Abstract
Tissue-specific promoters for gene therapy are typically too big for adeno-associated virus (AAV) vectors; thus, the exploration of small effective non-viral regulatory elements is of particular interest. Wild-type AAV can specifically integrate into a region on human chromosome 19 termed AAVS1. Earlier work has determined that a 347 bp fragment (Chr19) of AAVS1 has promoter and transcriptional enhancer activities. In this study, we further characterized this genetic regulation and investigated its application to AAV gene therapy in vitro and in vivo. The Chr19 347 bp fragment was dissected into three regulatory elements in human embryonic kidney cells: (i) TATA-independent promoter activity distributed throughout the fragment regardless of orientation, (ii) an orientation-dependent insulator function near the 5′ end and (iii) a 107 bp enhancer region near the 3′ end. The small enhancer region, coupled to the mini-CMV promoter, was used to drive the expression of several reporters following transduction by AAV2. In vivo data demonstrated enhanced transgene expression from the Chr19-mini-CMV promoter cassette after tail vein injection primarily in the liver at levels comparable to the chicken β-actin promoter and higher than the liver-specific TTR promoter (>2-fold). However, we did not observe this increase after muscle injection, suggesting tissue-specific enhancement. All of the results support identification of a small DNA fragment (347 bp) from AAV Chr19 integration site capable of providing efficient and enhanced liver-specific transcription when used in recombinant AAV vectors.
Keywords: AAV, chromosome 19, integration, cis-acting element, liver
Introduction
Adeno-associated virus (AAV) is a single-stranded DNA dependovirus of the family Parvoviridae. The 4.7 kb genome is flanked by 145 nucleotide inverted terminal repeats (ITRs). In human clinical trials, AAV has been utilized as a therapeutic gene delivery vector and has proven safe and effective. The advantages of recombinant AAV (rAAV) include long-term transgene expression, broad tissue tropism, its non-pathogenicity and ability to infect both non-dividing and dividing cells.1,2 However, rAAV transduction usually takes 6–8 weeks to reach peak transgene expression in vivo as transcription ensues following the conversion of the single-stranded DNA genome to a duplexed form. To overcome this rate-limiting step, self-complementary (sc) AAV vectors were developed and demonstrate faster and increased transgene expression compared to the conventional single-stranded context.3–6 However, scAAV also comes with a limitation; the transgene capacity is decreased from 4.7 kb (for single-stranded) to about 2.3 kb. This size constraint prevents the utilization of many strong, yet large, promoters (such as chicken β-actin (CBA)) and additional regulatory or enhancer elements. To extend the application of scAAV, investigations of small and strong and/or tissue-specific promoters/enhancers are imperative.
Adeno-associated virus replication requires coinfection by a helper virus such as adenovirus or herpes simplex virus. In the absence of such a helper, AAV genomes persist as episomes, although a small percentage integrate into a specific region of human chromosome 19 (AAVS1), a process mediated by the AAV Rep protein. AAVS1 is GC-rich and recent work demonstrated DNaseI hypersensitivity, suggesting open chromatin structure.7,8 Further studies demonstrated that the DNaseI hypersensitive region possessed promoter and transcriptional enhancer functions in cell lines.8 A different study also noted a transcriptional insulator in the same region of AAVS1.9
In this work, we further characterized a small region (347 bp, chromosome 19 (Chr19)) of AAVS1, dissecting several regulatory effects in cell culture. The enhancer function was then investigated for increased transgene expression following AAV transduction in vivo. Our results demonstrate that the enhancer from the 347 bp fragment increased transgene synthesis in a liver-specific manner. This study provides a small transcription regulatory element to enhance transgene expression, especially in the liver, perfectly suited for applications in which size is a limitation, such as scAAV.
Results
Chr19 promoter function in vitro
AAVS1 starts 427 bp upstream of the start codon of the myotonic dystrophy kinase-related Cdc-42-binding kinase (MRCK) α-kinase p85 gene10 (Figure 1). The region upstream of the p85 gene does not contain an obvious TATA box but, rather, is GC-rich (67%).7 Recent work demonstrated that this region is hypersensitive to treatment with DNaseI, suggesting relaxed or open chromatin structure with transcriptional regulation-like characteristics.8 It was also reported that this region conferred promoter activity to a reporter in 293 cells and to a lesser extent in HeLa cells.8 This transcriptional activity was independent of the fragment's orientation, and additional experiments demonstrated true enhancer function.8 Owing to the packaging limitations of AAV, strong yet small promoters are valuable and we hypothesized that this promoter/enhancer fragment could drive transgene expression following rAAV transduction.
Figure 1.
The diagram of Chr19 domain upstream of the p85 gene. A 347 bp region upstream of the p85 gene of human chromosome 19 was investigated for transcriptional regulation. Two sites involved in AAV integration are also depicted; (i) the terminal resolution site (TRS) and (ii) the AAV Rep protein-binding element (RBE). Primers used for genetic dissection of this region are also shown (F = forward, R = reverse). AAV, adeno-associated virus.
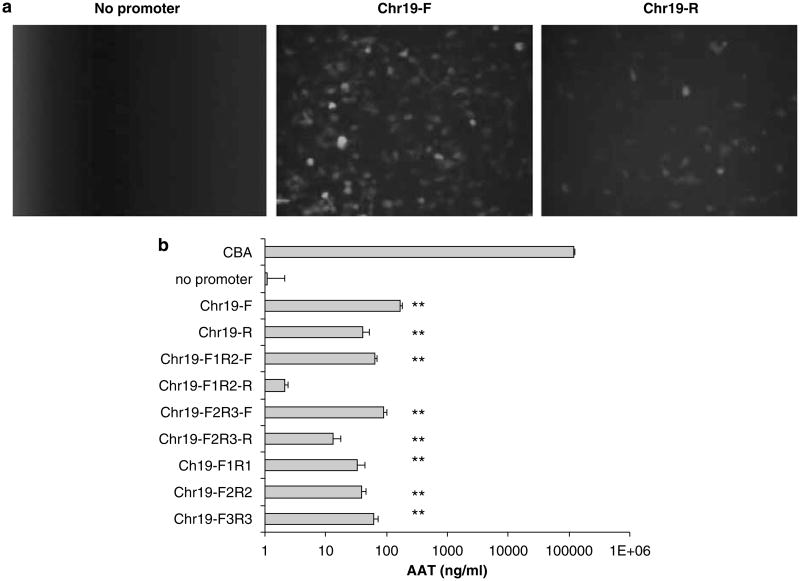
To confirm the promoter function of this sequence, we amplified a 347 bp fragment (named Chr19; Figure 1) from the chromosomal AAVS1 sequence and subsequently cloned it, in both orientations, upstream of a promoter-less green fluorescent protein (GFP)-coding sequence. In the absence of a promoter, no GFP+ cells were observed (Figure 2a). Consistent with earlier results, the plasmid constructs containing the Chr19 fragment resulted in significant GFP fluorescence in an orientation-independent manner (Figure 2a). To quantitate the efficiency of the Chr19 promoter, we chose the human α-1 antitrypsin gene (AAT) as a reporter. In 293 cells, AAT is efficiently secreted to the culture supernatant and is accurately quantitated by enzyme-linked immunosorbent assay. For these experiments, we used the CBA promoter as a positive control that is known to give robust expression in a variety of tissues and cell lines. The Chr19 fragment led to the detection of AAT in the culture supernatant at a level 50-fold over the no-promoter negative control in an orientation-independent manner (Figure 1).
Figure 2.
Chr19 promoter function in human embryonic kidney cells. 293 cells were transfected with plasmid constructs containing different promoter elements from the Chr19 region depicted in Figure 1. (a) The full-length (347 bp) Chr19 element was investigated for promoter activity, paired with gfp, in the forward (F) and reverse (R) orientations. Fluorescent microscopy was performed 24 h after the transfection of 5 μg of plasmid DNA in a 10 cm plate. (b) Promoter activity of the Chr19 region was further dissected using the α-1 antitrypsin (AAT) gene product as the reporter. The primers used for Chr19 region amplification are depicted in Figure 1. The AAT concentration was determined in culture supernatant by ELISA 24 h post-transfection of 5 μg plasmid DNA in a 24-well plate. The chicken β-actin promoter (CBA) was used as a relative control. The results were averaged from four independent experiments and standard deviation is shown. **P<0.01 vs baseline without promoter. ELISA, enzyme-linked immunosorbent assay.
Next, we genetically dissected the Chr19 sequence and its subfragments (Table 1 and Figure 1) were cloned upstream of the AAT transgene in the same context as above. AAT in the supernatant was observed from each construct regardless of orientation from the Chr19 fragment or subfragments (Figure 2b). The entire Chr19 fragment initiated higher transgene expression than any of its subfragments in the forward orientation (P<0.01 vs baseline without the promoter). When the Chr19 forward fragment was divided into two subfragments F1/R2 and F2/R3 with 120 bp overlap (F2/R2), the F2/R3 (227 bp) induced slightly higher AAT expression than the F1/R2 (240 bp). Of the three small Chr19 fragments without any overlap, F3/R3 (107 bp) demonstrated the highest gene expression, whereas the other two subfragments, F1/R1 and F2/R2 (120 bp each), had similar promoter activity. These results suggested that the promoter function was distributed over the entire Chr19 region, with the strongest activity in the F3/R3 fragment.
Table 1. The primers for PCR.
| Primer name | Sequence |
|---|---|
| Chr19 F1 | 5′-gaagatcttcCCCGGGGCAGTCTGCTATTCATCCC |
| Chr19 R1 | 5′-gaagatcttcTGAGGGCCTCCTCCGGGGAATGCTG |
| Chr19 F2 | 5′-gaagatcttcTCTGGCGATTTCCACTGGGCGCCTC |
| Chr19 R2 | 5′-gaagatcttcGGGGAGCGCTGGGAAATGGAGTCC |
| Chr19 F3 | 5′-gaagatcttcGCGACCTGCCCAGCACACCCTGGG |
| Chr19 R3 | 5′-gaagatcttcGGGCCGCCGGGAACTGCCGCTGG |
| Mini-CMV F | 5′-gaagatcttcCAAATGGGCGGTAGGCGTGTAC |
| Mini-CMV R | 5′-TCTGTCTTCTGGGCAGCATCTCC |
Sequences with lower case represent BglII recognition site.
We also examined the promoter function of Chr19 and its subfragments (F1/R2 and F2/R3) in the reverse orientation. It is interesting to note that promoter function was much lower than that in the forward orientation for Chr19 and the F2/R3 fragment (P<0.05). Minimal promoter function was found with the F1/R2 subfragment. The lower promoter activity of these reverse fragments implicated an insulator domain in the Chr19 region. The insulator function is apparent in the F1/R2 fragment with the majority in the F1/R1, as the F2/R3 fragment had higher promoter activity than the F1/R2 in the reverse orientation.
The Chr19 enhancer function in vitro
The Chr19 fragment, which demonstrates modest promoter activity (described above), also has been reported to function as a true transcriptional enhancer.8 To confirm this result, the Chr19 sequence was genetically dissected and cloned in opposing orientations upstream of the mini-CMV promoter driving expression of the AAT therapeutic reporter. Experiments using the smallest subfragments of Chr19 demonstrated the strongest effect with F3/R3, whose activity is not significantly different from that of the entire fragment (P>0.05), with decreasing enhancer function following the scheme F2/R2> F1/R1=mini-CMV (Figure 3). These data demonstrate that 107 bp of the Chr19 fragment (F3/R3) confers significant transcriptional enhancer activity in the forward orientation.
Figure 3.
Chr19 enhancer function in human embryonic kidney cells. (a) 293 cells were transfected with 0.5 μg of plasmid constructs containing different elements immediately upstream of the mini-CMV promoter. The primers used for Chr19 region amplification are depicted in Figure 1. The α-1 antitrypsin (AAT) gene was used as the reporter and AAT concentration was determined in culture supernatant by ELISA 24 h post-transfection. (b) The full-length Chr19 element–mini-CMV pairing was also investigated for AAT production when located between AAV's inverted terminal repeats (TRs) in a plasmid context. The result was the average of five separate experiments and standard deviation is shown. **P<0.01 or *P<0.05 vs the mini-CMV promoter. AAV, adeno-associated virus; ELISA, enzyme-linked immunosorbent assay.
When comparing transgene expression with the reverse orientation, Chr19 and the F1/R1, as well as the F1/R2, fragments did not augment AAT expression from the mini-CMV promoter (P>0.05 vs the mini-CMV promoter, Figure 3a). Contrary to the F1/R1 and the F1/R2 results, the fragments F2/R3, F2/R2 and F3/R3 increased AAT production similar to the level of enhancement determined for the forward orientations. These results can be explained by the presence of a transcriptional insulator located 5′ to the enhancer element of the Chr19 fragment. Collectively, these results of the Chr19 fragments further confirmed that the insulator region is mostly located in the F1/R1 sequence (Figure 3a).
The enhancer function of the Chr19 fragment through muscular injection of AAV vector
First, the Chr19-mini-CMV-AAT cassette was cloned between the AAV2 ITRs (in a plasmid) and used for transfection of 293 cells. Consistent with an earlier result, the ITRs demonstrated modest, yet significant, enhancer function11 (Figure 3b). Addition of the Chr19 fragment upstream of mini-CMV in either the forward or the reverse orientation enhanced AAT production four- and twofold, respectively (P<0.01 or <0.05 vs the mini-CMV promoter, respectively). In contrast to the pBR construct, the effect of the Chr19 insulator domain was not as dramatic in the ITR context, suggesting that the insulator only slightly inhibits the enhancer function from 5′-ITR but not 3′-ITR.
From our above results, the Chr19 has promoter function, but the activity is low compared to the CBA promoter (P<0.01). Besides the promoter function, Chr19 also demonstrated enhancer activity when combined with the mini-CMV promoter in cell culture, and additional enhancement was mediated by the AAV ITRs. Therefore, we chose to test the Chr19 enhancer function in conjunction with the mini-CMV promoter in vivo following AAV transduction. As the Chr19 fragment and its subfragments, F2/R3 as well as F3/R3 in the forward orientations, demonstrated similar enhancer activity without insulator function in 293 cells; these elements were investigated for their regulation of AAT synthesis following AAV2 transduction. Six weeks after the administration of AAV2/AAT vectors (5 × 1010 particles) with different promoter elements to the mouse gastronemius muscle, the AAT level in the blood was determined by enzyme-linked immunosorbent assay. In these experiments, the popular constitutive promoters CBA and CMV initiated similar transgene expression, both over 10-fold the activity of the mini-CMV promoter (P<0.01 vs the mini-CMV promoter, Figure 4). Inconsistent with the results obtained in 293 cells, Chr19 and its subfragments had little to no enhancer function compared to the mini-CMV promoter (P>0.05 vs the mini-CMV promoter). This result suggests that the Chr19 does not detectably function in mouse muscle.
Figure 4.
Chr19 enhancer function in muscle. Known promoters, chicken β-actin (CBA), CMV and mini-CMV were investigated for expression of the α-1 antitrypsin (AAT) gene after AAV2 transduction of mouse muscle. The Chr19 enhancer elements depicted in Figure 1 coupled to the mini-CMV promoter were also evaluated. Balb/C mice were injected with 5 × 1010 particles of AAV2/AAT vectors intramuscularly and 6 weeks later, the AAT level in the blood was measured by ELISA. The result was the average from five mice and standard deviation is shown. **P<0.01 vs the CBA promoter. AAV, adeno-associated virus; ELISA, enzyme-linked immunosorbent assay.
The Chr19 enhancer function in the liver
The liver is the biggest internal organ in the body holding about 30% of blood, and AAV2 vectors preferentially transduce liver cells very efficiently after systemic administration. Although the Chr19 fragment did not enhance promoter activity in mouse muscles, we investigated whether it had an effect in other tissues after systemic delivery. Six weeks post-tail vein injection of AAV2/AAT (with various promoters), AAT levels in the blood demonstrated that the CBA promoter induced the highest levels of circulating transgene product, over 10-fold when compared to the mini-CMV construct (P<0.01 vs the mini-CMV promoter, Figure 5). In contrast to the muscle data, the full-length CMV promoter induced similar or even lower AAT secretion in the blood than the mini-CMV promoter. This perhaps is because of silencing of the CMV promoter as noted with other viral vector delivery into the liver and may be associated with DNA CpG methylation.12–14 It was interesting to note that addition of the Chr19 fragment, or the F2/R3 subfragment, upstream of the mini-CMV promoter induced comparable transgene expression to the CBA promoter (P>0.05, Figure 5a). In contrast to the observations in 293 cells, the enhancer function of the F3/R3 fragment was not observed following tail vein injection (P<0.01, F3/R3 mini-CMV vs CBA). As the F2/R3 fragment displayed similar enhancer function to Chr19 through systemic administration, we presumed that the enhancer function of the Chr19 was contributed by the F2/R2 fragment. To confirm this hypothesis, we performed a head-to-head comparison of AAV2/Chr19-mini-CMV/AAT and AAV2/F2/R2-mini-CMV/AAT following tail vein injections. After six weeks, AAT levels in the blood were not statistically different in these two groups (P>0.05, Figure 5b). This result demonstrates the contribution of the F2/R2 fragment to Chr19 enhancer function after systemic injection in mice.
Figure 5.
Chr19 enhancer function in the liver. Known promoters, chicken β-actin (CBA), CMV, mini-CMV, U1a, U1b or transthyretin (TTR) were investigated for expression of the α-1 antitrypsin (AAT) or factor 9 (F9) genes after AAV2 transduction of mouse liver. The Chr19 enhancer elements depicted in Figure 1 coupled to the mini-CMV promoter were also evaluated. Balb/C mice were injected with 5 × 1010 (a) or 1 × 1010 particles (b) of AAV/AAT vectors through the tail vein. Six weeks later, the AAT level in the blood was determined. (c) A total of 4 × 1010 particles of AAV2/F9 were injected into the liver through the portal vein. Eight weeks postinjection, the F9 level in the blood was quantitated by ELISA. The results represent averaged data from five mice plus standard deviation. **P<0.01 or *P<0.05 vs the CBA promoter (a) or the Chr19-mini-CMV promoter (c). AAV, adeno-associated virus; ELISA, enzyme-linked immunosorbent assay.
To examine whether the Chr19 enhancer function also applies to another transgene and can be used to package scAAV construct, we made an scAAV construct with the Chr19-mini-CMV promoter to drive human F9 expression. For these experiments, we chose the liver-specific TTR promoter15 and two murine promoters smaller in size, U1a and U1b.16 After portal vein injection of AAV2/F9 (4 × 1010 particles), circulating F9 in the blood was detected by enzyme-linked immunosorbent assay 8 weeks postinjection (Figure 5c). Surprisingly, the Chr19-mini-CMV promoter resulted in twofold higher blood levels of F9 than the TTR promoter15 (P<0.05) and 10- or 20-fold higher than U1a or U1b promoter (P<0.01), respectively.16
Chr19 enhancer function in different tissues
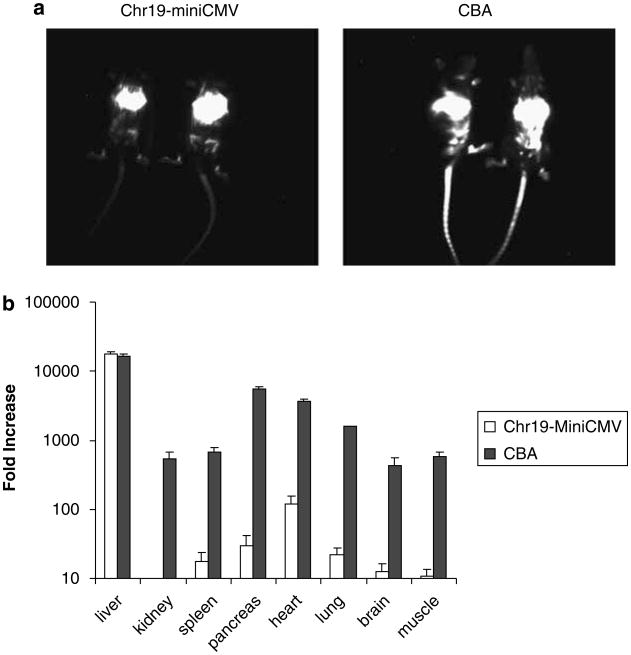
Different AAV serotypes have different tissue tropisms. Whereas the AAV2 capsid transduces muscle, liver and brain tissues efficiently, work in our lab and reports by others demonstrate the enhanced efficiency of AAV8 for transduction of various tissues in vivo.17–19 To examine the Chr19 enhancer function in different tissues, we used the firefly luciferase gene (luc). Packaged inside the AAV8 capsid, two constructs were compared following systemic delivery; Chr19-mini-CMV/luc and CBA/luc. The CBA promoter was chosen for this comparison because of its ability to initiate transgene expression in a variety of mouse tissues. Fifteen weeks after tail vein injection of 1 × 1011 particles of the AAV8 vector, luciferase activity was analyzed using whole-body live imaging. AAV8/CBA-luc vector administration induced strong and diffuse transgene expression, whereas AAV8/Chr19-mini-CMV/luc induced significant luciferase expression mostly localized to the liver area (Figure 6a). Sixteen weeks post-tail vein injection, different tissues were harvested and measured for luciferase activity. The values were normalized to the total protein recovered from the cognate tissue. Compared to mini-CMV promoter, the relative fold increase in different tissues was obtained (Figure 6). Consistent with the imaging result, after tail vein injection of AAV8/Ch19-mini-CMV/luc, a 10 000-fold increase in luciferase activity was achieved in the liver, comparable to AAV8/CBA/luc (Figure 6b). In addition, a 10- to 100-fold increase of luciferase activity was also observed in the spleen, pancreas, heart and lungs (Figure 6b). There was no change in luciferase expression in the kidney, brain and muscle. Demonstrating the ubiquitous nature of the CBA promoter, transgene expression was also increased about 100-fold in all other tissues in mice given AAV8/CBA/luc. These results confirm that the Chr19 fragment has enhancer function in selected tissues; the liver, heart, pancreas and lung; however, the effect was most obvious in the liver. This result for Chr19 enhancer function is consistent with the findings of p85 gene expression in different human tissues.10 Compared to the AAT results, the large increase in luciferase activity perhaps results from the sensitivity of detection of the reporter. This is evident when comparing the AAT expression in our studies to CAT expression in the study of Lamartina et al.8 in 293 cells. In this case, a 2-fold and 39-fold increase was determined for the same Chr19 enhancer function for AAT and CAT, respectively.
Figure 6.
Chr19 enhancer function in different tissues. Transgene synthesis was compared from either the chicken β-actin (CBA) or Chr19-mini-CMV promoters after systemic AAV8 administration. The luciferase gene was used as the reporter. A total of 1 × 1011 particles were injected into the tail vein of BalbC mice, and luciferase activity was determined 15 weeks later by live imaging (a). Sixteen weeks postinjection, mice were killed and luciferase activity in various tissues was determined as described in the text and normalized to total recovered protein from the cognate tissue. The data are presented as the relative fold increase, compared to values from the mini-CMV promoter (data not shown), to demonstrate the enhancement by the Chr19 fragment. AAV, adeno-associated virus.
Discussion
This study demonstrates several regulatory features of a small region of Chr19 and its application to AAV gene therapy in vitro and in vivo. Genetic dissection of Chr19 revealed three regulatory elements in vitro: (i) promoter activity distributed throughout 347 bp fragment regardless of orientation, (ii) a 107 bp enhancer region located downstream at the 3′ end and (iii) an orientation-dependent insulator function near the 5′ end. The minimal enhancer element (120 bp), which was coupled to small promoter and then packaged into the AAV2 capsid, was of particular interest. Chr19 did not demonstrate any enhancer function after intramuscular administration in a murine model. However, systemic administration revealed strong tissue-specific enhancer function, with the greatest activity observed in the liver. This finding is important and applicable to gene therapy strategies in which size is a limitation and liver expression is desired.
The AAVS1 region is located on the short arm of Chr19 and has several unique features. First, it contains a tetrad GACT (AAV Rep-binding element) repeat also found on the AAV2 ITR, which allows exclusive integration of the AAV genome in the presence of AAV's replication protein. Second, Chr19 is GC-rich (82% for the first 900 nucleotides), with more CpG islands relative to GpC islands. This has been noted for regions of transcriptional activity in vertebrates and is thought relevant to gene expression.20–22 Third, several putative transcription factor-binding sites are present in AAVS1 including CREB, AP-1, AP-2 and Sp1.7,8 Fourth, topoisomerase I is predicted to cleave at least two sites within AAVS1, one of which appears highly preferred.7 Fifth, Lamartina et al.8 have demonstrated open chromatin conformation of AAVS1 along with promoter activity of Chr19 in cultured cells. Combined, these attributes situate AAVS1 in an accessible position to be acted upon by both host and viral regulatory elements.
The cis-acting element function of Chr19 in vitro
In 293 cells, the 347 bp Chr19 promoter activity was detectable at a low level, rendering it individually deficient for strong transgene expression. However, after dissecting what functioned as an orientation-dependent insulator, a minimal fragment could be used to significantly increase product synthesis when connected to a known promoter (two were tested in this study; mini-CMV and CMV). Though not explicitly shown herein, this effect is attributed to transcription as (i) earlier investigations reported that the effect of Chr19 was at the transcriptional level, (ii) the only variable in several of the constructs was the addition of the small Chr19 or its subfragments and (iii) the effect was transgene-independent.
The enhancer function of Chr19 in vivo
Chr19, as an enhancer in mouse muscle, did not result in increased product synthesis. In contrast, a robust increase was noted in other tissues after systemic administration (Figure 6). Contrary to the findings in 293 cells, in which F3/R3 demonstrated similar augmented activity with the mini-CMV promoter as the entire Chr19, the F2/R2 fragment, but not the F3/R3, served as an enhancer in the liver. The discrepancies of Chr19 utility in different tissues are likely because of a different profile of cellular transcription factors, environment/genotype of cultured cells and/or comparison of plasmid results vs virus-delivered genomes. It is worth noting that the addition of the ITRs to the Chr19-mini-CMV element seemed to enhance transgene production in a synergistic manner (Figure 3b). The GC-rich ITRs have overlapping characteristics with known transcriptional enhancers, are bound by DNA-processing enzymes and mediate circularization of the AAV genomes after uncoating. Studies by our lab have also demonstrated that the ITRs confer transcription even in the absence of an obvious promoter element (C Li and RJ Samulski, unpublished data).
The insulator function of the Chr19 fragment
Insulators are DNA regulatory elements that can prevent the inappropriate interaction between adjacent chromatin domains. Insulators are divided into two types according to their function; an enhancer blocker or an enhancer barrier.23 An enhancer blocker is a cis-acting regulatory element that blocks enhancer transcription when localized in between the promoter and enhancer, but not otherwise. Barrier insulators prevent the spread of the heterochromatin in general. The insulator function of AAVS1 was first observed by Ogata et al.,9 who demonstrated that a 337 bp region of the Chr19 served as an insulator to block enhancer function. Our in vitro experiment demonstrated that the insulator function was primarily located at the 5′ end of Chr19, suggesting that it functions as a barrier insulator.
Concluding remarks
Chr19 has previously demonstrated enhancer/promoter functions in cultured cells.8 The study here applies these findings to a mouse model to show dramatic enhancer function, particularly, in the liver. Most importantly, we demonstrate that only 120 bp of Chr19 contributes to this effective enhancer function and, combined with the mini-CMV promoter, results in a total size of about 250 bp (Chr19F2/R2-mini-CMV promoter). This small promoter provides the opportunity to express larger transgenes in the scAAV format for gene therapy, especially, when liver expression is desired. Although all the data available support Chr19 fragment as a high-level and long-term liver tropic enhancer from AAV vector cassette, additional studies are required to determine expression past the 4-month time point established in this study. Future work will also focus on whether tandem copies of the Chr19F2/R2 sequence will have an additive effect on promoter enhancer function along with investigations of the required transcription factors as well as their prevalence in target tissues.
Materials and methods
Constructs
pBR322 (Invitrogen, Carlsbad, CA, USA) was used as the parent for all constructs without AAV2 ITRs. First, a BglII linker (NEB) was introduced into the EcoRI site of pBR322 to generate pBR322/BglII by blunt ligation in a manner that keeps the EcoRI restriction site intact. Insertion of the enhanced GFP-coding sequence and the SV40 polyA tail into the EcoRI site by blunt ligation resulted in pBR322/GFP. A 347 bp of Chr19 was amplified by PCR with primers Chr19 F1 and Chr19 R3 and cloned into the BglII site of pBR322/GFP to generate pBR-Chr19-F/GFP and pBR-Chr19-R/GFP in forward (F) or reverse (R) orientation, respectively.
We also investigated Chr19 function using the AAT gene as a reporter (generously provided by Dr Flotte, University of Florida). Promoter-less AAT cDNA and the bovine growth factor PolyA were subcloned into pBR322 using EcoRI and ClaI. The PCR products amplified from the pairs of primers (Figure 1, Table 1) F1/R3, F1/R2, F2/R3, F1/R1, F2/R2 and F3/R3 were digested with BglII and cloned into the BglII site of pBR/AAT to generate pBR/Chr19-F/AAT, pBR/Chr19-R/AAT, pBR/Chr19-F1R2-F/AAT, pBR/Chr19-F1R2-R/AAT, pBR/Chr19-F2R3-F/AAT, pBR/Chr19-F2R3-R/AAT, pBR/Chr19-F1R1/AAT, pBR/Chr19-F2R2/AAT and pBR/Chr19-F3R3/AAT, respectively. pTR2/CBA-hAAT was digested with EcoRI and BglII, blunted, then self-ligated to produce pTR2/AAT.
A DNA fragment containing the CMV minimal promoter (mini-CMV) and the CMV immediate-early enhancer was subcloned from the plasmid pTR2-EGFP and inserted into pTR2/CBA-hTTA after digestion with BglII and XbaI (pTR2/CMV-hAAT). The BglII+ClaI fragment from pTR2/CMV-hAAT was cloned into pBR322 to generate pBR/CMV/AAT. The mini-CMV promoter fragment was amplified by PCR with [mini-CMV-F and mini-CMV-R (Table 1)], digested with BglII+BamHI, then cloned into pBR/CMV-hAAT and pTR2/CMV-AAT to generate pBR/mini-CMV-hAAT and pTR/mini-CMV-AAT, respectively. The fragments from PCRs with different pairs of primers (Table 1) were cleaved by BglII and cloned into the BglII site of pBR/MiniCMV-hATT to produce pBR/Chr19-F-miniCMV/AAT, pBR/Chr19-R-miniCMV/AAT, pBR/Chr19F1R2-F-miniCMV/AAT, pBR/Chr19F1R2-R-miniCMV/AAT, pBR/Chr19F2R3-F-miniCMV/AAT, pBR/Chr19F2R3-R-miniCMV/AAT, pBR/Chr19F1R1-F-miniCMV/AAT, pBR/Chr19F1R1-R-miniCMV/AAT, pBR/Chr19F2R2-F-miniCMV/AAT, pBR/Chr19F2R2-R-miniCMV/AAT, pBR/Chr19F3R3-F-miniCMV/AAT and pBR/Chr19F3R3-R-miniCMV/AAT. The fragments from PCR with primer pairs (F1/R3, F2/R3, F2/R2 and F3/R3) were inserted into the BglII site of pTR2/MiniCMV-AAT to form pTR/Chr19-miniCMV/AAT, pTR/Chr19F2R3-miniCMV/AAT, pTR/Chr19F2R2-miniCMV/AAT and pTR/Chr19F3R3-miniCMV/AAT, respectively. All PCRs were performed using the Advantage-GCs Kit as recommended (Clontech, Mountain View, CA, USA). Templates for the mini-CMV promoter or Chr19/subfragment PCRs were pTR/CMV-hAAT and pRE2, respectively. The lengths of the PCR products are listed: 347 bp for F1/R3, 240 bp for F1/R2, 207 bp for F2/R3, 120 bp for F1/R1 and F2/R2, 107 bp for F3/R3 and 291 bp for mini-CMV. All constructs were verified by sequencing or endonuclease digestion (subclones).
To generate pTR/CBA-luc, pGL3 (Invitrogen) was digested with HindIII and XbaI to release the firefly luciferase gene that was then inserted into pTR/CBA-AAT, digested with EcoRI and NotI, by blunt ligation. Self-complimentary constructs, dspTR/U1a-F9, dspTR/U1b-F9 and dspTR/TTR-F9, have been described earlier.17 Double-stranded construct dspTR/Chr19-mini-CMV/F9 was generated by replacement of the TTR promoter in dspTR/TTR-F9 with the Chr19-mini-CMV promoter from pTR/Chr19-F-miniCMV/AAT.
Virus production
rAAV2 vectors were made by a calcium phosphate cotransfection method described earlier.24
Transfection
293 cells were seeded in 10 cm or 24-well plates at 50% confluence. The following day, plasmid transfections were performed by a calcium phosphate precipitate approach. Twenty-four hours later, the supernatant was harvested by pelleting out cell debris by centrifugation and AAT levels were determined in the supernatant.
Mice
Female Balb/c mice aged 5–6 weeks (The Jackson Laboratory, Bar Harbor, ME, USA) were used for this study. All mice were maintained in a specific pathogen-free facility at the UNC at Chapel Hill. The use and care of these animals were in accordance with institutional guidelines from the National Institutes of Health.
AAT and F9 detection
F9 and AAT levels in the blood or the supernatant from cell cultures were measured by ELSIA as described.25
Firefly luciferase assay
Mice were injected with 1 × 1011 particles of AAV2/luc vectors through the tail vein. At week 16 postadministration, the mice were killed and different tissues were collected. The luciferase activities were measured in tissue extracts. The tissues were homogenized in passive lysis buffer (Promega, Madison, WI, USA) and the homogenates were centrifuged for 30 min at 5000 r.p.m. at 4 °C to clear cell debris. The supernatant was further centrifuged for 15 min at 2000 r.p.m. at 4 °C. Ten microliters of supernatant was transferred to a 96-well plate. The Light Unit was measured following the addition of firefly luciferase substrate by a Victor 02 instrument (PerkinElmer, Waltham, MA, USA). The luciferase activities were calculated as the number of reference Light Units per 10 s per mg of protein. The protein was measured by a standard bicinchoninic acid assay with a commercial kit (Pierce, Rockford, IL, USA).
In vivo bioluminescence imaging of luciferase
The mice were anesthetized by intraperitoneal administration of 2.5% avertin. A solution of the luciferase substrate, luciferin-D (150 mg kg−1; Invitrogen), was then injected intraperitoneally. Five minutes later, mice were imaged for 5 min using a NightOwl cabinet with a charge-coupled device camera (Berthold Technologies, Bad Wildbad, Germany).
Statistical analysis
All data were shown as a mean±s.d. Student's T-test was used to determine significant differences between the groups with different promoters. A value of P<0.05 was considered significant.
Acknowledgments
This work was supported by the NIH research grants 5P01GM059299, 5P01HL066973 and 2P01HL051818, and a research grant from Alpha-1 Foundation.
References
- 1.Li C, Bowles DE, van Dyke T, Samulski RJ. Adeno-associated virus vectors: potential applications for cancer gene therapy. Cancer Gene Ther. 2005;12:913–925. doi: 10.1038/sj.cgt.7700876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Warrington KH, Jr, Herzog RW. Treatment of human disease by adeno-associated viral gene transfer. Hum Genet. 2006;119:571–603. doi: 10.1007/s00439-006-0165-6. [DOI] [PubMed] [Google Scholar]
- 3.Fu H, Muenzer J, Samulski RJ, Breese G, Sifford J, Zeng X, et al. Self-complementary adeno-associated virus serotype 2 vector: global distribution and broad dispersion of AAV-mediated transgene expression in mouse brain. Mol Ther. 2003;8:911–917. doi: 10.1016/j.ymthe.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 4.McCarty DM, Fu H, Monahan PE, Toulson CE, Naik P, Samulski RJ. Adeno-associated virus terminal repeat (TR) mutant generates self-complementary vectors to overcome the rate-limiting step to transduction in vivo. Gene Therapy. 2003;10:2112–2118. doi: 10.1038/sj.gt.3302134. [DOI] [PubMed] [Google Scholar]
- 5.McCarty DM, Monahan PE, Samulski RJ. Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Therapy. 2001;8:1248–1254. doi: 10.1038/sj.gt.3301514. [DOI] [PubMed] [Google Scholar]
- 6.Wang Z, Ma HI, Li J, Sun L, Zhang J, Xiao X. Rapid and highly efficient transduction by double-stranded adeno-associated virus vectors in vitro and in vivo. Gene Therapy. 2003;10:2105–2111. doi: 10.1038/sj.gt.3302133. [DOI] [PubMed] [Google Scholar]
- 7.Kotin RM, Linden RM, Berns KI. Characterization of a preferred site on human chromosome 19q for integration of adeno-associated virus DNA by non-homologous recombination. EMBO J. 1992;11:5071–5078. doi: 10.1002/j.1460-2075.1992.tb05614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamartina S, Sporeno E, Fattori E, Toniatti C. Characteristics of the adeno-associated virus preintegration site in human chromosome 19: open chromatin conformation and transcription-competent environment. J Virol. 2000;74:7671–7677. doi: 10.1128/jvi.74.16.7671-7677.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogata T, Kozuka T, Kanda T. Identification of an insulator in AAVS1, a preferred region for integration of adeno-associated virus DNA. J Virol. 2003;77:9000–9007. doi: 10.1128/JVI.77.16.9000-9007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan I, Ng CH, Lim L, Leung T. Phosphorylation of a novel myosin binding subunit of protein phosphatase 1 reveals a conserved mechanism in the regulation of actin cytoskeleton. J Biol Chem. 2001;276:21209–21216. doi: 10.1074/jbc.M102615200. [DOI] [PubMed] [Google Scholar]
- 11.Flotte TR, Solow R, Owens RA, Afione S, Zeitlin PL, Carter BJ. Gene expression from adeno-associated virus vectors in airway epithelial cells. Am J Respir Cell Mol Biol. 1992;7:349–356. doi: 10.1165/ajrcmb/7.3.349. [DOI] [PubMed] [Google Scholar]
- 12.Guo ZS, Wang LH, Eisensmith RC, Woo SL. Evaluation of promoter strength for hepatic gene expression in vivo following adenovirus-mediated gene transfer. Gene Therapy. 1996;3:802–810. [PubMed] [Google Scholar]
- 13.Prosch S, Stein J, Staak K, Liebenthal C, Volk HD, Kruger DH. Inactivation of the very strong HCMV immediate early promoter by DNA CpG methylation in vitro. Biol Chem Hoppe Seyler. 1996;377:195–201. doi: 10.1515/bchm3.1996.377.3.195. [DOI] [PubMed] [Google Scholar]
- 14.Loser P, Jennings GS, Strauss M, Sandig V. Reactivation of the previously silenced cytomegalovirus major immediate-early promoter in the mouse liver: involvement of NFkappaB. J Virol. 1998;72:180–190. doi: 10.1128/jvi.72.1.180-190.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costa RH, Lai E, Darnell JE., Jr Transcriptional control of the mouse prealbumin (transthyretin) gene: both promoter sequences and a distinct enhancer are cell specific. Mol Cell Biol. 1986;6:4697–4708. doi: 10.1128/mcb.6.12.4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bartlett JS, Sethna M, Ramamurthy L, Gowen SA, Samulski RJ, Marzluff WF. Efficient expression of protein coding genes from the murine U1 small nuclear RNA promoters. Proc Natl Acad Sci USA. 1996;93:8852–8857. doi: 10.1073/pnas.93.17.8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu Z, Sun J, Zhang T, Yin C, Yin F, Van Dyke T, et al. Optimization of self-complementary AAV vectors for liver-directed expression results in sustained correction of Hemophilia B at low vector dose. Mol Ther. 2007;16:280–289. doi: 10.1038/sj.mt.6300355. [DOI] [PubMed] [Google Scholar]
- 18.Wang Z, Zhu T, Qiao C, Zhou L, Wang B, Zhang J, et al. Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart. Nat Biotechnol. 2005;23:321–328. doi: 10.1038/nbt1073. [DOI] [PubMed] [Google Scholar]
- 19.Sun B, Zhang H, Franco LM, Young SP, Schneider A, Bird A, et al. Efficacy of an adeno-associated virus 8-pseudotyped vector in glycogen storage disease type II. Mol Ther. 2005;11:57–65. doi: 10.1016/j.ymthe.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Larsen F, Gundersen G, Lopez R, Prydz H. CpG islands as gene markers in the human genome. Genomics. 1992;13:1095–1107. doi: 10.1016/0888-7543(92)90024-m. [DOI] [PubMed] [Google Scholar]
- 21.Ponger L, Duret L, Mouchiroud D. Determinants of CpG islands: expression in early embryo and isochore structure. Genome Res. 2001;11:1854–1860. doi: 10.1101/gr.174501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamashita R, Suzuki Y, Sugano S, Nakai K. Genome-wide analysis reveals strong correlation between CpG islands with nearby transcription start sites of genes and their tissue specificity. Gene. 2005;350:129–136. doi: 10.1016/j.gene.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 23.Gaszner M, Felsenfeld G. Insulators: exploiting transcriptional and epigenetic mechanisms. Nat Rev Genet. 2006;7:703–713. doi: 10.1038/nrg1925. [DOI] [PubMed] [Google Scholar]
- 24.Xiao X, Li J, Samulski RJ. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J Virol. 1998;72:2224–2232. doi: 10.1128/jvi.72.3.2224-2232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li C, Hirsch M, Asokan A, Zeithaml B, Ma H, Kafri T, et al. Adeno-associated virus type 2 (AAV2) capsid-specific cytotoxic T lymphocytes eliminate only vector-transduced cells coexpressing the AAV2 capsid in vivo. J Virol. 2007;81:7540–7547. doi: 10.1128/JVI.00529-07. [DOI] [PMC free article] [PubMed] [Google Scholar]