Abstract
The HIV care continuum has received considerable attention in recent years, however, few care continua focus on the population of patients who are diagnosed during an inpatient hospital admission. We aimed to describe the HIV care continuum for patients newly diagnosed during hospitalization through 24-month follow-up. A retrospective chart review of HIV patients diagnosed at Grady Memorial Hospital from 2011 to 2012 was performed and records were matched to Georgia Department of Public Health HIV/AIDS surveillance data. Descriptive statistics and statistical tests of independence were utilized. Ninety-four new diagnoses were confirmed during the 2-year study period. Median age was 43 years (interquartile range [IQR] 30–51), 77% were male, 72% were non-Hispanic Black, 31% were men who have sex with men (MSM), and 77% were uninsured. Median CD4 count at diagnosis was 134 cells/μL (IQR 30–307). Eighty-four percent received their diagnosis before hospital discharge, 68% linked to care by 90 days, 73% were retained for 12 months, 48% were virologically suppressed by 12 months, 58% were retained for 24 continuous months, and 38% achieved continuous viral suppression (VS) during the initial 24 months after diagnosis. Late diagnosis is a persistent problem in hospitalized patients. Despite relative success with linkage to care and 12-month retention in care, a minority of patients maintained retention and VS for 24 continuous months.
Background
The national HIV/AIDS strategy for 2020 emphasizes the need for immediate linkage to care, improved retention in care, and timely antiretroviral therapy (ART) initiation to maximize the benefits of early therapy and reduce transmission risk.1 Ultimately, this will require a rapid and more seamless journey through the HIV care continuum.
For patients who succeed in navigating the care continuum in the United States, the median time from diagnosis to viral suppression (VS) is 18 months and for the subset with CD4 < 350 cells/μL it is 8 months.2 In San Francisco, a robust test-and-treat approach resulted in a decrease in median time from diagnosis to VS from 13 months in 2008 to 5 months in 2012.3 Rapid ART initiation after diagnosis is essential to achieving these results. Yet time to ART initiation often remains suboptimal.4,5 Many barriers exist that influence patients' ability to link, engage and be retained in HIV care, initiate ART, and achieve VS.
HIV-infected patients diagnosed during a hospitalization represent a unique opportunity for intervention. Patients who perceive themselves to be ill may be more likely to engage in care.6,7 Therefore, hospitalized patients may be more willing to engage in care because they are ill and already connected to the healthcare system. However, failure to facilitate engagement at that moment may have lasting consequences as patients who lack a perception of “being helped into care” are less likely to engage in care.8
Although many of the published care continua include patients who were diagnosed in the hospital, the care continuum has not been characterized specifically for this particular population. This gap in the literature may stem from several reasons: (1) hospital-based HIV testing and prevention initiatives focus heavily on linkage to care, but often lack resources to follow patients through retention and VS; (2) clinician researchers within hospitals may lack access to postdischarge data (or data outside of that hospital system); and (3) surveillance level data used to build large care continua often do not report place of diagnosis.
The impact of location of diagnosis on linkage to care shows that those diagnosed in the inpatient setting have higher linkage to care rates than those diagnosed in counseling and testing centers or correctional facilities, but lower linkage rates than outpatient medical clinic diagnoses.9 HIV diagnosis and timely linkage to care remain a critical aspect of the test-and-treat approach for HIV prevention, yet successful linkage to care is not sufficient for effective broad-scale treatment as prevention.10,11
At Grady Health System (GHS), we are uniquely situated to describe a full care continuum for patients who are newly diagnosed in the hospital. First, every new HIV diagnosis in the hospital is reported to a specialized social work team minimizing selection bias. Second, through collaboration with the Georgia Department of Public Health and taking advantage of mandatory laboratory reporting to the state, surveillance data supplement clinic level data from our hospital-affiliated Ryan White funded clinic, where the majority of patients diagnosed in the hospital are referred for outpatient care. Finally, the surveillance level data ensure that we capture data from patients who follow up outside of the GHS. We aimed to describe the HIV care continuum for patients newly diagnosed during hospitalization through the 24-month follow-up after hospital discharge.
Materials and Methods
Data source and study population
For patients ≥18 years of age and newly diagnosed with HIV during hospitalization at Grady Memorial Hospital (GMH) in Atlanta, Georgia, from January 2011 through December 2012, we describe the following steps of the HIV care continuum: diagnosis (positive serology with confirmatory testing), being informed of the test result, linkage to care, retention, and VS. Newly diagnosed patients were identified by patient self-report and verified by the GMH inpatient HIV social work team. A detailed chart review of the initial hospital admission provided demographic, social, biomedical, and systems-level data.
Postdischarge data, including visits with an HIV provider (defined as a provider with ART prescribing privileges), within the GMH outpatient system (Infectious Disease Program [IDP]), CD4 T-lymphocyte counts and HIV-1 RNA were abstracted for 24 months from the date of hospital discharge. Data were abstracted from electronic medical records into a Microsoft Office Access® by a single coauthor (J.J. Khoubian). Quality was assured through creating drop-down responses in the Access® database and accuracy was verified through cross checking 10% of the medical records by a second investigator (J.C.).
Patients were matched with the Georgia Department of Public Health Enhanced HIV/AIDS Reporting System (eHARS) for date of initial HIV diagnosis, CD4 T-lymphocyte counts, and HIV-1 RNA results in the state of Georgia. Death data were obtained from the Georgia bureau of Vital Statistics and the National Social Security Death Index.
We excluded patients who were found to have evidence of prior HIV diagnosis by medical record abstraction or in the eHARS database, patients who died before hospital discharge, patients with an undetectable HIV-1 RNA at the time of diagnosis (elite controllers), and patients with a primary residence outside of the United States.
Variables
Age, gender, race, ethnicity, HIV risk category, insurance status, cocaine use, homelessness, and mental illness were recorded based on data available at the time of hospital admission when the HIV diagnosis occurred. Race and ethnicity were initially captured in multiple categories and then combined into a dichotomous variable consisting of non-Hispanic Black versus non-Black. HIV risk category was recorded as men who have sex with men (MSM), heterosexual, injection drug use, or not reported, based on provider notes during the hospitalization. Insurance status was recorded as uninsured, public (Medicaid or Medicare), or private based on the patient's status at the time of admission to the hospital. All remaining variables were binary.
New HIV diagnosis was defined as having a positive serologic test during hospital admission and no prior evidence of HIV diagnosis after chart review and matching with the eHARS database. Receipt of HIV diagnosis was defined as evidence in the medical record that the patient was informed about the positive HIV serology before discharge from the hospital. Linkage to care, retention in care, and VS were each adapted from standard IOM definitions12 by using the date of discharge from the hospital rather than the date of diagnosis as the starting time point from where each was measured. This decision was made to account for variable lengths of hospital stays to ensure each patient had equivalent 12- and 24-month follow-up periods.
Linkage to care was defined at both 30 and 90 days after hospital discharge. Linkage to care was defined as either having evidence of a provider visit or HIV laboratories (CD4 T-lymphocyte count or HIV-1 RNA) in the GHS or eHARS systems before 30 or 90 days after hospital discharge. Time to linkage was calculated by the date of first IDP provider visit or eHARS laboratory (whichever was shorter duration) minus the date of discharge.
Twelve-month retention in care was defined as two provider visits (or CD4/VL values by eHARS) separated by greater than or equal to 90 days within 12 months from the date of discharge from the hospital. Twenty-four-month retention required meeting this definition during the patient's first and second years postdischarge. Twelve-month VS was defined as the last HIV-1 RNA value 12 months postdischarge being <200 copies/mL. Twenty-four-month VS required meeting the same definition during the patient's first and second year postdischarge. Patients with complete absence of any HIV-1 RNA results during the defined time periods of interest were considered to not be suppressed. Time to VS was calculated both from diagnosis and discharge until VS. Patients who were virologically suppressed at the time of discharge were excluded from the time from discharge to VS calculation.
Statistical analysis
SPSS™ IBM™ version 21 was used for the analysis. Descriptive statistics were utilized to characterize the population at each step of the continuum: diagnosis, receiving diagnosis, linkage, retention, and VS. Chi-square and Fisher exact tests of independence were utilized to evaluate the distribution of categorical covariates with respect to VS. Mann–Whitney tests distinguished medians. McNemar tests of independence were utilized to evaluate differences in retention and VS at 12 and 24 months.
Human subjects
The study was approved by the Emory University Institutional Review Board and the Grady Health System Research Oversight Committee.
Results
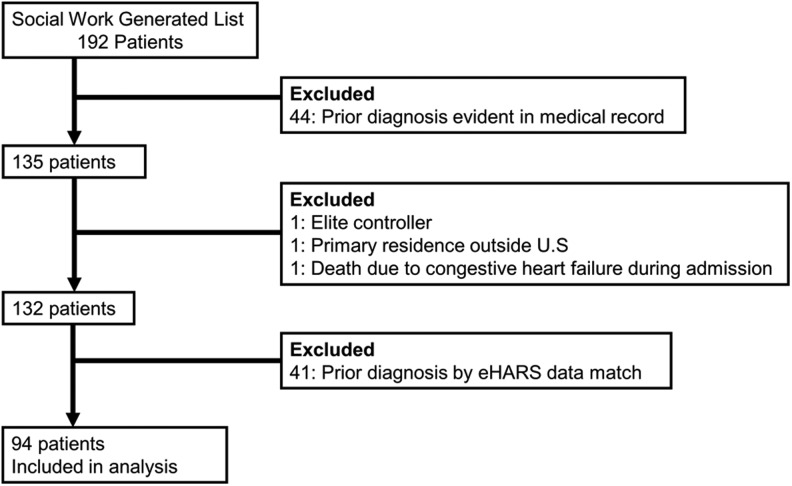
During 2011–2012, 172 patients were initially identified as newly diagnosed by the inpatient social work team. After medical record review, 132 patients were categorized as newly diagnosed with HIV, but after matching with the state eHARS database, only 94 individuals were confirmed as true new diagnoses during their inpatient admission (Fig. 1). The median age was 43 years (interquartile range [IQR] 30–51), 77% were male, 72% were Black, non-Hispanic, 77% were uninsured, and 31% were MSM during clinical interviews. The median CD4 count at diagnosis was 134 cells/μL (IQR 30–307) and a third of the patients had a CD4 count of <50 cells/μL. Median length of hospital stay was 7 days (IQR 4–18). Full demographic variables (Table 1), psychosocial and systems variables (Table 2), and clinical variables (Table 3) are presented for each stage of the care continuum.
FIG. 1.
Participant selection.
Table 1.
Demographic Characteristics Along the HIV Care Continuum for Patients Newly Diagnosed with HIV During an Inpatient Hospitalization at Grady Memorial Hospital, 2011–2012 (N = 94)
| Retention | Viral suppression | |||||||
|---|---|---|---|---|---|---|---|---|
| New diagnosis (N = 94) | Test result before DC (N = 79) | Linked by 30 days (N = 44) | Linked by 90 days (N = 64) | 12 Month (N = 69) | 24 Month (N = 51) | 12 Month (N = 45) | 24 Month (N = 36) | |
| Characteristics | N (%) or Median (IQR) | |||||||
| Demographic | ||||||||
| Age, years | 43 (30–51) | 41 (29–51) | 40 (29–51) | 41 (29–50) | 41 (29–50) | 41 (31–50) | 41 (31–49) | 42 (33–49) |
| 20–29 | 23 (24) | 20 (25) | 13 (30) | 19 (30) | 18 (26) | 11 (22) | 10 (22) | 6 (17) |
| 30–39 | 15 (16) | 13 (17) | 8 (18) | 10 (16) | 13 (19) | 11 (22) | 9 (20) | 8 (22) |
| 40–49 | 25 (27) | 23 (29) | 10 (23) | 18 (28) | 20 (29) | 16 (31) | 16 (36) | 14 (39) |
| ≥50 | 31 (33) | 23 (29) | 13 (29) | 17 (26) | 18 (26) | 13 (25) | 10 (22) | 8 (22) |
| Male | 72 (77) | 62 (79) | 34 (77) | 49 (77) | 54 (78) | 41 (80) | 38 (84) | 30 (83) |
| Black, non-Hispanic | 68 (72) | 58 (73) | 29 (66) | 45 (70) | 48 (70) | 34 (67) | 31 (69) | 23 (64) |
| HIV risk factor | ||||||||
| MSM | 29 (31) | 27 (34) | 16 (36) | 23 (36) | 23 (33) | 19 (37) | 17 (38) | 14 (39) |
| Heterosexual | 37 (39) | 30 (38) | 16 (36) | 26 (41) | 28 (41) | 22 (43) | 22 (49) | 16 (44) |
| Not reported | 28 (30) | 22 (28) | 12 (27) | 15 (23) | 18 (26) | 10 (20) | 6 (13) | 6 (17) |
| Insurance | ||||||||
| Uninsured | 72 (77) | 59 (75) | 34 (77) | 51 (79) | 56 (81) | 40 (78) | 32 (71) | 27 (75) |
| Public | 18 (19) | 16 (20) | 7 (16) | 10 (16) | 10 (15) | 8 (16) | 10 (22) | 7 (19) |
| Private | 4 (4) | 4 (5) | 3 (7) | 3 (5) | 3 (4) | 3 (6) | 3 (7) | 2 (6) |
DC, discharge; IQR, interquartile range; MSM, men who have sex with men; VS, viral suppression.
Table 2.
Psychosocial and Systems Level Characteristics Along the HIV Care Continuum for Patients Newly Diagnosed With HIV During an Inpatient Hospitalization at Grady Memorial Hospital, 2011–2012 (N = 94)
| Retention | Viral suppression | |||||||
|---|---|---|---|---|---|---|---|---|
| New diagnosis (N = 94) | Test result before DC (N = 79) | Linked by 30 days (N = 44) | Linked by 90 days (N = 64) | 12 Month (N = 69) | 24 month (N = 51) | 12 Month (N = 69) | 24 month (N = 51) | |
| Characteristics | N (%) or Median (IQR) | |||||||
| Psychosocial | ||||||||
| Language | ||||||||
| English | 83 (88) | 70 (89) | 39 (89) | 56 (87) | 61 (88) | 44 (86) | 38 (84) | 30 (83) |
| Non-English | 11 (12) | 9 (11) | 5 (11) | 8 (13) | 8 (12) | 7 (14) | 7 (16) | 6 (17) |
| Birth country | ||||||||
| Continental US | 73 (78) | 62 (78) | 35 (80) | 49 (77) | 51 (74) | 36 (70) | 30 (67) | 24 (67) |
| Noncontinental US | 21 (22) | 17 (22) | 9 (20) | 15 (23) | 18 (26) | 15 (30) | 15 (33) | 12 (33) |
| Active Cocaine | 13 (14) | 11 (14) | 5 (11) | 7 (11) | 8 (12) | 3 (6) | 2 (4) | 2 (6) |
| Homeless | 17 (18) | 14 (18) | 7 (16) | 9 (14) | 11 (16) | 7 (14) | 7 (16) | 4 (11) |
| Mental illness | 11 (12) | 9 (11) | 6 (14) | 10 (16) | 11 (16) | 9 (18) | 5 (11) | 5 (14) |
| Systems | ||||||||
| Year of diagnosis | ||||||||
| 2011 | 49 (52) | 40 (51) | 28 (64) | 35 (55) | 35 (51) | 25 (49) | 20 (44) | 16 (44) |
| 2012 | 45 (48) | 39 (49) | 16 (36) | 29 (45) | 34 (49) | 26 (51) | 25 (56) | 20 (56) |
| Admission service | ||||||||
| Non-medicine | 7 (7) | 7 (9) | 4 (9) | 5 (8) | 6 (9) | 4 (8) | 5 (11) | 5 (14) |
| Medicine | 87 (93) | 72 (91) | 40 (91) | 59 (92) | 63 (91) | 47 (92) | 40 (89) | 31 (86) |
| SW visit before discharge | 82 (87) | 77 (98) | 37 (84) | 56 (88) | 62 (90) | 46 (90) | 41 (91) | 32 (89) |
| Planned f/u appt | 49 (52) | 39 (49) | 21 (47) | 33 (52) | 36 (52) | 26 (51) | 26 (58) | 21 (58) |
| Length of stay, days | 7 (4–18) | 10 (5–20) | 6 (3–13) | 7 (3–17) | 7 (4–18) | 8 (4–18) | 10 (5–20) | 9 (4–18) |
SW, social worker; F/U, follow-up; Appt, appointment.
Table 3.
Clinical Characteristics Along the HIV Care Continuum for Patients Newly Diagnosed with HIV During an Inpatient Hospitalization at Grady Memorial Hospital, 2011–2012 (N = 94)
| Retention | Viral suppression | |||||||
|---|---|---|---|---|---|---|---|---|
| New diagnosis (N = 94) | Test result before DC (N = 79) | Linked by 30 days (N = 44) | Linked by 90 days (N = 64) | 12 Months (N = 69) | 24 Months (N = 51) | 12 Months (N = 69) | 24 Month (N = 51) | |
| Characteristics | N (%) or Median (IQR) | |||||||
| CD4 count (cells/μL) | 134 (30–307) | 95 (26–298) | 136 (31–308) | 86 (27–243) | 82 (28–232) | 74 (17–163) | 59 (20–139) | 57 (15–139) |
| CD4 < 50 cells/μL | 28 (31) | 26 (33) | 12 (27) | 22 (34) | 23 (33) | 20 (39) | 20 (40) | 17 (47) |
| CD4 < 200 cells/μL | 61 (65) | 55 (70) | 31 (71) | 47 (73) | 51 (74) | 42 (82) | 41 (82) | 32 (89) |
| HIV-1 RNA log 10 (copies/mL) | 5.36 (4.94–5.83) | 5.40 (4.99–5.85) | 5.33 (5.01–5.81) | 5.40 (5.00–5.84) | 5.40 (5.00–5.89) | 5.41 (5.02–5.89) | 5.48 (5.13–5.89) | 5.42 (5.11–5.86) |
| Opportunistic Infection | 37 (39) | 37 (47) | 16 (36) | 30 (47) | 33 (48) | 27 (53) | 25 (56) | 18 (50) |
| PCP | 15 (16) | 15 (19) | 7 (16) | 12 (19) | 13 (19) | 11 (22) | 10 (22) | 7 (19) |
| Cerebral Toxoplasmosis | 5 (5) | 5 (6) | 1 (2) | 3 (5) | 4 (6) | 3 (6) | 3 (7) | 3 (8) |
| Tuberculosis | 5 (5) | 5 (6) | 3 (7) | 4 (6) | 4 (6) | 3 (6) | 4 (9) | 2 (6) |
| Cryptococcosis | 3 (3) | 3 (4) | 1 (2) | 3 (5) | 3 (4) | 3 (6) | 3 (7) | 2 (6) |
| Lymphoma | 3 (3) | 3 (4) | 2 (5) | 3 (5) | 3 (4) | 2 (4) | 2 (4) | 2 (6) |
| AIDS at Diagnosis | 62 (66) | 56 (71) | 31 (71) | 47 (73) | 52 (75) | 43 (84) | 40 (89) | 32 (89) |
| ART at Discharge | 8 (9) | 8 (10) | 4 (9) | 7 (11) | 8 (12) | 7 (14) | 6 (13) | 5 (14) |
| Time to VS from discharge, days | 137 (78–266) | 131 (78–270 | 130 (69–263) | 124 (74–243) | 130 (76–262) | 127 (73–248) | 111 (73–217) | 101 (68–206) |
| Time to VS from diagnosis, days | 137 (94–267) | 132 (94–271) | 128 (87–254) | 127 (93–248) | 130 (93–254) | 128 (91–250) | 125 (91–237) | 114 (88–214) |
PCP, pneumocystis pneumonia; ART, antiretroviral therapy.
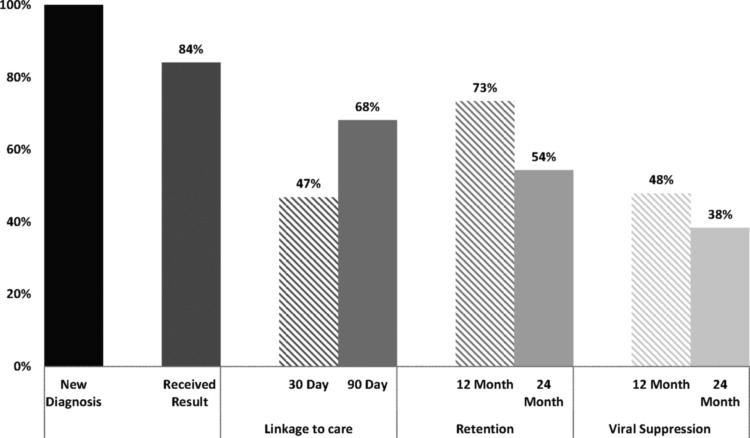
The full care continuum is presented in Figure 2. Fifteen patients (16%) with a positive HIV serology did not receive their results before discharge. In each case, the laboratory resulted after the patient was discharged. The HIV testing algorithm consisted of a third-generation enzyme immunoassay (EIA) with confirmation of positive EIA by Western blot. These tests were only processed Monday through Friday during daytime hours.
FIG. 2.
The HIV care continuum from diagnosis in the hospital through 24-month viral suppression.
A single patient left against medical advice before the test resulting, whereas the other 14 patients were discharged by the treating primary team before the HIV test results became available. Postdischarge attempts were made to contact each patient. Two were unable to be contacted as they were in jail. Five patients were reached by phone and attended clinic within 2 weeks after their hospital discharge to receive their results.
Six patients were notified of their diagnosis at the time of subsequent emergency department (ED) presentation or hospital admission from 2 months to 2 years later. When returning to the ED, one patient was diagnosed with pulmonary tuberculosis and one patient with cryptococcal meningitis. There was no evidence in the medical chart that the remaining two patients were notified of their diagnosis through 2 years of follow-up. The median length of stay for those who did not receive results was 2 days (IQR 1–7) compared to a median length of stay of 10 days (IQR 5–20) for those who did receive their results.
Sixty-four percent of patients with new HIV diagnoses linked to care within 90 days of the hospital discharge date. Of those who linked to care, 63% attended a provider visit at the Grady Infectious Diseases Clinic (IDP) and 37% linked based solely on eHARS laboratory values (no visit to IDP). Using a linkage definition of 30 days from hospital discharge, 44% of all new diagnoses were linked to care. For patients with a provider visit or eHARS laboratories postdischarge, the median time to linkage was 46 days (IQR 10–77).
Those who linked to care within 90 days had a median CD4 count of 87 cells/μL compared to 134 cells/μL for all new diagnoses. Of patients who did not receive their diagnosis as an inpatient, a smaller proportion linked to care (47%, n = 7) compared to those who did receive the news of their new diagnosis while inpatient (74%, n = 57), but this difference did not reach statistical significance (p = 0.052).
Of all new diagnoses, 73% were retained in care during the initial 12 months after discharge from the hospital, 59% were retained during the second year postdischarge, and 54% were continuously retained for 24 months. The 12- to 24-month decline (19%) in those retained was statistically significant (p < 0.0001).
The proportion of patients virologically suppressed at the end of year 1, end of year 2, and for 24 continuous months was 48%, 47%, and 38%, respectively. The 12- to 24-month decline (10%) in VS was statistically significant (p < 0.0001). A total of 54 patients (57%) achieved VS at any point during the follow-up period. The median time to VS was 137 days (94–267). For those who had 24-month continuous VS, the median time to VS was 114 days (89–215), and for those who did not achieve 24-month continuous VS, the median time to VS was 286 (160–537) (p = 0.01). For those who linked to care within 90 days, 47 achieved VS with a median time to VS of 127 days (93–248).
Eleven patients (11.7%) were completely lost to follow-up (LTFU) after their discharge, with no documented outpatient provider visits or surveillance laboratories in the subsequent 24 months. All were true new diagnoses and four did not receive their diagnosis before discharge. The median age of the LTFU group was 48 years (IQR 39–52), 82% were male, 82% were Black, and 36% were MSM.
Seven patients (7.4%) died during their 2-year follow-up period. We were not able to identify the cause of death. Three patients died within 100 days of their hospital discharge. The median time to death was 101 days (IQR 61–350). The median age of those who died was 48 years (IQR 35–52). The median CD4 count was 90 cells/μL (IQR 50–312) and four of the seven patients had AIDS at the time of their diagnosis. Six of the patients (85.7%) who died had received their diagnosis before discharge. Three of the patients who died linked to care within 90 days and all three of those patients had linked before 30 days from hospital discharge. None of the patients who died achieved VS at any point during the 2-year follow-up.
Discussion
This HIV care continuum of newly diagnosed hospitalized individuals comprised primarily uninsured Black men presenting with AIDS at the time of diagnosis. An average of 47 newly diagnosed HIV cases (two-thirds with AIDS) at a single tertiary care facility is another illustration of the serious state of the epidemic in the Southern United States. The United States saw minimal gains in CD4 count at diagnosis from 1997 to 2007 (steady around 300 cells/mm3), despite CDCs HIV testing recommendations and massive testing initiatives nationwide.13–15 Although this national average is higher than our hospitalized population (137 cells/mm3), these data, collectively, represent a continued problem of late diagnoses on a broad scale.
It was striking that 30% of patients initially thought to be new diagnoses, after the social work team and clinician review actually had a prior positive serology in the state of Georgia (Fig. 1). Data from testing programs support the notion that repeat positive tests are an ongoing issue.16,17 Presumably, this indicates that a proportion of patients do not receive their initial test results and/or they are not comfortable revealing their diagnosis to treating physicians.
The reason for repeat testing is typically not evident at the time of the encounter. Real-time matching of electronic medical record systems with state surveillance systems and health information exchange (HIE) may detect prior positive serology and prevent unnecessary repeat testing. However, until HIE is available on a broad scale, repeat testing provides an opportunity for linkage or relinkage to care.
Because our data revealed a disconnect between presumed new diagnoses and true new diagnoses, we elected to add a new step of the continuum that has not traditionally been used—that is, did the patient learn the result of his/her diagnosis? It is often assumed that the reported number of HIV diagnoses (new positive serology) is equivalent to the patient being aware of his/her diagnosis. In our analysis, 16% of patients with a positive serology failed to receive their results before discharge from the hospital. In each instance, this was because the test resulted after the patient was discharged. We realize, however, that the issue of patients not receiving results during an inpatient admission may not be representative of the rates of test result receipt in other types of testing programs. Reports from New Orleans and New York City show that 98%–99% received their test results.
Recent data suggest a striking difference in receipt of test results based on whether the test was rapid or traditional.18,19 Receiving test results in the hospital likely hinges on a number of factors, including, but not limited to the following: the type of test used, frequency that tests are processed, and length of the hospital admission. As laboratories conform to CDC guidelines with uptake of fourth-generation HIV-1/2 Ag/Ab combination immunoassay screening tests and confirmation HIV-1/HIV-2 rapid antibody differentiation immunoassays, we would anticipate a decrease in the turnaround time for confirmation.20 This may, in turn, minimize the number of patients discharged from the hospital with pending HIV test results.
In our analysis, the median length of stay for those who did not receive their diagnosis was 8 days shorter than for those who did receive the diagnosis. The failure of patients receiving test results was due to the laboratory not resulting before patient discharge rather than poor provider–patient communication. Nevertheless, communication of test results to patients is important. Hence, providers should know the anticipated length of hospital stay, the time it takes from ordering the test to receiving a result, and accurate contact information for the patient to maximize the likelihood that the patient will successfully receive his/her result.
The location where diagnosis occurs has an impact on linkage to care as patients diagnosed at sites with collocated testing and treatment services fare better.9 The closest HIV clinic (IDP) for uninsured Atlanta metro area residents with a CD4 count <200 cells/μL is less than 2 miles from the hospital. The Fulton County Health Department, where uninsured patients with a CD4 count >200 cells/μL are generally referred for care, is located directly across the street from GMH. In this setting, where clinics for uninsured patients are close in proximity to the location of diagnosis, only 68% were linked to care within 90 days, falling short of the 2015 National HIV/AIDS Strategy's (NHAS) goal of 85%.21 This suggests that close proximity alone is not sufficient to achieve optimal linkage to care, rather robust linkage programs and care coordination across institutions are necessary.
Linkage is critically important to successful HIV care through the end of the continuum; however, we urge caution in placing too much importance on a single step in the care continuum. As we look beyond linkage to retention and VS, especially longitudinally, we find interesting lessons about the care continuum.
One-year retention rates (73%) are comparable to those of nationally reported Ryan White HIV/AIDS Programs, but still fall short of the NHAS 2015 goal of 80%.21,22 Even if 12-month retention had met the NHAS goal, we could not have claimed success as just over half (54%) of patients were continuously retained for the entire 24-month follow-up. Similarly, VS rates declined from 48% at 12 months to 38% at 24 continuous months. This reaffirms the tremendous difficulty in maintaining retention and VS for extended periods of time.23
The phenomenon of patients cycling in and out of care (at times retained and at times not) has been termed “churn.”24 In our analysis, the length of follow-up is not long enough to classify patients into “churn,” but the concept of poor longitudinal rates compared with cross-sectional rates is evident. Further evidence that retention and VS are a dynamic process is described in the HIV Research Network (HIVRN), which suggests the need for a longitudinal approach to the care continuum.25
The single-center retrospective design of our study limits the generalizability of the findings. The small sample size limits the power of comparative analyses in this cohort. Nonetheless our findings shed important light on the state of HIV care for a particular population, those newly diagnosed, while hospitalized.
In conclusion, we found that a large number of patients are still newly diagnosed with HIV in the hospital and largely have late-stage disease at diagnosis. Although a reasonable proportion links to care by 3 months, this still needs improvement, and major concerns arise around longitudinal rates of retention and VS over the initial 2 years in care. By 24 months after diagnosis, only a minority of patients both achieved and maintained VS for the entire follow-up period. This means that 60% of patients, after being newly diagnosed, were a potential transmission risk at some point during their first 2 years in care.
The 38% of patients who maintained VS for 24 months represent the ideal scenario in which treatment as prevention can become a reality. Beyond cross-sectional defined goals of linkage, retention, or VS, a greater emphasis should be placed on long-term retention and VS from a metric, programmatic, and policy standpoint.
Acknowledgments
We are grateful to the Georgia Department of Public Health, HIV/AIDS Surveillance Branch and all of their staff, for their collaboration on this research. We would like to thank the Grady Memorial Hospital HIV Social Work team for their dedication to their patients and collaboration on this project. Finally, we are grateful to all the patients whose records were reviewed for this project.
This work was supported by the Center for AIDS Research at Emory University (NIH/NIAID 2P30 AI50409 and NIH/NIAID 1K23AI116388).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.White House Office of National AIDS Policy: National HIV/AIDS Strategy for the United States: Updated to 2020. Available at www.whitehouse.gov/sites/default/files/docs/national_hiv_aids_strategy_update_2020.pdf, accessed August18, 2015
- 2.Hall HI, Tang T, Westfall AO, Mugavero MJ: HIV care visits and time to viral suppression, 19 U.S. jurisdictions, and implications for treatment, prevention and the national HIV/AIDS strategy. PLoS One 2013;8:e84318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.San Francisco Department of Public Health. HIV Epidemiology Annual Report 2013. Available at www.sfdph.org/dph/files/reports/RptsHIVAIDS/HIVAIDAnnlRpt2013.pdf, accessed on August18, 2015
- 4.Adedinsewo DA, Wei SC, Robertson M, et al. : Timing of antiretroviral therapy initiation in a nationally representative sample of HIV-infected adults receiving medical care in the United States. AIDS Patient Care STDS 2014;28:613–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goswami ND CJ, Khoubian J, Huang Y, Armstrong WS, del Rio C: Time between HIV/AIDS diagnosis during hospitalization and ART initiation at a large public hospital the U.S. southeast. 10th International Conference on HIV Treatment and Prevention Adherence, June28–30, 2015, Miami, FL [Google Scholar]
- 6.Rangarajan S, Tram HN, Todd CS, et al. : Risk factors for delayed entrance into care after diagnosis among patients with late-stage HIV disease in southern Vietnam. PLoS One 2014;9:e108939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jenness SM, Myers JE, Neaigus A, Lulek J, Navejas M, Raj-Singh S: Delayed entry into HIV medical care after HIV diagnosis: Risk factors and research methods. AIDS Care 2012;24:1240–1248 [DOI] [PubMed] [Google Scholar]
- 8.Bell C, Metsch LR, Vogenthaler N, et al. : Never in care: Characteristics of HIV-infected crack cocaine users in 2 US cities who have never been to outpatient HIV care. J Acquir Immune Defic Syndr 2010;54:376–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yehia BR, Ketner E, Momplaisir F, et al. : Location of HIV diagnosis impacts linkage to medical care. J Acquir Immune Defic Syndr 2015;68:304–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Vital signs: HIV prevention through care and treatment—United States. MMWR Morb Mortal Wkly Rep 2011;60:1618–1623 [PubMed] [Google Scholar]
- 11.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ: The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis 2011;52:793–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Institute of Medicine: Monitoring HIV care in the United States: Indicators and data systems. Available at www.iom.edu/Reports/2012/Monitoring-HIV-Care-in-the-United-States.aspx, accessed February5, 2015 [PubMed]
- 13.Centers for Disease Control and Prevention: HIV/AIDS Surveillance Report, vol 25, Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2013. Available at www.cdc.gov/hiv/pdf/g-l/hiv_surveillance_report_vol_25.pdf#Page=17, accessed on July16, 2015 [Google Scholar]
- 14.Buchacz K, Armon C, Palella FJ, et al. : CD4 cell counts at HIV diagnosis among HIV outpatient study participants, 2000–2009. AIDS Res Treat 2012;2012:869841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lesko CR, Cole SR, Zinski A, Poole C, Mugavero MJ: A systematic review and meta-regression of temporal trends in adult CD4(+) cell count at presentation to HIV care, 1992–2011. Clin Infect Dis 2013;57:1027–1037 [DOI] [PubMed] [Google Scholar]
- 16.Lin X, Dietz PM, Rodriguez V, et al. : Routine HIV screening in two health-care settings—New York City and New Orleans, 2011–2013. MMWR Morb Mortal Wkly Rep 2014;63:537–541 [PMC free article] [PubMed] [Google Scholar]
- 17.Flash CA, Pasalar S, Hemmige V, et al. : Benefits of a routine opt-out HIV testing and linkage to care program for previously diagnosed patients in publicly funded emergency departments in Houston, TX. J Acquir Immune Defic Syndro 2015;69 Suppl 1:S8–S15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang YA, Hutchinson AB, Hollis ND, Sansom SL: Notification following new positive HIV test results. Int J STD AIDS 2015. [Epub ahead of print]; DOI: 10.1177/0956462415598090 [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Guo J, Lu W: Effects of rapid versus standard HIV voluntary counselling and testing on receipt rate of HIV test results: A meta-analysis. Int J STD AIDS 2015;26:196–205 [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention and Association of Public Health Laboratories: Laboratory testing for the diagnosis of HIV infection: Updated recommendations. Available at http://dx.doi.org/10.15620/cdc.23447, accessed March20, 2016 Published June 27, 2014
- 21.The White House Office of National AIDS Policy: National HIV/AIDS strategy for the United States. July 2010. Available at www.whitehouse.gov/sites/default/files/uploads/NHAS.pdf, accessed February12, 2015
- 22.Doshi RK, Milberg J, Isenberg D, et al. : High rates of retention and viral suppression in the US HIV safety net system: HIV care continuum in the Ryan White HIV/AIDS Program, 2011. Clin Infect Dis 2015;60:117–125 [DOI] [PubMed] [Google Scholar]
- 23.Colasanti J, Kelly J, Pennisi E, et al. : Continuous retention and viral suppression provide further insights into the HIV care continuum compared to the cross-sectional HIV care cascade. Clin Infect Dis 2015;62:648–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gill MJ, Krentz HB: Unappreciated epidemiology: The churn effect in a regional HIV care programme. Int J STD AIDS 2009;20:540–544 [DOI] [PubMed] [Google Scholar]
- 25.Yehia BR, Stephens-Shields AJ, Fleishman JA, et al. : The HIV care continuum: Changes over time in retention in care and viral suppression. PLoS One 2015;10:e0129376. [DOI] [PMC free article] [PubMed] [Google Scholar]