Abstract
Hyperglycemia, hyperlipidemia, and insulin resistance are hallmarks of obesity-induced type 2 diabetes, which is often caused by a high-fat diet (HFD). However, the molecular mechanisms underlying HFD-induced insulin resistance have not been elucidated in detail. In this study, we established a Drosophila model to investigate the molecular mechanisms of HFD-induced diabetes. HFD model flies recapitulate mammalian diabetic phenotypes including elevated triglyceride and circulating glucose levels, as well as insulin resistance. Expression of glass bottom boat (gbb), a Drosophila homolog of mammalian transforming growth factor-β (TGF-β), is elevated under HFD conditions. Furthermore, overexpression of gbb in the fat body produced obese and insulin-resistant phenotypes similar to those of HFD-fed flies, whereas inhibition of Gbb signaling significantly ameliorated HFD-induced metabolic phenotypes. We also discovered that tribbles, a negative regulator of AKT, is a target gene of Gbb signaling in the fat body. Overexpression of tribbles in flies in the fat body phenocopied the metabolic defects associated with HFD conditions or Gbb overexpression, whereas tribbles knockdown rescued these metabolic phenotypes. These results indicate that HFD-induced TGF-β/Gbb signaling provokes insulin resistance by increasing tribbles expression.
Obesity, defined as an excessive accumulation of lipid in fat tissue, is associated with an elevated risk of developing insulin resistance and metabolic abnormalities, including diabetes and cardiovascular disease1. Adipose tissue is not only the primary site for storage of excess nutrients, but also functions as an endocrine organ by secreting numerous cytokines, growth factors, and hormones that regulate metabolic homeostasis2,3. Obese individuals have high circulating levels of adipokines, adipose tissue–derived cytokines that contribute to the development of metabolic dysfunctions and inflammatory responses4,5,6. The adipokine transforming growth factor-β (TGF-β) was recently identified as a critical mediator of insulin resistance in obesity-induced metabolic diseases. Circulating TGF-β levels are significantly elevated in obese humans, ob/ob mice, and high-fat diet (HFD)-induced obese mice7,8. By regulating expression of its target genes, such as PGC-1α and PPAR-γ, elevated TGF-β/Smad3 signaling is associated with systemic insulin resistance and hepatic steatosis8,9. Systemic neutralization or inhibition of TGF-β in HFD-induced obese mice ameliorates these phenotypes, suggesting that TGF-β signaling makes a physiologically relevant contribution to the progression of metabolic disease10. However, the molecular mechanism underlying the link between TGF-β signaling in adipose tissue and the development of insulin resistance has not been elucidated.
The Drosophila genome contains a compact set of TGF-β signaling factors including seven ligands, four type I receptors, two type II receptors, and four Smad proteins. Therefore, Drosophila is regarded as a versatile model system for the study of TGF-β signaling11. The Drosophila protein Glass Bottom Boat (Gbb) is a TGF β family member that regulates growth, differentiation, and tissue morphogenesis12,13,14. gbb mutant larvae are transparent due to the reduction of lipid contents in the fat body, the functional counterpart of mammalian adipose and liver tissue15. Gbb signaling affects several aspects of metabolism and energy homeostasis. For example, fat-derived Gbb remotely controls the expression of neuronal Drosophila insulin–like peptide 2 (Dilp2) and regulates the transcription of genes involved in amino acid uptake, lipid hydrolysis, and the transport of these molecules in the fat body16. Because many metabolic pathways, including insulin signaling17,18,19, lipid metabolism20,21, and food intake22,23, are conserved between Drosophila and mammals, we used the Drosophila model to investigate the role of TGF-β/Gbb signaling in conditions of nutrient excess or obesity.
HFD-fed flies exhibited abnormal glucose and lipid levels and insulin resistance similar to those observed in obese mammals. The HFD-induced insulin resistance was mediated by the activity of the Gbb–tribbles pathway in the fat body. Thus, targeted inhibition of Gbb–tribbles signaling represents a new therapeutic strategy for treatment of obesity and its associated metabolic diseases.
Results
Induction of gbb expression mimics HFD phenotypes in Drosophila
Overgrowth of adipose tissue increases release of TGF-β family ligands in mammals8,24, but it remains unclear how these ligands are involved in metabolic phenotypes. To investigate the functions of TGF-β family ligands in metabolic disease, we fed adult w1118 flies a HFD containing 20% coconut oil for 14 days. As in mammals, dietary fat induces obesity and diabetic phenotypes in Drosophila. Whole-body triglyceride (TG) levels started to increase after HFD feeding: the TG level peaked 4 days after initiation of HFD and was maintained until day 14 (Figure S1A, top). The trehalose/glucose level did not change during the first 4 days, but started to increase on day 6 and reached a maximum at day 10 (Figure S1A, bottom). Expression of Dilp2 mRNA and secretion of the encoded protein were also increased by HFD feeding, peaking on day 4 and decreasing to control levels between day 6 and day 10 (Figure S1B). In flies subjected to long-term HFD, insulin-stimulated AKT phosphorylation (pAKT) in the fat body was significantly lower than in control flies (Figure S1C).
On day 14 of the HFD, we measured the expression levels of seven ligands of the TGF-β superfamily in the adult fly fat body. Of the factors we tested, only gbb expression was significantly upregulated by HFD feeding (Fig. 1A), particularly in the fat body (Figure S2A). Next, we investigated whether gbb was able to change the levels of TG in the fat body and trehalose/glucose in the hemolymph. gbb overexpression in the adult fat body (DCG > gbb) increased the levels of TG and trehalose/glucose compared with those in control flies (DCG-Gal4/ + ) (Fig. 1B). However, gbb overexpression in the gut (NP3084 > gbb) or muscle (MHC > gbb) did not change the level of TG (Figure S2B). Furthermore, the level of pAKT was significantly reduced in the fat-derived gbb (DCG > gbb) but not in the gut- or muscle-derived gbb (Figure S2C).
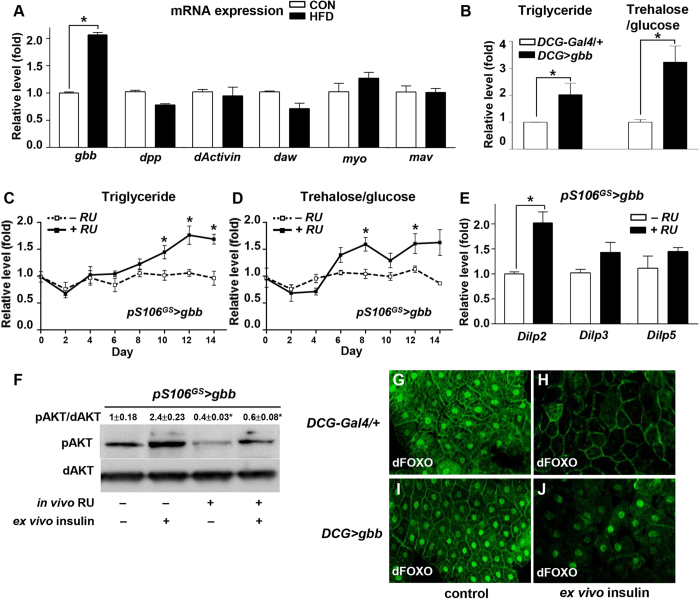
Figure 1. Induction of gbb in the fat body regulates metabolic phenotypes and insulin signaling.
(A) Expression levels of TGF-β ligands in HFD. gbb expression was increased in flies fed HFD relative to the level in flies fed a normal control diet. (B) Levels of triglyceride and trehalose/glucose in DCG > gbb were elevated relative to those in the DCG-Gal4 control. (C, D) During 14 days of HFD feeding, levels of triglyceride and trehalose/glucose in pS106GS > gbb + RU gradually increased relative to those in the −RU control. (E) pS106GS > gbb + RU increased Dilp2 expression relative to the level in the −RU control. (F) pS106GS > gbb + RU with or without insulin suppressed pAKT activation relative to that in the −RU control. (G–J) In the larval fat body, dFOXO was mainly localized in the nuclei in the DCG-Gal4 control (G) and in DCG > gbb (I). After insulin treatment of ex vivo cultured larval fat bodies, dFOXO was localized in the cytoplasm of the DCG-Gal4 control (H), but most dFOXO remained in nuclei in DCG > gbb (J). Data are presented as means ± s.e.m. from at least three independent experiments. *P < 0.05.
To determine whether induction of gbb in the adult flies mimics the effects of a HFD, we used the inducible gene switch pS106GS-Gal4 driver, which can be activated by the addition of the mifepristone/RU-486 (RU)25. pS106GS-Gal4 driver induced gbb expression only in the adult fat body (Figure S2D). Over 14 days, the levels of TG and trehalose/glucose gradually increased in flies overexpressing gbb in the adult fat body (pS106GS > gbb, + RU) relative to those in control flies (pS106GS > gbb, −RU) (Fig. 1C,D). These results demonstrate that HFD-induced obesity can activate gbb in the adult fat body, resulting in hyperlipidemia and hyperglycemia.
gbb overexpression in the adult fat body (pS106GS > gbb + RU) significantly increased expression of Dilp2, and slightly increased expression of Dilp3 and Dilp5, relative to the levels in −RU controls (Fig. 1E). To determine whether gbb regulates insulin signaling, we dissected adult fat body from pS106GS > gbb + RU or −RU flies and treated them with insulin ex vivo (Figure S2E). In the −RU controls, ex vivo insulin treatment increased the level of pAKT relative to that in untreated samples. By contrast, in samples obtained from + RU flies, which overexpressed gbb in the adult fat body, ex vivo insulin treatment did not increase the level of pAKT (Fig. 1F).
To further investigate the regulation of insulin signaling by gbb, we examined the subcellular localization of dFOXO in larval fat bodies using the DCG-Gal4 fat body–specific driver. In these experiments, dissected fat bodies were serum-starved and treated with human insulin ex vivo. In the starved DCG-Gal4 control and DCG > gbb fat bodies, dFOXO was mainly localized in the nuclei (Fig. 1G,I). Upon insulin stimulation, almost all dFOXO protein was re-localized to the cytoplasm in DCG-Gal4 control fat bodies (Fig. 1H), whereas in DCG > gbb fat bodies most dFOXO protein was still located in nuclei (Fig. 1J). These results indicate that gbb overexpression in the fat body inhibits insulin signaling, resulting in an insulin-resistant phenotype similar to that observed under long-term HFD feeding.
Inhibition of Gbb signaling rescues HFD-induced obesity and diabetic phenotypes
Next, we tested whether inhibition of Gbb signaling would rescue HFD-induced obesity and the insulin-resistant phenotype. Gbb signaling in the fat body was inhibited by gbb RNAi (DCG > gbb Ri), dominant negative (DN) form of type I receptors (DCG > sax DN, DCG > tkv DN), type II receptor (DCG > punt DN), or Gbb signaling transducer dMad RNAi (DCG > dMad Ri). The elevated TG level in the HFD DCG-Gal4/ + control was not observed in DCG > gbb Ri, DCG > sax DN, DCG > punt DN, and DCG > dMad Ri (Figure S3A). To confirm this result only in the adult fat body, we used RU-inducible gene switch system pS106GS-Gal4 and inhibited Gbb signaling by pS106GS > gbb RNAi, pS106GS > punt DN, or pS106GS > dMad RNAi. In the normal control diet (NCD) condition, inhibition of Gbb signaling did not change the levels of TG or trehalose/glucose relative to those in −RU controls. However, in the HFD condition, inhibition of Gbb signaling suppressed the elevation of TG and trehalose/glucose levels observed in the absence of RU (Fig. 2A,B). Next, we examined insulin signaling in the fat body by monitoring AKT activation. HFD feeding decreased the pAKT level in −RU controls, but this reduction in pAKT was restored when Gbb signaling was inhibited (HFD, + RU) (Fig. 2C–E). Elevated expression levels of dFOXO target genes d4E-BP and dInR in the HFD condition were also restored when Gbb signaling was inhibited (HFD, + RU) (Fig. 2F,G). Thus, inhibition of Gbb signaling in the fat body rescued HFD-induced obesity and insulin resistance. To further test the insulin-resistance phenotype under the HFD condition, we dissected fat bodies from flies in which Gbb signaling was inhibited and treated them with insulin ex vivo. The insulin-resistance phenotype (reflected by pAKT levels) in the HFD, −RU, + insulin condition was rescued in the HFD, + RU (Gbb signaling inhibited), + insulin condition (Figure S3B,C,D). These data provide further evidence that inhibition of Gbb signaling in the fat body can rescue the insulin-resistance phenotype.
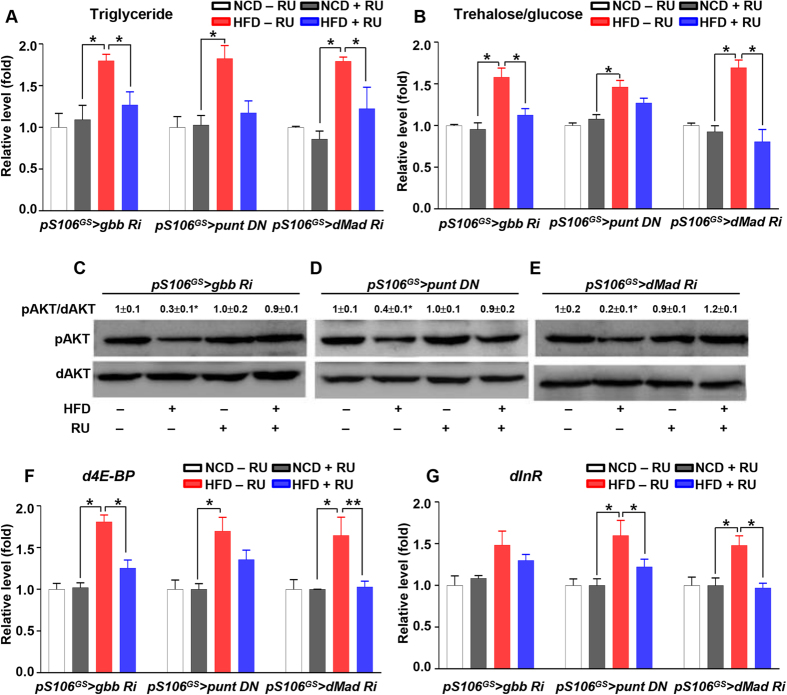
Figure 2. Inhibition of Gbb signaling rescues HFD-induced obesity and diabetic phenotype.
(A,B) In the HFD condition, pS106GS > gbb RNAi + RU, pS106GS > punt DN + RU, pS106GS > dMad RNAi + RU suppressed the elevated levels of triglyceride and trehalose/glucose observed in −RU to control levels (i.e., the levels in flies fed a normal control diet). (C–E) In the HFD condition, pS106GS > gbb RNAi + RU, pS106GS > punt DN + RU, pS106GS > dMad RNAi + RU restored the reduced pAKT levels observed in −RU to control levels. (F,G) In the HFD condition, pS106GS > gbb RNAi + RU, pS106GS > punt DN + RU pS106GS > dMad RNAi + RU suppressed the elevated expression of d4E-BP and dInR observed in −RU to control levels. Data are presented as means ± s.e.m. from at least three independent experiments. *P < 0.05.
tribbles is a downstream target of Gbb signaling
To determine how Gbb signaling negatively regulates insulin signaling, we measured the expression levels of negative regulators of insulin signaling in gbb-transfected cultured Drosophila S2 cells. Protein Tyrosine Phosphatase 61F (PTP61F) and dPten decrease the activity of dInR and phosphoinositide 3-kinase (PI3K), respectively19,26, and Tribbles-related protein (Trb) inhibits pAKT27,28. In gbb-overexpressing S2 cells, the expression level of trb, but not PTP61F and dPten, was higher than that in controls (Fig. 3A). trb expression was also elevated in fat body from gbb-overexpressing adult flies (pS106GS > gbb + RU), but reduced in the fat body of flies in which Gbb signaling was inhibited (pS106GS > gbb RNAi, pS106GS > punt DN, pS106GS > dMad RNAi + RU), relative to the expression level in −RU controls (Fig. 3B). Together, these in vitro and in vivo results demonstrate that trb is a downstream target gene of Gbb signaling.
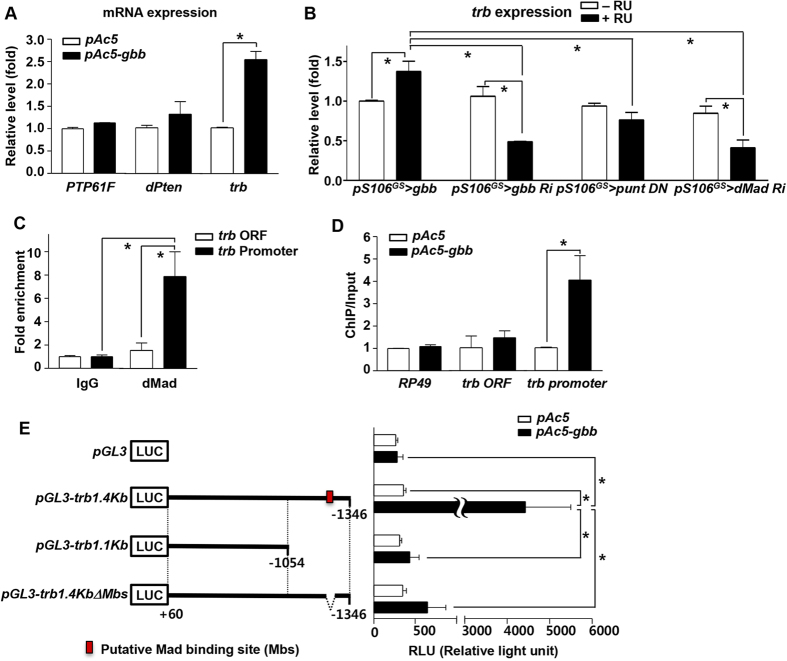
Figure 3. tribbles is a downstream target of Gbb signaling.
(A) In Drosophila S2 cells, gbb transfection induced expression of trb, not PTP61F and dPten. (B) Expression levels of trb increased in the fat bodies of pS106GS > gbb + RU flies relative to those in −RU controls, but decreased in pS106GS > gbb RNAi + RU, pS106GS > punt DN + RU, and pS106GS > dMad RNAi + RU. (C) In Drosophila S2 cells, dMad was associated with the trb promoter region, which contains putative dMad-binding sites, but not with the trb coding region (ORF). (D) gbb transfection enriched dMad binding at the trb promoter region, but had no effect on binding by nonspecific IgG (negative control). (E) Schematic representations of the pGL3 luciferase reporter vector (LUC) and trb upstream genomic DNA fragments containing the putative Mad binding site (red box) (left) and the luciferase activities by gbb overexpression (right). The significantly increased luciferases activity with pGL3-trb1.4kb was not observed with pGL3-trb1.1kb and pGL3-trb1.4kbΔMbs. Data are presented as means ± s.e.m. from at least three independent experiments. *P < 0.05.
To determine whether trb is a direct transcriptional target of Gbb signaling, we performed chromatin immunoprecipitation (ChIP) assays with dMad antibody, followed by PCR amplification of trb regulatory sequences, in Drosophila S2 cells. In particular, we amplified the putative promoter and the ORF of the trb gene, both of which contain potential Mad-binding consensus sequences (GTCT) (Figure S4A,B). In the ChIP-PCR assay, we observed an amplified band corresponding to the trb promoter, but no band corresponding to the ORF (Figure S4C). In a ChIP-qPCR assay, the trb promoter was enriched 8-fold with dMad antibody (Fig. 3C). In gbb-overexpressing S2 cells, the association level between dMad and the trb promoter was elevated, but this was not the case for the trb ORF (Fig. 3D). In the luciferase reporter assays, 1.4kb trb upstream genomic DNA fragment containing the putative Mad binding site increased the luciferase activity significantly when gbb was overexpressed. However, 1.1kb trb upstream genomic DNA fragment and 1.4kb trb upstream genomic DNA fragment missing only the putative Mad binding site didn’t increase the luciferase activity (Fig. 3E). These findings provide strong evidence that the Gbb signal transducer dMad directly binds to the trb promoter and regulates trb transcription.
tribbles negatively regulates insulin signaling
To determine whether Trb can inhibit pAKT in Drosophila, as it does in mammals, we measured pAKT levels in fat bodies from pS106GS > trb flies. pAKT was increased by ex vivo insulin treatment in −RU control fat bodies, whereas, in trb-overexpressing fat bodies (pS106GS > trb, + RU), the insulin stimulated pAKT level was not elevated relative to the no-insulin control (Fig. 4A). These results confirm that tribbles can inhibit pAKT in the Drosophila fat body. Likewise, in adipose tissue of HFD-fed mice, expression of TGF-β and Trb3 was elevated relative to the corresponding levels in NCD-fed mice (Fig. 4B). To determine whether TGF-β signaling can induce Trb3 expression and inhibit insulin signaling in mammals, we treated HepG2 cells with TGF-β ligand. trb expression was increased 3-fold over control levels by TGF-β treatment, but less than 2-fold by treatment with TGF-β and TGF-β receptor inhibitor (Fig. 4C). When we measured the pAKT level in HepG2 cells, insulin treatment alone increased the pAKT level by 5-fold relative to control, whereas treatment with insulin and TGF-β caused no increase in the pAKT level. Moreover, treatment with insulin, TGF-β, and TGF-β receptor inhibitor increased the pAKT level by 3-fold (Fig. 4D). These findings indicate that, in mammals, as in Drosophila, TGF-β signaling turns on the Trb3 gene and negatively regulates insulin signaling by blocking pAKT.
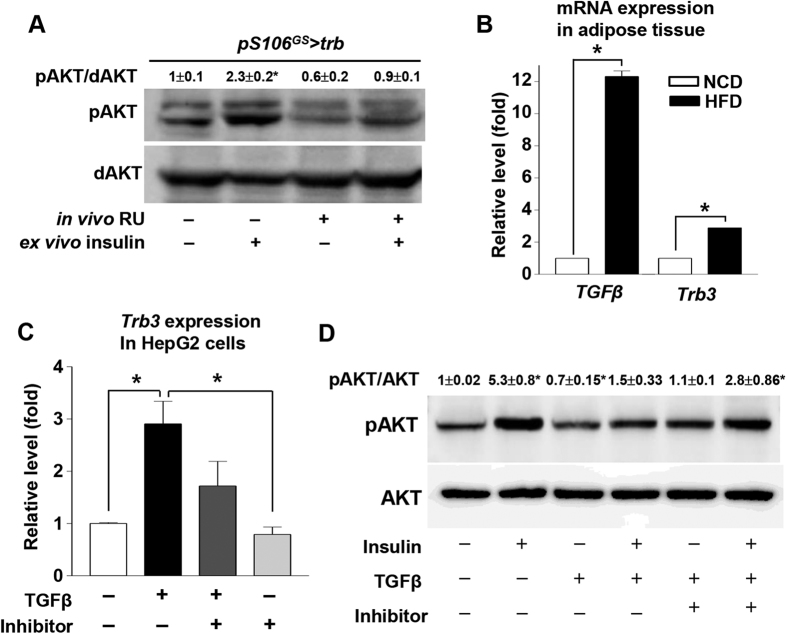
Figure 4. tribbles negatively regulates insulin signaling.
(A) Overexpression of trb in the fat body (pS106GS > trb + RU) decreased pAKT activation relative to that in −RU controls. (B) In mouse adipose tissue, HFD increased expression of TGF-β and Trb3 relative to the corresponding levels in mice fed a normal control diet. (C) In HepG2 cells, TGF-β induced Trb3 expression, which was restored by treatment with TGF-β receptor inhibitor. (D) TGF-β with or without insulin inhibited AKT activation, which was restored by treatment with TGF-β receptor inhibitor treatment. Data are presented as means ± s.e.m. from at least three independent experiments. *P < 0.05.
tribbles inhibition suppresses gbb-induced obesity and diabetic phenotype
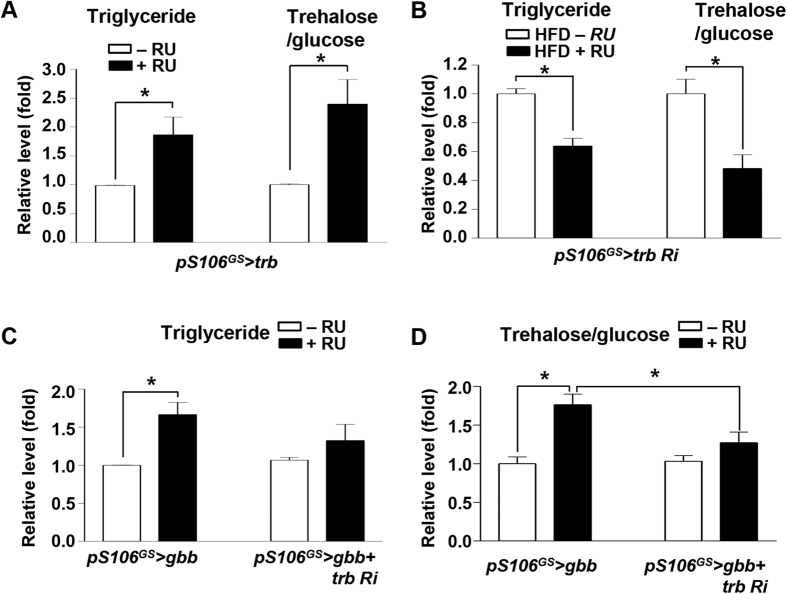
As with gbb, expression of trb increased in HFD flies (Figure S5A; compare with Fig. 1A). Therefore, we investigated the metabolic roles of trb. When trb was overexpressed in adult fat body (pS106GS > trb + RU), the levels of TG and trehalose/glucose were higher than those in −RU controls (Fig. 5A) and expression levels of dFOXO target genes d4E-BP and dInR were increased relative to those in −RU controls (Figure S5B). Inversely, when trb expression was knocked down in the adult fat body (pS106GS > trb RNAi + RU), the levels of TG and trehalose/glucose were reduced (Fig. 5B). To determine whether Gbb signaling regulates metabolism through trb, we knocked down trb in the adult fat body of gbb-overexpressing flies (pS106GS > gbb + trb RNAi) and monitored metabolic phenotypes. The levels of TG and trehalose/glucose in pS106GS > gbb + trb RNAi + RU conditions were lower than those in pS106GS > gbb + RU (Fig. 5C,D). Expression levels of dFOXO target genes d4E-BP and dInR were also lower in pS106GS > gbb + trb RNAi + RU than in pS106GS > gbb + RU (Figure S5C,D). These results strongly suggest that gbb regulates obesity and diabetic phenotypes by regulating trb expression in the fat body.
Figure 5. Inhibition of tribbles suppresses gbb-induced obesity and diabetic phenotype.
(A) Levels of triglyceride and trehalose/glucose in pS106GS > trb + RU increased relative to those in −RU controls. (B) In the HFD condition, knockdown of trb in the fat body suppressed the elevated levels of triglyceride and trehalose/glucose. (C,D) Knockdown of trb in gbb-overexpressing fat body (pS106GS > gbb + trb RNAi + RU) suppressed the elevated levels of triglyceride and trehalose/glucose observed in pS106GS > gbb + RU. Data are presented as means ± s.e.m. from at least three independent experiments. *P < 0.05.
Discussion
Abnormally high fat mass is a major risk factor for the development of diabetes. Previous studies emphasize that excess adiposity results in abnormal production of cytokines, growth factors, and hormones4,5, which in turn causes secondary diseases like insulin resistance6. In this study, we demonstrated that HFD-induced obesity triggered TGF-β signaling, which downregulates insulin signaling in the fat body. We also demonstrated the role of tribbles, a novel target of TGF-β/Gbb signaling, in the development of insulin resistance.
Drosophila models were used in several recent studies of diet-induced obesity, insulin resistance, hyperglycemia, and hyperinsulinemia29,30,31,32. In Drosophila larvae, a high-sugar diet induces type 2 diabetic phenotypes including hyperglycemia, high TG, and insulin resistance31. Likewise, in adult flies, HFD feeding also induces high TG and altered glucose metabolism, and in mammals it causes cardiac dysfunctions like diabetic cardiomyopathy29. In this study, we established a Drosophila model of obesity-induced insulin resistance, which has remarkable parallels with the mammalian system, and used it to observe and investigate the development of insulin resistance under chronic over-nutrition conditions. In addition, to study the Drosophila insulin-resistance phenotype in detail, we developed an ex vivo culture system (Figure S2E).
When we fed adult flies a HFD, their short- and long-term metabolic responses were different: for example, expression and secretion of Dilp2 was increased by short-term HFD but decreased by long-term HFD. Insulin signaling, which was assayed by monitoring pAKT activation and expression of the dFOXO target genes d4E-BP and dInR, was activated in short-term but not long-term HFD, whereas TG and trehalose/glucose levels in hemolymph were increased by long-term HFD (Figure S1). Because these pathological phenotypes in flies were very similar to the phenotypes associated with insulin-resistant diabetes in mammals, we conclude that HFD adult flies can be used as a model of type 2 diabetes.
In addition to increasing TG levels, HFD feeding in flies increased the expression of gbb. In mice, inhibition of TGF-β signaling by knockout of Smad3 protects against diet-induced obesity and diabetes8. Inhibition of TGF-β signaling may improve adipose function and reverse the effects of obesity on insulin resistance. The TGF-β/Smad3 signaling also plays a key role in adipogenesis33. However, it remains unclear how TGF-β signaling is related to the onset of diet-induced obesity and diabetes. In this study, we examined the effects of Drosophila TGF-β family ligands on obesity. Of the genes we tested, only gbb was upregulated by HFD (Fig. 1A). Gbb regulates lipid metabolism and controls energy homeostasis by responding to nutrient levels16; consequently, gbb mutants have extremely low levels of fat in the fat body, resembling a nutrient-deprived phenotype16. On the contrary, gbb overexpression increased the TG level, mimicking the effects of nutrient-rich conditions (Fig. 1B). These data suggest that TGF-β/Gbb signaling is involved in HFD-induced obesity. Indeed, overexpression of gbb in the fat body phenocopied the TG and trehalose/glucose levels in flies fed a HFD (Fig. 1C,D compared with Figure S1A). However, Dilp2 expression was increased by gbb overexpression in the fat body, consistent with the effects of short-term but not long-term HFD (Fig. 1E compared with Figure S1B).
We next focused on three negative regulators of insulin signaling, PTP1b, PTEN, and tribbles 3 (TRB3), which are involved in insulin resistance in obese mammals34,35,36. tribbles was upregulated in gbb-overexpressing cells and flies, (Fig. 3A,B). In mammals, Tribbles encodes an evolutionarily conserved kinase that plays multiple roles in development, tissue homeostasis, and metabolism37. A mammalian Tribbles homolog, Tribbles homolog 3 (TRB3), is highly expressed in liver tissue under fasting and diabetic conditions, and inhibits insulin signaling by direct binding to Akt and blocking phosphorylation-dependent Akt activation28. Indeed, the expression level of TRB3 is elevated in patients with type 2 diabetes and animal models of this disease38. In the systemic sclerosis model, TGF-β signaling can induce mammalian TRB3 and activates TGF-β signaling-mediated fibrosis39. Recent work showed that Drosophila tribbles, like mammalian TRB3, inhibits insulin-mediated growth by blocking Akt activation18. In this study, tribbles expression was increased in HFD conditions in both mice and flies (Figs 4B, S5A), as well as in TGF-β–treated human liver cells (Fig. 4C). tribbles knockdown rescued the diabetic phenotypes caused by HFD (Fig. 5B), consistent with previous findings in mammals. In addition, tribbles knockdown rescued the diabetic phenotypes caused by gbb overexpression (Fig. 5C,D). These data strongly suggest that the evolutionarily conserved tribbles gene is a novel downstream target of Gbb signaling, and that tribbles knockdown rescues diabetic phenotypes in flies. Therefore, future studies should seek to elucidate TGF-β–Trb3 signaling and its functions in mammalian adipocytes; the resultant findings could suggest new strategies for preventing type 2 diabetes.
In summary, we established a Drosophila insulin-resistance model and demonstrated that Gbb signaling in the fat body plays a critical role in obesity-mediated insulin resistance by regulating tribbles expression. These results provide insights regarding the function of Gbb/TGF-β signaling in metabolic disease, and suggest that this pathway represents a promising therapeutic target for treatment of obesity and diabetes.
Materials and Methods
Drosophila melanogaster stocks
Drosophila melanogaster were cultured and kept at 25 °C on a NCD containing 38% cornmeal, 20% yeast, 5% sugar, and 2% agar. w1118 flies were obtained from the Bloomington Stock Center, pS106GS–Gal4 from Dr. M. Tatar, DCG-Gal4 from Dr. J. Graff, UAS-gbb, UAS-dpp, UAS- UAS-dActβ, and UAS-punt DN from Dr. M. O’Conner, and UAS-tribbles from Dr. M. Milán. We also obtained UAS-gbb RNAi, UAS-dMad RNAi, and UAS-tribbles RNAi lines from the Vienna Drosophila Resource Center. In order to express pS106GS–Gal4, adult flies were cultured on an agar-based diet supplemented with 200 μM RU486 (Sigma).
Cell culture, stimulation, and transfection
Drosophila S2 cells purchased from Invitrogen were maintained at 26 °C in Schneider medium supplemented with 10% bovine calf serum. HepG2 cells were cultured in 4.5 g/l glucose Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum, 2% L-glutamine, 100 mU/ml penicillin, and 100 mg/ml streptomycin in a 5% CO2 atmosphere at 37 °C. The culture medium was changed every 2–3 days. Before peptide treatments, cells were starved for 8 h in serum-free medium containing 0.5% BSA and pretreated with chemical inhibitor or vehicle. TGF-β and TGF-β RI kinase inhibitor II (10 mM, Calbiochem) were used in these experiments. For transfection, cells were cultured in growth medium without antibiotics and transfected with small interfering RNA (siRNA) using Xtreme GENE HP (Roche). For overexpression of gbb, the full-length gbb cDNA was cloned into vector pAc5 (Invitrogen).
HFD and feeding
The HFD was prepared by the addition of 20% (vol/vol) coconut oil to the NCD; these proportions were used in all HFD experiments. The HFD was administered to male flies collected when they were 3–5 days old.
Measurement of body TG level
Thirty adult fly bodies were collected and homogenized, and TG levels were measured using the Triglyceride Determination Kit (Sigma). TG levels were normalized to total protein levels.
Measurement of trehalose/glucose level in hemolymph
Hemolymph was collected from 20 flies, and concentrations of trehalose/glucose were measured as previously described40.
RNA purification and quantitative RT-PCR analysis
Fat bodies from 20 flies were collected for RNA preparation. Total RNA extraction and quantitative RT-PCR were performed as previously described23. mRNA levels were expressed as the fold change relative to the corresponding level of rp49 mRNA, used as an internal normalization control. The comparative cycle threshold (Ct) method (User Bulletin 2, Applied Biosystems) was used to analyze the data. Primers used in this study are listed in Supplementary Table 1.
Ex vivo culture of adult fat bodies
Adult fat bodies were dissected in the Schneider’s medium, starved for 8 h in serum-free medium containing 0.5% BSA, and incubated for 1 h in Schneider medium containing human insulin (100 nM, GIBCO).
Western blot analysis and immunostaining
Total protein from adult fat bodies was isolated in Drosophila homogenate buffer (10 mM HEPES, 100 mM KCl, 1 mM EDTA, 5 mM DTT, 0.1% Triton X-100, 10% glycerol, and protease inhibitor cocktail [Sigma]). Western blots were performed as previously described22. Phospho-AKT and dAKT primary antibodies (both used at 1:1000) were obtained from Cell Signaling, and horseradish peroxidase–conjugated anti-rabbit IgG secondary antibody (1:2000) was obtained from Santa Cruz Biotechnology. Immunostaining was performed as previously described41 with dFOXO primary antibody (1:200; gift from Dr. M. Tatar) and the anti-rabbit IgG Alexa 488 secondary antibody (1:200, Molecular Probes).
ChIP
Cells were fixed in 0.5% formaldehyde for 15 min. ChIP analysis was performed as described23. After nuclei were isolated and chromatin was sonicated, an aliquot of DNA was removed as the input control, and the remainder was incubated with either normal mouse IgG (2 μg, Santa Cruz Biotechnology) or mouse anti-dMad (2 μg, Santa Cruz Biotechnology). Traditional PCR and qPCR were performed using input DNA and immunoprecipitated DNA. Primers used in this study are listed in Supplementary Table 2.
Luciferase reporter assay in Drosophila S2 cells
For luciferase assays, 1.4 kb Drosophila trb upstream genomic DNA (from +60 to −1346) and 1.1 kb (from +60 to −1054) were amplified by PCR, subcloned into the pGL3 luciferase reporter vector (Promega), and generated pGL3-trb1.4kb and pGL3-trb1.1kb. pGL3-trb1.4KbΔMbs, the deletion construct of the putative Mad binding sequence (GACATCCGTCTG) in pGL3-trb1.4Kb, was generated by overlap extension PCR using primers sitting on flanking sequences of the deletion42. These DNA constructs and pAc5.1A-gbb or pAc5.1A protein expression vector were transfected in Drosophila S2 cells with Xtreme GENE HP (Roche). After 48 h, cells were harvested and measured the luciferase activity using Luciferase reporter assay system (Promega) according to manufacturer’s instructions. The assays were repeated three times. Primers used in this study are listed in Supplementary Table 2.
Statistical analysis
Each experiment was repeated at least three times, and the data were presented as mean ± s.e.m. Student’s t-test was used for statistical analyses, and P < 0.05 was accepted as statistically significant.
Additional Information
How to cite this article: Hong, S.-H. et al. High fat diet-induced TGF-β/Gbb signaling provokes insulin resistance through the tribbles expression. Sci. Rep. 6, 30265; doi: 10.1038/srep30265 (2016).
Supplementary Material
Acknowledgments
We thank the members of the Yu laboratory for helpful comments. This work was supported by grants from KRIBB Research Initiative Program, National Research Council of Science & Technology (CRC-15-04-KIST), National Research Foundation of Korea (2014M3A9D8034462, 2015R1A5A1009024, 2015R1C1A2A01053705), and the Cooperative Research Program for Agriculture Science & Technology Development, Korea (PJ01168101).
Footnotes
Author Contributions S.-H.H., K.-S.L. and K.Y. designed the experiments, S.-H.H. and M.K. performed the experiments, and S.-H.H., K.-S.L. and K.Y. analyzed the data. S.-H.H., K.-S.L. and K.Y. wrote the paper.
References
- Samuel V. T. & Shulman G. I. Mechanisms for insulin resistance: common threads and missing links. Cell 148, 852–871, doi: 10.1016/j.cell.2012.02.017 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havel P. J. Update on adipocyte hormones: regulation of energy balance and carbohydrate/lipid metabolism. Diabetes 53 Suppl 1, S143–151 (2004). [DOI] [PubMed] [Google Scholar]
- Rosen E. D. & Spiegelman B. M. Adipocytes as regulators of energy balance and glucose homeostasis. Nature 444, 847–853, doi: 10.1038/nature05483 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelman B. M. & Flier J. S. Adipogenesis and obesity: rounding out the big picture. Cell 87, 377–389 (1996). [DOI] [PubMed] [Google Scholar]
- Guilherme A., Virbasius J. V., Puri V. & Czech M. P. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nature reviews. Molecular cell biology 9, 367–377, doi: 10.1038/nrm2391 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ferranti S. & Mozaffarian D. The perfect storm: obesity, adipocyte dysfunction, and metabolic consequences. Clinical chemistry 54, 945–955, doi: 10.1373/clinchem.2007.100156 (2008). [DOI] [PubMed] [Google Scholar]
- Samad F., Yamamoto K., Pandey M. & Loskutoff D. J. Elevated expression of transforming growth factor-beta in adipose tissue from obese mice. Molecular medicine 3, 37–48 (1997). [PMC free article] [PubMed] [Google Scholar]
- Yadav H. et al. Protection from obesity and diabetes by blockade of TGF-beta/Smad3 signaling. Cell metabolism 14, 67–79, doi: 10.1016/j.cmet.2011.04.013 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan C. K. et al. Smad3 deficiency in mice protects against insulin resistance and obesity induced by a high-fat diet. Diabetes 60, 464–476, doi: 10.2337/db10-0801 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan C. K., Chong H. C., Tan E. H. & Tan N. S. Getting ‘Smad’ about obesity and diabetes. Nutrition & diabetes 2, e29, doi: 10.1038/nutd.2012.1 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson A. J. & O’Connor M. B. Strategies for exploring TGF-beta signaling in Drosophila. Methods 68, 183–193, doi: 10.1016/j.ymeth.2014.03.016 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker L., Stathakis D. G. & Arora K. Regulation of BMP and activin signaling in Drosophila. Progress in molecular and subcellular biology 34, 73–101 (2004). [DOI] [PubMed] [Google Scholar]
- O’Connor M. B., Umulis D., Othmer H. G. & Blair S. S. Shaping BMP morphogen gradients in the Drosophila embryo and pupal wing. Development 133, 183–193, doi: 10.1242/dev.02214 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe B. D. et al. The BMP homolog Gbb provides a retrograde signal that regulates synaptic growth at the Drosophila neuromuscular junction. Neuron 39, 241–254 (2003). [DOI] [PubMed] [Google Scholar]
- Khalsa O., Yoon J. W., Torres-Schumann S. & Wharton K. A. TGF-beta/BMP superfamily members, Gbb-60A and Dpp, cooperate to provide pattern information and establish cell identity in the Drosophila wing. Development 125, 2723–2734 (1998). [DOI] [PubMed] [Google Scholar]
- Ballard S. L., Jarolimova J. & Wharton K. A. Gbb/BMP signaling is required to maintain energy homeostasis in Drosophila. Developmental biology 337, 375–385, doi: 10.1016/j.ydbio.2009.11.011 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldham S. & Hafen E. Insulin/IGF and target of rapamycin signaling: a TOR de force in growth control. Trends in cell biology 13, 79–85 (2003). [DOI] [PubMed] [Google Scholar]
- Das R., Sebo Z., Pence L. & Dobens L. L. Drosophila tribbles antagonizes insulin signaling-mediated growth and metabolism via interactions with Akt kinase. PloS one 9, e109530, doi: 10.1371/journal.pone.0109530 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. L. et al. Dock/Nck facilitates PTP61F/PTP1B regulation of insulin signalling. The Biochemical journal 439, 151–159, doi: 10.1042/BJ20110799 (2011). [DOI] [PubMed] [Google Scholar]
- Gronke S. et al. Brummer lipase is an evolutionary conserved fat storage regulator in Drosophila. Cell metabolism 1, 323–330, doi: 10.1016/j.cmet.2005.04.003 (2005). [DOI] [PubMed] [Google Scholar]
- Canavoso L. E., Jouni Z. E., Karnas K. J., Pennington J. E. & Wells M. A. Fat metabolism in insects. Annual review of nutrition 21, 23–46, doi: 10.1146/annurev.nutr.21.1.23 (2001). [DOI] [PubMed] [Google Scholar]
- Lee K. S., You K. H., Choo J. K., Han Y. M. & Yu K. Drosophila short neuropeptide F regulates food intake and body size. The Journal of biological chemistry 279, 50781–50789, doi: 10.1074/jbc.M407842200 (2004). [DOI] [PubMed] [Google Scholar]
- Hong S. H. et al. Minibrain/Dyrk1a regulates food intake through the Sir2-FOXO-sNPF/NPY pathway in Drosophila and mammals. PLoS genetics 8, e1002857, doi: 10.1371/journal.pgen.1002857 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son J. W. et al. Association of serum bone morphogenetic protein 4 levels with obesity and metabolic syndrome in non-diabetic individuals. Endocrine journal 58, 39–46 (2011). [DOI] [PubMed] [Google Scholar]
- Hwangbo D. S., Gershman B., Tu M. P., Palmer M. & Tatar M. Drosophila dFOXO controls lifespan and regulates insulin signalling in brain and fat body. Nature 429, 562–566, doi: 10.1038/nature02549 (2004). [DOI] [PubMed] [Google Scholar]
- Scanga S. E. et al. The conserved PI3’K/PTEN/Akt signaling pathway regulates both cell size and survival in Drosophila. Oncogene 19, 3971–3977, doi: 10.1038/sj.onc.1203739 (2000). [DOI] [PubMed] [Google Scholar]
- Naiki T., Saijou E., Miyaoka Y., Sekine K. & Miyajima A. TRB2, a mouse Tribbles ortholog, suppresses adipocyte differentiation by inhibiting AKT and C/EBPbeta. The Journal of biological chemistry 282, 24075–24082, doi: 10.1074/jbc.M701409200 (2007). [DOI] [PubMed] [Google Scholar]
- Du K., Herzig S., Kulkarni R. N. & Montminy M. TRB3: a tribbles homolog that inhibits Akt/PKB activation by insulin in liver. Science 300, 1574–1577, doi: 10.1126/science.1079817 (2003). [DOI] [PubMed] [Google Scholar]
- Birse R. T. et al. High-fat-diet-induced obesity and heart dysfunction are regulated by the TOR pathway in Drosophila. Cell metabolism 12, 533–544, doi: 10.1016/j.cmet.2010.09.014 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na J. et al. A Drosophila model of high sugar diet-induced cardiomyopathy. PLoS genetics 9, e1003175, doi: 10.1371/journal.pgen.1003175 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musselman L. P. et al. A high-sugar diet produces obesity and insulin resistance in wild-type Drosophila. Disease models & mechanisms 4, 842–849, doi: 10.1242/dmm.007948 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pospisilik J. A. et al. Drosophila genome-wide obesity screen reveals hedgehog as a determinant of brown versus white adipose cell fate. Cell 140, 148–160, doi: 10.1016/j.cell.2009.12.027 (2010). [DOI] [PubMed] [Google Scholar]
- Tsurutani Y. et al. The roles of transforming growth factor-beta and Smad3 signaling in adipocyte differentiation and obesity. Biochemical and biophysical research communications 407, 68–73, doi: 10.1016/j.bbrc.2011.02.106 (2011). [DOI] [PubMed] [Google Scholar]
- Zinker B. A. et al. PTP1B antisense oligonucleotide lowers PTP1B protein, normalizes blood glucose, and improves insulin sensitivity in diabetic mice. Proceedings of the National Academy of Sciences of the United States of America 99, 11357–11362, doi: 10.1073/pnas.142298199 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal A. et al. PTEN mutations as a cause of constitutive insulin sensitivity and obesity. The New England journal of medicine 367, 1002–1011, doi: 10.1056/NEJMoa1113966 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh H. J. et al. Tribbles 3 mediates endoplasmic reticulum stress-induced insulin resistance in skeletal muscle. Nature communications 4, 1871, doi: 10.1038/ncomms2851 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata J., Curado S., Ephrussi A. & Rorth P. Tribbles coordinates mitosis and morphogenesis in Drosophila by regulating string/CDC25 proteolysis. Cell 101, 511–522 (2000). [DOI] [PubMed] [Google Scholar]
- Oberkofler H. et al. Aberrant hepatic TRIB3 gene expression in insulin-resistant obese humans. Diabetologia 53, 1971–1975, doi: 10.1007/s00125-010-1772-2 (2010). [DOI] [PubMed] [Google Scholar]
- Tomcik M. et al. Tribbles homologue 3 stimulates canonical TGF-beta signalling to regulate fibroblast activation and tissue fibrosis. Annals of the rheumatic diseases, doi: 10.1136/annrheumdis-2014-206234 (2015). [DOI] [PubMed] [Google Scholar]
- Broughton S. et al. Reduction of DILP2 in Drosophila triages a metabolic phenotype from lifespan revealing redundancy and compensation among DILPs. PloS one 3, e3721, doi: 10.1371/journal.pone.0003721 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S. et al. Drosophila short neuropeptide F signalling regulates growth by ERK-mediated insulin signalling. Nature cell biology 10, 468–475, doi: 10.1038/ncb1710 (2008). [DOI] [PubMed] [Google Scholar]
- Ho S. N., Hunt H. D., Horton R. M., Pullen J. K. & Pease L. R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77, 51–59 (1989). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.