Abstract
Mitofusin-2 (Mfn2) is essential for embryonic development, anti-apoptotic events, protection against free radical-induced lesions, and mitochondrial fusion in many cells. However, little is known about its mechanism and function during oocyte maturation. In this study, we found that Mfn2 was expressed in the cytoplasm during different stages of mouse oocyte maturation. Mfn2 was mainly associated with α-tubulin during oocyte maturation. Knockdown of Mfn2 by specific siRNA injection into oocytes caused the mitochondrial morphology and quantity to change, resulting in severely defective spindles and misaligned chromosomes. This led to metaphase I arrest and the failure of first polar body extrusion. Furthermore, Mfn2 depletion from GV stage oocytes caused the redistribution of p38 MAPK in oocyte cytoplasm. These findings provide insights into potential mechanisms of Mfn2-mediated cellular alterations, which may have significant implications for oocyte maturation.
The mitofusin-2 (Mfn2) gene, also called the hyperplasia suppressor gene, was identified by screening vascular smooth muscle cell cDNA libraries of Wistar–Kyoto and spontaneously hypertensive rats using the differential display technique. Mfn2 is a powerful suppressor of cell proliferation in vivo and in vitro. The anti-proliferative effect of Mfn2 is mediated by inhibition of ERK/mitogen-activated protein kinase (MAPK) signalling and subsequent cell-cycle arrest1.
Mitochondria are the most abundant organelles in mammalian oocytes and early embryos2. Because mitochondria produce ATP through aerobic respiration, they became a driving force in evolution3. Optimal mitochondrial function is ensured by a quality-control system tightly coupled to fusion and fission. The fusion of mitochondria plays a crucial role in embryonic development4. Mfn2 participates in mitochondrial fusion and plays an essential role in metabolic homeostasis5,6. Increasing evidence indicates that Mfn2 not only plays critical roles in energy metabolism, endoplasmic reticulum stress, and signal transduction, but also has a close relationship with blastocyst formation and early embryonic development7,8,9,10,11. However, the role and mechanism of Mfn2 in regulating oocyte maturation is still unknown.
Oocyte maturation is a complex and precisely synchronized process affected by many factors. Oocyte maturation refers to the meiotic process that takes place from the germinal vesicle (GV) stage to the metaphase II stage. The first indication for this process is the disappearance of the GV as observed under the light microscope. This change is called germinal vesicle breakdown (GVBD). After GVBD, oocytes pass through metaphase of the first meiotic division and entry into the second meiotic division. Thereafter, oocytes are arrested at metaphase of the second meiotic division until fertilization takes place12.
Cell division involves precise spindle organization and chromosome segregation. Functional analysis of p38 MAPK in mouse oocytes suggests that this kinase regulates spindle assembly and accurate chromosome segregation through phosphorylation of MAPK-activated protein kinase13,14. In porcine oocytes, p38 MAPK contributes to the transition of metaphase I to metaphase II15. Previous study showed that Mfn2 could affect p38 mitogen-activated protein kinases (MAPKs) pathway and have relationship with p38 MAPK phosphorylation in somatic cells16,17. However, the interactions remain unclear between Mfn2 and p38 MAPK during oocyte maturation.
Here, we demonstrate that Mfn2 is indispensable for the meiotic progression and mitochondrial morphology as well as the localization of p38 MARK in mouse oocytes.
Results
Expression and subcellular localization of Mfn2 during oocyte meiotic maturation
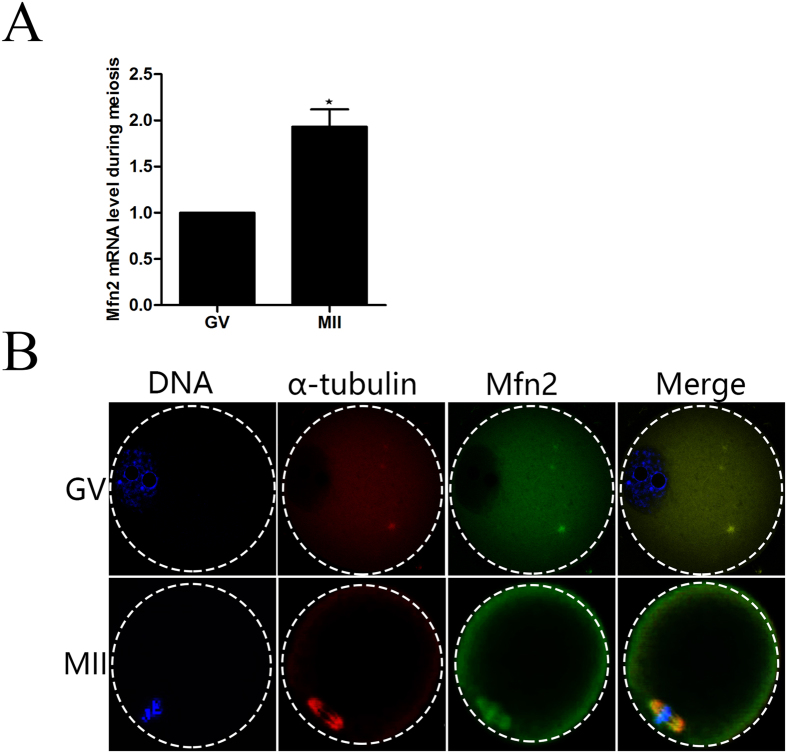
To investigate the role of Mfn2 during meiosis, the expression and subcellular localization of this protein was examined. Expression of Mfn2 in GV and ovulated MII oocytes was detected by the quantitative real-time polymerase chain reaction (qRT-PCR). The mRNA level of Mfn2 was moderate at GV stages, and reached the highest level at MII stages (Fig. 1A) (P < 0.05). To assess the subcellular location of Mfn2 protein during meiotic maturation, GV and ovulated MII mouse oocytes were immunolabelled with anti-α-tubulin and anti-Mfn2 antibodies to visualize the spindle and Mfn2, respectively. Oocytes were co-stained with Hoechst 33342 to visualize the chromosomes. In GV oocytes, Mfn2 was distributed mainly in the cytoplasm. Interestingly, Mfn2 accumulated in the sub-membrane region and was concentrated in the region of the spindle and chromosomes in MII oocytes (Fig. 1B).
Figure 1. Expression and subcellular localization of Mfn2 during mouse oocyte meiotic maturation.
(A) Expression of Mfn2 mRNA was determined by qT-PCR analysis. (B) Confocal microscopy showing the subcellular localization of Mfn2 (green), α-tubulin (red), and DNA (blue) in mouse oocytes at the GV and MII stages.
Knockdown of Mfn2 causes MI arrest and reduces PB1 extrusion
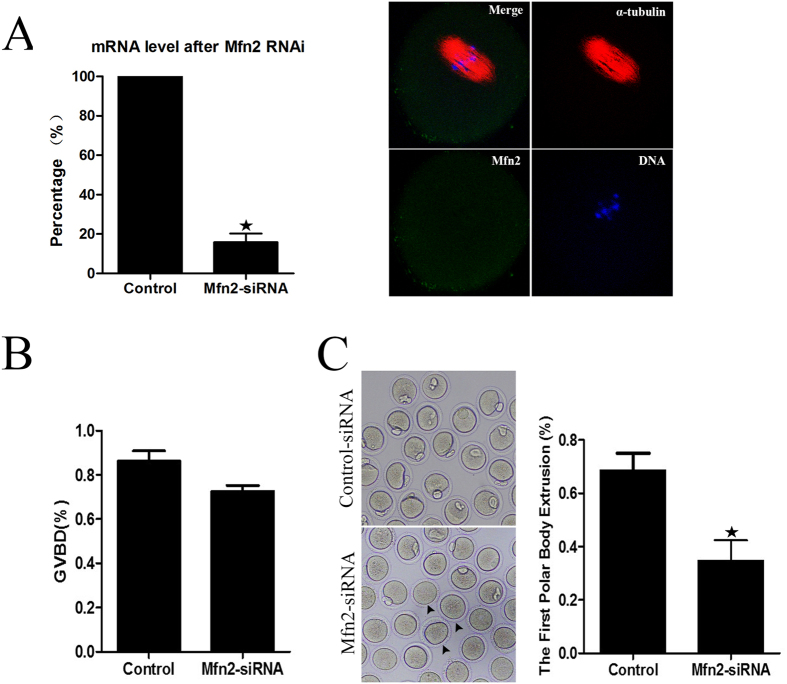
To determine whether Mfn2 functions in regulating mouse oocyte maturation, Mfn2-siRNA was microinjected into the cytoplasm of GV oocytes. The efficiency of siRNA was determined by measuring Mfn2 mRNA levels by qRT-PCR. Compared with the control, the mRNA level of Mfn2 in siRNA- injected oocytes was significantly decreased (15.8 ± 7.7 vs. 100%, n = 150, p < 0.05). Immunofluorescent staining results showed that, after RNAi, there was minimal specific localization of Mfn2 around spindles, implying successful Mfn2 downregulation by siRNA (Fig. 2A). Compared with the control, the extent of GVBD in siRNA- injected oocytes was significantly decreased (86.3 ± 8.03 vs. 72.7 ± 4.35%, n = 186, p < 0.05, Fig. 2B). After culturing for 14 h, the extrusion of PB1 in the siRNA group (34.9 ± 13.1%, n = 152) was considerably lower than that in the control group (68.7 ± 10.9%, n = 158, p < 0.05, Fig. 2C).
Figure 2. Mfn2 siRNA inhibits the GVBD and first polar body extrusion.
(A) Levels of Mfn2 mRNA and protein in oocytes injected with siRNA. (B) Percentages of GVBD in siRNA-injected and control oocytes. (C) Percentages of first polar body extrusion in siRNA-injected and control oocytes. Data are expressed as means ± SEM of at least three independent experiments. *Significantly different from control (p < 0.05).
Depletion of Mfn2 induces spindle defects and chromosome misalignment during oocyte maturation
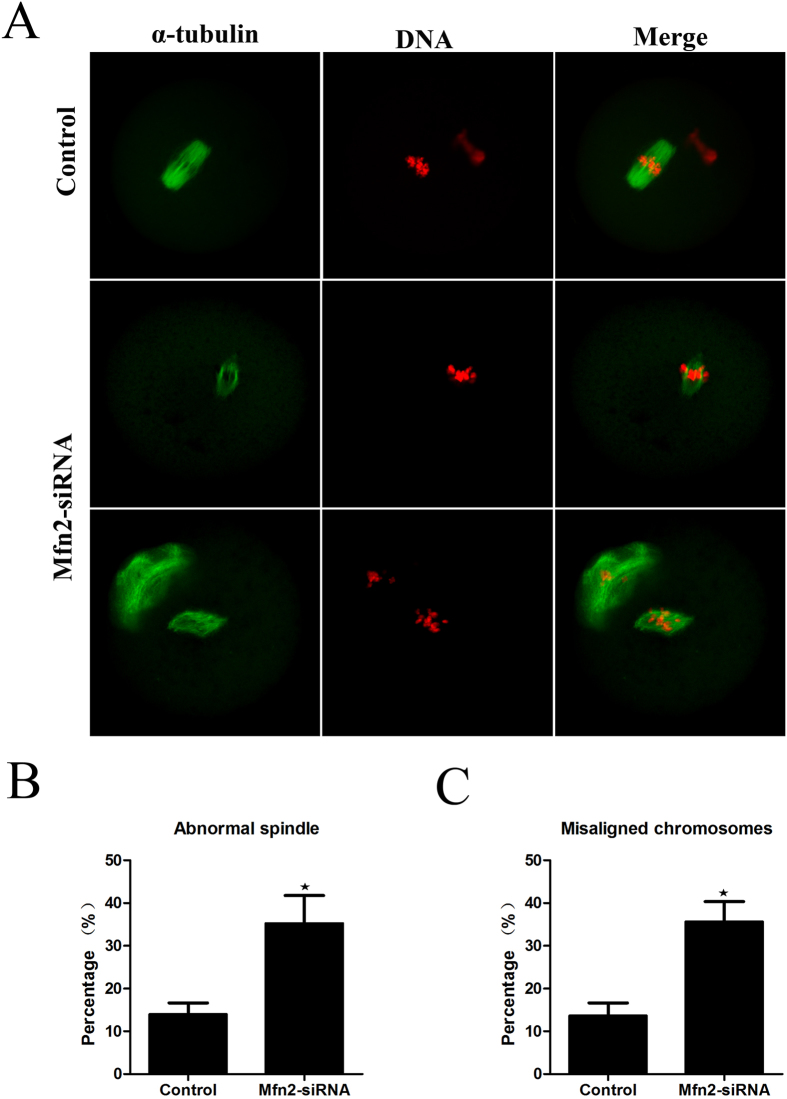
To explore the possible effects of Mfn2 on the organization of the spindle and chromatin during meiotic maturation, we examined spindle morphology and chromosome distribution by using confocal microscopy. As shown in Fig. 3A, downregulation of Mfn2 resulted in significant defects in spindle formation and chromosome alignment. The extent of abnormal spindle formation in the experiment group was (35.6 ± 11.4%) (n = 172), which was considerably higher than the control group (14.4 ± 4.86%, p < 0.05) (n = 168) (Fig. 3B). Mfn2-depleted oocytes displayed severe defects in chromosome alignment, showing lagging and irregularly scattered chromosomes (Fig. 3A). The incidence of misaligned chromosomes in the experimental group was 35.8 ± 7.99% (n = 172), much higher than the control group (14.1 ± 5.44%, p < 0.05) (n = 168) (Fig. 3C). These data suggest that Mfn2 is required for regulating spindle organization and chromosome alignment.
Figure 3. Mfn2 siRNA causes defects of the meiotic spindle and chromosome alignment.
(A) Oocytes microinjected with Mfn2 or control siRNA were incubated in M2 medium containing 2.5 μM milrinone for 24 h, and then transferred to milrinone-free M2 for 12 h. This was followed by immunostaining with an α-tubulin antibody (green) and Hoechst 33342 (red). (B,C) The percentages of oocytes with abnormal spindles or misaligned chromosomes in siRNA-injected and control oocytes. Data are expressed as means ± SEM of at least three independent experiments. *Significantly different from control (p < 0.05).
Downregulation of Mfn2 causes the redistribution of mitochondria
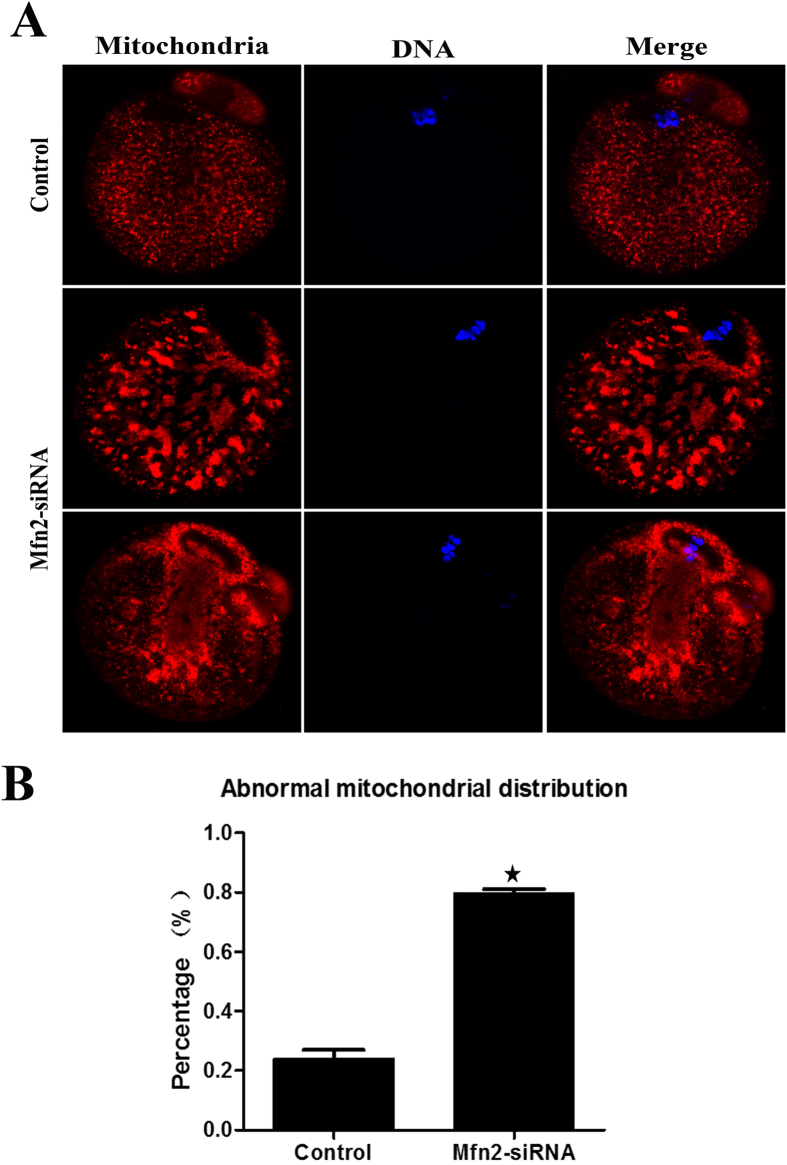
In order to determine whether Mfn2 influences the spatial remodelling of mitochondria during oocyte maturation, we compared the mitochondrial distribution patterns between oocytes from Mfn2-depleted and control oocytes. A homogeneous distribution of mitochondria throughout the entire ooplasm was observed by immunofluorescence microscopy in control oocytes (Fig. 4A). Increased mitochondrial clustering was readily observed in Mfn2-depleted oocytes (79.6 ± 2.46%, n = 126) as compared with control oocytes (24.1 ± 5.06%, n = 122, P < 0.05) (Fig. 4B). These results suggest that depletion of Mfn2 can disrupt mitochondrial distribution during oocyte maturation.
Figure 4. Mfn2 siRNA causes the redistribution of mitochondria.
(A) Oocytes microinjected with Mfn2 or control siRNA were incubated in M2 medium containing 2.5 μM milrinone for 24 h, and then transferred to milrinone-free M2 medium for 12 h. This was followed by immunostaining with MitoTracker (red) and Hoechst 33342 (blue). (B) The percentages of oocytes with abnormal distribution of mitochondria in the Mfn2 siRNA and control groups. Data are expressed as means ± SEM of at least three independent experiments. *Significantly different from control (p < 0.05).
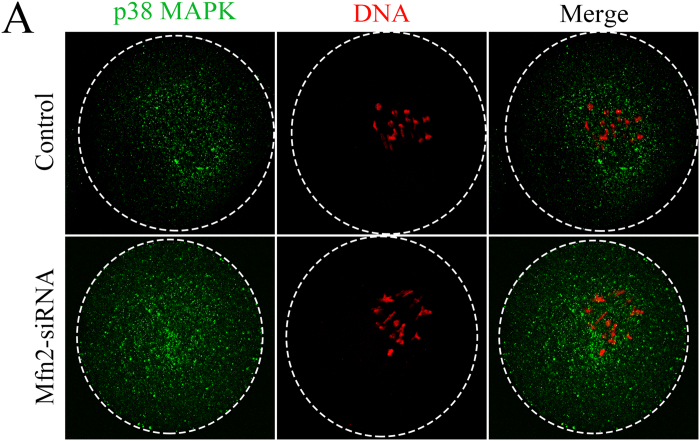
Depletion of Mfn2 causes the location of p38 MAPK to become scattered
To further investigate the mechanism and signal transduction pathway of Mfn2 during oocyte meiotic maturation, we used a specific Mfn2-siRNA to deplete most of the endogenous Mfn2 in oocytes. Immunofluorescence microscopy showed that p38 MAPK was scattered in an irregular pattern in the cytoplasm of oocytes after Mfn2 depletion. In contrast, in control oocytes, p38 MAPK accumulated mainly in the spindle region (Fig. 5). This correlation strongly suggests that a deficiency of Mfn2 may be linked directly to abnormal p38 MAPK distribution that then retards oocyte maturation.
Figure 5. Changes in the distribution of p38 MAPK in oocytes injected with Mfn2 or control siRNA.
Confocal microscopy showing the subcellular localization of p38 MAPK (green) and DNA (red) in oocytes injected with Mfn2 or control siRNA.
Discussion
Increasing data imply that Mfn2 may play critical roles in female mammalian reproduction18,19. In the current study, we show that Mfn2 is expressed in mouse oocytes from the GV and MII stages (Fig. 1A). In mouse oocytes, the subcellular localization of Mfn2 was obvious at the spindle at the meiotic metaphase. The localization of Mfn2 completely overlapped with that of spindle at the meiotic metaphase stages (Fig. 1B), suggesting that Mfn2 may participate in the spindle formation during meiotic maturation. The localization during oocyte meiosis was similar to that in previous study11. GVBD is a step in the development of oocytes, marking their maturation. Our data show that depletion of Mfn2 from the GV oocyte leads to less GVBD, less extrusion of the PB1, and developmental retardation. Our study also shows that, in MII oocytes, Mfn2 locates to the spindle, binds to microtubules, and functions as a major factor of meiotic spindle assembly and chromosome segregation. We extended our studies to examine spindle and chromosome organization during oocyte meiosis. Knockdown of Mfn2 in meiotic oocytes led to spindle organization defects and chromosome misalignment, thus leading to cell cycle arrest at the MI phase. These results suggest that the main function of Mfn2 in meiosis is to organize microtubules to form the spindle and segregate the chromosomes.
Early studies suggested that microtubules were the major component of cytoskeletal systems responsible for regulating the distribution of mitochondria in mammalian cells20,21. This finding compelled us to consider the possibility of microtubule-mitochondria binding at the level of protein-protein interactions. Several studies in mammalian species have shown that mitochondria undergo stage-specific changes in distribution during oocyte maturation22. Such a spatial remodelling of mitochondria may allow maturing oocytes to cater to differing energy requirements and provide a means for environmental sensing. In our study, the normal distribution pattern of mitochondria during meiotic maturation was disrupted in Mfn2-depleted oocytes resulting in clustered mitochondria. The spatial remodelling of mitochondria during oocyte maturation may reflect different ATP requirements at different developmental stages23.
Mammalian oocytes undergo intracytoplasmic mitochondria translocation during maturation and fertilization. This translocation is a microtubule-mediated cellular event. It is generally thought that mitochondrial spatial remodelling may be indicative of the energy requirement of various key events, such as GVBD and metaphase spindle formation23. Therefore, inadequate redistribution of mitochondria may be an important factor contributing to maturation delay and spindle/chromosome disorganization. The redistribution and changeability of mitochondria suggests that, in addition to its function in regulating mitochondrial fusion, Mfn2 is involved in other roles that control mitochondrial morphology and distribution.
These findings prompted us to investigate the effects of Mfn2 on the status of mitochondria, and spindle and chromosome organization in mouse oocytes, and then explore the relationship between these effects. Previously, the regulation of meiotic spindle organization and chromosome segregation in mammalian oocytes has not been studied as well as that in mitotic somatic cells. In the current study, we found a higher percentage of chromosome failure, spindle shape changes, and mitochondrial clustering in Mfn2 knockdown oocytes, suggesting this as a cause of oocyte maturation retardation. This correlation strongly suggests that deficient chromosome alignment may be directly linked to abnormal mitochondrial distribution, and is consistent with previous reports showing the involvement of mitochondrial function in spindle assembly and genomic stability of germ cells24,25.
Mfn2 plays an important role in spindle integrity and cell cycle progression in meiotic oocytes. p38 MAPK is one of the MAPKs involved in cell differentiation and apoptosis. p38 MAPK is a microtubule-associated protein and is required for stabilizing spindle assembly in mouse oocytes. p38α is required for the recruitment of γ-tubulin to the microtubule organizing centre and stabilization of spindle bipolarity. Depletion of p38α may compromise meiotic spindle organization and chromosome alignment via the p38α/MAPK-activated protein kinase signalling pathway.
Although MAPKs have been implicated in oocyte maturation in several species26,27, there is very limited information regarding the relationship between p38 MAPK and Mfn2 during oocyte meiosis. In the current study, the function of Mfn2 in oocyte maturation was found to rely on the p38-MAPK signalling pathways as shown by our findings of abnormal localization and expression of p38-MAPK after microinjecting Mfn2 siRNA into GV oocytes. Collectively, our observations indicated that Mfn2 was present in different oocyte developmental stages. Mfn2 knockdown oocytes displayed a higher frequency of spindle defects and chromosome misalignment in meiosis. Mfn2 affected the maturation of mammalian oocytes, possibly through changes in mitochondrial function and the p38 MAPK signalling pathway.
In general, Mfn2 dysfunction has emerged as a key factor in a myriad of diseases and metabolic disorders28,29,30,31,32,33. The current study provides new information about how Mfn2 functions impinge on mouse oocyte maturation. Further studies are needed to elucidate the precise mechanism and biological significance of Mfn2 in oocyte maturation.
Materials and Methods
Ethics statement
This study was approved by the Animal Care and Use Committee of the Institute of Zoology, Chinese Academy of Sciences. All animal manipulations were performed according to the guidelines of the Animal Care and Use Committee.
Collection and culture of mouse oocytes
Oocytes were collected from 4–6 week-old ICR mice. To obtain GV oocytes, females were primed with 5 IU of pregnant mare serum gonadotropin and sacrificed after 48 h. By puncturing the fully grown follicles, GV oocytes were released from the ovaries into pre-warmed M2 medium. MII oocytes were collected as described previously34,35. After specific treatments, oocytes were washed thoroughly and cultured in M2 medium, undergoing GV, GVBD, MI, and MII stages.
Immunofluorescence analysis
Oocytes were washed three times with phosphate-buffered saline (PBS) and then fixed in 4% paraformaldehyde in PBS at 4 °C for 1 h. Fixed cells were washed 3 times with PBST (PBS supplemented with 0.1% Tween 20) and incubated in 0.1% Triton X-100 in PBS at room temperature for 30 min. After washing with PBST, oocytes were blocked with 5% bovine serum albumin in PBS at room temperature for 1 h and transferred into diluted media containing a rabbit anti-Mfn2 antibody (1:50, a kind gift of Professor Chen Kuang-Hueih), a monoclonal anti-tubulin antibody (1:100), or a rabbit anti-p-p38 antibody (1:100) either at room temperature for 2 h or overnight at 4 °C. Finally, the labelled oocytes were washed with PBST and stained with FITC-labelled goat-anti-rabbit IgG (1:100) and TRITC-labelled goat-anti-mouse IgG (1:100) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at room temperature for 2 h. For mitochondrial staining, oocytes were incubated for 30 min at 37 °C in M2 medium supplemented with 200 nM MitoTracker Red. The oocytes were finally stained with Hoechst 333342 after three washes in washing buffer and were mounted on glass slides for immunofluorescence microscopy. Photos were captured using a confocal laser-scanning microscope (Zeiss LSM 780, Oberkochen, Germany).
siRNA microinjection
All injections were carried out according to the procedures described previously36. The small interfering RNA (siRNA) of Mfn2 (sequence: UCCUCAAGGUUUAUAAGAATT) (GenePharma, Shanghai, China), or the siRNA control, was microinjected (25 μM) into the cytoplasm of fully grown GV oocytes with an Eppendorf microinjection instrument (Hamburg, Germany) and completed within 30 min. Oocytes were kept in M2 medium supplemented with 2.5 μM milrinone (Sigma-Aldrich, St. Louis, MO, USA) to prevent GV breakdown and to allow Mfn2-siRNA to complete its role during this period. After 24 h, the oocytes were cleaned thoroughly to resume meiosis. Each experiment was repeated three to five times.
qRT-PCR
Total RNA was extracted from GV and MII mouse oocytes using an RNeasy micro-purification kit (Qiagen, Valencia, CA, USA) and then reverse transcribed to cDNA with an oligo dT primer using a Prime Script 1st Strand cDNA Synthesis Kit (TaKaRa Bio, Shiga, Japan). The full length Mfn2 coding sequence was amplified by PCR with the following primers: Forward: 5′-CCCCTGGCTCATACCCTAAT-3′, Reverse: 5′-AAGTAGGAGTGGCTGCCTGA-3′. Actin was selected as the reference gene. The SYBR Premix Ex Tag2 kit (TaKaRa Bio) was used in an ABI Prism 7500 sequence detection system (Life Technologies, Carlsbad, CA, USA). Relative gene expression was calculated by the 2ΔΔCt method.
Statistical analysis
Data (means ± SEM) were from at least three replicates per experiment and analysed by ANOVA using SPSS software (IBM, Chicago, IL, USA) followed by Fisher’s LSD test. Differences at p < 0.05 were considered to be statistically significant
Additional Information
How to cite this article: Zhang, J.-H. et al. Mitofusin-2 is required for mouse oocyte meiotic maturation. Sci. Rep. 6, 30970; doi: 10.1038/srep30970 (2016).
Acknowledgments
This work was supported by Grants from the National Natural Science Foundation of China (NSFC 30770247) and the International Cooperation Projects of MOE (2011DFA30920).
Footnotes
Author Contributions T.Z., K.W., F.-F.M., N.Y., T.A. and Y.-F.L. performed the experiments; S.-H.G. and J.-H.Z. analyzed the data; J.-W.H., X.-Y.Y. and G.-J.J. designed the experiments and wrote the manuscript. All authors reviewed the manuscript.
References
- Chen K. H. et al. Dysregulation of HSG triggers vascular proliferative disorders. Nature cell biology 6, 872–883 (2004). [DOI] [PubMed] [Google Scholar]
- Gibson T. C., Kubisch H. M. & Brenner C. A. Mitochondrial DNA deletions in rhesus macaque oocytes and embryos. Mol Hum Reprod 11, 785–789 (2005). [DOI] [PubMed] [Google Scholar]
- Friedman J. R. & Nunnari J. Mitochondrial form and function. Nature 505, 335–343 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H. et al. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J. Cell Biol. 160, 189–200 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara N., Eura Y. & Mihara K. Mitofusin 1 and 2 play distinct roles in mitochondrial fusion reactions via GTPase activity. J. Cell Sci. 117, 6535–6546 (2004). [DOI] [PubMed] [Google Scholar]
- Sebastián D. et al. Mitofusin 2 (Mfn2) links mitochondrial and endoplasmic reticulum function with insulin signalling and is essential for normal glucose homeostasis. Proc Natl Acad Sci USA 109, 5523–5528 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorzano A., Hernández-A M. I., Sebastián D. & Muñoz J. P. Mitofusin 2 as a driver that controls energy metabolism and insulin signalling. Antioxid Redox Sign. 22, 1020–1031 (2015). [DOI] [PubMed] [Google Scholar]
- Schneeberger M. et al. Mitofusin 2 in POMC neurons connects ER stress with leptin resistance and energy imbalance. Cell 155, 172–187 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara A., Cipolat S., Chen Y., Dorn G. W. & Scorrano L. Mitochondrial fusion directs cardiomyocyte differentiation via calcineurin and Notch signalling. Science 342, 734–737 (2013). [DOI] [PubMed] [Google Scholar]
- Zhao N., Zhang Y., Liu Q. & Xiang W. Mfn2 Affects embryo development via mitochondrial dysfunction and apoptosis. PLoS One 10, e0125680 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang G. J. et al. Expression of HSG is essential for mouse blastocyst formation. Biochem Biophys Res Commun. 335, 351–355 (2005). [DOI] [PubMed] [Google Scholar]
- Masui Y. & Clarke H. J. Oocyte maturation. Int Rev Cytol. 57, 185–282 (1979). [DOI] [PubMed] [Google Scholar]
- Yuan J. et al. MAPK activated protein kinase 2 is required for mouse meiotic spindle assembly and kinetochore-microtubule attachment. PLoS One 5, e11247 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou X. H. et al. p38α MAPK is associated protein regulating spindle assembly, spindle length and accurate chromosome segregation during mouse oocyte meiotic maturation. Cell Cycle 9, 4130–4143 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa-Diaz L. G. & Miyano T. Activation of p38 MAPK during porcine oocyte maturation. Biol Reprod 71, 691–696 (2007). [DOI] [PubMed] [Google Scholar]
- Liu C. et al. Mitofusin 2 decreases intracellular lipids in macrophages by regulating peroxisome proliferator-activated receptor-γ. Biochem Biophys Res Commun. 450, 500–506 (2014). [DOI] [PubMed] [Google Scholar]
- Li L. et al. p38 MAP kinase-dependent phosphorylation of the Gp78 E3 ubiquitin the ligase controls ER-mitochondria association and mitochondria motility. Mol Biol Cell 26, 3828–3840 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakai T., Harada Y., Miyado K. & Kono T. Mitochondrial dynamics controlled by mitofusins define organelle positioning and movement during mouse oocyte maturation. Mol Hum Reprod 20, 1090–1100 (2014). [DOI] [PubMed] [Google Scholar]
- Pang W. et al. Low expression of Mfn2 is associated with mitochondrial damage and apoptosis in the placental villi of early unexplained miscarriage. Placenta. 34, 613–618 (2013). [DOI] [PubMed] [Google Scholar]
- Sobajima T., Aoki F. & Kohmoto K. Activation of mitogen-activated protein kinase during meiotic maturation in mouse oocytes. J Reprod Fertil. 97, 389–394 (1993). [DOI] [PubMed] [Google Scholar]
- Heggeness M. H., Simon M. & Singer S. J. Association of mitochondria with microtubules in cultured cells. Proc Natl Acad Sci USA 75, 3863–3866 (1978). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. M. et al. Mitoguardin-1 and -2 promote maturation and the developmental potential of mouse oocytes by maintaining mitochondrial dynamics and functions. Oncotarget 12, 1155–1167 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van B. J., Davis P., Mathwig V. & Alexander S. Domains of high-polarized and low-polarized mitochondria may occur in mouse and human oocytes and early embryos. Hum Reprod 17, 393–406 (2002). [DOI] [PubMed] [Google Scholar]
- Zhang X. et al. Deficit of mitochondria-derived ATP during oxidative stress impairs mouse MII oocyte spindles. Cell Res. 16, 841–850 (2006). [DOI] [PubMed] [Google Scholar]
- Liu L., Trimarchi J. R., Smith P. J. & Keefe D. L. Mitochondrial dysfunction leads to telomere attrition and genomic instability. Aging Cell 1, 40–46 (2002). [DOI] [PubMed] [Google Scholar]
- Gotoh. Y. et al. In vitro effects on microtubule dynamics of purified Xenopus M phase-activated MAP kinase. Nature 349, 251–254 (1991). [DOI] [PubMed] [Google Scholar]
- Stricker S. A. & Smythe T. L. Endoplasmic reticulum reorganizations and Ca2+ signalling in maturing and fertilizedoocytes of marine protostome worms: the roles of MAPKs and MPF. Development 130, 2867–2879 (2003). [DOI] [PubMed] [Google Scholar]
- Lv H. et al. A cohort study of Han Chinese MFN2-related Charcot-Marie-Tooth 2A. J Neurol Sci. 358, 153–157 (2015). [DOI] [PubMed] [Google Scholar]
- Sawyer S. L. et al. Homozygous mutations in MFN2 cause multiple symmetric lipomatosis associated with neuropathy. Hum Mol Genet 24, 5109–5114 (2015). [DOI] [PubMed] [Google Scholar]
- Lowell B. B. & Shulman G. I. Mitochondrial dysfunction and type 2 diabetes. Science 307, 384–387 (2005). [DOI] [PubMed] [Google Scholar]
- Bach D. et al. Expression of Mfn2, the Charcot-Marie-Tooth neuropathy type 2A gene, in human skeletal muscle: effects of type 2 diabetes, obesity, weight loss, and the regulatory role of tumor necrosis factor alpha and interleukin-6. Diabetes 54, 2685–2693 (2005). [DOI] [PubMed] [Google Scholar]
- Sebastian D. et al. Mitofusin 2 (Mfn2) links mitochondrial and endoplasmic reticulum function with insulin signalling and is essential for normal glucose homeostasis. Proc Natl Acad Sci USA 109, 5523–5528 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneeberger M. et al. Mitofusin 2 in POMC neurons connects ER stress with leptin resistance and energy imbalance. Cell 155, 172–187 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T. et al. Nuf2 is required for chromosome segregation during mouse oocyte meiotic maturation. Cell Cycle 14, 2701–2710 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T. et al. SIRT1, 2, 3 protect mouse oocytes from postovulatory aging. Aging 8, 685–696 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang T. S. et al. Ca2+ oscillations induced by a cytosolic sperm protein factor are mediated by a maternal machinery that functions only once in mammalian eggs, Development 127, 1141–1150 (2000). [DOI] [PubMed] [Google Scholar]