Abstract
Brain microglia become dysregulated during aging and express proinflammatory cytokines that play a role in cognitive aging. Recent studies suggest the flavonoid luteolin reduces neuroinflammation and improves learning and memory in aged mice. However, if dietary luteolin reduces microglia activity in the brain of senescent mice is not known. We hypothesized that feeding aged mice a diet with luteolin would reduce microglia activity. Adult (3–6 months) and aged (22–24 months) mice were fed American Institute of Nutrition (AIN)-93M or AIN-93M with luteolin (6 g/kg) for 4 weeks and injected intraperitoneally with saline or lipopolysaccharide (LPS) before microglia were isolated and stained for major histocompatibility complex (MHC) class II, interleukin (IL)-1β, and IL-6 for flow cytometry. In saline-treated mice fed control diet, aging increased the proportion of microglia that stained for MHC class II (<3% for adults vs. 23% for aged), IL-1β (<2% for adults vs. 25% for aged), and IL-6 (<2% for adults vs. 25% for aged), indicating an age-related increase in proinflammatory microglia. In saline-treated aged mice fed luteolin, the proportion of microglia that stained for MHC class II, IL-1β, and IL-6 was reduced by nearly half (to 12%, 13%, and 12%, respectively). Interestingly, luteolin significantly reduced the proportion of microglia that stained for IL-1β and IL-6 in LPS-treated adult mice but not aged. Collectively, the results show that a diet supplemented with luteolin inhibited brain microglia activity during aging and activation by LPS in adults. Therefore, luteolin may inhibit neuroinflammation and improve cognition in the otherwise healthy aged by constraining brain microglia.
Introduction
Microglia are the resident macrophages of the central nervous system. Under healthy conditions, “resting” microglia randomly extend and contract arms with filopodia-like protrusions to survey the microenvironment.1 In response to insult, however, microglia become activated toward a proinflammatory profile. In this state, they direct the movement of the protrusions toward the insult,1 take on a deramified morphology that enables motility,2 and/or express major histocompatibility complex (MHC) class II and other markers indicative of inflammation.3
During aging the percentage of brain microglia that express MHC class II increases and signs of neuroinflammation emerge. For example, <3% of microglia isolated from the brain of young adult mice stained positive for MHC class II compared to >25% of microglia from brains of aged mice.4 Most of the MHC class II-positive microglia from aged mice were also interleukin (IL)-1β-positive.4 This is consistent with a prior study where the proportion of IL-6-positive microglia was higher if the donor mouse was 22–24 months old compared to 6-months or 1 week old.5 A recent study suggests that microglia from aged mice retain a prominent pro-inflammatory profile and are less sensitive to the anti-inflammatory effects of IL-4.6 Reducing the proportion of microglia that are activated is a priority for reducing age-related neuroinflammation.
Flavonoids are naturally occurring polyphenolic compounds present in plants. The major sources of flavonoids in the human diet include fruits, vegetables, tea, wine, and cocoa.7 Significant evidence has emerged to indicate that consuming a diet rich in flavonoids may inhibit cognitive aging. For example, in a prospective study of individuals aged 65 years or older, dietary flavonoid intake was associated with improved cognitive function over a 10-year period.8 Furthermore, data from the Chicago Health and Aging Project suggested that adherence to a Mediterranean diet reduced the rate of cognitive decline.9 Numerous other studies have yielded consistent results with older rats or mice showing improved cognitive function when fed a flavonoid-rich diet.10–13
A recent study of healthy aged mice found improved learning and memory and reduced expression of inflammatory genes in the hippocampus when the flavonoid luteolin was included in the diet.10 Luteolin inhibits several transcription factors that mediate inflammatory genes (e.g., NF-κB14 and activator protein-1 [AP-1]15) in microglia and is a potent activator of nuclear factor erythroid 2-related factor 2, which induces expression of genes encoding antioxidant enzymes.16 Thus, dietary luteolin may improve cognitive function in the aged by reducing exaggerated brain microglial cell activity. Indirect support for a microglia-dependent mechanism comes from a recent in vitro study where luteolin stimulated the formation of filopodia and caused ramification of BV-2 cells (a microglia cell line).17 Hence, the flavonoid luteolin is a naturally occurring immunomodulator that may be effective in reducing inflammatory microglia in the senescent brain.
In the present study, we hypothesized that feeding a diet with luteolin would reduce primed microglial cell activity in the brain of aged mice, as well as lipopolysaccharide (LPS)-induced activation in aged and adult mice.
Materials and Methods
Animals and treatments
Adult (3–6 month old, n = 39) and aged (22–24 month old, n = 42) male Balb/c mice from our in-house breeding colony were used. Mice were housed in polypropylene cages and maintained at 21°C under a reverse-phase 12-hour light/12-hour dark cycle with ad libitum access to water and rodent chow.
Luteolin supplementation was determined as previously described.10 In short, before starting the experiment, all mice were provided American Institute of Nutrition (AIN)-93M diet (Research Diets, New Brunswick, NJ)18,19 for a 1-week acclimation period. Thereafter, control mice continued on AIN-93M diet, while luteolin-fed animals were switched to AIN-93M containing 6 g luteolin/kg diet. Luteolin was purchased from Shaanxi Sciphar Biotechnology (Xian, China) and homogeneously blended into the AIN-93G control diet, pelleted, and preserved in a manner to ensure the stability of luteolin. Food intake was measured daily, while body weight was measured weekly for the duration of the study. Food intake and body weight changes were similar for the treatment groups.
After 4 weeks, mice from each dietary treatment were injected intraperitoneally (i.p.) with saline or 0.03 mg/kg (about 1 μg/mouse) Escherichia coli LPS (serotype 0127:B8; Sigma, St. Louis, MO). Thus, the eight treatments comprised the 2 × 2 × 2 factorial arrangement of age (adult vs. aged), diet (control vs. luteolin supplemented), and LPS (saline vs. 1 μg). Mice were killed 4 hours after injection for microglia isolation (described below). Food intake during the 4-hour postinjection period was determined. All procedures were in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee.
Microglia isolation
Microglia from whole brain were isolated as described previously, with few modifications.4 Mice were euthanized and whole brains were collected, placed in sterile phosphate-buffered saline (PBS), and then homogenized by passage through a 70-μm cell strainer in Dulbecco's PBS (DPBS) supplemented with 0.2% glucose. Homogenates were centrifuged (600 g for 6 minutes at 10°C) and resulting pellets were resuspended in a 70% isotonic Percoll (GE-Healthcare) supplemented with phenol red (0.01%) at room temperature. The suspension was centrifuged (2000 g for 20 minutes) on a discontinuous Percoll density gradient and microglia were collected from the interphase between the 70% and 50% Percoll layers. Cells were washed with DPBS and then resuspended in PBS-0.5% BSA/0.01% sodium azide solution (flow buffer). Each isolation yielded ∼3 × 105 viable cells from both adult and aged mouse brains.
Extracellular and intracellular flow cytometric analysis
Flow cytometric analysis of microglial surface and intracellular markers was performed based on the BD Cytofix/Cytoperm Plus Fixation/Permeabilization protocol (BD Biosciences, San Jose, CA), as described previously, with a few modifications.4 In brief, isolated cells were incubated in DMEM (BioWhittaker, Cambrex, MD) with 10% fetal bovine serum (Hyclone, Logan, UT), 200 mM glutamine, 100 U/mL penicillin/streptomycin (Invitrogen, Carlsbad, CA), and Brefeldin A (BD Biosciences) at 37°C in a humidified incubator under 5% CO2, for 4 hours. After washing and blocking Fc receptors with the anti-CD16/CD32 antibody (eBioscience, San Diego, CA), cells were incubated with anti-CD11b-allophycocyanin (APC), anti-CD45− fluorescein isothiocyanate (FITC), and anti-MHC-II-R-phycoerythrin (PE) antibodies (eBioscience). Next, cells were fixed and permeabilized with the BD Cytofix/Cytoperm™ solution, washed and resuspended in the BD Perm/Wash™ buffer, and incubated with either anti-IL-1β-PE or anti-IL-6-PE (eBioscience) for 30 minutes. Cells were washed in the BD Perm/ Wash™ buffer and resuspended in a flow buffer.
Expression of surface and intracellular antigens was determined using a Becton-Dickinson LSR II Flow Cytometer (Red Oaks, CA). Thirty thousand events were collected and microglia were identified by CD11b+ and CD45+low expression.20 Gating was determined based on fluorescently labeled isotype antibodies for APC, FITC, PE (eBiosciences), and unstained samples as controls. Flow data were analyzed using FCS Express software (De Novo Software, Los Angeles, CA).
Statistical analysis
StatView and Statistical Analysis System software (SAS Inst., Cary, NC) were used for data analysis. All data were subjected to a three-way ANOVA to determine significance of main factors (age, luteolin, and LPS) and all main factor interactions. When appropriate, post hoc Student's t test of least square means was used to determine if treatment means were significantly different from one another (p < 0.05). All data are presented as mean ± SEM.
Results
Microglia isolation
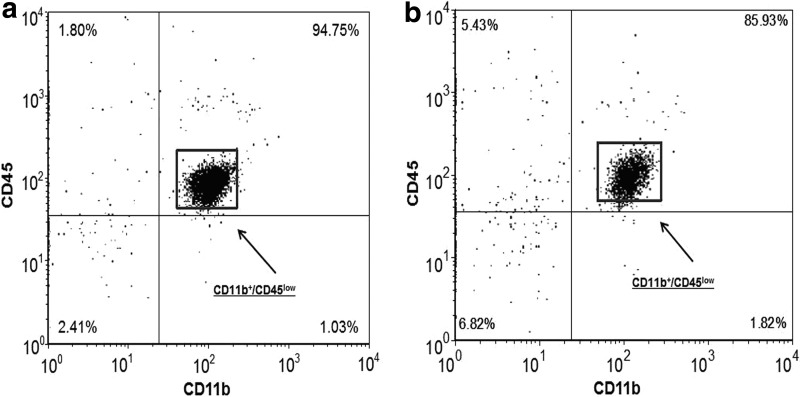
Approximately 90%–95% of the viable cells isolated from adult mice (n = 39) were microglia (CD11b+/CD45low) (Fig. 1a), while 85%–90% of the viable cells isolated from aged mice (n = 42) were microglia (Fig. 1b). In the case of both adult and aged mice, less than 3% of the viable cells isolated were macrophages (CD11b+/CD45high). Based on these findings, the expression of proinflammatory markers was investigated in CD11b+ stained cells (i.e., microglia) from adult and aged mice and analyzed using flow cytometry.
FIG. 1.
Isolation of microglia from adult and aged mouse brain. Representative dot blots of isolated cells that were incubated with antibodies for extracellular markers CD11b and CD45 and analyzed by flow cytometry. Microglia were identified by CD11b+/CD45low staining. (a) Approximately 90%–95% of the viable cells isolated from adults were CD11b+/CD45low, (b) while 85%–90% of the viable cells isolated from aged mice were CD11b+/CD45low.
Effects of dietary luteolin on microglia in brain of aged mice
Adult and aged mice on the two dietary treatments were injected i.p. with saline or LPS and microglia were isolated and stained for MHC class II, IL-1β, and IL-6. Only one significant three-way interaction was detected for IL-1β (age × luteolin × LPS, n = 8–11, p = 0.05), so for clarity of presentation, data from saline-treated mice are presented separate from LPS-treated mice.
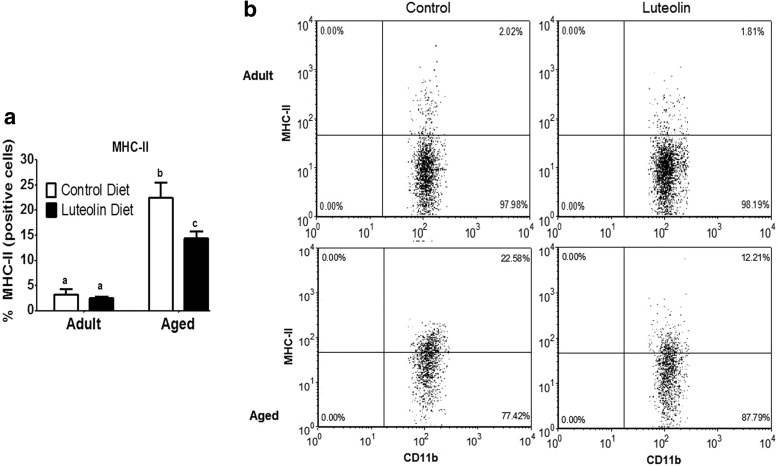
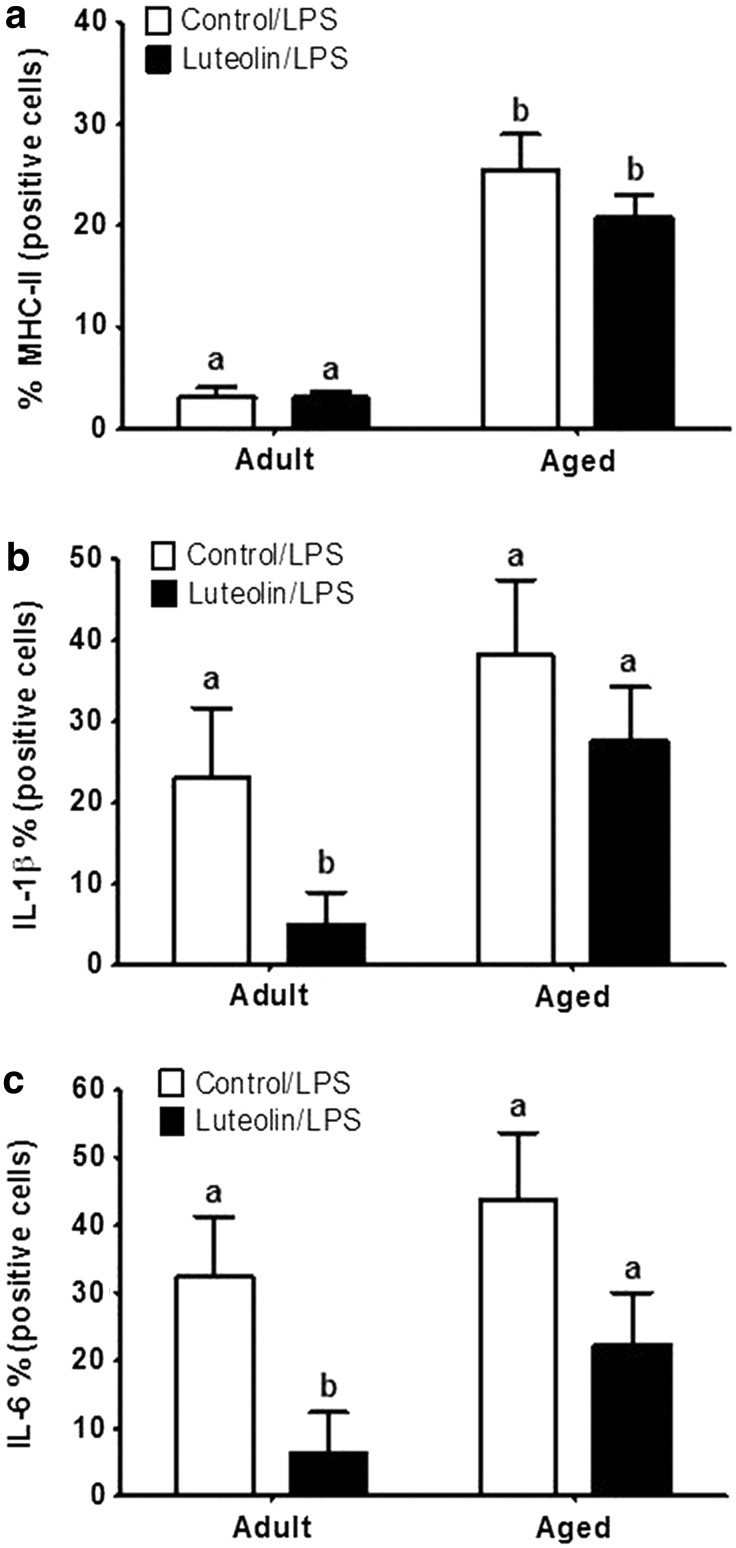
Figure 2 shows the mean percentage of MHC class II-positive microglia from saline-treated adult and aged mice fed control and luteolin-supplemented diets. In saline-treated mice fed control diet, aging increased the proportion of microglia that stained positive for MHC class II (<3% for adults vs. 22% for aged, n = 8–11 p < 0.001). In saline-treated adult mice, dietary luteolin did not affect the proportion of microglia that were MHC class II-positive (2% for adult control vs. 2% for adult luteolin, n = 8–11), but in saline-treated aged mice, the proportion of microglia that stained positive for MHC class II was reduced by luteolin (22% for aged control vs. 12% for aged luteolin, n = 9–11, age × diet, p < 0.05).
FIG. 2.
(a) Percentage of major histocompatibility complex (MHC) class II-positive microglia isolated from brains of adult and aged mice after 4-week consumption of control or luteolin-supplemented diet and (b) representative two-color dot blots from each condition. Values are mean ± SEM (n = 8–11). Labeled means without a common letter differ, p < 0.001.
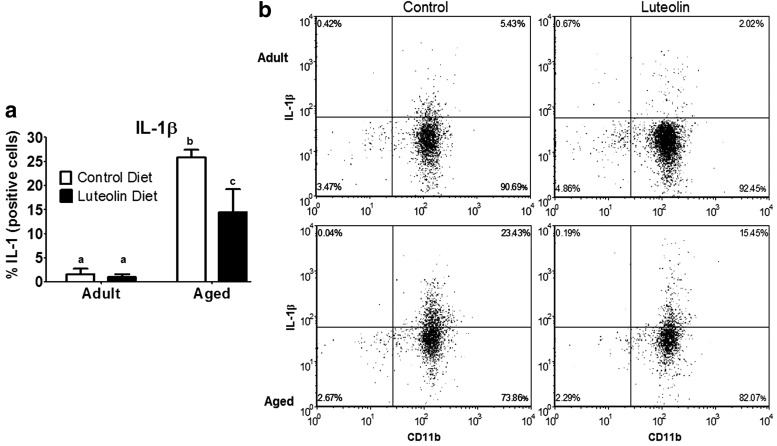
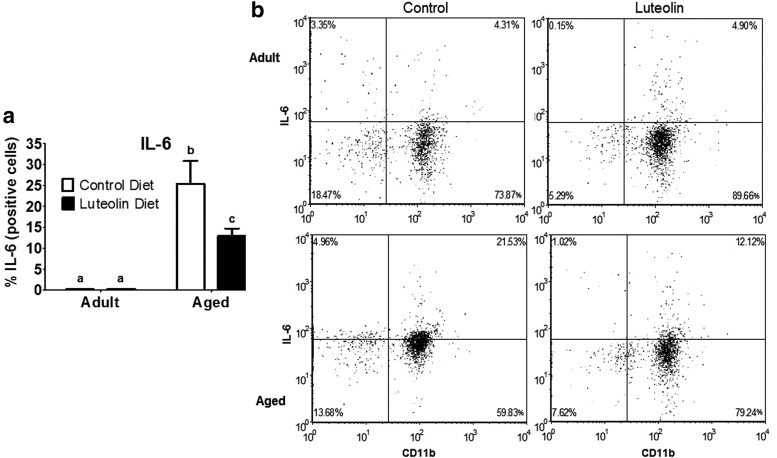
Similar results were evident when microglia were stained for IL-1β and IL-6 (Figs. 3 and 4). In saline-treated mice fed control diet, aging increased the proportion of microglia that stained positive for IL-1β (<2% for adults vs. 25% for aged, n = 8–11, p < 0.0001) and IL-6 (<2% for adults vs. 25% for aged, n = 8–11, p < 0.001). In saline-treated adult mice, dietary luteolin did not affect the proportion of microglia that were IL-1β- or IL-6-positive (<2% for adult control vs. <2% for adult luteolin, n = 8–11, for IL-1β and IL-6), but in saline-treated aged mice, the proportion of microglia that stained positive for IL-1β (25% for aged control vs. 14% for aged luteolin age × diet, n = 9–11, p < 0.05) and IL-6 (25% for aged control vs. 13% for aged luteolin age × diet, n = 9–11, p < 0.05) was reduced by luteolin.
FIG. 3.
(a) Percentage of interleukin (IL)-1β-positive microglia isolated from brains of adult and aged mice after 4-week consumption of control or luteolin-supplemented diet and (b) representative two-color dot blots from each condition. Values are mean ± SEM (n = 8–11). Labeled means without a common letter differ, p < 0.05.
FIG. 4.
(a) Percentage of IL-6-positive microglia isolated from brains of adult and aged mice after 4-week consumption of control or luteolin-supplemented diet and (b) representative two-color dot blots from each condition. Values are mean ± SEM (n = 8–11). Labeled means without a common letter differ, p < 0.05.
Effects of dietary luteolin on the response to peripheral immune stimulation
Figure 5 shows the effects of age and diet on the proportion of microglia expressing the inflammatory markers after injection of LPS. As observed in other similar studies, LPS did not affect MHC class II expression by microglia in either the adult or aged brain (Fig. 5a), although a trend for an age × diet × LPS interaction (n = 8–11, p < 0.06) suggested the reduction of microglia expressing MHC class II in aged mice fed luteolin was reduced when the immune system was stimulated. Peripheral injection of LPS increased the proportion of microglia that stained positive for IL-1β and IL-6 (Fig. 5b, c). The number of cytokine-positive microglia trended higher when derived from aged mice, but the LPS × age interaction was not significant (p = 0.19 and p = 0.20 for IL-1β and IL-6, respectively, n = 8–11), which suggests that the dose of LPS used did not elicit the maximal inflammatory response in both age groups. Interestingly, feeding luteolin reduced the proportion of microglia that stained positive for IL-1β and IL-6 after LPS injection in adults (p < 0.04 and p < 0.01, respectively, n = 8–11) and only trended to do so in the aged (p = 0.12 and p = 0.08, respectively, n = 8–11).
FIG. 5.
Percentage of MHC class II-positive (a), IL-1β-positive (b), and IL-6-positive (c) microglia isolated from brains of adult and aged mice after 4-week consumption of control or luteolin-supplemented diet and injection of lipopolysaccharide (LPS). Values are mean ± SEM (n = 8–11). Labeled means without a common letter differ, p < 0.04.
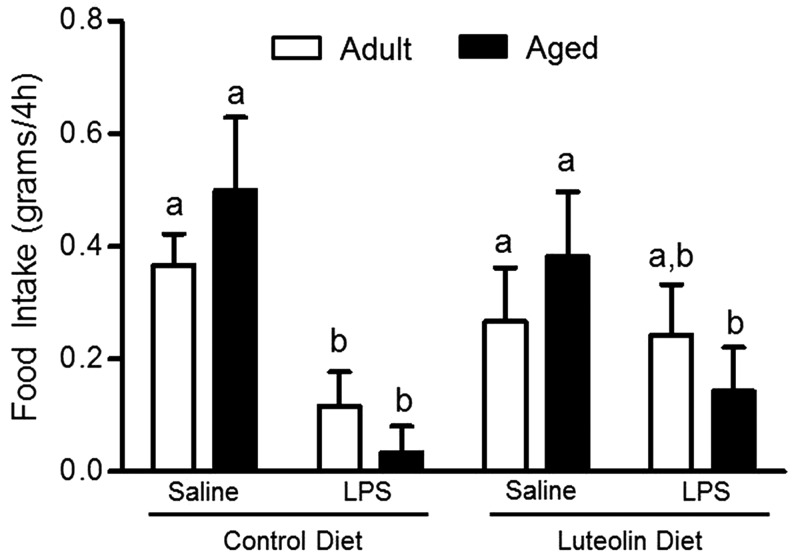
In adult and aged mice fed control diet, food intake was reduced in the 4-hour period after LPS injection (p < 0.01, n = 8–11; Fig. 6). The age × diet × LPS interaction was not significant (p = 0.15). There was a trend for luteolin to inhibit the LPS-induced decrease in food intake (luteolin × LPS interaction) in adult (n = 8–11, p = 0.10) but not aged (n = 9–11, p = 0.16) mice.
FIG. 6.
Food intake of adult and aged mice after 4-week consumption of control or luteolin-supplemented diet and injection of LPS. Food intake was measured for 4 hours after injection. Bars represent the mean ± SEM (n = 8–11). Labeled means without a common letter differ, p < 0.01.
Discussion
Microglial cell activity and signs of inflammation increase in the brain of senescent mice.5,21–24 Although evidence indicates that luteolin is an immunomodulator that may be effective in reducing age-related inflammation,10 whether ingesting a diet supplemented with luteolin reduces brain microglial cell activity had not been examined before. The important results show that providing mice a diet supplemented with luteolin inhibited increases in brain microglial cell activity and proinflammatory cytokine production in aged mice and after stimulation of the peripheral immune system in adult mice. Therefore, consuming a diet supplemented with luteolin or perhaps other flavonoids may constrain brain microglial cells and inhibit neuroinflammation.
Reducing proinflammatory cytokine production by microglia may be critical for preventing behavioral pathology in the elderly. Evidence suggests primed microglia in the senescent brain not only constitutively produce proinflammatory cytokines but also produce excessive levels of proinflammatory cytokines in response to peripheral infection,6,21,23,25 tissue trauma caused by surgery,26,27 and acute psychological stress.28 Results of the present study show a similar trend toward an age-related increase of IL-1β-positive microglia after LPS (23% in adult mice vs. 39% in aged mice), but the age × LPS interaction did not reach significance perhaps because the dose of LPS was too low. To avoid masking potential effects of luteolin, the dose of LPS used in this study was lower than what has been used previously.4
The heightened production of proinflammatory cytokines by microglia is thought to underlie severe behavioral deficits seen in aged rodents after peripheral immune stimulation.29 Consistent with this notion, intracisternal injection of IL-1 receptor antagonist (IL-1ra) reduced neuroinflammation and prevented postoperative cognitive decline in aged rats,26 and intracerebroventricular injection of IL-1ra inhibited behavioral deficits caused by peripheral injection of LPS in aged mice.30 Thus, by reducing the number of proinflammatory microglia in the senescent brain and the sensitivity of microglia to peripheral immune stimulation, supplemental flavonoids may provide a pragmatic way to improve the likelihood for successful aging.
In the present study, supplemental levels of luteolin were investigated. Based on an average daily food intake of 3–4 g, we estimate mice provided the luteolin-supplemented diet ingested 18–24 mg/d luteolin. If adjusted for body weight, this far exceeds what a person could obtain eating a diet rich in fruits and vegetables and thus needs to be supplemented. However, fruits and vegetables contain a variety of flavonoids and other bioactives that may interact at lower concentrations.
In a previous mouse study, plasma levels of luteolin were assayed by HPLC.10 In addition to luteolin, several unidentifiable peaks were detected in the plasma from mice fed luteolin. This suggests the presence of luteolin metabolites such as luteolin monoglucuronide, the main metabolite of luteolin or perhaps phenolic acid compounds generated in the large intestine. Studies have found that these metabolites may play an important role in the anti-inflammatory effects of luteolin.31,32 For example, feeding rats dietary supplements of fruit and vegetable extracts for 8 m beginning at 6 m of age prevented neurochemical and behavioral signs of brain aging.33 Rats provided fruit and vegetable extracts had increased dopamine release by striatal slices, increased percentage of cerebellar Purkinje neurons that responded to the β-adrenergic agonist, isoproterenol, and improved spatial learning compared to age-matched rats fed control diet.33
Another study by the same group indicated that feeding older rats dietary supplements of fruit and vegetable extracts could reverse signs of brain aging.11 More recently, a 7-week supplementation with a blueberry diet (2% w/w) improved spatial learning and memory of young rats.34 All of these observations in rodents help understand the positive correlation between flavonoid intake and cognitive function reported over a 10-year period for men 65 years of age or older,8 and both men and women aged 50–69 years.13 As a result, flavonoids have been suggested as novel naturally occurring agents for preventing neuroinflammation, cognitive aging, and age-related neurodegenerative diseases.35 The results of the present study suggest microglial cells as an important target of luteolin and perhaps other flavonoids that affect similar intracellular pathways.
That luteolin would target microglia is not unexpected. We previously showed in a microglia cell line (BV-2) that luteolin reduced LPS-stimulated IL-6 production by inhibiting c-Jun N-terminal kinase phosphorylation and activation of AP-1.15 Another group, also using BV-2 cells, showed that luteolin induced global changes in the transcriptome leading to an anti-inflammatory phenotype.17 A recent study with aged mice found improved learning and memory and reduced expression of inflammatory genes in the hippocampus when luteolin was included in the diet.10
The present study was limited in scope with the specific objective to determine if dietary luteolin reduced microglial activation and expression of proinflammatory cytokines. From the present study we cannot say that ingested luteolin, which is detectable in blood,10 accessed the brain and acted directly on microglia. Although the presence of luteolin in the brain was not determined, evidence suggests that flavonoids generally can penetrate the blood–brain barrier.35 Thus, it is reasonable to predict that circulating luteolin had access to the microglial cell compartment. In addition, although the presence of peripheral cytokines was not determined, evidence suggests if luteolin reduced immune-to-brain signaling by an undefined peripheral mechanism, this too could explain the reduced signs of inflammation in the brain. In any case, the practical conclusion is that ingestion of a luteolin-supplemented diet reduced the number of primed proinflammatory microglia in the brain of aged mice even after stimulation of the peripheral immune system.
Acknowledgments
The authors thank Dr. Saeybyeol Jang for assistance with the experimental diet formulation and Dr. Barbara Pilas for her technical expertise and assistance with flow cytometry. This research was supported by NIH grant R01-AG16710.
Authors' Contributions
R.W.J. and M.D.B. conceptualized the project, developed the research plan, and wrote the article. M.D.B. conducted the experiments, and collected and analyzed data. J.L.R. and R.A. helped conduct the experiments. All authors read and approved the final manuscript.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 2005;308:1314–1318 [DOI] [PubMed] [Google Scholar]
- 2.Perry VH, Nicoll JA, Holmes C. Microglia in neurodegenerative disease. Nat Rev Neurol 2010;6:193–201 [DOI] [PubMed] [Google Scholar]
- 3.Varnum MM, Ikezu T. The classification of microglial activation phenotypes on neurodegeneration and regeneration in Alzheimer's disease brain. Arch Immunol Ther Exp 2012;60:251–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henry CJ, Huang Y, Wynne AM, Godbout JP. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1beta and anti-inflammatory IL-10 cytokines. Brain Behav Immun 2009;23:309–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ye SM, Johnson RW. Increased interleukin-6 expression by microglia from brain of aged mice. J Neuroimmunol 1999;93:139–148 [DOI] [PubMed] [Google Scholar]
- 6.Fenn AM, Henry CJ, Huang Y, Dugan A, Godbout JP. Lipopolysaccharide-induced interleukin (IL)-4 receptor-alpha expression and corresponding sensitivity to the M2 promoting effects of IL-4 are impaired in microglia of aged mice. Brain Behav Immun 2012;26:766–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harnly JM, Doherty RF, Beecher GR, Holden JM, Haytowitz DB, Bhagwat S, Gebhardt S. Flavonoid content of U.S. fruits, vegetables, and nuts. J Agric Food Chem 2006;54:9966–9977 [DOI] [PubMed] [Google Scholar]
- 8.Letenneur L, Proust-Lima C, Le Gouge A, Dartigues JF, Barberger-Gateau P. Flavonoid intake and cognitive decline over a 10-year period. Am J Epidemiol 2007;165:1364–1371 [DOI] [PubMed] [Google Scholar]
- 9.Tangney CC, Kwasny MJ, Li H, Wilson RS, Evans DA, Morris MC. Adherence to a Mediterranean-type dietary pattern and cognitive decline in a community population. Am J Clin Nutr 2011;93:601–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jang S, Dilger RN, Johnson RW. Luteolin inhibits microglia and alters hippocampal-dependent spatial working memory in aged mice. J Nutr 2010;140:1892–1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joseph JA, Shukitt-Hale B, Denisova NA, Bielinski D, Martin A, McEwen JJ, Bickford PC. Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplementation. J Neurosci 1999;19:8114–8121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams CM, El Mohsen MA, Vauzour D, Rendeiro C, Butler LT, Ellis JA, Whiteman M, Spencer JP. Blueberry-induced changes in spatial working memory correlate with changes in hippocampal CREB phosphorylation and brain-derived neurotrophic factor (BDNF) levels. Free Radic Biol Med 2008;45:295–305 [DOI] [PubMed] [Google Scholar]
- 13.Brickman AM, Khan UA, Provenzano FA, Yeung LK, Suzuki W, Schroeter H, Wall M, Sloan RP, Small SA. Enhancing dentate gyrus function with dietary flavanols improves cognition in older adults. Nat Neurosci 2014;17:1798–1803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xagorari A, Papapetropoulos A, Mauromatis A, Economou M, Fotsis T, Roussos C. Luteolin inhibits an endotoxin-stimulated phosphorylation cascade and proinflammatory cytokine production in macrophages. J Pharmacol Exp Ther 2001;296:181–187 [PubMed] [Google Scholar]
- 15.Jang S, Kelley KW, Johnson RW. Luteolin reduces IL-6 production in microglia by inhibiting JNK phosphorylation and activation of AP-1. Proc Natl Acad Sci U S A 2008;105:7534–7539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim JH, Park HS, Choi JK, Lee IS, Choi HJ. Isoorientin induces Nrf2 pathway-driven antioxidant response through phosphatidylinositol 3-kinase signaling. Arch Pharm Res 2007;30:1590–1598 [DOI] [PubMed] [Google Scholar]
- 17.Dirscherl K, Karlstetter M, Ebert S, Kraus D, Hlawatsch J, Walczak Y, Moehle C, Fuchshofer R, Langmann T. Luteolin triggers global changes in the microglial transcriptome leading to a unique anti-inflammatory and neuroprotective phenotype. J Neuroinflammation 2010;7:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reeves PG, Nielsen FH, Fahey GC., Jr. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 1993;123:1939–1951 [DOI] [PubMed] [Google Scholar]
- 19.Reeves PG, Rossow KL, Lindlauf J. Development and testing of the AIN-93 purified diets for rodents: Results on growth, kidney calcification and bone mineralization in rats and mice. J Nutr 1993;123:1923–1931 [DOI] [PubMed] [Google Scholar]
- 20.Ford AL, Goodsall AL, Hickey WF, Sedgwick JD. Normal adult ramified microglia separated from other central nervous system macrophages by flow cytometric sorting. Phenotypic differences defined and direct ex vivo antigen presentation to myelin basic protein-reactive CD4+ T cells compared. J Immunol 1995;154:4309–4321 [PubMed] [Google Scholar]
- 21.Chen J, Buchanan JB, Sparkman NL, Godbout JP, Freund GG, Johnson RW. Neuroinflammation and disruption in working memory in aged mice after acute stimulation of the peripheral innate immune system. Brain Behav Immun 2008;22:301–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corona AW, Fenn AM, Godbout JP. Cognitive and behavioral consequences of impaired immunoregulation in aging. J Neuroimmune Pharmacol 2012;7:7–23 [DOI] [PubMed] [Google Scholar]
- 23.Frank MG, Barrientos RM, Biedenkapp JC, Rudy JW, Watkins LR, Maier SF. mRNA up-regulation of MHC II and pivotal pro-inflammatory genes in normal brain aging. Neurobiol Aging 2006;27:717–722 [DOI] [PubMed] [Google Scholar]
- 24.Frank MG, Barrientos RM, Watkins LR, Maier SF. Aging sensitizes rapidly isolated hippocampal microglia to LPS ex vivo. J Neuroimmunol 2010;226:181–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. FASEB J 2005;19:1329–1331 [DOI] [PubMed] [Google Scholar]
- 26.Barrientos RM, Hein AM, Frank MG, Watkins LR, Maier SF. Intracisternal interleukin-1 receptor antagonist prevents postoperative cognitive decline and neuroinflammatory response in aged rats. J Neurosci 2012;32:14641–14648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosczyk HA, Sparkman NL, Johnson RW. Neuroinflammation and cognitive function in aged mice following minor surgery. Exp Gerontol 2008;43:840–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buchanan JB, Sparkman NL, Chen J, Johnson RW. Cognitive and neuroinflammatory consequences of mild repeated stress are exacerbated in aged mice. Psychoneuroendocrinology 2008;33:755–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Norden DM, Godbout JP. Microglia of the aged brain: Primed to be activated and resistant to regulation. Neuropathol Appl Neurobiol 2013;39:19–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abraham J, Johnson RW. Central inhibition of interleukin-1beta ameliorates sickness behavior in aged mice. Brain Behav Immun 2009;23:396–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spilsbury A, Vauzour D, Spencer JP, Rattray M. Regulation of NF-kappaB activity in astrocytes: Effects of flavonoids at dietary-relevant concentrations. Biochem Biophys Res Commun 2012;418:578–583 [DOI] [PubMed] [Google Scholar]
- 32.Nabavi SF, Braidy N, Gortzi O, Sobarzo-Sanchez E, Daglia M, Skalicka-Wozniak K, Nabavi SM. Luteolin as an anti-inflammatory and neuroprotective agent: A brief review. Brain Res Bull 2015;119(Pt A):1–11 [DOI] [PubMed] [Google Scholar]
- 33.Joseph JA, Shukitt-Hale B, Denisova NA, Prior RL, Cao G, Martin A, Taglialatela G, Bickford PC. Long-term dietary strawberry, spinach, or vitamin E supplementation retards the onset of age-related neuronal signal-transduction and cognitive behavioral deficits. J Neurosci 1998;18:8047–8055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rendeiro C, Vauzour D, Kean RJ, Butler LT, Rattray M, Spencer JP, Williams CM. Blueberry supplementation induces spatial memory improvements and region-specific regulation of hippocampal BDNF mRNA expression in young rats. Psychopharmacology 2012;223:319–330 [DOI] [PubMed] [Google Scholar]
- 35.Spencer JP, Vafeiadou K, Williams RJ, Vauzour D. Neuroinflammation: Modulation by flavonoids and mechanisms of action. Mol Aspects Med 2012;33:83–97 [DOI] [PubMed] [Google Scholar]