Abstract
We evaluated the possible alterations produced by liver cholestasis (LC), a model of decompensated liver cirrhosis in sympathetic, sensory and nitrergic nerve function in rat superior mesenteric arteries (SMA). The vasoconstrictor response to electrical field stimulation (EFS) was greater in LC animals. Alpha-adrenoceptor antagonist phentolamine and P2 purinoceptor antagonist suramin decreased this response in LC animals more than in control animals. Both non-specific nitric oxide synthase (NOS) L-NAME and calcitonin gene related peptide (CGRP) (8-37) increased the vasoconstrictor response to EFS more strongly in LC than in control segments. Vasomotor responses to noradrenaline (NA) or CGRP were greater in LC segments, while NO analogue DEA-NO induced a similar vasodilation in both experimental groups. The release of NA was not modified, while those of ATP, nitrite and CGRP were increased in segments from LC. Alpha 1 adrenoceptor, Rho kinase (ROCK) 1 and 2 and total myosin phosphatase (MYPT) expressions were not modified, while alpha 2B adrenoceptor, nNOS expression and nNOS and MYPT phosphorylation were increased by LC. Together, these alterations might counteract the increased splanchnic vasodilation observed in the last phases of decompensated liver cirrhosis.
Liver cirrhosis is a common disease currently ranked as one of the 10 most common causes of death in the Western world (Stewart and Day, 2003). It courses with portal hypertension (PH) related to augmented hepatic vascular resistance, caused by an increase in vasoconstrictor factors like thromboxane A2 (TXA2)1, and a reduction in nitric oxide (NO) bioavailability2. This pathology is also associated with the development of hyperdynamic circulation3, in which peripheral arterial vasodilation, mainly in the splanchnic circulation, plays a major role4. This decreased splanchnic vascular resistance is associated to alterations in different endothelial factors in these vessels, such as increased release and sensitivity of vasodilator factors like NO5,6 and prostaglandin I2 (PGI2)7,8 as well as decreased response to factors like noradrenaline (NA)9, phenylephrine or U-4661910,11. Endothelial factor participation is known to change over time in the course of liver cirrhosis. Thus, although the increase in splanchnic and systemic circulation is maintained in decompensated liver cirrhosis and long-term PH models, NO involvement decreases, leading to greater participation by PGI212,13,14,15.
Apart from endothelial factors, vascular tone is also determined by the participation of perivascular innervation. Rat mesenteric arteries possess rich sympathetic16, nitrergic17,18 and sensory19 innervations, which respectively release NA and ATP, NO and calcitonin gene related peptide (CGRP), when electrically stimulated. These neurotransmitters participate in vasomotor tone modulation through their vasoconstrictor and vasodilator actions on smooth muscle cells. We have reported that different physiopathological situations, such as aging20,21, hypertension22,23 or diabetes24,25 can modify the participation of the different neurotransmitters involved in the maintenance of vascular tone. In line with this, in a model of mild liver cirrhosis induced by carbon tetrachloride treatment, several studies have reported decreased sympathetic innervation function26,27,28,29,30, increased NO release from neuronal NO synthase (nNOS)29,31 and augmented sensory innervation function32. Additionally, we have previously described a rearrangement of participation by the different components of perivascular mesenteric innervation in a model of long-term PH21. However, to the best of our knowledge, possible alterations in the function of perivascular innervation components in decompensated liver cirrhosis have yet to be studied.
In light of this background, the aim of this study was to analyse the possible alterations in the vasomotor response to electric field stimulation (EFS) in rat superior mesenteric artery, and how sympathetic, sensory and nitrergic components could be affected in decompensated liver cirrhosis induced by liver cholestasis.
Materials and Methods
Animals
Male Wistar rats were obtained and housed in the Animal Facility of the Universidad Autónoma de Madrid (Registration number EX-021U). The research conforms to the European Commission Directive 86/609 CEE Art. 21 (1995), and the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). This study has been approved by the ethical committee of the Universidad Autónoma de Madrid.
Rats (Initial weight: 294.5 ± 2.6 g) were divided into two groups: Sham-operated (SO; n = 10), in which the common bile duct was dissected; and microsurgical liver cholestasis (LC; n = 10), in which the extrahepatic biliary tract was resected33,34. Surgery was performed under aseptic but not sterile conditions. Briefly, rats were anaesthetised with Ketamine hydrochloride (100 mg/kg) and Xylazine (12 mg/kg) i.m. In the SO group, the bile duct and its lobular branches were dissected. In the LC group, the extrahepatic bile tract was resected using a binocular operatory microscope (Zeiss, OPMI 1-FR). First, the common bile duct was ligated (silk 4/0) and sectioned close to the beginning of its intrapancreatic portion. Dissection and sectioning between ligatures of all biliary branches that drain the hepatic lobes is possible using a binocular operatory microscope (Zeiss, OPMI 1-FR). The dissection and section of the bile ducts from the four liver lobes of the rat must be done without injuring either the portal or, and most especially, the arterial vascularization of these lobes. The abdomen was closed in two layers by continuous running sutures using an absorbable suture (3/0 polyglycolic acid) and silk (3/0). Buprenorphine s.c. (0.05 mg/kg/8 hours) was administered postoperatively for analgesia the first 24 hours after the surgery.
Rats were housed at a constant room temperature, humidity and 12 h light/dark cycle and had free access to tap water and standard rat chow. Mean arterial pressure (MAP) was measured using the tail-cuff method21,32, 8–9 weeks after the surgery was performed. Afterwards, animals were sacrificed by CO2 inhalation; ascetic liquid was collected, and liver, spleen and the first branch of the mesenteric artery were removed and placed in cold Krebs−Henseleit solution (KHS, in mmol/L: NaCl 115; CaCl2 2.5; KCl 4.6; KH2PO4 1.2; MgSO4∙7H2O 1.2; NaHCO3 25; glucose 11.1, Na2 EDTA 0.03) at 4 °C.
Portal Vein Pressure Measurement
Splenic pulp pressure, an indirect measurement of portal pressure (PP) was measured by inserting a fluid filled 20-gauge needle into the splenic parenchyma15,21,32. The needle was joined to a PE-50 tube, and then connected to a pressure recorder (PowerLab 200 ML 201) and to a transducer (Sensonor SN-844) with a Chart V 4.0 computer program (ADI Instruments); these were calibrated before each experiment. The pressure reading was considered satisfactory when a stable recording was produced and respiratory variations were observed. Previous studies have demonstrated the excellent correlation between splenic pulp pressure and PP35.
Vascular Reactivity
The method used for isometric tension recording has been described in full elsewhere18,36. Mesenteric segments from SO and LC animals were suspended in an organ bath containing 5 mL of KHS at 37 °C and continuously bubbled with a 95% O2 to 5% CO2 mixture (pH of 7.4); two parallel stainless steel pins were introduced through the lumen of the vascular segment: one was fixed to the bath wall, and the other connected to a force transducer (Grass FTO3C; Quincy, Mass., USA); this, in turn, was connected to a model 7D Grass polygraph. Some experiments were performed in endothelium-denuded segments to eliminate the main source of vasoactive substances, including endothelial NO. This avoided possible actions by different drugs on endothelial cells that could lead to result misinterpretation. Endothelium was removed by gently rubbing the luminal surface of the segments with a thin wooden stick. The segments were subjected to a tension of 0.5 g, which was readjusted every 15 min during a 90-min equilibration period before drug administration. After this, the vessels were exposed to 75 mmol/L KCl to check their functional integrity. Endothelium removal did not alter the contractions elicited by 75 mmol/L KCl. After a washout period, the presence or absence of vascular endothelium was tested by the inability of 10 μmol/L acetylcholine (Ach) to relax segments precontracted with NA. Arteries which relaxed either more than 90% or less than 10% of the previous tone obtained by NA were respectively considered to be endothelium-intact or endothelium-denuded.
For electrical field stimulation (EFS) experiments, segments were mounted between two platinum electrodes 0.5 cm apart and connected to a stimulator (Grass, model S44) modified to supply adequate current strength. The parameters used for EFS were 200 mA, 0.3 ms, with ascending frequencies from 1 to 16 Hz, for 30 s at each frequency, at an interval of 1 min between each stimulus, the time required to recover basal tone. To prove the neuronal origin of the EFS-induced contractile response, segments were incubated with nerve impulse blocker tetrodotoxin (TTX, 0.1 μmol/L). A washout period of at least 1 h was necessary to avoid desensitisation between consecutive curves. Two successive frequency-response curves separated by 1-hour intervals produced similar contractile responses.
To determine the participation of sympathetic innervation in the EFS-induced response in segments from the SO and LC animals, 1 μmol/L phentolamine, an alpha-adrenoceptor antagonist, 0.1 mmol/L suramin, a non-specific P2 purinergic receptor antagonist, or a combination of phentolamine plus suramin, were added to the bath 30 min before performing the frequency-response curve. Additionally, the vasoconstrictor response to exogenous NA (1 nmol/L-10 μmol/L) was tested in segments from both rat groups.
To analyse the participation of NO in the EFS-induced response in segments from SO and LC animals, 0.1 mmol/L Nω-nitro-L-arginine methyl ester (L-NAME), a non-specific inhibitor of nitric oxide synthase (NOS), was added to the bath 30 min before performing the second frequency–response curve. The vasodilator response to the NO donor, diethylamine NONOate, (DEA-NO, 0.1 nmol/L–0.1 mmol/L) was determined in NA-precontracted segments from the two groups.
To study the possible participation of sensory innervation in the EFS-induced response, 0.5 μmol/L CGRP (8-37), a CGRP receptor antagonist, was added to the bath 30 min before performing the second frequency–response curve. The vasodilator response to exogenous CGRP (0.1 nmol/L–0.1 μmol/L) was determined in NA-precontracted segments from both experimental groups.
NA, ATP, nitrite and CGRP release
To measure the release of NA, ATP, nitrite and CGRP, we used a Noradrenaline research EIA (Labor Diagnostica Nord, Gmbh & Co., KG), an ATP Colorimetric/Fluorometric Assay kit (Abcam, Cambridge, UK), Nitric Oxide Assay Kit (Abcam, Cambridge, UK) and a rat CGRP enzyme immunoassay kit (Spibio, Bertin group), following the manufacturers’ instructions. For sample collecting, endothelium-denuded segments of rat mesenteric arteries from all experimental groups were preincubated for 30 minutes in 5 mL of KHS at 37 °C and continuously gassed with a 95% O2–5% CO2 mixture (stabilisation period). This was followed by two washout periods of 10 min in a bath of 0.4 mL of KHS. Then, the medium was collected to measure basal release. Next the organ bath was refilled and cumulative EFS periods of 30 s at 1, 2, 4, 8 and 16 Hz were applied at 1 min intervals. Afterwards, the medium was collected to measure EFS-induced neurotransmitter release. Samples were immediately frozen in liquid nitrogen and conserved at −70 °C until experiments were performed. The EFS-induced neurotransmitter release was calculated by subtracting basal release from that evoked by EFS. Results were expressed as pg NA/mL mg tissue, pmol ATP/mL mg tissue, pmol nitrite/mg tissue and pg CGRP/mL mg tissue.
Western blot analysis
Western blot analysis was performed as previously described37. For these experiments, we used a mouse monoclonal antibody against nNOS (1:2000, BD Biosciences), a rabbit polyclonal antibody against nNOS phosphorylated In Ser1417 (P-nNOS, 1:2000, Abcam), a rabbit monoclonal antibody against Rho kinase 1 (ROCK1, 1:1000, Abcam), a mouse monoclonal antibody against ROCK2 (1:500, Abcam), a rabbit polyclonal antibody against myosin phosphatase (MYPT, 1:2000, Abcam), a rabbit polyclonal antibody against MYPT phosphorylated In Thr696 (P-MYPT, 1:500, Abcam), and a monoclonal anti-β-actin-peroxidase antibody (1:50000, Sigma-Aldrich, Spain). Appropriate positive controls were used for each blot.
Drugs used
L -NA hydrochloride, ACh chloride, diethylamine NONOate diethyilammonium salt, 8-37 CGRP, rat CGRP, TTX, L -NAME hydrochloride, phentolamine, and suramin were from Sigma-Aldrich, Spain. Stock solutions (10 mmol/L) of drugs were made in distilled water, except for NA, which was dissolved in a NaCl (0.9%)-ascorbic acid (0.01% w/v) solution. These solutions were kept at –20 °C and appropriate dilutions were made in KHS on the day of the experiment.
Data Analysis
The responses elicited by EFS and NA were expressed as a percentage of the initial contraction elicited by 75 mmol/L KCl for comparison between experimental groups. To determine differences in the effect of endothelium removal, phentolamine, suramin, L-NAME or CGRP(8–37) on the responses to EFS, we analysed the differences between areas under the curve (dAUC), which were expressed as the percentage of increase or decrease in the area under the curve produced by each drug. The relaxations induced by either DEA-NO or CGRP were expressed as a percentage of the initial contraction elicited by NA. Results are given as mean ± S.E.M. Statistical analysis was performed by comparing the curve obtained in the presence of the different substances with the previous or control curve by means of two-way analysis of variance (ANOVA) followed by a Fisher’s post hoc test, using GraphPad Prism 6.0 software (CA, USA). For dAUC and NA, ATP, nitrite and CGRP release data, the statistical analysis was done using Student’s t-test. P < 0.05 was considered significant.
Results
Animal evolution
All LC animals showed jaundice and choluria. Paraesophageal, splenorenal and pararectal collateral vessels developed in LC animals (Data not shown). Body weight gain was lower in LC animals. Diminished mean blood pressure, PH, spleen and liver hypertrophy, and extravasation of ascetic fluid were also present in LC animals (Table 1).
Table 1. Effect of liver cholestasis on body weight (BW), body weight gain (BWG), portal pressure (PP), liver weight to body weight ratio (LW/BW), spleen weight to body weight ratio (SW/BW) and ascetic fluid extravasation in Wistar rats.
| BW (g) | BWG (g) | PP (mm Hg) | LW/BW (%) | SW/BW (%) | Ascetic fluid (mL) | |
|---|---|---|---|---|---|---|
| SO | 412.5 ± 9.3 | 43.8 ± 5.6 | 8.3 ± 2.1 | 3.06 ± 0.06 | 0.25 ± 0.03 | — |
| LC | 305.4 ± 10.5* | 22.4 ± 6.1* | 14.7 ± 2.4* | 5.74 ± 0.34* | 0.80 ± 0.19* | 6.4 ± 1.4 |
Results are expressed as means ± S.E.M. ∗P < 0.05 versus SO. n = 10 animals each group.
Vasomotor Response to KCl
In endothelium-intact mesenteric segments, the vasoconstrictor response to 75 mmol/L KCl was similar in both experimental groups (SO: 1516 +53.9 mg; LC: 1541 + 82.8 mg; P > 0.05). Endothelium removal did not alter KCl-induced vasoconstriction (SO: 1505 + 83.8 mg; LC: 1477 + 65.8 mg; P > 0.05).
Vascular Responses to EFS
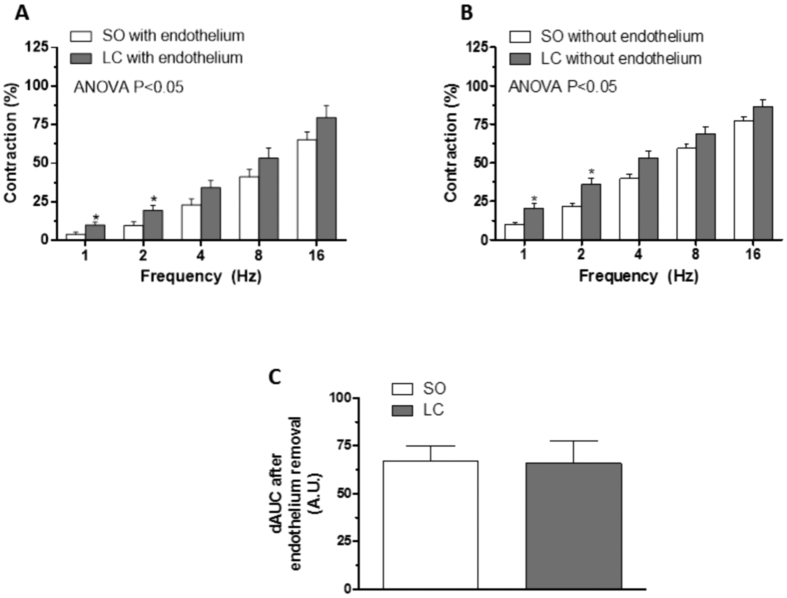
The application of EFS induced a frequency-dependent contractile response in endothelium-intact mesenteric segments from both the SO and LC groups. This vasoconstriction was greater in segments from LC rats compared to SO animals (Fig. 1A). Endothelium removal increased EFS-induced contractile response similarly in segments from both experimental groups (Fig. 1B,C). EFS-induced contractions were practically abolished in segments from both experimental groups by the neurotoxin TTX (0.1 mmol/L) (Table 2).
Figure 1. Vasoconstrictor response to EFS.
EFS-induced vasoconstriction in mesenteric segments with (A) or without (B) endothelium from Sham-operated (SO) or liver cholestasis (LC) rats. Results (mean ± SEM) are expressed as a percentage of the initial contraction elicited by KCl. n = 10 animals per group. *P < 0.05 (Fisher post hoc test) (C) Differences of area under the curve (dAUC) in the absence or presence of endothelium. *P < 0.05 SO vs. LC rats (Student’s t-test). dAUC values are expressed as arbitrary units.
Table 2. EFS-induced contraction after preincubation with 0.1 μmol/L TTX or 1 μmol/L phentolamine plus 0.1 mmol/L suramin, in mesenteric segments from SO and LC rats.
| 1 Hz | 2 Hz | 4 Hz | 8 Hz | 16 Hz | |
|---|---|---|---|---|---|
| SO | 10.1 ± 1.4 | 21.7 ± 2.3 | 39.7 ± 2.8 | 59.2 ± 2.8 | 77.1 ± 2.9 |
| + TTX | 0 | 0 | 0 | 0.5 ± 0.17* | 1.3 ± 0.31* |
| +phentolamine + suramin | 0 | 0 | 0.2 ± 0.12* | 0.8 ± 0.20* | 2.5 ± 0.23* |
| LC | 20.7± 3.1 | 35.9 ± 4.2 | 52.9 ± 4.7 | 68.8 ± 4.8 | 86.4 ± 4.8 |
| + TTX | 0 | 0 | 0 | 0.17 ± 0.09* | 1.57 ± 0.14* |
| +phentolamine + suramin | 0 | 0 | 0.1 ± 0.03* | 1.2 ± 0.5* | 2.6 ± 1.5* |
Results (means ± SEM) are expressed as percentages of the response elicited by 75 mM KCl. Zeros are used when contraction was not detected. *P < 0.05 vs. conditions without drug. n = 6-10 animals each group.
Participation of the sympathetic component of mesenteric vascular innervation
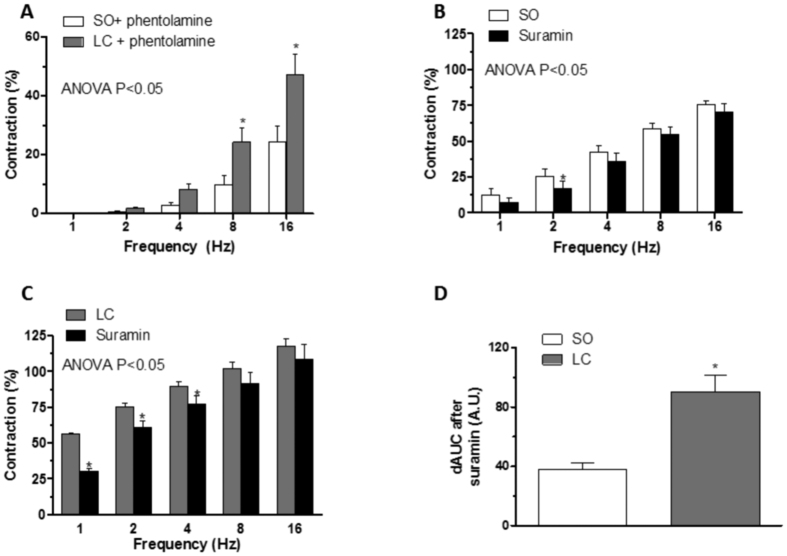
Preincubation with the alpha-adrenergic antagonist phentolamine (1 μmol/L) decreased the vasoconstrictor response induced by EFS in endothelium-denuded segments from both rat groups (Fig. 2A,B). This decrease was greater in mesenteric segments from LC animals (Fig. 2C). NA-induced vasoconstriction was greater in mesenteric segments from the LC group than in segments from SO animals (Fig. 2D). EFS-induced NA release was similar in both groups (Table 3).
Figure 2. Effect of liver cholestasis on sympathetic innervation function.
Effect of preincubation with 1 μmol/L phentolamine on vasoconstrictor response induced by EFS in endothelium-denuded mesenteric segments from Sham-operated (SO, A) and liver cholestasis (LC, B) rats. Results (mean ± S.E.M.) were expressed as a percentage of the initial contraction elicited by KCl. n = 6-8 animals per group. *P < 0.05 (Fisher post hoc test) (C) Differences of area under the curve (dAUC) in the absence or presence of 1 μmol/L phentolamine. *P < 0.05 SO vs. LC rats (Student’s t test). dAUC values are expressed as arbitrary units. (D) Vasoconstrictor response to NA in segments of SO and LC rats. Results (mean ± S.E.M.) were expressed as a percentage of the initial contraction elicited by KCl. n = 6 animals per group. *P < 0.05 (Fisher post hoc test).
Table 3. Effect of liver cholestasis (LC) on EFS-induced NA, ATP, nitrite and CGRP release.
| NA release (pg/mL mg tissue) | ATP release (pmol/mL mg tissue) | Nitrite release (pmol/mg tissue) | CGRP release (pg /mL mg tissue) | |
|---|---|---|---|---|
| SO | 0.87 ± 0.09 | 11.8 ± 3.1 | 1.3 ± 0.4 | 0.03 ± 0.01 |
| LC | 0.81 ± 0.31 | 68.1 ± 14.5* | 15.4 ± 4.6* | 0.21 ± 0.02* |
Results were calculated by subtracting basal release from that evoked by EFS, and expressed as means ± S.E.M. ∗P < 0.05 versus SO. n = 10 animals each group.
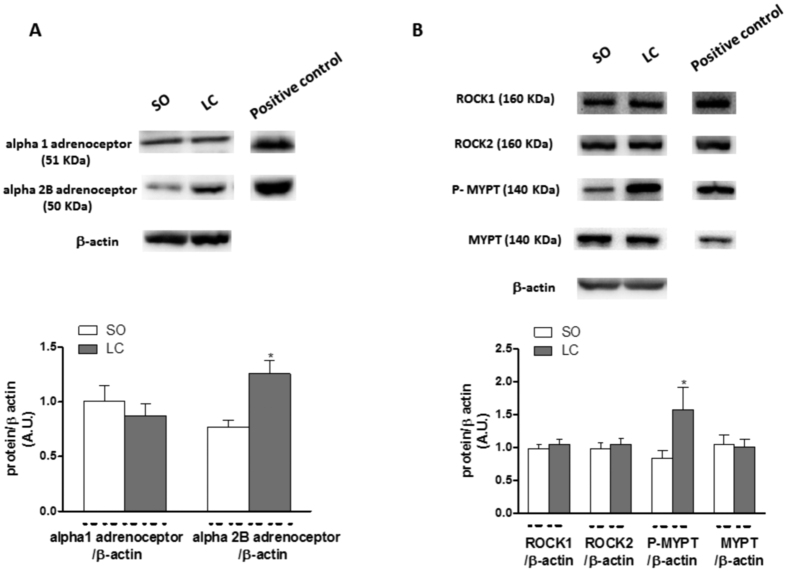
Alpha1-adrenoceptor expression was similar in segments from both groups, while alpha2B-adrenoceptor expression was greater in arteries from LC animals (Fig. 3A).
Figure 3. Effect of liver cholestasis on alpha adrenoceptor signalling transduction pathway.
(A) Effect of liver cholestasis on alpha 1- adrenoceptor and alpha 2B- adrenoceptor expression. The blot is representative of six separate segments from each group. Rat brain homogenates were used as a positive control. Lower panel shows relation between alpha 1-adrenoceptor or alpha 2B-adrenoceptor expression and β-actin. Results (mean ± SEM) are expressed as a ratio of the signal obtained for each protein and the signal obtained for β-actin. *P < 0.05 SO vs. LC rats (Student’s t-test). (B) Effect of liver cholestasis on ROCK1, ROCK2, P-MYPT and MYPT expression. The blot is representative of six separate segments from each group. HeLa cell lysates were used as a positive control. Lower panel shows relation between ROCK1, ROCK2, P-MYPT or MYPT expression and β-actin. Results (mean ± SEM) are expressed as a ratio of the signal obtained for each protein and the signal obtained for β-actin. *P < 0.05 SO vs. LC rats (Student’s t-test).
Both ROCK1 and ROCK2 expressions were similar in arteries from the two rat groups, as well as MYPT expression, while the phosphorylation of MYPT in Thr 696 was higher in arteries from LC rats (Fig. 3B).
When analysing the remnant phentolamine-resistant contractile response, we observed that it was greater in mesenteric segments from LC animals (Fig. 4A). Preincubation with non-specific P2 purinergic receptor antagonist suramin (0.1 mmol/L) decreased EFS-induced vasoconstrictor response in segments from both groups, but more markedly in arteries from LC animals (Fig. 4B–D). The combined preincubation with phentolamine plus suramin practically abolished EFS-induced vasoconstriction in segments from both experimental groups (Table 2). Related to these results, the EFS-induced ATP release was greater in mesenteric segments from LC animals (Table 3).
Figure 4. Effect of liver cholestasis on ATP function.
(A) Remnant EFS-induced vasoconstriction after preincubation with phentolamine. Effect of preincubation with 0.1 mmol/L suramin on vasoconstrictor response induced by EFS in endothelium-denuded mesenteric segments from Sham-operated (SO, B) and liver cholestasis (LC, C) rats. Results (mean ± S.E.M.) were expressed as a percentage of the initial contraction elicited by KCl. n = 6-8 animals per group. *P < 0.05 (Fisher post hoc test) (D) Differences of area under the curve (dAUC) in the absence or presence of 0.1 mmol/L suramin. *P < 0.05 SO vs. LC rats (Student’s t-test). dAUC values are expressed as arbitrary units.
Participation of the nitrergic component in vascular responses to EFS
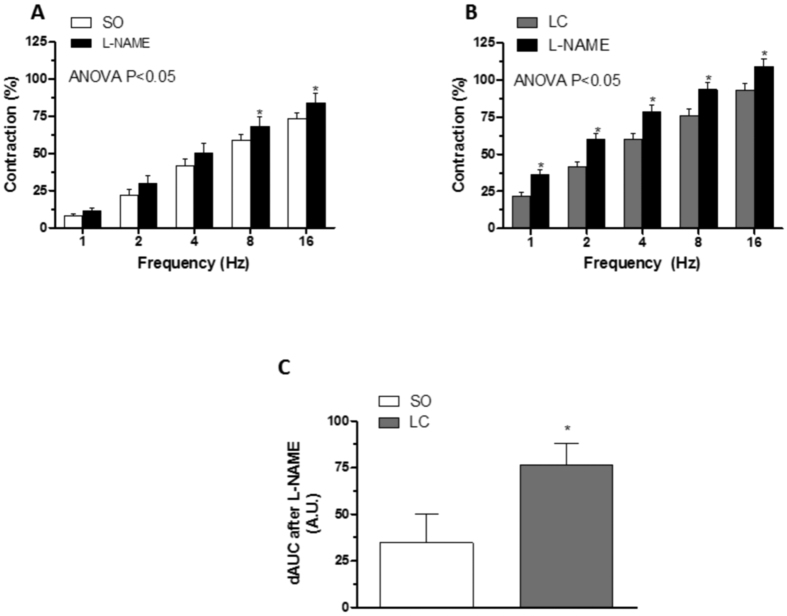
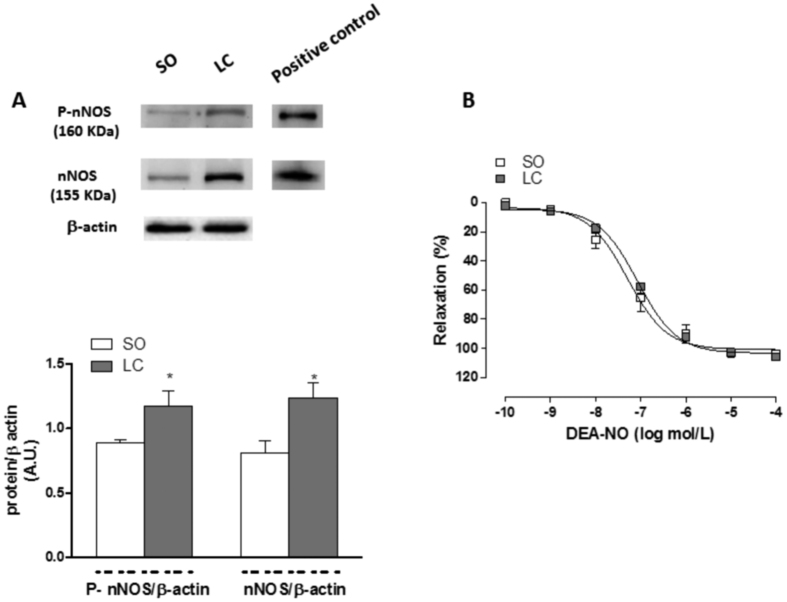
Preincubation with unspecific NOS inhibitor L-NAME (0.1 mmol/L) significantly increased the EFS-contractile response in endothelium-denuded segments from both groups (Fig. 5A,B). This increase was greater in segments from LC animals (Fig. 5C). EFS induced nitrite release in segments from both groups. This release was higher in LC mesenteric segments (Table 3). Both the expression and the phosphorylation of nNOS in Ser1417 were increased in mesenteric homogenates from LC animals compared to expression in homogenates from the SO group (Fig. 6A). LC did not alter the vasodilator response to DEA-NO (NA pre-contraction: SO: 1033 ± 74.1 mg; LC: 1046 ± 65.4 mg; P > 0.05) (Fig. 6B).
Figure 5. Effect of liver cholestasis on nitrergic innervation function.
Effect of preincubation with 0.1 mmol/L L-NAME on the vasoconstrictor response induced by EFS in mesenteric segments from Sham-operated (SO, A) and liver cholestasis (LC, B) rats. Results (mean ± S.E.M.) are expressed as a percentage of the previous contraction elicited by KCl. n = 6-8 animals per group *P < 0.05 (Fisher post hoc test). (C) Differences of area under the curve (dAUC) in the absence or presence of 0.1 mmol/L L-NAME; dAUC values are expressed as arbitrary units.*P < 0.05 SO vs. LC rats (Student’s t-test).
Figure 6. Effect of liver cholestasis on neuronal nitric oxide synthase expression and activation, and on DEA-NO-induced vasodilation.
(A) Effect of liver cholestasis on nNOS and P-nNOS expression. The blot is representative of six separate segments from each group. Rat brain homogenates were used as a positive control. Lower panel shows relation between nNOS or P-nNOS expression and β-actin. Results (mean ± SEM) are expressed as a ratio of the signal obtained for each protein and the signal obtained for β-actin. *P < 0.05 SO vs. LC rats (Student’s t-test). (B) Vasodilator response to NO donor DEA-NO in NA-precontracted mesenteric segments from Sham-operated (SO) and liver cholestasis (LC) rats. Results (mean ± S.E.M.) are expressed as a percentage of the previous tone elicited by exogenous NA. n = 7 animals per group.
Participation of the sensory component in vascular responses to EFS
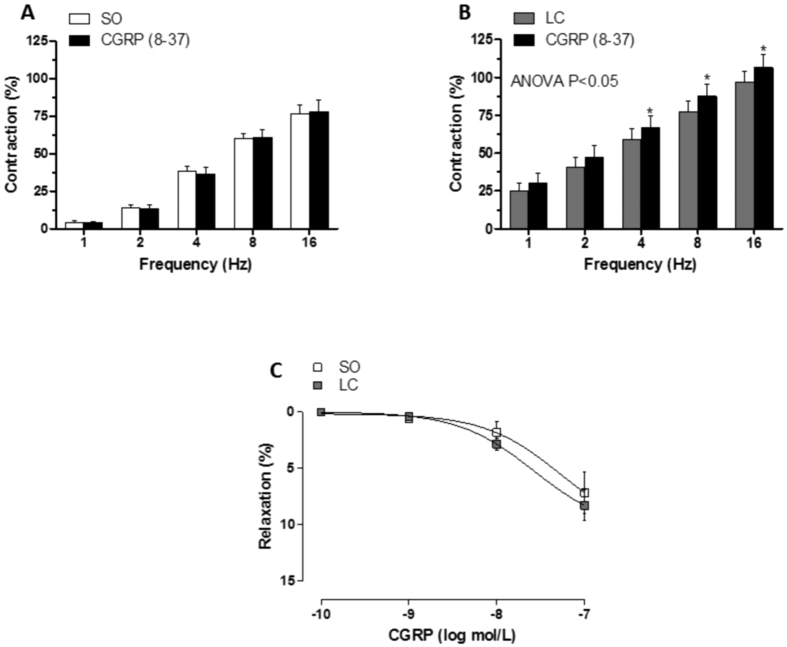
Preincubation with the CGRP receptor antagonist CGRP (8–37) (0.5 μmol/L) increased the EFS-induced contraction only in segments from LC animals (Fig. 7A,B). Vasodilator response to CGRP was similar in mesenteric segments from both experimental groups (NA precontraction: SO: 1095 ± 98.7 mg; LC: 1123 ± 62.1 mg; P > 0.05) (Fig. 7C). EFS-induced CGRP release was greater in segments from LC animals (Table 3).
Figure 7. Effect of liver cholestasis on sensory innervation function.
Effect of preincubation with 0.5 μmol/L CGRP (8-37) on the vasoconstrictor response induced by EFS in mesenteric segments from Sham-operated (SO, A) and liver cholestasis (LC, B) rats. Results (mean ± S.E.M.) are expressed as a percentage of the previous contraction elicited by KCl. n = 8 animals per group. *P < 0.05 (Fisher post hoc test) (B) Vasodilator response to exogenous CGRP in NA-precontracted mesenteric segments from SO and LC rats. Results (mean ± S.E.M.) are expressed as a percentage of the previous tone elicited by exogenous NA. n = 6 animals per group.
Discussion
The present study in an experimental model of obstructive LC shows an increase in EFS-induced vasoconstriction in superior mesenteric artery that is the net effect of 1) increased sensitivity to NA through enhanced alpha 2B adrenoceptor expression and ROCK activity, and augmented ATP release, and 2) elevated neuronal NO and CGRP releases from the nitrergic and sensory innervations, respectively.
As we have previously described in this experimental model, LC animals develop liver fibrosis and hepatomegaly, together with a mean arterial pressure decrease, PH, enlarged spleen and collateral portosystemic circulation, as well as hepatomegaly, liver encephalopathy and ascites38,39,40; these alterations make this an appropriate experimental model for studying alterations associated to decompensated liver cirrhosis.
The maintenance and worsening of PH during the progression of cirrhosis is due to the development of two synergistic mechanisms: increased intrahepatic vascular resistance and increased splanchnic blood flow. The characteristic decrease in splanchnic bed vascular resistance observed in liver cirrhosis is associated to modifications in both endothelial and/or neuronal function41. Focussing on the latter, the decreases in EFS-induced contraction reported in mesenteric segments from rats subjected to different mild liver cirrhosis models have been attributed to altered sympathetic, nitrergic and sensory function26,27,28,32,42. However, in this model of decompensated LC we have observed an augmented EFS-induced contractile response in endothelium-intact mesenteric segments. A possible explanation for the observed differences is that LC alters the intrinsic vascular contractile machinery. However we observed that the vasoconstrictor response after exposition to a depolarizing solution of KCl was not different in segments from the two experimental groups, which ruled out this possibility and contrasted to previous reports showing hypo reactivity to KCl in cirrhotic animals43.
Several groups have described time-dependent alterations in endothelial function in PH and liver cirrhosis12,13,14,15. Endothelium-derived factors are known to affect the response to several vasomotor substances, including neurotransmitters44,45,46,47. To determine a possible different influence by endothelium in decompensated cirrhosis, we compared EFS-induced vasoconstriction in endothelium-intact and endothelium-denuded segments. Endothelium removal increased vasoconstrictor response to EFS similarly in both study groups, indicating that the role of endothelium in modulating the EFS vasoconstrictor response is similar in both control and LC animals and suggesting that the differences in EFS-induced vasoconstriction would be due to neuronal alterations. This was confirmed by the fact that the nerve impulse blocker TTX abolished EFS-induced vasoconstriction in segments from both SO and LC animals. Taken together, these data indicate that the increased contractile response observed in LC segments is due to modifications in perivascular innervation function and produced by an endothelium-independent mechanism. We therefore performed the following experiments in endothelium-denuded mesenteric segments.
Several studies have shown that cirrhosis produces alterations in perivascular innervation components. Regarding sympathetic function, both increases and decreases in sympathetic discharge have been described in cirrhosis30,42,48. Thus, our next objective was to analyse the possible participation of sympathetic innervation in the increased vasoconstrictor response observed in LC rats. After incubating segments with the alpha-adrenoceptor antagonist phentolamine, we observed that the vasoconstrictor response elicited by EFS was significantly reduced in segments from both SO and LC animals. This decrease was higher in LC rats, suggesting a different participation by the adrenergic component in the two experimental groups. This effect could be associated to either an alteration in NA release and/or NA-induced vasoconstriction. Although increases and decreases in NA release have both been reported in cirrhosis27,29,48, in our experimental conditions we observed no differences in EFS-induced NA release. Regarding vasoconstrictor response to exogenous NA, previous reports showed both hypo- and hyper-reactivity to NA in mesenteric vessels from cirrhotic animals12,26,49,50, the difference depending on the time after cirrhosis onset. In the present study, we found increased NA-induced vasoconstriction in segments from LC animals. This vasoconstrictor response is largely mediated by activation of post-synaptic alpha1 and alpha 2B adrenoceptors in this vascular bed51,52. We found no alterations in alpha 1 adrenoceptor expression, as previously described53, while alpha 2B expression was increased in mesenteric segments from LC animals. Among the components of the signal transduction pathway activated by alpha adrenoceptors, ROCK plays a relevant role in inducing vasoconstriction. Hyperactivation of the ROCK pathway has been reported to enhance phosphorylation and subsequent inactivation of MYPT and this leads to smooth muscle cell contraction54. In decompensated LC, we observed no differences in ROCK1 and ROCK2 isoform expression. Nevertheless, ROCK activity was greater in LC animals, since we observed an increase in the phosphorylation of MYPT. This result indicates that ROCK pathway hyperactivity is one of the possible underlying mechanisms responsible for increased NA-induced constriction and, consequently, for the augmented adrenergic function observed in decompensated LC. This result contrasts with observations in mild liver cirrhosis, where the characteristic splanchnic hypocontractility to NA is produced by a downregulated and defective ROCK activation55, and reinforces the idea of a rearrangement in the participation of the different vasoactive factors over time.
Sympathetic vasomotor reflexes have been described as involving simultaneous NA and ATP release, both acting on vascular smooth muscle cells56,57,58. The importance of ATP as a functional sympathetic neurotransmitter in blood vessels depends on the species, the vascular bed, the type of blood vessel and the pathology analysed59. In this sense, changes in purinergic cardiovascular signalling have been described in liver pathologies60,61. We have observed a substantial phentolamine-resistant contractile response, greater in mesenteric segments from LC rats, which was abolished after adding the P2-purinoceptor antagonist suramin. What is more, suramin alone also decreased EFS-induced vasoconstriction in both rat groups, but to a greater extent in arteries from LC animals. Both results indicate a greater participation by the sympathetic co-transmitter ATP in LC rats. Purinergic contribution has been reported to depend on previous vascular tone, and is higher when tone is increased37,57,59,62,63,64. Taking this into account, the increased vasoconstrictor response to EFS in LC rats could be responsible for the enhanced ATP release observed in these animals. We have demonstrated that the alterations in NA and ATP releases in this artery not only appear in the same way, as previously described57,58, but also in the opposite sense, or even one changing and other remaining unaltered37,62,64, indicating a complex interaction between the two neurotransmitters that would depend on the pathophysiological situation. These data show that greater NA-induced vasoconstriction, through augmented alpha 2B adrenoceptor expression and enhanced ROCK activity, and increased ATP release contribute to the increased sympathetic participation and, consequently, to the enhanced EFS-induced vasoconstriction in LC animals.
The pivotal role played by endothelial NO in the high splanchnic vasodilation observed in PH and liver cirrhosis has been widely demonstrated7,65, and it either increases vasodilation by itself, or it leads to a hypo responsiveness to constrictor factors such as NA14. Regarding neuronal NO, earlier reports have shown increased levels in mesenteric arteries and veins in both PH and compensated cirrhosis, and that this participation decreases through time21,66. To evaluate possible alterations in nitrergic innervation in LC, segments from both experimental groups were incubated with non-specific NOS inhibitor L-NAME. We observed increased EFS-induced constrictor response in segments from both groups, but the response was stronger in arteries from LC animals, indicating a greater role by nitrergic innervation. This increase in nitrergic participation can be due to an alteration in the synthesis of and/or the vasodilator response to nitrergic neurotransmitter NO. The analysis of EFS-induced nitrites, the stable end metabolic product of NO, showed an increase in segments from LC animals, which agrees with the augmented nNOS expression and phosphorylation that we observed. The vasodilator response to the NO donor DEA-NO was similar in both SO and LC rats, contrasting with the increase observed in other cirrhosis models67,68. Together, these observations indicate that the nitrergic innervation participation in segments from LC rats is enhanced through increased NO release. The great amount of interactions described between NA, ATP and NO highlight the complexity of the relationship between these vasoactive factors. Thus, the simultaneous increase in ATP and NO releases observed in this artery in this and other studies37,65 agrees with previous observations in endothelium and central nervous system69,70,71. However, a direct relationship between both neurotransmitters does not always appear, since we have also observed increased ATP together with decreased NO release62. Additionally, we have previously reported that modifications in NO release are independent of alterations in NA vasoconstriction37,63,64,72. Once again, these results seem to confirm that the possible interaction between the different components of perivascular innervation depends on the pathophysiological situation under study, and highlight the need for studying innervation function in different PH and cirrhosis models.
A recent review reports that, despite the wide distribution of sensory innervation in different vascular beds, sensory participation is only revealed in adverse cardiovascular events, and acts as a compensating and/or protector mechanism73, in agreement with previous reports from our groups21,32,72. Here we observed that preincubation with the CGRP antagonist CGRP (8-37) did not modify the vasoconstrictor response to EFS in mesenteric rings from SO rats, although it increased it in segments from LC rats. These results indicate a role for sensory innervation in EFS-induced contraction only in decompensated cirrhosis, as previously observed in the compensated phase of this pathology32. Our next objective was to determine whether this participation was associated with an increase in CGRP release and/or vasodilation. EFS-induced CGRP release was higher in mesenteric segments from LC rats. Additionally, exogenous CGRP produced no differences in the vasodilation in segments from either rat group, ruling out alterations in sensitivity to CGRP. This result contrasts with previous observations in compensated liver cirrhosis, where an increased CGRP vasodilator response was observed in this artery32, once more reinforcing the idea that the mechanisms implicated in the alterations of splanchnic hyperdynamic circulation depend on the time since onset and on the severity of the pathology, and that CGRP only has a relevant role in pathological situations.
Among other causes, vascular complications associated to PH and liver cirrhosis are due to inflammatory mechanisms38, for instance, cyclooxygenase (COX) is overexpressed in smooth muscle cells from different vascular beds74,75. We have previously described that the COX derivates TXA2 and PGI2 play a role in modulating sympathetic and nitrergic function in this artery. In this sense, an increase in COX activity and subsequently TXA2 expression leads to augmented NA vasoconstriction76. Additionally, over-release of endogenous PGI2 enhances neuronal NO release77. Thus, it is possible that COX derivatives could modulate the innervation function in decompensated LC.
Altogether, the results of the present study demonstrate that, in superior mesenteric arteries, decompensated LC produces a) increased vasoconstrictor response to NA, due to enhanced alpha 2B adrenoceptor expression and ROCK activity; b) augmented ATP release; c) raised release of the nitrergic neurotransmitter NO; and d) enhanced release of the sensory neurotransmitter CGRP. The net effect of these alterations is an increase in the EFS-induced vasoconstrictor response and this might be a counteracting mechanism to the increased splanchnic vasodilation observed in the last phases of decompensated liver cirrhosis.
Additional Information
How to cite this article: Sastre, E. et al. Decompensated liver cirrhosis and neural regulation of mesenteric vascular tone in rats: role of sympathetic, nitrergic and sensory innervations. Sci. Rep. 6, 31076; doi: 10.1038/srep31076 (2016).
Acknowledgments
This study was supported by Ministerio de Economía y Competitividad (SAF2012-38530) and by Instituto de Salud Carlos III (ISCIII)-Fondo Europeo de Desarrollo Regional (FEDER) (RD12/0042/0024). E. Sastre received a FPI-UAM fellowship.
Footnotes
Author Contributions E.S., L.C., G.B. and J.B.-R. conceived and designed the experiments. I.P., M.A.A. and J.A. performed the surgery. E.S., L.C., P.L. and J.B.-R. performed the experiments. E.S., L.C., P.L. and J.B.-R. analysed the data. I.P., M.A.A., J.A., G.B. and J.B.-R. contributed reagents/materials/analysis tools. M.A.A., J.A., G.B. and J.B.-R. wrote the manuscript. All authors revised the manuscript.
References
- Stewart S. F. & Day C. P. The management of alcoholic liver disease. J Hepatol 38 (Suppl.), 2–13 (2003). [DOI] [PubMed] [Google Scholar]
- Gracia-Sancho J. et al. Enhanced vasoconstrictor prostanoid production by sinusoidal endothelial cells increases portal perfusion pressure in cirrhotic rat livers. J Hepatol 47(2), 220–227 (2007). [DOI] [PubMed] [Google Scholar]
- Gracia-Sancho J. et al. Increased oxidative stress in cirrhotic rat livers: A potential mechanism contributing to reduced nitric oxide bioavailability. Hepatology 47(4), 1248–1256 (2008). [DOI] [PubMed] [Google Scholar]
- Henriksen J. H. & Moller S. Hemodynamics, distribution of blood volume, and kinetics of vasoactive substances in cirrhosis. The Kidney in Liver Disease. Philadelphia, PA: Hanley & Belfus, 241–258 (1996). [Google Scholar]
- Schrier R. W. et al. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology 8, 1151–1157 (1988). [DOI] [PubMed] [Google Scholar]
- Morales-Ruiz M. et al. Increased nitric oxide synthase expression in arterial vessels of cirrhotic rats with ascites. Hepatology 24(6), 1481–1486 (1996). [DOI] [PubMed] [Google Scholar]
- Iwakiri Y. The molecules: mechanisms of arterial vasodilatation observed in the splanchnic and systemic circulation in portal hypertension. J Clin Gastroenterol. 41(10 Suppl 3), S288–S294 (1996). [DOI] [PubMed] [Google Scholar]
- Vaughan R. B., Angus J. A. & Angus P. W. Vasoconstrictor responses are normal but prostanoid-mediated vasodilatation is enhanced in human cirrhotic mesenteric arteries. J Gastroenterol Hepatol. 20(8), 1158–1164 (2005). [DOI] [PubMed] [Google Scholar]
- Battaglia S., Angus P. & Chin-Dusting J. P. Role of the endothelium on vasoactive agents in patients with liver cirrhosis. J Gastroenterol Hepatol 21(7), 1189–1193 (2006). [DOI] [PubMed] [Google Scholar]
- Van Obbergh L. et al. The endothelial and non-endothelial mechanism responsible for attenuated vasoconstriction in cirrhotic rats. Exp Physiol. 80(4), 609–617 (1995). [DOI] [PubMed] [Google Scholar]
- Ebrahimkhani M. R. et al. Opioid Receptor Blockade Improves Mesenteric Responsiveness in Biliary Cirrhosis. Dig Dis Sci 53(11), 3007–3011 (2008). [DOI] [PubMed] [Google Scholar]
- Bomzon A., Gali D., Better O. S. & Blendis L. M. Reversible suppression of the vascular contractile response in rats with obstructive jaundice. J Lab Clin Med 105(5), 568–572 (1985). [PubMed] [Google Scholar]
- Sitzmann J. V., Campbell K., Wu Y. & St Clair C. Prostacyclin production in short-term, chronic, and long-term experimental portal hypertension. Surgery 115, 290–294 (1994). [PubMed] [Google Scholar]
- Michielsen P. P., Boeckxstaens G. E., Sys S. U., Herman A. G. & Pelckmans P. A. The role of increased nitric oxide in the vascular hyporeactivity to noradrenaline in long-term portal vein ligated rats. J.Hepatol. 23, 341–347 (1995). [PubMed] [Google Scholar]
- Blanco-Rivero J., Aller M. A., Arias J., Ferrer M. & Balfagón G. Long-term portal hypertension increases the vasodilator response to acetylcholine in rat aorta: role of prostaglandin I2. Clin Sci (Lond). 117(10), 365–374 (2009). [DOI] [PubMed] [Google Scholar]
- Li Y. J. & Duckles S. P. Effect of endothelium on the actions of sympathetic and sensory nerves in the perfused rat mesentery. Eur. J. Pharmacol. 210, 23–40 (1992). [DOI] [PubMed] [Google Scholar]
- Yu X. J., Li Y. J. & Deng H. W. The regulatory effect of bradykinin on the actions of sensory nerves in the perfused rat mesentery is mediated by nitric oxide, Eur. J. Pharmacol. 241, 35–40 (1993). [DOI] [PubMed] [Google Scholar]
- Marín J. & Balfagón G. Effect of clenbuterol on non-endothelial nitric oxide release in rat mesenteric arteries and the involvement of β-adrenoceptors. Br. J. Pharmacol. 124(3), 473–478 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki H. et al. Calcitonin gene related peptide acts as a novel vasodilator neurotransmitter in mesenteric resistance vessels of the rat, Nature 335: 164–167 (1988). [DOI] [PubMed] [Google Scholar]
- Ferrer M., Sánchez M., Minoves N., Salaices M. & Balfagón G. et al. Aging increases neuronal nitric oxide release and O2.- generation in mesenteric arteries from spontaneously hypertensive rats. J Vasc Res 40(6), 509–519 (2003). [DOI] [PubMed] [Google Scholar]
- Sastre E. et al. Effect of short- and long-term portal hypertension on adrenergic, nitrergic and sensory functioning in rat mesenteric artery. Clin Sci (Lond) 122(7), 337–348 (2012). [DOI] [PubMed] [Google Scholar]
- Marín J. Ferrer M. & Balfagon G. Role of protein kinase C in electrical-stimulation-induced neuronal nitric oxide release in mesenteric arteries from hypertensive rats. Clin Sci (Lond). 99(4), 277–283 (2000). [PubMed] [Google Scholar]
- Ferrer M., Alonso M. J., Salaices M., MArín J. & Balfagón G. Angiotensin II increases neurogenic nitric oxide metabolism in mesenteric arteries from hypertensive rats, Life Sci. 68, 1169–1179 (2001). [DOI] [PubMed] [Google Scholar]
- Ferrer M., Marín J. & Balfagon G. Diabetes alters neuronal nitric oxide release from rat mesenteric arteries. Role of protein kinase C. Life Sci. 66(4), 337–345 (2000). [DOI] [PubMed] [Google Scholar]
- Sastre E. et al. Biphasic Effect of Diabetes on Neuronal Nitric Oxide Release in Rat Mesenteric Arteries. PLoS One. 11(6), e0156793 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob G., Said O., Finberg J. & Bomzon A. Peripheral vascular neuroeffector mechanisms in experimental cholestasis. Am J Physiol. 265(3 Pt 1), G579–G586 (1993). [DOI] [PubMed] [Google Scholar]
- Kirstetter P. et al. Vascular properties of isoflurane: comparison between normal and cirrhotic rats. Br J Anaesth 81(6), 968–969 (1998). [DOI] [PubMed] [Google Scholar]
- Coll M. et al. Down-regulation of genes related to the adrenergic system may contribute to splanchnic vasodilation in rat portal hypertension. J Hepatol 49, 43–51 (2008). [DOI] [PubMed] [Google Scholar]
- Gatta A., Bolognesi M. & Merkel C. Vasoactive factors and hemodynamic mechanisms in the pathophysiology of portal hypertension in cirrhosis. Mol Aspects Med. 29(1–2), 119–129 (2008). [DOI] [PubMed] [Google Scholar]
- Coll M. et al. Atrophy of mesenteric sympathetic innervation may contribute to splanchnic vasodilation in rat portal hypertension. Liver Int 30, 593–602 (2010). [DOI] [PubMed] [Google Scholar]
- Xu L. et al. Neuronal nitric oxide synthase and systemic vasodilation in rats with cirrhosis. Am J Physiol Renal Physiol. 279(6), F1110–F1115 (2000). [DOI] [PubMed] [Google Scholar]
- Blanco-Rivero J. et al. Cirrhosis decreases vasoconstrictor response to electrical field stimulation in rat mesenteric artery: role of calcitonin gene-related peptide. Exp Physiol. 96(3), 275–286 (2011). [DOI] [PubMed] [Google Scholar]
- Aller M. A., Lorente L., Alonso S. & Arias J. A model of cholestasis in the rat, using a microsurgical technique. Scand J Gastroenterol 28(1), 10–14 (1993). [DOI] [PubMed] [Google Scholar]
- Aller M. A. et al. Comparative study of macro- and microsurgical extrahepatic cholestasis in the rat. Microsurgery 24(6), 442–447 (2004). [DOI] [PubMed] [Google Scholar]
- Kravetz D., Sikuler E. & Groszmann R. J. Splanchnic and systemic hemodynamics in portal hypertensive rats during hemorrhage and blood volume restitution. Gastroenterology 90(5 Pt 1), 1232–1240 (1986). [DOI] [PubMed] [Google Scholar]
- Nielsen K. C. & Owman C. Contractile response and amine receptor mechanisms in isolated middle cerebral artery of the cat. Brain Res. 27(1), 33–42 (1971). [DOI] [PubMed] [Google Scholar]
- Blanco-Rivero J. et al. Rosuvastatin restored adrenergic and nitrergic function in mesenteric arteries from obese rats. Br J Pharmacol 162(1), 271–285 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aller M. A. et al. Experimental obstructive cholestasis: the wound-like inflammatory liver response. Fibrogenesis Tissue Repair. 1(1), 6, 10.1186/1755-1536-1-6 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Patán F. et al. Biliary fibrosis in microsurgical extrahepatic cholestasis in the rat. Microsurgery. 28(5), 361–366 (2008). [DOI] [PubMed] [Google Scholar]
- Aller M. A. et al. A half century (1961-2011) of applying microsurgery to experimental liver research. World J Hepatol 4(7), 199–208 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwakiri Y. Pathophysiology of portal hypertension. Clin Liver Dis. 18(2), 281–291 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevic V., Mathie R. T., Moore K. P. & Burnstock G. Vasoconstrictor responsiveness of the rat mesenteric arterial bed in cirrhosis. Br J Pharmacol 118(2), 435–441 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. Y. et al. Role of Ca2+-dependent potassium channels in in vitro anandamide-mediated mesenteric vasorelaxation in rats with biliary cirrhosis. Liver Int 27(8), 1045–1055 (2007). [DOI] [PubMed] [Google Scholar]
- Thorin E. & Atkinson J. Modulation by the endothelium of sympathetic vasoconstriction in an in vitro preparation of the rat tail artery. Br J Pharmacol 111(1), 351–357 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolo L. L., Westfall T. C. & Macarthur H. Modulation of neurotransmitter release by NO is altered in mesenteric arterial bed of spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 287(4), H1842–H1847 (2004). [DOI] [PubMed] [Google Scholar]
- Kolo L. L., Westfall T. C. & Macarthur H. Nitric oxide decreases the biological activity of norepinephrine resulting in altered vascular tone in the rat mesenteric arterial bed. Am J Physiol Heart Circ Physiol 286(1), H296–H303 (2004). [DOI] [PubMed] [Google Scholar]
- Sousa J. B., Fresco P. & Diniz C. Endothelial dysfunction impairs vascular neurotransmission in tail arteries. Neurochem Int. 80, 7–13 (2015). [DOI] [PubMed] [Google Scholar]
- Henriksen J. H. et al. Splanchnic and renal elimination and release of catecholamines in cirrhosis. Evidence of enhanced sympathetic nervous activity in patients with decompensated cirrhosis. Gut. 25(10), 1034–1043 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W., Benjamin I. S., Moore K., Portmann B. & Alexander B. The action of nitric oxide on hepatic haemodynamics during secondary biliary cirrhosis in the rat. Eur J Pharmacol. 7, 461(1), 41–48 (2003). [DOI] [PubMed] [Google Scholar]
- Chen W. et al. Desensitization of G-protein-coupled receptors induces vascular hypocontractility in response to norepinephrine in the mesenteric arteries of cirrhotic patients and rats. Hepatobiliary Pancreat Dis Int. 12(3), 295–304 (2013). [DOI] [PubMed] [Google Scholar]
- Docherty J. R. Subtypes of functional alpha1- and alpha2-adrenoceptors. Eur J Pharmacol. 361(1), 1–15 (1998). [DOI] [PubMed] [Google Scholar]
- Civantos-Calzada B. & Aleixandre de Artiñano A. Alpha-adrenoceptor subtypes. Pharmacol Res. 44(3), 195–208 (2001). [DOI] [PubMed] [Google Scholar]
- Chen W., Liu D. J., Huo Y. M., Wu Z. Y. & Sun Y. W. Reactive oxygen species are involved in regulating hypocontractility of mesenteric artery to norepinephrine in cirrhotic rats with portal hypertension. Int J Biol Sci. 10(4), 386–395 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo A. P. & Somlyo A. V. Ca2+ sensitivity of smooth muscle and non-muscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 83, 1325–1358 (2003). [DOI] [PubMed] [Google Scholar]
- Hennenberg M. et al. Defective RhoA/Rho-kinase signaling contributes to vascular hypocontractility and vasodilation in cirrhotic rats. Gastroenterology 130, 838e54 (2006). [DOI] [PubMed] [Google Scholar]
- Racchi H. et al. Adenosine 5′-triphosphate and neuropeptide Y are co-transmitters in conjunction with noradrenaline in the human saphenous vein. Br J Pharmacol. 126(5), 1175–1185 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goonetilleke L., Ralevic V. & Dunn W. R. Influence of pressure on adenosine triphosphate function as a sympathetic neurotransmitter in small mesenteric arteries from the spontaneously hypertensive rat. J Hypertens. 31(2), 312–320 (2013). [DOI] [PubMed] [Google Scholar]
- Sousa J. B. et al. Lack of endogenous adenosine tonus on sympathetic neurotransmission in spontaneously hypertensive rat mesenteric artery. PLoS One. 9(8), e105540 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatarat A., Dunn W. R. & Ralevic V. Raised tone reveals ATP as a sympathetic neurotransmitter in the porcine mesenteric arterial bed. Purinergic Signal. 10(4), 639–649 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp R. et al. Evaluation of the mitochondrial respiration of cardiac myocytes in rats submitted to mechanical bile duct obstruction. Acta Cir Bras. 23 Suppl 1, 66–71 (2008). [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purinergic signalling in the gastrointestinal tract and related organs in health and disease. Purinergic Signal. 10(1), 3–50 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Rivero J., Sastre E., Caracuel L., Granado M. & Balfagón G. Breast feeding increases vasoconstriction induced by electrical field stimulation in rat mesenteric artery. Role of neuronal nitric oxide and ATP. PLoS One 8(1), e53802 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastre E., Blanco-Rivero J., Caracuel L., Callejo M. & Balfagón G. Alterations in perivascular sympathetic and nitrergic innervation function induced by late pregnancy in rat mesenteric arteries. PLoS One 10(5), e0126017 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastre E., Caracuel L., Balfagón G. & Blanco-Rivero J. Aerobic exercise training increases nitrergic innervation function and decreases sympathetic innervation function in mesenteric artery from rats fed a high-fat diet. J Hypertens 33(9), 1819–1830 (2015). [DOI] [PubMed] [Google Scholar]
- Wiest R. Splanchnic and systemic vasodilation: the experimental models. J Clin Gastroenterol. 41 Suppl 3, S272–S287 (2007). [DOI] [PubMed] [Google Scholar]
- Li Y., Liu H., Gaskari S. A., Tyberg J. V. & Lee S. S. Altered mesenteric venous capacitance and volume pooling in cirrhotic rats are mediated by nitric oxide. Am J Physiol Gastrointest Liver Physiol. 295(2), G252–G259 (2008). [DOI] [PubMed] [Google Scholar]
- Mathie R. T. et al. Mesenteric vasodilator responses in cirrhotic rats: a role for nitric oxide? Hepatology 23(1), 130–136 (1996). [DOI] [PubMed] [Google Scholar]
- Safka V. et al. Vascular hyporesponsiveness to vasodilators in rats with cirrhosis. J Hepatol. 26(2), 382–386 (1997). [DOI] [PubMed] [Google Scholar]
- Boric M. P. et al. Rise in endothelium-derived NO after stimulation of rat perivascular sympathetic mesenteric nerves. Am J Physiol 277(3 Pt 2), H1027–H1035 (1999). [DOI] [PubMed] [Google Scholar]
- Buvinic S., Briones R. & Huidobro-Toro J. P. P2Y(1) and P2Y(2) receptors are coupled to the NO/cGMP pathway to vasodilate the rat arterial mesenteric bed. Br J Pharmacol 136, 847–856 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codocedo J. F., Godoy J. A., Poblete M. I., Inestrosa N. C. & Huidobro-Toro J. P. ATP induces NO production in hippocampal neurons by P2X(7) receptor activation independent of glutamate signaling. PLoS One 8, e57626 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Queiroz D. B. et al. Alterations in perivascular innervation function in mesenteric arteries from offspring of diabetic rats. Br J Pharmacol. 172(19), 4699–4713 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell F. A., King R., Smillie S. J., Kodji X. & Brain S. D. Calcitonin gene-related peptide: physiology and pathophysiology. Physiol Rev 94(4), 1099–1142 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier F. E., Blanco-Rivero J., Sastre E., Badimón L. & Balfagón G. Simultaneous inhibition of TXA(2) and PGI(2) synthesis increases NO release in mesenteric resistance arteries from cirrhotic rats. Clin Sci (Lond). 119(7), 283–292 (2010). [DOI] [PubMed] [Google Scholar]
- Liu D. J. et al. Prostacyclin decreases splanchnic vascular contractility in cirrhotic rats. Hepatobiliary Pancreat Dis Int. 13(4), 416–422 (2014). [DOI] [PubMed] [Google Scholar]
- Aras-López R. et al. Dexamethasone decreases contraction to electrical field stimulation in mesenteric arteries from spontaneously hypertensive rats through decreases in thromboxane A2 release. J Pharmacol Exp Ther. 322(3), 1129–1136 (2007). [DOI] [PubMed] [Google Scholar]
- Ferrer M., Salaices M. & Balfagón G. Endogenous prostacyclin increases neuronal nitric oxide release in mesenteric artery from spontaneously hypertensive rats. Eur J Pharmacol. 506(2), 151–156 (2004). [DOI] [PubMed] [Google Scholar]