection. AIDS 2010;24:1835–1840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heeney JL, Dalgleish AG, Weiss RA: Origins of HIV and the evolution of resistance to AIDS. Science 2006;313:462–466 [DOI] [PubMed] [Google Scholar]
- 30.Emu B, Sinclair E, Hatano H, et al. : HLA class I-restricted T-cell responses may contribute to the control of human immunodeficiency virus infection, but such responses are not always necessary for long-term virus control. J Virol 2008;82:5398–5407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brodie SJ, Patterson BK, Lewinsohn DA, et al. : HIV-specific cytotoxic T lymphocytes traffic to lymph nodes and localize at sites of HIV replication and cell death. J Clin Invest 2000;105:1407–1417 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Shacklett BL, Cox CA, Sandberg JK, Stollman NH, Jacobson MA, Nixon DF: Trafficking of human immunodeficiency virus type 1-specific CD8+ T cells to gut-associated lymphoid tissue during chronic infection. J Virol 2003;77:5621–5631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shacklett BL, Beadle TJ, Pacheco PA, et al. : Characterization of HIV-1-specific cytotoxic T lymphocytes expressing the mucosal lymphocyte integrin CD103 in rectal and duodenal lymphoid tissue of HIV-1-infected subjects. Virology 10 2000;270:317–327 [DOI] [PubMed] [Google Scholar]
- 34.Ferre AL, Hunt PW, Critchfield JW, et al. : Mucosal immune responses to HIV-1 in elite controllers: A potential correlate of immune control. Blood 2009;113:3978–3989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferre AL, Lemongello D, Hunt PW, et al. : Immunodominant HIV-specific CD8+ T-cell responses are common to blood and gastrointestinal mucosa, and Gag-specific responses dominate in rectal mucosa of HIV controllers. J Virol 2010;84:10354–10365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dahl V, Peterson J, Spudich S, et al. : Single-copy assay quantification of HIV-1 RNA in paired cerebrospinal fluid and plasma samples from elite controllers. AIDS 2013;27:1145–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Probasco JC, Deeks SG, Lee E, et al. : Cerebrospinal fluid in HIV-1 systemic viral controllers: absence of HIV-1 RNA and intrathecal inflammation. AIDS 2010;24:1001–1005 [DOI] [PubMed] [Google Scholar]
- 38.Hunt PW: Natural control of HIV-1 replication and long-term nonprogression: Overlapping but distinct phenotypes. J Infect Dis 2009;200:1636–1638 [DOI] [PubMed] [Google Scholar]
- 39.Krishnan S, Wilson EM, Sheikh V, et al. : Evidence for innate immune system activation in HIV type 1-infected elite controllers. J Infect Dis 2014;209:931–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okulicz JF, Marconi VC, Landrum ML, et al. : Clinical outcomes of elite controllers, viremic controllers, and long-term nonprogressors in the US Department of Defense HIV natural history study. J Infect Dis 2009;200:1714–1723 [DOI] [PubMed] [Google Scholar]
- 41.Pereyra F, Lo J, Triant VA, et al. : Increased coronary atherosclerosis and immune activation in HIV-1 elite controllers. AIDS 2012;26:2409–2412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roederer M: Compensation in flow cytometry. Curr Protoc Cytom 2002;Chapter 1:Unit 1.14 [DOI] [PubMed]
- 43.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A: Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 1999;401:708–712 [DOI] [PubMed] [Google Scholar]
- 44.Champagne P, Ogg GS, King AS, et al. : Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature 2001;410:106–111 [DOI] [PubMed] [Google Scholar]
- 45.Richardson MW, Jadlowsky J, Didigu CA, Doms RW, Riley JL: Kruppel-like Factor 2 Modulates CCR5 Expression and Susceptibility to HIV-1 Infection. J Immunol 2012;189:3815–3821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eggena MP, Barugahare B, Okello M, et al. : T cell activation in HIV-seropositive Ugandans: Differential associations with viral load, CD4+ T cell depletion, and coinfection. J Infect Dis 2005;191:694–701 [DOI] [PubMed] [Google Scholar]
- 47.Giorgi JV, Lyles RH, Matud JL, et al. : Predictive value of immunologic and virologic markers after long or short duration of HIV-1 infection. J Acquir Immune Defic Syndr 2002;29:346–355 [DOI] [PubMed] [Google Scholar]
- 48.Huber C, Batchelor JR, Fuchs D, et al. : Immune response-associated production of neopterin. Release from macrophages primarily under control of interferon-gamma. J Exp Med 1984;160:310–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Betts MR, Nason MC, West SM, et al. : HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 2006;107:4781–4789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Emu B, Sinclair E, Favre D, et al. : Phenotypic, functional, and kinetic parameters associated with apparent T-cell control of human immunodeficiency virus replication in individuals with and without antiretroviral treatment. J Virol 2005;79:14169–14178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sankaran S, Guadalupe M, Reay E, et al. : Gut mucosal T cell responses and gene expression correlate with protection against disease in long-term HIV-1-infected nonprogressors. Proc Natl Acad Sci U S A 2005;102:9860–9865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fukazawa Y, Lum R, Okoye AA, et al. : B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat Med 2015;21:132–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li Q, Skinner PJ, Ha SJ, et al. : Visualizing antigen-specific and infected cells in situ predicts outcomes in early viral infection. Science 2009;323:1726–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spudich SS, Nilsson AC, Lollo ND, et al. : Cerebrospinal fluid HIV infection and pleocytosis: Relation to systemic infection and antiretroviral treatment. BMC Infect Dis 2005;5:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dahl V, Peterson J, Spudich S, et al. : Single-copy assay quantification of HIV-1 RNA in paired cerebrospinal fluid and plasma samples from elite controllers. AIDS 2013;27:1145–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hsue PY, Hunt PW, Schnell A, et al. : Role of viral replication, antiretroviral therapy, and immunodeficiency in HIV-associated atherosclerosis. AIDS 2009;23:1059–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hunt PW, Brenchley J, Sinclair E, et al. : Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis 2008;197:126–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peterson J, Gisslen M, Zetterberg H, et al. : Cerebrospinal fluid (CSF) neuronal biomarkers across the spectrum of HIV infection: hierarchy of injury and detection. PLoS One 2014;9:e116081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Deng H, Liu R, Ellmeier W, et al. : Identification of a major co-receptor for primary isolates of HIV-1. Nature 1996;381:661–666 [DOI] [PubMed] [Google Scholar]
- 60.Dragic T, Litwin V, Allaway GP, et al. : HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature 1996;381:667–673 [DOI] [PubMed] [Google Scholar]
- 61.Alkhatib G, Combadiere C, Broder CC, et al. : CC CKR5: A RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science 1996;272:1955–1958 [DOI] [PubMed] [Google Scholar]
- 62.Wu L, Paxton WA, Kassam N, et al. : CCR5 levels and expression pattern correlate with infectability by macrophage-tropic HIV-1, in vitro. J Exp Med 1997;185:1681–1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Olson TS, Ley K: Chemokines and chemokine receptors in leukocyte trafficking. Am J Physiol Regul Integr Comp Physiol 2002;283:R7–R28 [DOI] [PubMed] [Google Scholar]
- 64.Cyster JG: Chemokines and cell migration in secondary lymphoid organs. Science 1999;286:2098–2102 [DOI] [PubMed] [Google Scholar]
- 65.Arimilli S, Ferlin W, Solvason N, Deshpande S, Howard M, Mocci S: Chemokines in autoimmune diseases. Immunol Rev 2000;177:43–51 [DOI] [PubMed] [Google Scholar]
- 66.Glass WG, Lim JK, Cholera R, Pletnev AG, Gao JL, Murphy PM: Chemokine receptor CCR5 promotes leukocyte trafficking to the brain and survival in West Nile virus infection. J Exp Med 2005;202:1087–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sanders VJ, Pittman CA, White MG, Wang G, Wiley CA, Achim CL: Chemokines and receptors in HIV encephalitis. AIDS 1998;12:1021–1026 [PubMed] [Google Scholar]
- 68.Kaech SM, Cui W: Transcriptional control of effector and memory CD8+ T cell differentiation. Nat Rev Immunol 2012;12:749–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Grevenynghe J, Procopio FA, He Z, et al. : Transcription factor FOXO3a controls the persistence of memory CD4(+) T cells during HIV infection. Nat Med 2008;14:266–274 [DOI] [PubMed] [Google Scholar]
- 70.Ahmed R, Bevan MJ, Reiner SL, Fearon DT: The precursors of memory: Models and controversies. Nat Rev Immunol 2009;9:662–668 [DOI] [PubMed] [Google Scholar]
- 71.Kivisakk P, Mahad DJ, Callahan MK, et al. : Human cerebrospinal fluid central memory CD4+ T cells: Evidence for trafficking through choroid plexus and meninges via P-selectin. Proc Natl Acad Sci U S A 2003;100:8389–8394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kivisakk P, Mahad DJ, Callahan MK, et al. : Expression of CCR7 in multiple sclerosis: Implications for CNS immunity. Ann Neurol 2004;55:627–638 [DOI] [PubMed] [Google Scholar]
- 73.Giunti D, Borsellino G, Benelli R, et al. : Phenotypic and functional analysis of T cells homing into the CSF of subjects with inflammatory diseases of the CNS. J Leukoc Biol 2003;73:584–590 [DOI] [PubMed] [Google Scholar]
- 74.Schneidewind A, Brockman MA, Yang R, et al. : Escape from the dominant HLA-B27-restricted cytotoxic T-lymphocyte response in Gag is associated with a dramatic reduction in human immunodeficiency virus type 1 replication. J Virol 2007;81:12382–12393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Goulder PJ, Phillips RE, Colbert RA, et al. : Late escape from an immunodominant cytotoxic T-lymphocyte response associated with progression to AIDS. Nat Med 1997;3:212–217 [DOI] [PubMed] [Google Scholar]
- 76.Goulder PJ, Bunce M, Krausa P, et al. : Novel, cross-restricted, conserved, and immunodominant cytotoxic T lymphocyte epitopes in slow progressors in HIV type 1 infection. AIDS Res Hum Retroviruses 1996;12:1691–1698 [DOI] [PubMed] [Google Scholar]
- 77.Miura T, Brockman MA, Schneidewind A, et al. : HLA-B57/B*5801 human immunodeficiency virus type 1 elite controllers select for rare gag variants associated with reduced viral replication capacity and strong cytotoxic T-lymphocyte [corrected] recognition. J Virol 2009;83:2743–2755 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.