Abstract
With the average life span of humans on the rise, aging in the world has drawn considerable attentions. The monoamine neurotransmitters and neurotrophic factors in brain areas are involved in learning and memory processes and are an essential part of normal synaptic neurotransmission and plasticity. In the present study, the effect of Zhuang Jing Decoction (ZJD) on the learning and memory ability in aging rats was examined in vivo using Morris water maze. Furthermore, the levels of monoamine neurotransmitters and neurotrophic factors in brain were detected by high-performance liquid chromatography with a fluorescence detector and enzyme-linked immunosorbent assay, respectively. These data showed that oral administration with ZJD at the dose of 30 g·kg−1 exerted an improved effect on learning and memory ability in aging rats. The results revealed that ZJD could effectively adjust the monoamine neurotransmitters and neurotrophic factors, restore the balance of the level of monoamine neurotransmitters and neurotrophic factors in brain, and finally attenuate the degeneration of learning and memory ability. These findings suggested that ZJD might be a potential agent as cognitive-enhancing drug in improving learning and memory ability. It may exert through regulating the levels of monoamine neurotransmitters and neurotrophic factors in brain, which demonstrated that ZJD had certain antiaging effects.
Introduction
Globally, people aged over 65 will be close to 500,000,000. According to the sixth census data released in 2010,1 the number of people aged over 65 in China is 118,831,709, accounting for 8.87%. China has entered the aging society; therefore, research on the mechanisms of aging and antiaging medicine has become a focus of public medical service.2
Changes in synaptic structure and synapse loss with age by neuroanatomical region have been thoroughly reviewed, and the evidence is robust.3 A recent study found that age-dependent declines in cognition and memory may best be explained as a decline in synaptic stability.4 The monoamine neurotransmitters in brain areas are involved in learning and memory processes and are an essential part of normal synaptic neurotransmission and plasticity. To the best of our knowledge, the neurotransmitters namely catecholamines (norepinephrine [NE], dopamine [DA]) and serotonin (5-hydroxytryptamine [(5-HT]) play critical role in emotions, sleep, arousal, and cognitive function.5,6 Neurotrophic factors are critical for neuronal differentiation, maturation, and survival. However, as the aging, the balance of neurotrophic factors is disturbed. Level of basic fibroblast growth factor is upregulated, whereas the levels of neurotrophic factors are reduced in the hippocampus, the frontal cortex, and the parietal cortex.7 Obviously, monoamine neurotransmitters and neurotrophic factors in brain areas play important roles in learning and memory processes.
Nowadays, many research results have found that the Chinese medicine compound can improve learning and memory ability.8,9 Zhuang Jing Decoction (ZJD), a traditional Chinese medicine (TCM) formula consisting of Talinum crassifolium, Gynostemma pentaphylla, Euonymus fortunei, and Curculigo capitulata root, has been reported to be effective in the treatment of perimenopausal women syndrome, which demonstrated that the ZJD may have certain effects on antiaging.10 Moreover, it has been reported that some of the components had beneficial effects on learning and memory ability.11,12 This study was designed to investigate the effect of ZJD on learning and memory ability, monoamine neurotransmitters, and neurotrophic factors in aging rats. Our data indicated that ZJD treatment might act through regulating the levels of monoamine neurotransmitters and neurotrophic factors in the brain, which played a role in improving learning and memory ability in aging rats.
Materials and Methods
Animals
Forty aging Sprague-Dawley (SD) female rats (12 months old, weighing 300 ± 20 g) and 10 young SD female rats (5 months old, weighing 260 ± 20 g), specific pathogen-free grade, were provided by Youjiang Medical College for Nationalities of Guangxi laboratory animal center: SCXK ( ) 2012-0003. Animal cares were in keeping with the international standards established by the National Institutes of Health “Guide for the Care and Use of Laboratory Animals”.13 All the animals were housed under conventional conditions at a controlled temperature (23°C ± 2°C), humidity(55% ± 10%), and maintained under a natural light/dark cycle. Rats had free access to a standard laboratory diet and water during this experiment.
) 2012-0003. Animal cares were in keeping with the international standards established by the National Institutes of Health “Guide for the Care and Use of Laboratory Animals”.13 All the animals were housed under conventional conditions at a controlled temperature (23°C ± 2°C), humidity(55% ± 10%), and maintained under a natural light/dark cycle. Rats had free access to a standard laboratory diet and water during this experiment.
Experimental drugs
ZJD is composed of Talinum crassifolium (30 g), Gynostemma pentaphylla (25 g), Euonymus fortunei (20 g), and Curculigo capitulata root (10 g). The herbs were decocted by boiling in distilled water at 100°C for 30 minutes. The solution was then freeze-dried under a vacuum and made into a powder, which was stored in aliquots at −20°C. The powder was dissolved in distilled water when administrated to rats. Each rat received 3 mL liquid daily through the oral gavage needle (10 cm) and injector (5 mL).
Reagents
Standard substance: noradrenaline (batch No. 100169-201103), dopamine (batch No. 100070-201006), and serotonin (batch No. 111656-50679) (National Institute for the Young control of Pharmaceutical and Biological Products). Mobile phase: acetonitrile, purified water; potassium dihydrogen phosphate; EDTA-2Na (Wuhan Boster Bioengineering Company Limited). Enzyme-linked immunosorbent assay (ELISA) kits: Brain derived neurotrophic factor (BDNF) (31.2 pg/mL →2000 pg/mL) Emax immunoassay kit; Nerve growth factor (NGF) (31.2 pg/mL →2000 pg/mL) Emax immunoassay kit (batch No. 190987822) (Wuhan Boster Bioengineering Company Limited).
Modeling
The method of natural aging was used to select appropriate rats. After adapting to the new housing environment for a week, the vaginal exfoliated cells smear method was taken to monitored the estrous cycle of each rat (12 months old) for 2 weeks. Rats with irregular estrous cycles and persistent anestrus were selected as aging rats.14
Grouping and treating
Five-month-old SD female rats were chosen as the young control group. The 12-month-old SD female rats were divided into four groups in accordance with the random number table: aging group, low-dose ZJD (low dose), medium-dose ZJD (med dose), high-dose ZJD (high dose), 10 rats in each group. The young control group and aging group were given saline, 10 mL·kg−1, the low-, medium-, and high-dose ZJD groups. All the rats were administered three doses of ZJD (7.5 g·kg−1 [low dose], 15 g·kg−1 [medium dose], 30 g·kg−1 [high dose]) through oral gavage once daily for 8 weeks.
Morris water maze test
The Morris water maze test consists of a circular pool (diameter: 180 cm; height: 60 cm; depth of water: 32 cm) with a featureless inner surface and a hidden escape platform (diameter: 10 cm) submerged 2 cm below the surface of the water and invisible from the surface of the water. There were some specific graphics on the pool wall available to the rats. The water was darkened with 50 mL of prepared ink and maintained at 22°C ± 1°C. A video camera recorded each rat, and a tracking system (Institute of Material Medical, Chinese Academy of Medical Sciences, China) analyzed each rat's path. The dependent measure was swim distance (m), with less swim distance interpreted as better performance. The animals were acclimatized before starting swim trails. After each trial, the rats were moved temporarily into the heated cage until their fur was dried. The test was given starting from day 50 to day 55. All animals received four trials per day for five consecutive days with approximately an equal interval between trials.15 The probe trial was performed 24 hours after the hidden platform experiment on day 56. The platform in the pool was removed, and rats were put into the pool from the original point with the head toward the pool wall. The rats swimming trajectories within 100 seconds were systematically recorded and analyzed.16 The dependent measures for the probe trial were the percentage of total swim distance (cm) in the target quadrant compared with the opposite quadrant.
Monoamine neurotransmitters assay
After the Morris water maze test, each rat was rapidly decapitated. The cerebral cortex was removed, and the hippocampus was dissected bilaterally on ice. The tissue samples were put into the liquid nitrogen tank, then stored at −80°C.17 PBS buffer was added at the ratio of 1:5 followed by the same volume of 5% perchloric acid. Then, the tissue was homogenized in ice for 30 seconds and centrifuged at 12,000 r/min for 15 minutes below 4°C. Finally, the supernatants were filtered through 0.2 μm microporous membranes and used for the analysis of monoamine neurotransmitters by using high-performance liquid chromatography with fluorescence detector (HPLC-FD).18,19 Chromatographic conditions: mobile phase (the ratio of aqueous phase and acetonitrile is 90 to 10. Preparation: 1 L aqueous phase containing 0.2 mol monopotassium phosphate, 0.1 mol EDTA ·2Na, was dissolved by ultrasound then filtered through a 0.2 μm microporous membrane and reserved after ultrasonic degassing), the pH is adjusted to three with phosphoric acid; the column temperature was 28°C, with the flow speed 0.5 mL/min; the emission wavelength of 338 nm, the excitation wavelength of 220 nm. In this chromatographic condition, NE, DA, and 5-HT are separated from the baseline.
ELISA for neurotrophic factors
The hippocampus level of NGF and BDNF was determined through the ELISA double-antibody sandwich method. The operation is according to the ELISA kit instructions. Used 96-well plates, and the absorbance wavelength was 450 nm.
Statistics
All statistical analyses were performed using Statistic Package for Social Science (SPSS) 17.0 software. The differences of Morris water maze test in escape latency were analyzed by repeated measures analysis of variance (ANOVA) followed by the least significant difference (LSD) post hoc test. Other data were analyzed by the one-way ANOVA followed by LSD post hoc test, or the nonparametric test where appropriate. A value of p < 0.05 was considered as statistically significant.
Results
ZJD improved the learning and memory ability
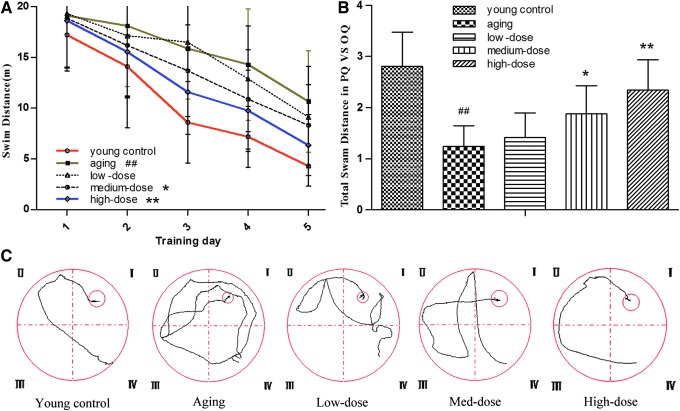
The space exploration experiment was performed to evaluate the effect of ZJD on SD aging rats. As depicted in Figure 1, the rats in the aging group had a poor performance on finding the platform when compared with the young control group (p < 0.01). However, the treatment of the high-dose group significantly improved spatial learning and memory ability. The swim distance of rats treated with high-dose ZJD was significantly shorter (p < 0.01). What's more, the high-dose group explored longer distance in the target quadrant (p < 0.01) in the probe trial, which indicated that these rats remembered the location of the hidden platform and found it purposively. The result suggested that ZJD could improve the spatial learning and memory ability in aging rats.
FIG. 1.
Use Morris water maze test to evaluate the effect of Zhuang Jing Decoction (ZJD) on learning and memory ability in aging rats. (A) Swim distance in water maze during the platform trial. (B) Swim distance in the target quadrant versus the opposite quadrant during the probe trial. (C) Representative tracings of the animal's path during the retention test. These dates revealed that ZJD improved the learning and memory ability in aging rats. Data are shown as the mean ± SE. ##p < 0.01 versus the young control group. *p < 0.05 and **p < 0.01 versus the aging group. Color images available online at www.liebertpub.com/rej
ZJD regulated the level of monoamine neurotransmitters in hippocampus and cerebral cortex
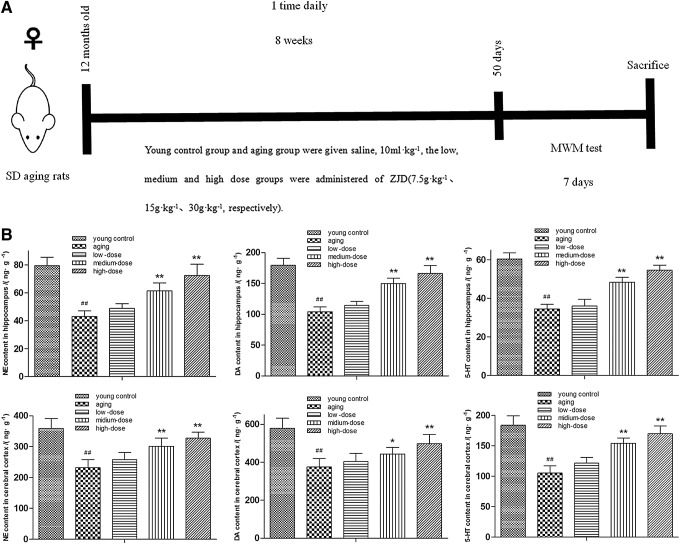
As shown in Figure 2, a prominent decrease of the monoamine neurotransmitter level in the hippocampus and cerebral cortex was observed in the aging group when compared with the young control group (p < 0.01). However, this decrease in the monoamine neurotransmitter level was markedly attenuated by ZJD treatment (p < 0.01), namely, ZJD could adjust the expression level of monoamine neurotransmitters in the brain in aging rats.
FIG. 2.
Use high-performance liquid chromatography with fluorescence detector to detect the effect of ZJD on levels of monoamine neurotransmitters in the hippocampus and cerebral cortex in aging rats. (A) Scheme of experimental design. (B) The level of norepinephrine (NE), dopamine (DA), and 5-hydroxytryptamine (5-HT) in the hippocampus; the level of NE, DA, and 5-HT in the cerebral cortex. These dates revealed that ZJD improved the levels of monoamine neurotransmitters of brain in aging rats. Data are shown as the mean ± SE. #p < 0.05 and ##p < 0.01 versus the young control group. **p < 0.01 versus the aging group.
ZJD enhanced the expression level of neurotrophic factors in hippocampus
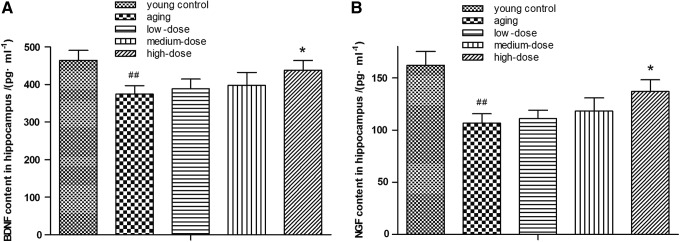
Emerging evidence indicated that neurotrophic factors could contribute to the reduction on the risk of the progression of cognitive impairment in neurotrophic systems, affecting neuronal survival and function and improving synaptic plasticity and long-term memory.20 As shown in Figure 3, we observed that the expression level of neurotrophic factors in the hippocampus tissue had a significant decrease in the aging group compared with the young control group (p < 0.01). After being treated with ZJD, the content of neurotrophic factors was increased significantly (p < 0.05). These findings indicated that ZJD could improve the expression level of neurotrophic factors in the hippocampus in aging rats.
FIG. 3.
Use enzyme-linked immunosorbent assay to determine the effect of ZJD on levels of neurotrophic factors in the hippocampus in aging rats. (A) The levels of BDNF and NGF in hippocampus. These dates revealed that ZJD enhanced the levels of neurotrophic factors of brain in aging rats. Data are shown as the mean ± SE. ##p < 0.01 versus the young control group. *p < 0.05 versus the aging group. BDNF, brain derived neurotrophic factor; NGF, nerve growth factor.
Discussion
Aging initiates under the condition of normal biological maturity, with the increase of age, gradual decline of capacity of body systems, falling of the internal environmental stability and the ability of stress reaction, and structural constituent progressively degenerative changes. It is an irreversible trend and finally results as death.21 In TCM, the theory “the kidney nourishes marrow and brain is the sea of marrow,” which was recorded in the ancient book of The Medical Classic of the Yellow Emperor (Huang Di Nei Jing), has been instructing traditional Chinese medical practitioners in preventing and treating dementia in aged people for thousands of years.22
In this study, the Morris water maze test, which is widely used to evaluate the spatial learning and memory ability of animals, was used to explore the antiaging effects of ZJD on learning and memory ability in aging rats.23,24 The MWM result revealed that the high-dose ZJD obviously improved the learning and memory ability in aging rats.
Learning and memory ability is one of the advanced neural functions of the central nervous system and is closely associated with monoamine neurotransmitters.25,26 Monoamine neurotransmitters are the endogenous chemicals, which are released at the end of a nerve fiber by the arrival of a nerve impulse, by diffusing across the synapse or junction, and effect the transfer of nerve impulse to another nerve fiber. 5-HT, DA, and NE were important monoamine neurotransmitters in the central nervous system and were closely related to neural functions and aging.27 More specifically, 5-HT excites the functions of learning and memory28,29 and can trigger facilitation, and DA has an excitatory effect on overall behavior and participates in the reappearance of memory trace. NE regulates excitation of the cerebral cortex and influences awakening, sensation, emotions, and advanced cognitive functions; increased excitability of NE improves learning and memory.30
It was reported that the initial decline of memory function with age is due to changes in synaptic function rather than a loss of neurons.31 Synapse loss in the hippocampal region significantly correlates with the severity of their cognitive symptoms.32 As one of the widely distributed neurotrophic factors in the brain, BDNF involved in neuronal growth, maintenance, and in different aspects of activity-dependent synaptic physiology by acting in neuronal plasticity and mediating long-term potentiation and memory fixation.33 Recent studies have shown that decreased levels of BDNF correlate with the severity of cognitive impairment, suggesting that reduced BDNF availability may be an early cofactor involved in aging.34 NGF is a potential maintenance factor for cholinergic neuron function. It not only may increase the sprouting of cholinergic neurons but also may rescue the cholinergic neuron from lesion-induced atrophy to enhance the neuronal survival.35,36 Spatial memory impairments in aging rats were reversed by NGF and neurotrophins 3 and 4/5, but not by BDNF, which indicated that NGF might play a critical role in the cognition function.37 Our finding demonstrated that ZJD may increase the levels of monoamine neurotransmitters and neurotrophic factors in brain in aging rats, and the effects were related to the concentration of ZJD.
In conclusion, our preliminary observation indicated that chronic administration with ZJD significantly improved learning and memory ability in aging rats. ZJD treatment increased monoamine neurotransmitter levels in the brain and enhanced the expression of neurotrophic factors in the hippocampus of the aging rats. Therefore, ZJD may have potential as a cognitive-enhancing drug for antiaging. However, further investigation to confirm our findings and to explore possible mechanisms of action is warranted.
Acknowledgments
The authors would like to thank Professor Cenhan Huang (Youjiang Medical University for Nationalities) for providing the ZJD. This study was supported by Guangxi Provincial Natural Science Fund of China (No. 2010GXNSFA013265), South China Chinese Medicine Collaborative Innovation Center (No. A1-AFD01514A05), and Characteristic Key Discipline Construction Fund of Chinese Internal Medicine of Guangzhou University of Chinese Medicine (2013–2015).
Author Disclosure Statement
No competing financial interests exist. The authors alone are responsible for the content and writing of the article.
References
- 1.The sixth national population census office of the state council, the national bureau of statistics of population and employment statistics division. 2010 the sixth national population census data. Beijing: China Statistical Publishing House; 2011; 07, Version 1 [Google Scholar]
- 2.Pearce DJ, Anjos-Afonso F, Ridler CM, et al. . Age-dependent increase in side population distribution within hematopoiesis: Implications for our understanding of the mechanism of ageing. Stem Cells 2007;25:828–835 [DOI] [PubMed] [Google Scholar]
- 3.Petralia RS, Mattson MP, Yao PJ. Communication breakdown: The impact of ageing on synapse structure. Ageing Res 2014;14:31–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grillo FW, Song S, Teles-Grilo Ruivo LM, et al. . Increased axonal bouton dynamics in the aging mouse cortex. Proc Natl Acad Sci U S A 2013;110:E1514–E1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McEwen BS. Sex, stress and the hippocampus: allostasis, allostatic load and the aging process. Neurobiol Aging 2002;23:921–939 [DOI] [PubMed] [Google Scholar]
- 6.Oliveira L, Graeff FG, Pereira SRC, Oliveira-Silva IF, Franco GC, Ribeiro AM. Correlations among central serotonergic parameters and age-related emotional and cognitive changes assessed through the elevated T-maze and the Morris water maze. Age 2010;32:187–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schindowski K, Bretteville A, Leroy K, Begard S, Brion JP, Hamdane M, Buee L. Alzheimer's disease-like tau neuropathology leads to memory deficits and loss of functional synapses in a novel mutated tau transgenic mouse without any motor deficits. Am J Pathol 2006;169:599–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hou XQ, Zhang L, Yang C, et al. . Alleviating effects of BSYZ formula on IBO-induced cholinergic impairments in rat. Rejuvenation Res 2015;18:111–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lan Z, Liu J, Chen L, et al. . Danggui-Shaoyao-San ameliorates cognition deficits and attenuates oxidative stress-related neuronal apoptosis in D-galactose-induced senescent rats. J Ethnopharmacol 2012;141:386–395 [DOI] [PubMed] [Google Scholar]
- 10.Liu YP, Huang CH. 58 cases treatment of perimenopausal syndrome by ZJD. J Liaoning Tradit Chin Med 2008;3:1875–1876 [Google Scholar]
- 11.Zhang X, Wang J, Xing Y, Gong L, Li H, Wu Z, Li Y, Wang J, Wang Y, Dong L, Li S. Effects of ginsenoside Rg1 or 17β-estradiol on a cognitively impaired, ovariectomized rat aging of Alzheimer's disease. Neuroscience 2012;220:191–200 [DOI] [PubMed] [Google Scholar]
- 12.Zhou L, Hou Y, Yang Q, Du X, Li M, Yuan M, Zhou Z: Tetrahydroxystilbene glucoside improves the learning and memory of amyloid-β (1–42)-injected rats and may be connected to synaptic changes in the hippocampus. Can J Physiol Pharmacol 2012;90:1446–1455 [DOI] [PubMed] [Google Scholar]
- 13.National Research Council Guide for the Care and Use of Laboratory Animals, 7th edition. Washington, DC: Public Health Service, 1996 [Google Scholar]
- 14.Sun JF, Zhao Y, Wu SH. Perimenopausal syndrome comparative analysis of experimental animal aging. J Shanxi Med Univ 2007;38:43–45 [Google Scholar]
- 15.Chen LX, Guo LL, Li L. The application of circular Morris water maze and related testing index analysis. J Liaoning Univ Chin Med 2008;10:55–57 [Google Scholar]
- 16.Liu HX, Zhang JJ, Zheng P, Zhang Y. Altered expression of MAP-2, GAP-43, and synaptophysin in the hippocampus of rats with chronic cerebral hypoperfusion correlates with cognitive impairment. Brain Res Mol Brain Res 2005;139:169–177 [DOI] [PubMed] [Google Scholar]
- 17.Ma SS, Qi Y, Wang Y, et al. . Effects of continuous sleep deprivation on cognitive, neurotransmitters and hippocampal formation in rats. Navy Med J 2013;34:82–85 [Google Scholar]
- 18.Wei YF, Wang YH, Zhang N, et al. . High performance liquid fluorescent assay detects 5 kinds of monoamine neurotransmitter in plasma and brain tissues of rats. Chin Arch Tradit Chin Med 2009;27:1942–1943 [Google Scholar]
- 19.Zhang LL, Feng F, Ye XM, et al. . High performance liquid fluorescent assay detects Monoamine neurotransmitter in the brain tissues and its related substances in rats. J China Med Univ 2010;41:367–371 [Google Scholar]
- 20.Weinstein G, Beiser AS, Choi SH, Preis SR, Chen TC, Vorgas D, et al. . Serum brain derived neurotrophic factor and the risk for dementia: The Framingham Heart Study. JAMA Neurol 2014;71:55–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zou CL, ed. Current Biology. BeiJing: Chinese Zhi Gong’ Publishing House, 2000, pp. 394–396 [Google Scholar]
- 22.Li L, Wei HF, Zhang L, Chu J, Zhao L. Modern biological basis of Chinese medical theory that “kidney nourishes marrow and brain is sea of marrow”. Zhongguo Zhong Yao Za Zhi 2006;31:1397–1400, 1417. [PubMed] [Google Scholar]
- 23.Gupta R, Gupta LK. Improvement in long term and visuo-spatial memory following chronic pioglitazone in mouse aging of Alzheimer's disease. Pharmacol Biochem Behav 2012;102:184–190 [DOI] [PubMed] [Google Scholar]
- 24.Kwon SH, Lee HK, Kim JA, Hong SI, Kim HC, Jo TH, Park YI, Lee CK, Kim YB, Lee SY, Jang CG. Neuroprotective effects of chlorogenic acid on scopolamine-induced amnesia via anti-acetylcholinesterase and anti-oxidative activities in rats. Eur J Pharmacol 2010;649:210–217 [DOI] [PubMed] [Google Scholar]
- 25.Zhu L, Zhang R, Li TL. Effects of Acanthopanax on learning and memory ability and hippocampal monoamine neurotransmitters in sleep-deprived rats. Chin J Exp Formula Chin Med 2012;18:219–222 [Google Scholar]
- 26.Birthelmer A, Stemmelin J, Jackisch R, et al. . Presynaptic modulation of acetylcholine, noradrenalin, and serotonin release in the hippocampus of aged rats with various levels of memory impairments. Brain Res Bull 2003;60:283–296 [DOI] [PubMed] [Google Scholar]
- 27.Nazarali AJ, Reynolds GP. Monoamine neurotransmitters and their metabolites in brain regions in Alzheimer's disease: A postmortem study. Cell Mol Neurobiol 1992;12:581–587 [DOI] [PubMed] [Google Scholar]
- 28.Yan JJ, Liu M, Hu Y, et al. . Effect and mechanism of dingzhixiao wan on scopolamine-induced learning-memory impairment in rats. Zhongguo Zhong Yao Za Zhi 2012;37:3293–3296 [PubMed] [Google Scholar]
- 29.Meneses A, Liy-Salmeron G. Liy-Salmeron, Serotonin and emotion, learning and memory. Rev Neurosci 2012;23:543–553 [DOI] [PubMed] [Google Scholar]
- 30.Harley CW. Norepinephrine and dopamine as learning signals. Neural Plast 2004;11:191–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lesné S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH. A specific amyloid- protein assembly in the brain impairs memory. Nature 2006;440:352–357 [DOI] [PubMed] [Google Scholar]
- 32.Scheff SW, Price DA, Schmitt FA, Mufson EJ. Hippocampal synaptic loss in early Alzheimer's disease and mild cognitive impairment. Neurobiol Aging 2006;27:1372–1384 [DOI] [PubMed] [Google Scholar]
- 33.Numakawa T, Adachi N, Richards M, Chiba S, Kunugi H. Brain-derived neurotrophic factor and glucocorticoids: Reciprocal influence on the central nervous system. Neuroscience 2013;239:157–172 [DOI] [PubMed] [Google Scholar]
- 34.Diógenes MJ, Costenla AR, Lopes LV, et al. . Enhancement of LTP in aged rats is dependent on endogenous BDNF. Neuropsychopharmacology 2011;36:1823–1836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Niewiadomska G, Komorowski S, Baksalerska-Pazera M. Amelioration of cholinergic neurons dysfunction in aged rats depends on the continuous supply of NGF. Neurobiol Aging 2002;23:601–613 [DOI] [PubMed] [Google Scholar]
- 36.Niewiadomska G, Mietelska-Porowska A, Mazurkiewicz M. The cholinergic system, nerve growth factor and the cytoskeleton. Behav Brain Res 2011;221:515–526 [DOI] [PubMed] [Google Scholar]
- 37.Fischer W, Sirevaag A, Wiegand SJ, Lindsay RM, Bjorklund A. Reversal of spatial memory impairments in aged rats by nerve growth factor and neurotrophins 3 and 4/5 but not by brain-derived neurotrophic factor. Proc Natl Acad Sci U S A 1994;91:8607–8611 [DOI] [PMC free article] [PubMed] [Google Scholar]