Abstract
Background: When exogenous testosterone or treatments to elevate testosterone (human chorionic gonadotropin [HCG] or Clomid) are prescribed for men who have antecedent thrombophilia, deep venous thrombosis and pulmonary embolism often occur and may recur despite adequate anticoagulation if testosterone therapy is continued. Case Presentation: A 55-year-old white male was referred to us because of 4 thrombotic events, 3 despite adequate anticoagulation over a 5-year period. We assessed interactions between thrombophilia, exogenous testosterone therapy, and recurrent thrombosis. In 2009, despite low-normal serum testosterone 334 ng/dL (lower normal limit [LNL] 300 ng/dL), he was given testosterone (TT) cypionate (50 mg/week) and human chorionic gonadotropin (HCG; 500 units/week) for presumed hypogonadism. Ten months later, with supranormal serum T (1385 ng/dL, upper normal limit [UNL] 827 ng/dL) and estradiol (E2) 45 pg/mL (UNL 41 pg/mL), he had a pulmonary embolus (PE) and was then anticoagulated for 2 years (enoxaparin, then warfarin). Four years later, on TT-HCG, he had his first deep venous thrombosis (DVT). TT was stopped and HCG continued; he was anticoagulated (enoxaparin, then warfarin, then apixaban, then fondaparinux). One year after his first DVT, on HCG, still on fondaparinux, he had a second DVT (5/315), was anticoagulated (enoxaparin + warfarin), with a Greenfield filter placed, but 8 days later had a second PE. Thrombophilia testing revealed the lupus anticoagulant. After stopping HCG, and maintained on warfarin, he has been free of further DVT-PE for 9 months. Conclusion: When DVT-PE occur on TT or HCG, in the presence of thrombophilia, TT-HCG should be stopped, lest DVT-PE reoccur despite concurrent anticoagulation.
Keywords: testosterone, human chorionic gonadotropin, Clomid, deep venous thrombosis, pulmonary embolus, anticoagulation
Case Report
A white male, age 55, was referred to us (June 8, 2015) because of 4 thrombotic events, 3 despite adequate anticoagulation over a 5-year period. We assessed interactions between thrombophilia, exogenous testosterone therapy, and recurrent thrombosis.
A previously healthy 55-year-old skilled welder developed acromegaly and had removal of a growth-hormone secreting pituitary adenoma in 2002. Seven years later (July 2009), after complaints of fatigue and increasing muscle weakness, he was found to have low-normal serum testosterone 334 ng/dL (lower normal limit [LNL] 300 ng/dL) and high estradiol [E2] 45 pg/mL (upper normal limit [UNL] 41 pg/mL), and despite these findings, he was started on 50 mg intramuscular testosterone therapy (TT) cypionate/week and intramuscular human chorionic gonadotropin (HCG) 250 IU twice/week. On TT-HCG, both serum total T and E2 became supranormal (T: 1385 ng/dL, UNL 827 ng/dL; E2: 45 pg/mL, UNL 41).
Ten months after starting TT-HCG, in May 2010, he sustained multiple pulmonary embolus PE (Figure 1). Sonography for deep venous thrombosis (DVT) was negative, and he was treated with enoxaparin and then warfarin, which was continued for 2 years (Figure 1). At the time of the PE, the injected testosterone cypionate was replaced by testosterone gel 50 mg/day, which was continued along with the HCG for 10 months, after which the gel was changed back to the injections (testosterone cypionate 50 mg/week; (Figure 1).
Figure 1.
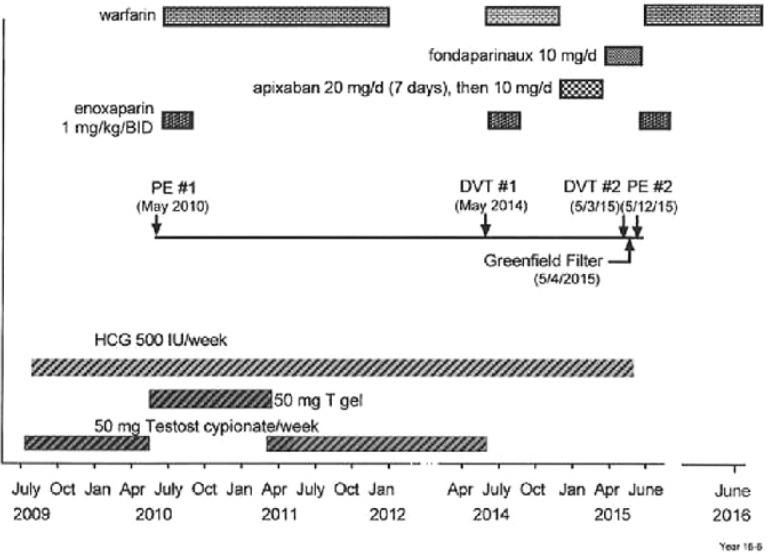
Recurrent pulmonary embolism (PE) and deep venous thrombosis (DVT) despite anticoagulation when testosterone-human chorionic hormone (HCG) therapy was continued in a patient later shown to have the lupus anticoagulant. No further DVT or PE in 9 months after stopping HCG.
On TT-HCG (March 18, 2014), his serum E2 was high (54 pg/mL, UNL 41), and total T was normal (543 ng/dL, laboratory normal range 300-827).
In May 2014, 4 years from the initial PE, on TT-HCG but no anticoagulation, DVT #1 in the right leg was diagnosed. TT was stopped, but HCG was continued (Figure 1). Anticoagulation with enoxaparin and warfarin (8 months) was given, then apixaban (20 mg/day for 7 days, then 5 mg BID) and then fondaparinux 10 mg/day for another 4 months (Figure 1).
On May 3, 2015, 1 year after his first DVT, despite having been on enoxaparin, then warfarin, then apixaban, and then fondaparinux, while still on HCG, his second DVT occurred (Figure 1). Venous Doppler revealed a 20″ long nonocclusive thrombus in the right leg. A Greenfield filter was placed (May 4, 2015), enoxaparin and warfarin were re-instituted, and the HCG was stopped on our consultation (May 10, 2015). However, his second PE occurred 8 days after placement of the Greenfield filter (Figure 1) while still receiving both enoxaparin and warfarin (Figure 1). After stopping the HCG, and maintained on enoxaparin and warfarin together, and then warfarin alone for 9 months, he has had no recurrent DVT-PE (Figure 1).
We evaluated the patient for the first time in Cincinnati on May 28, 2015. Five years from his first PE, our tests of thrombophilia and hypofibrinolysis revealed the presence of the lupus anticoagulant, measured while on warfarin, with DRVVT 57.9 seconds (reference <55) and hexagonal phospholipid neutral 14.
Being off the HCG for 2 weeks and off TT for a year, serum T was normal (622 ng/dL; laboratory normal range 348-1197 ng/dL), and E2 was normal (19.1 pg/mL; laboratory normal range 7.6-42.6 pg/mL).
Discussion
TT is often indiscriminately prescribed1 without consideration of risks.2 As in this case, many men given TT do not meet well-defined diagnostic and therapeutic criteria.3 Before TT-HCG, our patient was found to have low-normal serum T (334 ng/dL; LNL 300 ng/dL) and high E2 (45 pg/mL, UNL 41 pg/mL). Despite these findings, he was started on 50 mg testosterone cypionate and HCG 500 IU per week. On TT-HCG serum total T became supranormal (1385 ng/dL, UNL 827), and serum E2 was high (45 pg/mL; UNL 41), both speculatively contributing to his DVT-PE events, interacting with his lupus anticoagulant.
In 36 hypogonadal patients in our center studied before and on conventional TT (50-60 mg gel/day) 11% developed supranormal serum T (>800 ng/mL) on TT.4 In these 36 patients, E2 was supranormal (>42.6 pg/mL) before TT in 6%, and on TT in 14%. Conventional TT can lead to supranormal T and E2 in hypogonadal patients.
We have previously described 11 of 67 patients with thrombophilia given exogenous TT5,6 who sustained a first venous thromboembolic event (VTE) and then, while continuing TT, and despite adequate anticoagulation, sustained a second VTE. Moreover, of these 11 patients, with TT further continued, 6 men4-6 had a third VTE, despite continuing adequate anticoagulation. Recurrent PEs and DVTs despite anticoagulation in our current case when TT-HCG or HCG alone were continued parallels our previous 11 patients’4-6 recurrent thrombotic events. After stopping HCG, with continuation of warfarin, in the current study, the subject had no further DVT-PE over the subsequent 9 months of follow-up.
In the current case, the sole thrombophilia identified was the lupus anticoagulant, which was present during warfarin anticoagulation. Recent studies have suggested that despite concurrent warfarin, the lupus anticoagulant can be detected by dilute Russel’s Viper Venom time.7,8 However, the lupus anticoagulant can be transient and should be remeasured over time for optimal confirmation.
In 2014, both the US Food and Drug Administration9 and Canada Health,10 based on postmarketing data, required manufacturers to add a warning to testosterone product labels about the potential risks of VTE, including DVT and PE. As a result, there is now broad public concern regarding this issue.
TT and/or HCG are conventionally used in the treatment of hypogonadism.11 High serum E2 is common during TT therapy5,6,12,13 via aromatization from exogenous T and may provide a direct stimulus to thrombosis, particularly when, as in the current case, TT supplementation leads to supranormal serum T, and subsequently to supranormal E2. TT can also promote VTE via increased blood pressure,14 polycythemia,15,16 decrements in high-density lipoprotein cholesterol,14,16 blood hyperviscosity, and platelet aggregation.16-18 Intramuscular TT increases platelet thromboxane A2 receptor density and platelet aggregation,19 increasing adhesion to the coronary artery endothelium and thrombus formation, with subsequent plaque rupture and acute coronary syndrome.20 Dihydrotestosterone can promote acute coronary events through enhanced monocyte activation.21,22
From 2001 to 2011, androgen use in men age ≥40 has increased more than 3-fold.23 Despite the example of thrombotic and cardiovascular events related to sex-hormone therapy in postmenopausal women from the Women’s Health Initiative,24,25 TT is rapidly increasing, often indiscriminately, without understanding of its long-term effects.1,26
Use of TT in young men with classic forms of hypogonadism is considered effective and there is little disagreement regarding its use in this patient population.3 However, T levels fall with age,27,28 with chronic disease,29,30 and with obesity29 but not with smoking.31,32 With aging, accumulation of obesity, and development of diabetes, more and more men have lower T, but do not meet diagnostic criteria3 for hypogonadism. Moreover, most normal ranges come from healthy younger men.33 Differences in T assay methods34,35 and adverse muscle symptoms at different T levels further interfere with efficient diagnosis.36
Use of TT in men affected by late onset hypogonadism (LOH),37,38 where low T has no definable etiology beyond aging and/or chronic disease, is controversial, with concerns over its effectiveness and safety.2,39-42 Indiscriminate use of TT for LOH is a real issue,2,4,5,43-45 as this form of therapy is administered to almost 1 in 25 American men over the age of 60.46
Increasing use of TT may have thrombotic12,13,47-50 and cardiovascular ramifications.2,51,52 Adverse or intermediate cardiovascular disease (CVD) outcomes have been widely reported after TT was started.2,14,16,41-43,51,53,54 In contrast, 2 studies demonstrated significant CVD event reduction on TT.39,40 A placebo-controlled clinical trial demonstrated that in patients age 65 or older, raising low testosterone to mid-normal range improved sexual function, mood, and symptoms of depression, but failed to improve vitality and walking distance.55 The sample size was too small provide conclusions about TT risks.55
We have compared 67 patients who sustained VTE4 after starting TT to 76 controls, not taking TT, who sustained DVT/PE. Of the 67 VTE TT patients, 16 (24%) were found to be heterozygous for the factor V Leiden mutation versus 7 of the 76 (7%) VTE controls not on TT, P = .004. The 67 patients with VTE on TT also were more likely than the 76 VTE no-TT controls to have the lupus anticoagulant, 9/64 (14%) versus 2/76, 3%, P = .023.4 When screening men4 for thrombophilia before starting TT, we suggest that minimal tests include the factor V Leiden mutation and the lupus anticoagulant, while more extensive tests would also include PCR for the G20210A prothrombin gene mutation, factors VIII and XI, and homocysteine.
In contrast to our clinical case-control series,4 in an observational population study by Baillargeon et al,56 TT use was not associated with increased VTE events. Having filled a prescription for TT was not significantly associated with increased risk of VTE.56 In a retrospective study of male adults with low serum T at a low-moderate baseline risk of DVT/PE, and after excluding men with prior history of DVT/PE, cancer, hypercoagulable state, and chronic anticoagulation, Sharma et al57 did not detect a significant association between TT and risk of DVT/PE. However, almost all of our 67 subjects4 with VTE after starting TT would have been excluded by Sharma et al57 because of antecedent DVT/PE, hypercoagulable state, and chronic anticoagulation, biasing against recognizing a significant association4 between TT and DVT-PE events.57
Particularly since our recent retrospective study of men and women hospitalized for VTE44 revealed that personal and family history were nonspecific and insensitive to effectively identify subjects in whom TT and estrogen replacement therapy should not be given, prescreening for thrombophilia is realistic. However, it is expensive, with average laboratory costs in our center $1200. On the other hand, direct hospital costs for VTE events are about $15 000,58 and higher for re-admission, and there are high direct and indirect costs of post–phlebitic syndrome. It would be valuable to do a pharmacoeconomic study of the number of quality of life years realized by prescreening for coagulation factors before starting TT, and the balance between the cost of hospitalization for VTE and dollars saved by prescreening. This analysis should provide an incremental cost-effectiveness ratio within a society willingness-to-pay threshold.
Why TT induces VTE in thrombophilic patients despite correct use of anticoagulation is unknown, but we speculate that testosterone with subsequent aromatization to estradiol must interact with underlying coagulation factors to overwhelm the expected protection from the anticoagulant.
A limitation of our study is that there is only one set of lupus anticoagulant data. The lupus anticoagulant may be transient,59 and therefore we do not know that it was present during the entire time frame that the patient was studied. Even if the lupus anticoagulant was only transient, based on this case and in 11 similar cases (of 67 previously reported),4 we would never continue TT despite concurrent anticoagulants in patients with previous VTE, particularly if they had familial thrombophilia or repetitively demonstrated lupus anticoagulant.
Conclusions
When DVT-PE occurs in men or women48 given exogenous TT and/or HCG, and especially in the presence of thrombophilia-hypofibrinolysis,4-6 we believe that continued or subsequent use of TT is contraindicated. As in this case, and in the presence of thrombophilia, continuation of anticoagulation therapy during TT repetitively fails to prevent recurrent DVT-PE, and to date, the only solution is to stop the TT. Moreover, we suggest that screening for thrombophilia be done before starting TT in order to avoid interaction of TT with underlying thrombophilia with resultant VTE. If thrombophilia is found before starting TT, this substantially changes estimations of risk-benefit ratios related to TT.
Footnotes
Authors’ Note: This study followed a protocol approved by our institutional review board. Signed informed consent was obtained.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Supported in part by the Lipoprotein Research Fund of the Jewish Hospital of Cincinnati.
References
- 1. Anawalt BD. Guidelines for testosterone therapy for men: how to avoid a mad (t)ea party by getting personal. J Clin Endocrinol Metab. 2010;95:2614-2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vigen R, O’Donnell CI, Baron AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310:1829-1836. [DOI] [PubMed] [Google Scholar]
- 3. Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536-2559. [DOI] [PubMed] [Google Scholar]
- 4. Glueck CJ, Prince M, Patel N, et al. Thrombophilia in 67 patients with thrombotic events after starting testosterone therapy [published online November 30, 2015]. Clin Appl Thromb Hemost. doi: 10.1177/1076029615619486. [DOI] [PubMed] [Google Scholar]
- 5. Freedman J, Glueck CJ, Prince M, Riaz R, Wang P. Testosterone, thrombophilia, thrombosis. Transl Res. 2015;165:537-548. [DOI] [PubMed] [Google Scholar]
- 6. Glueck CJ, Wang P. Testosterone therapy, thrombosis, thrombophilia, cardiovascular events. Metabolism. 2014;63:989-994. [DOI] [PubMed] [Google Scholar]
- 7. Kumano O, Ieko M, Naito S, et al. Verification of the guidelines for lupus anticoagulant detection: usefulness of index for circulating anticoagulant in APTT mixing test. Thromb Res. 2014;134:503-509. [DOI] [PubMed] [Google Scholar]
- 8. Olteanu H, Downes KA, Patel J, Praprotnik D, Sarode R. Warfarin does not interfere with lupus anticoagulant detection by dilute Russell’s viper venom time. Clin Lab. 2009;55:138-142. [PubMed] [Google Scholar]
- 9. Food and Drug Administration. MEDWATCH: Testosterone products. FDA/CDER statement. Risk of venous thromboembolism. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm402054.htm. Published June 20, 2014. Accessed July 15, 2016.
- 10. Health Canada. Summary safety review. Testosterone replacement products and cardiovascular risk. http://www.hc-sc.gc.ca/dhp-mps/medeff/reviews-examens/testosterone-eng.php. Published July 15, 2014. Accessed July 15, 2016.
- 11. Crosnoe-Shipley LE, Elkelany OO, Rahnema CD, Kim ED. Treatment of hypogonadotropic male hypogonadism: case-based scenarios. World J Nephrol. 2015;4:245-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Glueck CJ, Goldenberg N, Budhani S, et al. Thrombotic events after starting exogenous testosterone in men with previously undiagnosed familial thrombophilia. Transl Res. 2011;158:225-234. [DOI] [PubMed] [Google Scholar]
- 13. Glueck CJ, Friedman J, Hafeez A, Hassan A, Wang P. Testosterone, thrombophilia, thrombosis. Blood Coagul Fibrinolysis. 2014;25:683-687. [DOI] [PubMed] [Google Scholar]
- 14. Spitzer M, Huang G, Basaria S, Travison TG, Bhasin S. Risks and benefits of testosterone therapy in older men. Nat Rev Endocrinol. 2013;9:414-424. [DOI] [PubMed] [Google Scholar]
- 15. Marchioli R, Finazzi G, Specchia G, et al. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med. 2013;368:22-33. [DOI] [PubMed] [Google Scholar]
- 16. Fernandez-Balsells MM, Murad MH, Lane M, et al. Clinical review 1: adverse effects of testosterone therapy in adult men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2010;95:2560-2575. [DOI] [PubMed] [Google Scholar]
- 17. Baskurt OK, Meiselman HJ. Iatrogenic hyperviscosity and thrombosis. Semin Thromb Hemost. 2012;38:854-864. [DOI] [PubMed] [Google Scholar]
- 18. Peerschke EI, Silver RT, Weksler BB, Yin W, Bernhardt B, Varon D. Examination of platelet function in whole blood under dynamic flow conditions with the cone and plate(let) analyzer: effect of erythrocytosis and thrombocytosis. Am J Clin Pathol. 2007;127:422-428. [DOI] [PubMed] [Google Scholar]
- 19. Ajayi AA, Mathur R, Halushka PV. Testosterone increases human platelet thromboxane A2 receptor density and aggregation responses. Circulation. 1995;91:2742-2747. [DOI] [PubMed] [Google Scholar]
- 20. Stapleton PA, James ME, Goodwill AG, Frisbee JC. Obesity and vascular dysfunction. Pathophysiology. 2008;15:79-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Death AK, McGrath KC, Sader MA, et al. Dihydrotestosterone promotes vascular cell adhesion molecule-1 expression in male human endothelial cells via a nuclear factor-kappaB-dependent pathway. Endocrinology. 2004;145:1889-1897. [DOI] [PubMed] [Google Scholar]
- 22. Pamukcu B, Lip GY, Devitt A, Griffiths H, Shantsila E. The role of monocytes in atherosclerotic coronary artery disease. Ann Med. 2010;42:394-403. [DOI] [PubMed] [Google Scholar]
- 23. Baillargeon J, Urban RJ, Ottenbacher KJ, Pierson KS, Goodwin JS. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med. 2013;173:1465-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333. [DOI] [PubMed] [Google Scholar]
- 25. Valdiviezo C, Lawson S, Ouyang P. An update on menopausal hormone replacement therapy in women and cardiovascular disease. Curr Opin Endocrinol Diabetes Obes. 2013;20:148-155. [DOI] [PubMed] [Google Scholar]
- 26. Vitry AI, Mintzes B. Disease mongering and low testosterone in men: the tale of two regulatory failures. Med J Aust. 2012;196:619-621. [DOI] [PubMed] [Google Scholar]
- 27. Layton JB, Li D, Meier CR, et al. Testosterone lab testing and initiation in the United Kingdom and the United States, 2000-2011. J Clin Endocrinol Metab. 2014;99:835-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Araujo AB, Esche GR, Kupelian V, et al. Prevalence of symptomatic androgen deficiency in men. J Clin Endocrinol Metab. 2007;92:4241-4247. [DOI] [PubMed] [Google Scholar]
- 29. Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87:589-598. [DOI] [PubMed] [Google Scholar]
- 30. Wu FC, Tajar A, Pye SR, et al. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab. 2008;93:2737-2745. [DOI] [PubMed] [Google Scholar]
- 31. Svartberg J, Jorde R. Endogenous testosterone levels and smoking in men. The fifth Tromso study. Int J Androl. 2007;30:137-143. [DOI] [PubMed] [Google Scholar]
- 32. Wang W, Yang X, Liang J, et al. Cigarette smoking has a positive and independent effect on testosterone levels. Hormones (Athens). 2013;12:567-577. [DOI] [PubMed] [Google Scholar]
- 33. Lazarou S, Reyes-Vallejo L, Morgentaler A. Wide variability in laboratory reference values for serum testosterone. J Sex Med. 2006;3:1085-1089. [DOI] [PubMed] [Google Scholar]
- 34. McShane LM, Dorgan JF, Greenhut S, Damato JJ. Reliability and validity of serum sex hormone measurements. Cancer Epidemiol Biomarkers Prev. 1996;5:923-928. [PubMed] [Google Scholar]
- 35. Yun YM, Botelho JC, Chandler DW, et al. Performance criteria for testosterone measurements based on biological variation in adult males: recommendations from the Partnership for the Accurate Testing of Hormones. Clin Chem. 2012;58:1703-1710. [DOI] [PubMed] [Google Scholar]
- 36. Finkelstein JS, Yu EW, Burnett-Bowie SA. Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med. 2013;369:2457. [DOI] [PubMed] [Google Scholar]
- 37. Corona G, Maseroli E, Rastrelli G, et al. Is late-onset hypogonadotropic hypogonadism a specific age-dependent disease, or merely an epiphenomenon caused by accumulating disease-burden? Minerva Endocrinol. 2016;41:196-210. [PubMed] [Google Scholar]
- 38. Rastrelli G, Corona G, Tarocchi M, Mannucci E, Maggi M. How to define hypogonadism? Results from a population of men consulting for sexual dysfunction. J Endocrinol Invest. 2016;39:473-484. [DOI] [PubMed] [Google Scholar]
- 39. Wallis CJ, Lo K, Lee Y, et al. Survival and cardiovascular events in men treated with testosterone replacement therapy: an intention-to-treat observational cohort study. Lancet Diabetes Endocrinol. 2016;4:498-506. [DOI] [PubMed] [Google Scholar]
- 40. Sharma R, Oni OA, Gupta K, et al. Normalization of testosterone level is associated with reduced incidence of myocardial infarction and mortality in men. Eur Heart J. 2015;36:2706-2715. [DOI] [PubMed] [Google Scholar]
- 41. Layton JB, Meier CR, Sharpless JL, Sturmer T, Jick SS, Brookhart MA. Comparative safety of testosterone dosage forms. JAMA Intern Med. 2015;175:1187-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Anderson JL, May HT, Lappe DL, et al. Impact of testosterone replacement therapy on myocardial infarction, stroke, and death in men with low testosterone concentrations in an integrated health care system. Am J Cardiol. 2016;117:794-799. [DOI] [PubMed] [Google Scholar]
- 43. Basaria S, Harman SM, Travison TG, et al. Effects of testosterone administration for 3 years on subclinical atherosclerosis progression in older men with low or low-normal testosterone levels: a randomized clinical trial. JAMA. 2015;314:570-581. [DOI] [PubMed] [Google Scholar]
- 44. Prince M, Glueck CJ, Shah P, et al. Hospitalization for pulmonary embolism associated with antecedent testosterone or estrogen therapy in patients found to have familial and acquired thrombophilia. BMC Hematol. 2016;16:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Glueck CJ, Riaz R, Prince M, Freiberg RA, Wang P. Testosterone therapy can interact with thrombophilia, leading to osteonecrosis. Orthopedics. 2015;38:e1073-e1078. [DOI] [PubMed] [Google Scholar]
- 46. Stanworth RD, Jones TH. Testosterone for the aging male; current evidence and recommended practice. Clin Interv Aging. 2008;3:25-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Glueck CJ, Richardson-Royer C, Schultz R, et al. Testosterone therapy, thrombophilia-hypofibrinolysis, and hospitalization for deep venous thrombosis-pulmonary embolus: an exploratory, hypothesis-generating study. Clin Appl Thromb Hemost. 2014;20:244-249. [DOI] [PubMed] [Google Scholar]
- 48. Glueck CJ, Bowe D, Valdez A, Wang P. Thrombosis in three postmenopausal women receiving testosterone therapy for low libido. Womens Health (Lond Engl). 2013;9:405-410. [DOI] [PubMed] [Google Scholar]
- 49. Glueck CJ, Richardson-Royer C, Schultz R, et al. Testosterone, thrombophilia, and thrombosis. Clin Appl Thromb Hemost. 2014;20:22-30. [DOI] [PubMed] [Google Scholar]
- 50. Pandit RS, Glueck CJ. Testosterone, anastrozole, factor V Leiden heterozygosity and osteonecrosis of the jaws. Blood Coagul Fibrinolysis. 2014;25:286-288. [DOI] [PubMed] [Google Scholar]
- 51. Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363:109-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Finkle WD, Greenland S, Ridgeway GK, et al. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS One. 2014;9(1):e85805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Anderson RA, Ludlam CA, Wu FC. Haemostatic effects of supraphysiological levels of testosterone in normal men. Thromb Haemost. 1995;74:693-697. [PubMed] [Google Scholar]
- 54. Xu L, Freeman G, Cowling BJ, Schooling CM. Testosterone therapy and cardiovascular events among men: a systematic review and meta-analysis of placebo-controlled randomized trials. BMC Med. 2013;11:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Snyder PJ, Bhasin S, Cunningham GR, et al. Effects of testosterone treatment in older men. N Engl J Med. 2016;374:611-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Baillargeon J, Urban RJ, Morgentaler A, et al. Risk of venous thromboembolism in men receiving testosterone therapy. Mayo Clin Proc. 2015;90:1038-1045. [DOI] [PubMed] [Google Scholar]
- 57. Sharma R, Oni OA, Chen G, et al. Association between testosterone replacement therapy and the incidence of deep vein thrombosis and pulmonary embolism: a retrospective cohort study of the Veterans Administration database [published online May 11, 2016]. Chest. doi: 10.1016/j.chest.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 58. Fernandez MM, Hogue S, Preblick R, Kwong WJ. Review of the cost of venous thromboembolism. Clinicoecon Outcomes Res. 2015;7:451-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pahus SH, Hansen AT, Hvas AM. Thrombophilia testing in young patients with ischemic stroke. Thromb Res. 2016;137:108-112. [DOI] [PubMed] [Google Scholar]


