Abstract
Background
Cyanobacteria are major primary producers in extreme cold ecosystems. Many lineages of cyanobacteria thrive in these harsh environments, but it is not fully understood how they survive in these conditions and whether they have evolved specific mechanisms of cold adaptation. Phormidesmis priestleyi is a cyanobacterium found throughout the cold biosphere (Arctic, Antarctic and alpine habitats). Genome sequencing of P. priestleyi BC1401, an isolate from a cryoconite hole on the Greenland Ice Sheet, has allowed for the examination of genes involved in cold shock response and production of extracellular polymeric substances (EPS). EPSs likely enable cyanobacteria to buffer the effects of extreme cold and by identifying mechanisms for EPS production in P. priestleyi BC1401 this study lays the way for investigating transcription and regulation of EPS production in an ecologically important cold tolerant cyanobacterium.
Results
We sequenced the draft genome of P. priestleyi BC1401 and implemented a new de Bruijn graph visualisation approach combined with BLAST analysis to separate cyanobacterial contigs from a simple metagenome generated from non-axenic cultures. Comparison of known cold adaptation genes in P. priestleyi BC1401 with three relatives from other environments revealed no clear differences between lineages. Genes involved in EPS biosynthesis were identified from the Wzy- and ABC-dependent pathways. The numbers of genes involved in cell wall and membrane biogenesis in P. priestleyi BC1401 were typical relative to the genome size. A gene cluster implicated in biofilm formation was found homologous to the Wps system, although the intracellular signalling pathways by which this could be regulated remain unclear.
Conclusions
Results show that the genomic characteristics and complement of known cold shock genes in P. priestleyi BC1401 are comparable to related lineages from a wide variety of habitats, although as yet uncharacterised cold shock genes in this organism may still exist. EPS production by P. priestleyi BC1401 likely contributes to its ability to survive efficiently in cold environments, yet this mechanism is widely distributed throughout the cyanobacterial phylum. Discovering how these EPS related mechanisms are regulated may help explain why P. priestleyi BC1401 is so successful in cold environments where related lineages are not.
Electronic supplementary material
The online version of this article (doi:10.1186/s12864-016-2846-4) contains supplementary material, which is available to authorized users.
Keywords: Cyanobacteria, Cryosphere, Cryoconite, Cold-adaptation, Exopolysaccharides, Biofilms, ABC-transporters, c-di-GMP
Background
Cyanobacteria are photosynthetic prokaryotes that have thrived on our planet for at least 2.33–2.4 Ga [1, 2]. In the cryosphere cyanobacteria are major primary producers found in many types of habitats (e.g., lakes, lithic substrates and cryoconite [3]) where potential environmental pressures include exposure to high levels of ultraviolet radiation during the summer, complete absence of light during the winter at the Poles, limited nutrient availability, rapid freeze-thaw cycles and low water activity [4].
Microorganisms (including bacteria, archaea and unicellular eukaryotes) have numerous adaptations that enable them to survive at low temperatures. Generic cold-shock mechanisms exist (many of which have been characterised in mesophiles like Escherichia coli) that are responsible for maintaining cell membrane fluidity, destabilising nucleic acid secondary structures and helping to maintain chromosome structure in cold conditions (reviewed in Barria et al. [5]). Other mechanisms that have been proposed include the production of exopolysaccharides (EPSs), which play a major role in protection from extreme cold environments (e.g., Bacteria: Colwellia psychrerythraea 34H [6], Pseudomonas sp. ID1 [7] and Pseudoalteromonas sp. SM20310 [8]; Archaea: Methanococcoides burtonii and Halorubrum lacusprofundi [9]; Diatoms: Synedropsis sp., Fragilariopsis curta and F. Cylindrus [10]. In cyanobacteria, EPSs are known to enable desiccation resistance in arid habitats [11–13] and contribute to biofilm formation [14, 15]. In cold environments EPSs may have a role in freeze-thaw tolerance [12, 13]. EPSs likely provide a site for the localisation of ultraviolet protective compounds such as scytonemin and mycosporine-like amino acids (MAAs) and allow for the scavenging of metal cations in oligotrophic conditions [16]. EPSs also serve as a carbon source for the microbial web [17–19].
While all of these mechanisms can be found in the majority of prokaryotes, little is understood about how they might differ in cyanobacteria from cold environments compared to their temperate relatives. Many cyanobacteria commonly found in the cryosphere are also found in a variety of diverse habitats (e.g., deserts: Chroococcidiopsis [20] and Nostoc [21]; caves: Chroococcidiopsis, Nostoc, Phormidium, Leptolyngbya and Pseudanabaena [22]; soil crusts: Phormidium, Leptolyngbya and Pseudanabaena [23]) where their persistence is due to a non-specific resilience to extreme environments. Some lineages appear more localised to the cryosphere and as such may be more specialised to surviving in the cold. One such organism is the EPS producing, non-heterocystous filamentous cyanobacteria Phormidesmis priestleyi. Analysis of small subunit (SSU) rRNA gene sequences has shown that P. priestleyi can be found throughout the global cryosphere [24, 25]. Trait evolution analyses have predicted that P. priestleyi had a cold tolerant ancestor [25].
In the Antarctic, P. priestleyi commonly grows in de-glaciated regions where it is typically found associated with moving water and glacial run-off [26]. In the Arctic and Alpine regions P. priestleyi SSU rRNA gene sequences have been recovered from surface ice on glaciers in Svalbard [27], oligotrophic lakes in the Pyrenees [28], glaciers on the Tibetan Plateau [29] and meltwater lakes on ice shelves in the Canadian High Arctic [24]. On the Greenland Ice Sheet (GrIS) it can be found in cryoconite holes; melt water pools on the surface of glaciers formed by reduced local albedo that contain inorganic and organic particulate matter [30]. Cyanobacterial filaments constitute a large proportion of organic matter present in cryoconite granules and it is believed that cyanobacteria help to mediate the formation of cryoconite through the aggregation of particulate matter [31, 32] thus directly influencing the ‘biocryomorphology’ of ice surfaces [33].
In this study we sequenced the genome of P. priestleyi BC1401 using a recently developed de Bruijn graph assembly viewer to remove non-cyanobacterial sequences from the assembly. We used BLAST analyses to investigate the presence of known cold stress related genes in this and closely related genomes. We identified genes for putative mechanisms responsible for the regulation, production and export of polysaccharides using BLAST and Pfam domain searches. P. priestleyi BC1401 is the first published draft genome of a cyanobacterium isolated from the cryosphere.
Methods
Cryoconite material was collected during the summer of 2014 from nearby the University of Utrecht’s S6 weather station on the GrIS (67°04′ N, 49°23′ W) ~1000 m above sea level and ~30 km from the ice margin, before being transported back to Bristol in a chilled container (~2–4 °C). In the laboratory, small amounts of cryoconite were transferred to petri dishes of sterile liquid BG-11 [34] and incubated at 4 °C for 4–6 weeks.
Axenic cultures have typically been used for the sequencing of single strains of cyanobacteria. However, several problems are associated with this approach. Firstly, certain strains may be reliant upon their commensal biota for successful growth e.g. due to utilisation of secondary metabolites [35]. Second, the process is labour intensive and hard to include within the timeframe of a small scale research program. Finally, cultures will undergo many generations during the process of growth and purification; this might result in significant molecular divergence of cultured strains from wild-type organisms. The methods shown here have allowed for the sequencing of a unialgal strain along with its commensal microbiota with the successful removal of contaminating (non-cyanobacteria) sequences during the assembly process. To obtain unialgal strains individual cyanobacterial filaments were separated using a microscope and glass needle, transferred to culture tubes containing sterile liquid BG-11, and incubated at 15 °C. After a further 4–6 weeks, a successful culture was transferred back to petri dishes of sterile liquid BG-11 and grown at 4 °C until sufficient biomass was available to extract gDNA. Harvested cells were stored in sterile 1.5 ml tubes at −20 °C.
DNA extraction and sequencing
Extraction of DNA from cyanobacteria can often be inhibited by the presence of EPSs and thick cell walls. In order to address this issue we used a simple chemical/mechanical lysis method to rupture cells prior to extraction. Approximately 0.5 ml cellular material was defrosted and centrifuged at 13,000 G for 30 s in a 1.5 ml tube. The supernatant was removed and the pellet washed in 500 μl Milli-Q water. This process was repeated three times before the pellet was transferred to a clean 1.5 ml tube. The pellet was then re-suspended in 200 μl SoluLyse (Genlantis, San Diego, CA) (see Hall et al. [36]) and incubated at room temperature for 15 min. The entire contents of the tube were then transferred to a MO BIO (MO BIO Laboratories, Cambridge, UK) 0.7 mm bead beading tube and vortexed at full power for 5 min. gDNA was then extracted from the lysate using Machery-Nagel AXG20 (Machery-Nagel, Düren, Germany) gravity flow columns according to the manufacturers protocol (including the optional step of addition of lysozyme). Integrity of high molecular weight gDNA was assessed using gel imaging (1 % agarose gel for 1.5 h) and quantified using QUBIT (Invitrogen, Carlsbad, CA) assay before being sent for sequencing. Library preparation was done using the Illumina TruSeq Nano DNA Library Preparation Kit (Illumina, San Diego, CA) according to the manufacturers instructions with a final average library size distribution (including adapters) of 650 bp. Sequencing was done using Illumina Hi-Seq 2500 (one lane) to generate a total of 83,595,840 100 bp paired-end reads with an insert size of ~600 bp. Data was processed using RTA v1.18.64, with default filter and quality settings. The reads were demultiplexed with CASAVA v1.8.4 (allowing no mismatch).
Assembly
Before genome analysis could be carried out it was necessary to separate the cyanobacterial genome from non-cyanobacterial sequences. An ideal prokaryotic assembly produced by a de Bruijn graph based assembler such as SPAdes [37] would produce a single chromosome where there is only one possible path between nodes (i.e., individual contigs). However, ambiguities often remain in an assembly (e.g., due to repeated regions or duplicated genes) that result in de Bruijn graphs with multiple edges (i.e., possible alignments between contigs). While these multi-edges prevent the formation of a single conclusive chromosome, they can be used to determine relationships between clusters of smaller contigs. While this information is usually discarded it can be used to isolate individual genomes from a metagenome, and validated where contigs can be simultaneously defined by some other shared characteristic such as read depth, BLAST similarity or sequence composition. Visualisation of this information has recently been made available in the de Bruijn graph viewer Bandage [38].
Prior to assembly Illumina TruSeq-3-PE adapters were trimmed and reads filtered for quality using Trimmomatic v0.32 [39] using the following parameters: Leading:20, Trailing:20, SlidingWindow:4:20, MinLen:50. Error correction and assembly was done using SPAdes v3.5.0 [37] with k-mer lengths of 67, 77, 87 and 97 and a coverage cut-off of 20. SPAdes FASTG files were then opened in Bandage v0.07 [38] and a BLAST database generated for the entire assembly. One thousand and fifty-four core cyanobacterial clusters of orthologous groups of proteins (core CyOGs) (see Mulkidjanian et al. [40], Supplementary Information) exclusive to cyanobacteria and plastids were searched against this database using tBLASTn v2.2.30+ with an e-value threshold of 1e-10. Cyanobacterial contigs were identified as graph nodes containing core CyOGs and connected nodes with similar read depth. Additional unconnected nodes with similar coverage to confirmed cyanobacterial contigs were checked manually by performing further tBLASTn searches against the entire NCBI GenBank database (e-value threshold of 1e-10); those that did not contain putative cyanobacterial genes were discarded. Finally, contigs with read depth <10 were discarded to remove remaining non-cyanobacterial contigs and contigs with length <200 bp were discarded to bring into line with NCBI standards. Raw reads were mapped to the resulting draft assembly using BWA [41]. Reads that did not map to the cyanobacterial assembly were discarded, revealing an overall coverage of the P. priestleyi BC1401 genome of 340.55×.
The draft genome was submitted to JGI IMG/ER [42] for annotation (GOLD Analysis Project ID: Ga0078185). Based on sequence similarity, the assembled genome was aligned to its nearest neighbour with a finished genome, Leptolygnbya boryana PCC 6306 with Contiguator v2.7.4 [43] using BLASTn with an e-value of 1e-10, a contig length threshold of 1000, a hit length threshold of 1000 and a contig coverage threshold of 10 %. Identification of specific genes of interest was done using BLASTp (e-value threshold of 1e-5) and further searches for Pfam domains were made if BLAST searches yielded ambiguous results. These analyses were performed using tools available in JGI IMG/ER [42].
To infer structural homology of proteins where certain domains were absent, genes were aligned in Jalview v2.9.0b2 [44] and protein structures modelled using the Phyre2 web portal [45].
Genome plots were produced in Circos v0.68 [46] and gene diagrams were made using FancyGene v1.4 [47]. All plots were manually edited using Inkscape v0.91 [48].
This Whole Genome Shotgun project has been deposited at DDBJ/ENA/GenBank under the accession LXYR00000000. The version described in this paper is version LXYR01000000. Genome sequence and annotation data are available at the JGI IMG/ER database [49].
Phylogenetic analysis
A SSU rRNA dataset was constructed including P. priestleyi BC1401 and a further 130 cyanobacteria genomes as used by Sánchez-Baracaldo [50]. Additionally, the SSU rRNA sequence P. priestleyi ANT.L61.2 [51] was included as a reference for an Antarctic strain of P. priestleyi. An alignment of 1,712 characters was generated using SATé 2.2.7 [52] (using MAFFT v6.717 [53], MUSCLE v3.7 [54], FASTTREE v2.1.4 [55] with the CAT approximation and decomposition strategy set to ‘longest’). Phylogenetic reconstruction was carried out using RaxML v8.1.11 [56] using the GTR + G model. The tree was constrained using the 130 taxa phylogenomic (135 protein and two rRNA: LSU and SSU) tree described in Sánchez-Baracaldo [50]. Trees were visualised using FigTree v1.4.0 [57] and manually edited in Inkscape v0.91 [48].
Results and discussion
Our phylogenetic analyses indicate that P. priestleyi BC1401 is sister to Phormidesmis ANT.L61.2 from the McMurdo Dry Valleys, Antarctica. These two lineages are sister to L. boryana PCC 6306, Oscillatoriales cyanobacterium JSC-12 and Geitlerinema sp. PCC 7407 (Fig. 1). These strains are nested within a group of small cell diameter filamentous lineages within the Microcyanobacteria [50]. Genome statistics and sampling locations for all complete genomes used in this study are shown in Table 1.
Fig. 1.
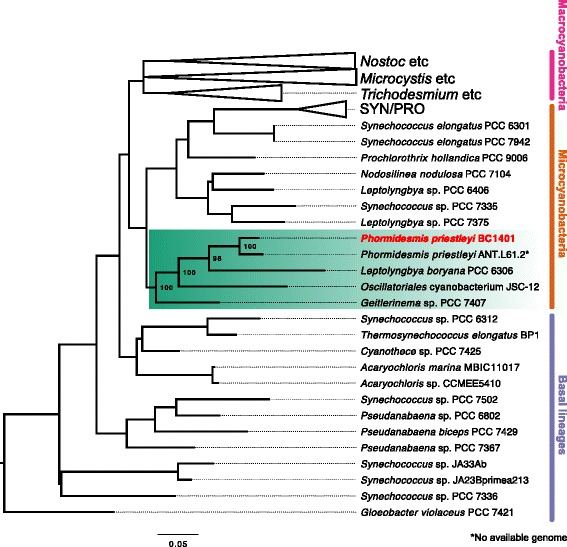
SSU rRNA maximum likelihood phylogeny showing relatives of P. priestleyi BC1401. Tree topology was enforced using a phylogenomic constraint tree containing 130 taxa (135 protein and two rRNA: LSU and SSU) [50]. The tree is divided into ‘Basal lineages’, ‘Microcyanobacteria’ and ‘Macrocyanobacteria’ as described in Sánchez-Baracaldo [50]. The clade containing P. priestleyi BC1401 and related lineages is highlighted in green with bootstrap support (1000 replicates) shown
Table 1.
Genome characteristics of strains used in this study
| Phormidesmis priestleyi BC1401 | Leptolyngbya boryana PCC 6306 | Oscillatoriales cyanobacterium JSC-12 | Geitlerinema sp. PCC 7407 | |
|---|---|---|---|---|
| Geographic location | Greenland Ice Sheet | Unknown | Yellowstone, USA | Unknown |
| Habitat | Cryoconite | Temperate lake | Hot spring | Unknown |
| Environmental temperature | ~0–3 °C | ~4–20 °C | ~37–54 °C | Unknown |
| Genome size(bp) | 5,551,896 | 7,262,454 | 5,530,391 | 4,681,111 |
| DNA coding (%) | 83.8 | 87.93 | 86.41 | 84.37 |
| DNA G+C (%) | 49.16 | 47.01 | 47.47 | 58.46 |
| DNA scaffolds | 213 | 5 | 1 | 1 |
| Scaffold size (bp) | 213–275,582 | 27,440–6,255,543 | 5,530,391 | 4,681,111 |
| N50 | 79,760 | 6,255,543 | 5,530,391 | 4,681,111 |
| Total genes | 5,612 | 6,911 | 5,081 | 3,913 |
| PEGs | 5,507 | 6,827 | 5,024 | 3,854 |
The de Bruijn graph of the entire raw assembly consisted of 752 nodes with 439 edges and a total length of 21,126,715 bp representing a metagenome containing both P. priestleyi BC1401 and its associated microbiota. The main cyanobacterial portion of the graph was determined as a cluster of 268 nodes linked by 355 edges (5,524,226 bp) with a mean depth of 16.5. Of these, 49 nodes with 3 edges (3,795,178 bp) and a mean depth of 14.1 had positive BLAST hits for core CyOGs. All BLAST hits for core CyOGs were contained within the main cyanobacterial portion of the graph [see Additional file 1]. The final assembled draft genome of P. priestleyi BC1401 constituted a total of 213 contigs containing 5,507 protein-encoding genes (PEGs). Contigs ranged from 213 to 2,755,82 bp and the assembly had an N50 of 79,760 bases. Genome size was estimated to be 5.55 Mb with an overall GC content of 49.16 %. The sequenced genome size of P. priestleyi BC1401 was found to be smaller than that of its closet relative with a complete genome, (SSU rRNA gene identity = 92 %) L. boryana PCC 6306 (size = 7.26 Mb, GC = 47.01 %), while having slightly higher GC content. Despite the smaller size in relation to L. boryana PCC 6306 all 1,054 core CyOGs were present in the P. priestleyi BC1401 draft genome suggesting a nearly complete assembly. Eighty-five contigs were successfully mapped to L. boryana PCC 6306 (4.77 Mb, 85.97 % of the total draft genome) (Fig. 2) leaving 128 smaller contigs (0.78 Mb, 14.03 % of the total draft genome) unmapped. A plot displaying all unmapped contigs can be seen in Additional file 2. Forty-five of these contigs were below 1,000 bp in length and 77 were dropped from the alignment due to low coverage.
Fig. 2.
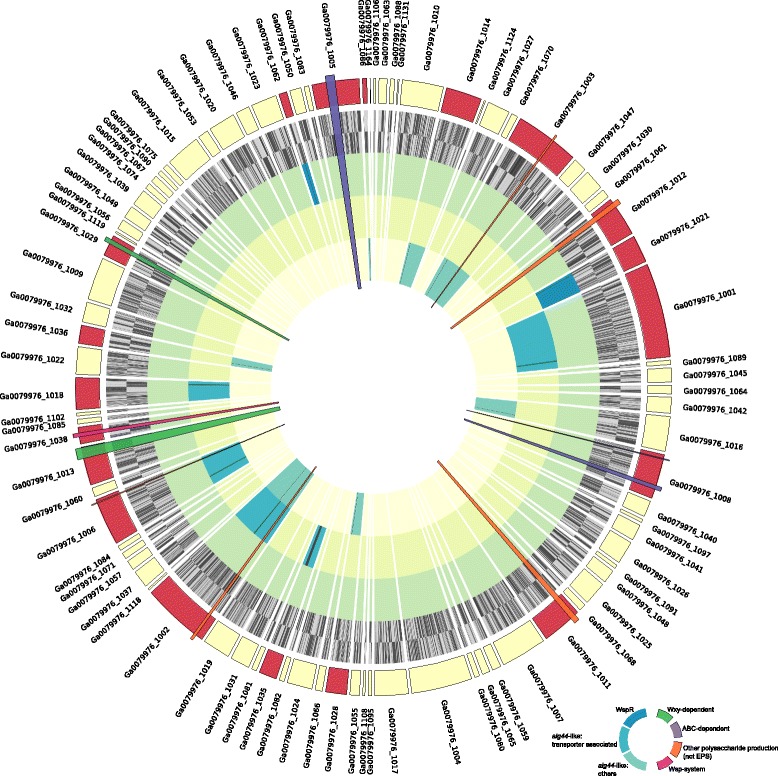
Circular plot of the P. priestleyi BC1401 genome ordered according to L. boryana PCC 6306. Rings are as follows (outer - inner): 1) Mapped contigs, contigs containing genes putatively involved in EPS biosynthesis and export are highlighted in red; 2) Annotated genes are shown in dark grey, outer = plus strand, inner = minus strand; 3) putative wspR homologues are shown in dark grey, contigs are highlighted in blue; 4) alg44-like genes that are components of ABC-transporters are shown in dark grey, contigs are highlighted in blue; 5) other alg44-like genes are shown in dark grey, contigs are highlighted in blue. Wedges show locations of putative gene clusters involved in Wzy-, ABC-dependent-, Wsp- and other non EPS specific polysaccharide biosynthesis/export pathways
Cold adaptation
Molecular evolution related to cold adaptation can leave distinct signatures on organism’s genomes (e.g., Bacteria: Psychrobacter arcticus 273–4 [58], Colwellia psychrerythraea 34H [59]; Archaea: Halobrum lacusprofundi [60], Methanogenium frigidum and Methanococcoides burtonii [61]. In particular, the tendency of certain amino acids to influence protein structures at low temperatures can lead to the substitution of one amino acid for another. For example the amino acid arginine readily forms H-bonds in protein secondary structures; in low temperature environments this can inhibit protein flexibility and prevent optimal protein function. As such, cold adapted organisms may substitute arginine residues with lysine to reduce structural stability at low temperatures as seen in Alteromonas haloplanctis [62]. Similarly, a reduction in proline content can also increase the flexibility of a protein’s secondary structure [63, 64] (e.g., Psychrobacter immobilis A5 [65]). The percentage of proline residues in the hypothetical proteome of P. priestleyi BC1401 (Pro% = 4.76) was similar to that of L. boryana PCC 6306 (Pro% = 4.66) and Oscillatoriales cyanobacterium JSC-12 (Pro% = 4.78) and was slightly higher in Geitlerinema sp. PCC 7407 (Pro% = 5.44). The ratio of arginine to lysine in BC1401 (Arg:Lys = 1.4) was also comparable to PCC 6306 (Arg:Lys = 1.45) and JSC-12 (Arg:Lys = 1.49), while being slightly higher in PCC 7404 (Arg:Lys = 2.22). Given the large temperature ranges experienced by each of these strains (Table 1) it appears that there is no obvious signal of increased genomic adaptation to the cold in BC1401 compared to other cyanobacteria typically found in warmer habitats.
Temperature stress in bacteria is managed by cold shock or heat shock mechanisms, allowing cells to function at low temperatures or to survive above their thermal optimum. In bacteria, many genes have been identified that encode proteins related to cellular responses to cold stress (e.g., pyruvate dehydrogenases, DNA gyrases, chaperones and fatty acid desaturases) [5, 66]. Several of the proteins implicated in cold shock also act as heat shock proteins. Since the physiological optimum of cryoconite phototrophs is above that encountered in the environment [67], cold stress is likely very important in P. priestleyi BC1401. However, if organisms are acclimated to growing at low temperatures then in the event of sudden warming a heat stress response may still occur. The numbers of BLAST hits in the P. priestleyi BC1401 genome for genes implicated in cold shock response as compared to its three closest relatives with complete genomes (Leptolyngbya boryana PCC 6306, Oscillatoriales cyanobacterium JSC-12 and Geitlerinema sp. PCC 7407) are shown in Table 2. All genes listed were present in all genomes with the exception of the following: csp-family genes were absent from Oscillatoriales cyanobacterium JSC-12, otsA was found only in Geitlerinema sp. PCC7407, and yfiaA was absent from L. boryana PCC6306. Otherwise, copy number variation between the four genomes was minimal with no clear increase in cold stress genes in P. priestleyi BC1401. This included genes for chaperones that are also involved in heat shock response (dnaK, dnaJ) and no difference in copy number among the compared cyanobacterial genomes was found for the heat shock specific gene grpE [68]. Even so, it is possible that currently uncharacterised genes involved in cold tolerance (e.g. production of ice nucleation proteins or resistance to osmotic stress [69]) may yet remain unidentified within the P. priestleyi BC1401 genome.
Table 2.
Numbers of BLAST hits for genes implicated in bacterial cold shock response in all strains used in this study
| Gene | Number of BLAST hits | |||
|---|---|---|---|---|
| Phormidesmis priestleyi BC1401 | Leptolyngbya boryana PCC 6306 | Oscillatoriales cyanobacterium JSC12 | Geitlerinema sp. PCC7407 | |
| aceE | 1 | 2 | 2 | 1 |
| aceF | 1 | 2 | 1 | 1 |
| csp-family | 1 | 1 | 0 | 1 |
| deaD | 1 | 1 | 1 | 1 |
| desAB | 2 | 2 | 1 | 2 |
| dnaA | 1 | 1 | 1 | 1 |
| gyrA | 2 | 2 | 2 | 2 |
| dnaK a | 5 | 5 | 6 | 4 |
| dnaJ a | 1 | 1 | 1 | 1 |
| hupB | 4 | 3 | 3 | 1 |
| infA/IF-1 | 1 | 1 | 1 | 1 |
| infB/IF-2 | 1 | 1 | 1 | 1 |
| infC/IF-3 | 1 | 1 | 2 | 2 |
| nusA | 1 | 1 | 1 | 1 |
| otsA | 0 | 0 | 0 | 1 |
| pnp | 1 | 1 | 1 | 1 |
| rnr | 1 | 1 | 1 | 1 |
| rbfA | 1 | 1 | 1 | 1 |
| recA | 1 | 1 | 1 | 1 |
| tig | 1 | 1 | 1 | 1 |
| yfiA | 1 | 0 | 1 | 1 |
| Total | 29 | 29 | 29 | 27 |
The apparent absence of differentiation of cold shock genes between P. priestleyi BC1401 and temperate lineages could be related to the tendency of polar cyanobacteria to be psycrotrophs rather than psychrophiles, exhibiting optimal growth at far higher temperatures than the low ambient temperatures that they are likely to experience in the environment [70]. In light of this, it may be that more universal mechanisms such as the production of EPSs are responsible for the success of cyanobacteria in cold environments.
EPS production mechanisms in P. priestleyi BC1401
While not fully characterised experimentally in cyanobacteria, the genes responsible for EPS biosynthesis and export in cyanobacteria have been clearly outlined in Pereira et al. [71]. These genes encode the constituent proteins of several specific pathways in gram-negative bacteria that were initially studied in E. coli (reviewed in Cuthbertson et al. [72]). In the following sections, we report the distribution of these genes throughout the genome of P. priestleyi BC1401.
Production of prokaryotic EPSs within the cell typically occurs in three main steps [15]. First, oligosaccharides are synthesised within the cytoplasm. Second, repeating units of oligosaccharides are assembled and attached to a lipid carrier by glycosyltransferases. Finally, repeating units are polymerised and translocated out of the cell. This final step is carried out by one of three main systems: the Wzy-dependent, ATP-binding cassette (ABC)-dependent and synthase dependent pathways [16, 64, 73].
In the Wzy-dependent pathway, lipid linked oligosaccharide units are translocated from the cytoplasm to the periplasm by Wzx where they are polymerised by Wzy. Export then occurs via the transmembrane polysaccharide co-polymerase (PCP) Wzc and outer membrane transporter (OPX) Wza. Two gene clusters in P. priestleyi BC1401 followed the scheme for a Wzy-dependent EPS export system, one on contig Ga0079976_1029 and a second on Ga0079976_1013 (Fig. 3a). Both clusters included genes containing domains that are conserved in the genes wza, wzc, wzx, and wzy. In both clusters, wza and wzc were adjacent to each other, while the arrangement of the remainder of associated genes differed considerably. Both clusters included genes containing domains involved in biosynthesis (WcaA domains) and assembly (glycosyltransferases, RfaB domains). Two genes in the cluster on contig Ga0079976_1013 are putatively annotated as exotosins, the glucuronosyltransferase domain of which has been shown to be homologous to the mur3 gene in Arabidopsis thaliana [74]. mur3 is responsible for modification of xyloglucans (involved in crosslinking of cellulose matrices) and the genes found here may encode proteins responsible for similar functions that contribute to the structure of the EPS matrix.
Fig. 3.

Gene diagrams of putative a) Wzy- and b) ABC-dependent clusters in P. priestleyi BC1401. Schematics of the organisation of proteins within the cell membrane are shown (based on Pereira et al. [71])
In the ABC-dependent pathway, polymerisation occurs in the cytoplasm and the ABC-transporter KpsTM transfers assembled polysaccharides across the cytoplasmic membrane, before they are exported from the cell by the PCP/OPX proteins KpsD and KpsE. Two clusters in P. priestleyi BC1401 contained components for the ABC-dependent pathway (Fig. 3b). Gene clusters on contigs Ga0079976_1005 and Ga0079976_1008 contain genes with domains found in kpsD, kpsE, kpsM and kpsT. On contig Ga0079976_1005, kpsM was annotated as two smaller genes and all of the kps_ genes were clustered close together, whereas on Ga0079976_1008 kpsD and kpsE were located ~80kbp upstream of the kpsM and kpsT homologues. As with the Wzy-dependent clusters, several biosynthetic and assembly genes were also present, suggesting relatively self-contained modules for EPS synthesis and export in P. priestleyi BC1401.
In the synthase-dependent pathway, a single protein, Alg8 (which is regulated by Alg44) carries out synthesis, polymerisation and translocation from the cytoplasm to the periplasm. Export is via an outer membrane porin, AlgE which is scaffolded to the membrane by AlgK. Despite several alg8 homologs being found in the genome, no clear synthase-dependent pathways were identified in P. priestleyi BC1401. This is consistent with the findings of Peireira et al. [71] who were unable to confidently identify key components of the synthase-dependent pathway throughout the entire phylum of cyanobacteria.
Out of the known prokaryotic EPS export pathways P. priestleyi BC1401 contained two Wzy- and two ABC-dependent gene clusters and no complete synthase dependent gene clusters, similar to many other cyanobacteria irrespective of the environment [71]. Despite the importance of EPS production for survival in cold environments, there was no indication that the genome of P. priestleyi BC1401 had any relative increase in EPS related genes. EPS genes are contained within the cluster of orthologous genes (COG) category ‘Cell wall/membrane/envelope biogenesis’. A total of 225 genes representing 7.61 % of the P. priestleyi BC1401 genome were included in the ‘Cell wall/membrane/envelope biogenesis’ category (see Fig. 4a) compared to 6.91 % in L. boryana PCC 6306, 7.17 % in Oscillatoriales cyanobacterium JSC-12 and 8.05 % in Geitlerinema sp. PCC 7407. Peireira et al. [71] indicated that genome size rather than habitat had the largest effect on number of EPS related genes. P. priestleyi BC1401 appears to be no exception to this with the number of ‘Cell wall/membrane/envelope biogenesis’ genes falling well within the expected distribution of genomes that size (see Fig. 4b).
Fig. 4.
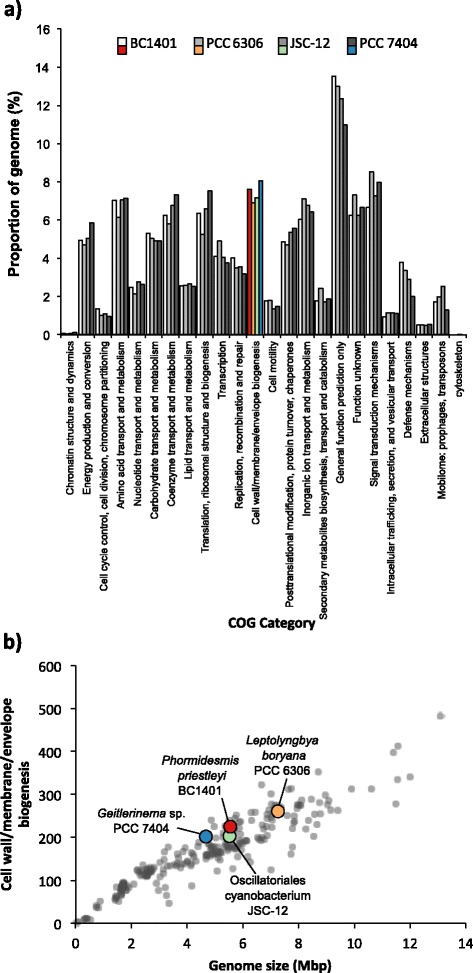
Plots showing proportion of genes involved in COG category ‘Cell wall/membrane/envelope biogenesis’ in P. priestleyi BC1401, L. boryana PCC 6306, Oscillatoriales cyanobacterium JSC-12 and Geitlerinema sp. PCC 7407. a Proportion of all COGs, ‘Cell wall/membrane/envelope biogenesis’ is highlighted and b) The number of ‘Cell wall/membrane/envelope biogenesis’ genes compared to genome size in the context of 265 other cyanobacterial genomes publicly available on JGI IMG/ER
Environmental sensing
While it is clear that many cyanobacteria produce EPSs, the way in which EPS production is regulated in cyanobacteria is not yet understood. The bacterial second messenger bis (3′-5′)-cyclic dimeric guanosine monophosphate (c-di-GMP) has been implicated in a large number of signalling pathways related to EPS biosynthesis and biofilm formation [75, 76]. It is synthesised by proteins containing the conserved GGDEF/GGEEF domain and binds to a wide variety of cellular receptors, which are normally proteins containing PilZ domains. Synthesis of c-di-GMP can be regulated by the Wsp system (first identified in Pseudomonas fluorescens), a chemosensory pathway thought to modulate c-di-GMP levels in response to surface adhesion causing biofilm formation and a transition from motile to sessile lifestyle [77–79]. A nearly complete cassette for the Wsp chemosensory pathway was found in P. priestleyi BC1401, located on contig Ga0079976_1038 (Fig. 5). A gene for a cyclic nucleotide binding protein is followed on the forward strand by wspB, wspC, wspA, wspD, wspE, wspF. In the position normally occupied by the response regulator WspR was a class 3 adenylate cyaclase. Whereas WspR is a GGDEF/GGEEF domain containing diguanylate cyclase, class 3 adenylate cyclases are cyclase homology domain containing mononucleotidyl cyclases. Searches for GGDEF/GGEEF pfam domains within the P. priestleyi BC1401 genome revealed six potential wspR homologues. In-silico modelling of their structures using Phyre2 revealed two proteins featuring homology to WspR with 100 % confidence over an alignment length of >90 % (Table 3, Fig. 6). One of these is a single gene on contig Ga0079976_1021 and the second is on contig Ga0079976_1062. A further five genes contained GGDEF/GGEEF domains but showed low sequence similarity for the remainder of the protein (Table 3).
Fig. 5.
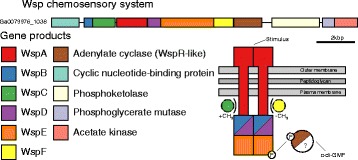
Gene diagrams of putative Wsp-chemosensory system in P. priestleyi BC1401. Schematics of the organisation of proteins within the cell membrane are shown (based on Belas [79])
Table 3.
Locations of genes with GGDEF/GGEEF domains in the P. priestleyi BC1401 draft genome and similarity of the gene product to WspR based upon modelling of protein structure in Phyre2 [45]
| BC1401 gene | Gene Product Name | Length (aa) | Confidence | %ID | Alignment length |
|---|---|---|---|---|---|
| Ga0079976_102110 | Response regulator receiver modulated diguanylate cyclasea | 331 | 100 % | 38 % | 93 % |
| Ga0079976_106215 | Response regulator receiver modulated diguanylate cyclasea | 312 | 100 % | 39 % | 97 % |
| Ga0079976_102431 | Response regulator receiver modulated diguanylate cyclase/phosphodiesterase | 595 | 100 % | 27 % | 50 % |
| Ga0079976_101496 | Response regulator receiver modulated diguanylate cyclase/phosphodiesterase | 613 | 100 % | 27 % | 51 % |
| Ga0079976_10199 | PAS domain S-box-containing protein/diguanylate cyclase (GGDEF) domain-containing protein | 725 | 100 % | 29 % | 23 % |
| Ga0079976_10297 | Diguanylate cyclase (GGDEF) domain-containing protein | 530 | 100 % | 34 % | 21 % |
| Ga0079976_101051 | PAS domain S-box-containing protein/diguanylate cyclase (GGDEF) domain-containing protein | 732 | 100 % | 34 % | 22 % |
Putative wspR homologues are marked with a
Fig. 6.
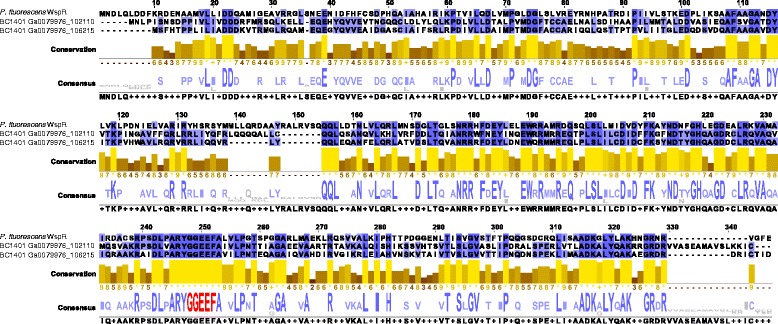
Alignment of two putative WspR homologues in P. priestleyi BC1401 with the WspR of Pseudomonas fluorescens. The GGDEF/GGEEF domain is highlighted in red
For a standard WspR regulated Wsp-system to influence EPS production and biofilm formation, a receptor must exist for synthesised c-di-GMP. Somewhat unexpectedly, genes containing the typical c-di-GMP binding PilZ domains were apparently absent from the P. priestleyi BC1401 draft genome. Blast searches for homologues of the gene for Alg44, the c-di-GMP binding component of the absent synthase dependent pathway, revealed 14 genes that shared the HlyD domain of alg44 while the PilZ domain appeared to be missing (Table 4). Five of these were clustered together with ABC-transporter genes; one such cluster was homologous to DevBCA, the efflux transporter responsible for formation of the heterocyst envelope in diazotrophic cyanobacteria [80, 81]. It should be stressed that ABC-transporters are involved in a wide variety of cellular processes and are not necessarily linked to EPS. Other genes have also been implicated in c-di-GMP binding. For example, non-enzymatic GGDEF/GGEEF domains (e.g., PopA and PelD) have also been shown to operate as c-di-GMP effectors [72]. At least one such protein may exist in P. priestleyi BC1401 where a GGDEF/GGEEF domain was identified but the GGDEF/GGEEF motif itself was absent (Ga0079976_10297). However, despite the clear existence of a well-defined Wsp-like chemosensory system, the exact nature of the system’s response regulators and effectors remain unclear. The close BLAST similarity of Wsp genes in P. priestleyi BC1401 to those in several other cyanobacteria suggest that this may be an important pathway across the cyanobacteria and warrants further investigation if we are to understand the way in which cyanobacteria interact with their environment.
Table 4.
Locations of putative alg44 homologues lacking PilZ domains in the P. priestleyi BC1401 draft genome
| BC1401 Gene | Gene product name | Length (aa) | Domains |
|---|---|---|---|
| Ga0079976_1001251 | HlyD family secretion proteina | 466 | Biotin_lipoyl_2(pfam13533)HlyD_D23(pfam16576) |
| Ga0079976_100279 | HlyD family secretion proteinab | 395 | HlyD_3(pfam13437) Biotin_lipoyl_2(pfam13533) |
| Ga0079976_100288 | HlyD family secretion protein | 477 | HlyD_3(pfam13437) Biotin_lipoyl_2(pfam13533) |
| Ga0079976_100319 | RND family efflux transporter, MFP subunit | 484 | HlyD_D23(pfam16576) |
| Ga0079976_100646 | HlyD family secretion proteina | 385 | HlyD_3(pfam13437) Biotin_lipoyl_2(pfam13533) |
| Ga0079976_10149 | membrane fusion protein, multidrug efflux system | 463 | Biotin_lipoyl_2(pfam13533) HlyD_D23(pfam16576) |
| Ga0079976_101677 | RND family efflux transporter, MFP subunit | 480 | HlyD_D23(pfam16576) |
| Ga0079976_101876 | HlyD family secretion proteina | 438 | Biotin_lipoyl_2(pfam13533)HlyD_D23(pfam16576) |
| Ga0079976_102849 | membrane fusion protein, multidrug efflux system | 556 | HlyD_D23(pfam16576) |
| Ga0079976_103537 | HlyD family secretion proteina | 434 | HlyD_3(pfam13437) Biotin_lipoyl_2(pfam13533) |
| Ga0079976_103615 | RND family efflux transporter, MFP subunit | 438 | Biotin_lipoyl_2(pfam13533)HlyD_D23(pfam16576) |
| Ga0079976_104331 | HlyD family secretion protein | 509 | HlyD_3(pfam13437) Biotin_lipoyl_2(pfam13533) |
| Ga0079976_106912 | RND family efflux transporter, MFP subunit | 443 | HlyD_3(pfam13437) Biotin_lipoyl_2(pfam13533) |
| Ga0079976_10868 | membrane fusion protein, cobalt-zinc-cadmium efflux | 530 | HlyD_D23(pfam16576) |
Genes associated with ABC-transport genes are marked a. Genes associated with DevBCA-type exporters are markedb
The mechanisms of EPS production and regulation described here are by no means unique to P. priestleyi BC1401 and can be found throughout the cyanobacterial phylum [71]. However, they are also not ubiquitous and lineage specific characteristics may mean that certain cyanobacteria are predisposed to exploit certain environments or interact with their environment in particular ways. For example, the Wsp system is not present in all cyanobacteria, being identified in L. boryana PCC 6306 and Geitlerinema sp. PCC 7407 but not in Oscillatoriales cyanobacterium JSC-12. This implies that closely related lineages may interact with their environment in very different ways. In terms of P. priestleyi BC1401, the presence of the Wsp system may have considerable implications for the mechanisms of cryoconite formation. Physical contact of the cell surface of P. priestleyi with particulate matter may result in the activation of biofilm formation and EPS production mechanisms, thus initiating the first stages of cryoconite aggregation. Further investigation into the regulation and expression of this and the EPS production mechanisms will help us to understand how cyanobacteria influence ice sheet surfaces.
Conclusions
The work presented here represents a first step in understanding the molecular underpinnings of adaptation of cyanobacteria to cold environments and raises many questions. The Arctic cyanobacterium P. priestleyi BC1401 is closely related to the Antarctic P. priestleyi based upon SSU similarity and likely represents a cryosphere specific lineage. A standard complement of cold shock genes and a lack of cold biased molecular evolution, as has been seen in other prokaryotes, suggests that P. priestleyi BC1401 does not require these characteristics to tolerate survival in cold environments. Instead, it is likely that the production of EPSs buffers it from the extreme conditions that it experiences. Why P. priestleyi is found in the cryosphere while closely related lineages bearing similar genome characteristics are not is not yet known. This is intriguing since the EPS synthesis and export mechanisms in P. priestleyi BC1401 appear to follow the same scheme that has been reported from throughout the cyanobacterial phylum. Any differences may then be a result of regulation and differential expression under the conditions experienced in cold environments. As a result, future work should include targeted transcriptomics to understand how these genes are expressed and what implications that may have for both the organism and the environment it inhabits. Furthermore, the Wsp chemosensory pathway represents a possible link between the environment and production of EPS and understanding how this operates in P. priestleyi BC1401 may help us to better understand the principles of cryoconite formation.
Abbreviations
ABC, ATP-binding cassette; AF, alignment fraction; C-di-GMP, bis (3′-5′)-cyclic dimeric guanosine monophosphate; COG, clusters of orthologous genes; CyOG, cyanobacterial clusters of orthologous groups of proteins; EPS, exopolysaccharide; gANI, genomic average nucleotide identity; GrIS, Greenland Ice Sheet; MAA, mycosporine-like amino acid; OPX, outer membrane transporter; PCP, polysaccharide co-polymerase; PEG, protein-encoding genes; SSU, small subunit
Acknowledgements
We thank Jane Coghill and Christy Waterfall at the Bristol Genomics Facility, Annette Richer for help with culturing, Karen Cameron for assistance in the field, Marek Stibal and Jason Box for facilitating fieldwork on the Greenland ice sheet. No permission was required for collection of samples used in this study.
Funding
This research was carried out as part of the NERC GW4+ Doctoral Training Partnership in Bristol supporting NAMC. Funding support for this work came from a NERC grant (NE/J02399X/1) awarded to AMA and a Royal Society Dorothy Hodgkin Fellowship for PS-B.
Availability of data and materials
This Whole Genome Shotgun project has been deposited at DDBJ/ENA/GenBank under the accession LXYR00000000. The version described in this paper is version LXYR01000000. Genome sequence and annotation data are available at the JGI IMG/ER database and is available on GOLD under Analysis Project ID: Ga0078185. Phylogenetic data is deposited in the TreeBASE database and can be accessed at the following URL: http://purl.org/phylo/treebase/phylows/study/TB2:S19421.
Authors’ contributions
PSB, AMA and NAMC devised the overall project. NAMC performed fieldwork and isolated P. priestley BC1401, assembled the genome of P. priestley BC1401 with guidance from GB, and analysed the data. NAMC and PSB wrote the manuscript, which was improved by GB and AMA. All authors have read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Additional files
Visualisation of the assembled P. priestleyi BC1401 metagenome. Output from Bandage v0.07 [38]. Contigs with a depth <10 are shown in blue, contigs with a depth >10 are shown in orange. Positive BLAST hits (minimum e-value = 1e-10) for core CyOGs as determined by Mulkidjanian et al. [40] are indicated with overlapping labels. All of the contigs containing core CyOGs and the majority of contigs with coverage >10 are all contained within a single subgraph. (PDF 1463 kb)
Circos plot showing P. priestleyi BC1401 contigs that did not map to L. boryana PCC6306. Contigs are ordered according to size in an anticlockwise direction. (PDF 20 kb)
Contributor Information
Nathan A. M. Chrismas, Email: n.a.m.chrismas@bristol.ac.uk
Gary Barker, Email: gary.barker@bristol.ac.uk.
Alexandre M. Anesio, http://a.m.anesio.bristol.ac.uk
Patricia Sánchez-Baracaldo, Email: p.sanchez-baraclado@bristol.ac.uk.
References
- 1.Bekker A, Holland HD, Wang P-L, Rumble D, Stein HJ, Hannah JL, Coetzee LL, Beukes NJ. Dating the rise of atmospheric oxygen. Nature. 2004;427:117–120. doi: 10.1038/nature02260. [DOI] [PubMed] [Google Scholar]
- 2.Schirrmeister BE, Sánchez-Baracaldo P, Wacey D. Cyanobacterial evolution during the Precambrian. Int J Astrobiol. 2016;FirstView:1–18. [Google Scholar]
- 3.Quesada A, Vincent WF. Cyanobacteria in the cryosphere: snow, ice and extreme cold. In: Whitton BA, editor. Ecology of cyanobacteria II. Netherlands: Springer; 2012. pp. 387–399. [Google Scholar]
- 4.Laybourn-Parry J, Tranter M, Hodson AJ. The ecology of snow and ice environments. 1. Oxford: OUP; 2012. [Google Scholar]
- 5.Barria C, Malecki M, Arraiano CM. Bacterial adaptation to cold. Microbiology. 2013;159:2437–2443. doi: 10.1099/mic.0.052209-0. [DOI] [PubMed] [Google Scholar]
- 6.Marx JG, Carpenter SD, Deming JW. Production of cryoprotectant extracellular polysaccharide substances (EPS) by the marine psychrophilic bacterium Colwellia psychrerythraea strain 34H under extreme conditions. Can J Microbiol. 2009;55:63–72. doi: 10.1139/W08-130. [DOI] [PubMed] [Google Scholar]
- 7.Carrión O, Delgado L, Mercade E. New emulsifying and cryoprotective exopolysaccharide from Antarctic Pseudomonas sp. ID1. Carbohyd Polym. 2015;117:1028–1034. doi: 10.1016/j.carbpol.2014.08.060. [DOI] [PubMed] [Google Scholar]
- 8.Liu S-B, Chen X-L, He H-L, Zhang X-Y, Xie B-B, Yu Y, Chen B, Zhou B-C, Zhang Y-Z. Structure and ecological roles of a novel exopolysaccharide from the arctic sea ice bacterium Pseudoalteromonas sp. strain SM20310. Appl Environ Microbiol. 2013;79:224–230. doi: 10.1128/AEM.01801-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reid IN, Sparks WB, Lubow S, McGrath M, Livio M, Valenti J, Sowers KR, Shukla HD, MacAuley S, Miller T, Suvanasuthi R, Belas R, Colman A, Robb FT, DasSarma P, Müller JA, Coker JA, Cavicchioli R, Chen F, DasSarma S. Terrestrial models for extraterrestrial life: methanogens and halophiles at Martian temperatures. Int J Astrobiol. 2006;5:89–97. doi: 10.1017/S1473550406002916. [DOI] [Google Scholar]
- 10.Aslam SN, Cresswell-Maynard T, Thomas DN, Underwood GJC. Production and characterization of the intra- and extracellular carbohydrates and polymeric substances (EPS) of three sea-ice diatom species, and evidence for a cryoprotective role for EPS. J Phycol. 2012;48:1494–1509. doi: 10.1111/jpy.12004. [DOI] [PubMed] [Google Scholar]
- 11.Hill DR, Keenan TW, Helm RF, Potts M, Crowe LM, Crowe JH. Extracellular polysaccharide of Nostoc commune (Cyanobacteria) inhibits fusion of membrane vesicles during desiccation. J Appl Phycol. 1997;9:237–248. doi: 10.1023/A:1007965229567. [DOI] [Google Scholar]
- 12.Tamaru Y, Takani Y, Yoshida T, Sakamoto T. Crucial role of extracellular polysaccharides in desiccation and freezing tolerance in the terrestrial cyanobacterium Nostoc commune. Appl Environ Microbiol. 2005;71:7327–7333. doi: 10.1128/AEM.71.11.7327-7333.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knowles EJ, Castenholz RW. Effect of exogenous extracellular polysaccharides on the desiccation and freezing tolerance of rock-inhabiting phototrophic microorganisms: effect of EPS on tolerance of rock-inhabiting phototrophs. FEMS Microbiol Ecol. 2008;66:261–270. doi: 10.1111/j.1574-6941.2008.00568.x. [DOI] [PubMed] [Google Scholar]
- 14.Zippel B, Neu TR. Characterization of glycoconjugates of extracellular polymeric substances in Tufa-Associated biofilms by using fluorescence lectin-binding analysis. Appl Environ Microbiol. 2011;77:505–516. doi: 10.1128/AEM.01660-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rossi F, De Philippis R. Role of cyanobacterial exopolysaccharides in phototrophic biofilms and in complex microbial mats. Life. 2015;5:1218–38. doi: 10.3390/life5021218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pereira S, Zille A, Micheletti E, Moradas-Ferreira P, De Philippis R, Tamagnini P. Complexity of cyanobacterial exopolysaccharides: composition, structures, inducing factors and putative genes involved in their biosynthesis and assembly. Fems Microbiol Rev. 2009;33:917–941. doi: 10.1111/j.1574-6976.2009.00183.x. [DOI] [PubMed] [Google Scholar]
- 17.Decho A. Microbial exopolymer secretions in ocean environments - their role(s) in food webs and marine processes. Oceanogr Mar Biol. 1990;28:73–153. [Google Scholar]
- 18.Bhaskar PV, Bhosle NB. Microbial extracellular polymeric substances in marine biogeochemical processes. Curr Sci. 2005;88:45–53. [Google Scholar]
- 19.Stibal M, Šabacká M, Žárský J. Biological processes on glacier and ice sheet surfaces. Nat Geosci. 2012;5:771–774. doi: 10.1038/ngeo1611. [DOI] [Google Scholar]
- 20.Bahl J, Lau MCY, Smith GJD, Vijaykrishna D, Cary SC, Lacap DC, Lee CK, Papke RT, Warren-Rhodes KA, Wong FKY, McKay CP, Pointing SB. Ancient origins determine global biogeography of hot and cold desert cyanobacteria. Nat Commun. 2011;2:163. doi: 10.1038/ncomms1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dodds WK, Gudder DA, Mollenhauer D. The ecology of Nostoc. J Phycol. 1995;31:2–18. doi: 10.1111/j.0022-3646.1995.00002.x. [DOI] [Google Scholar]
- 22.Lamprinou V, Danielidis D, Economou-Amilli A, Pantazidou A. Distribution survey of Cyanobacteria in three Greek caves of Peloponnese. Int J Spel. 2012;41(2):267–272. doi: 10.5038/1827-806X.41.2.12. [DOI] [Google Scholar]
- 23.Dojani S, Kauff F, Weber B, Büdel B. Genotypic and phenotypic diversity of cyanobacteria in biological soil crusts of the Succulent Karoo and Nama Karoo of southern Africa. Microb Ecol. 2014;67:286–301. doi: 10.1007/s00248-013-0301-5. [DOI] [PubMed] [Google Scholar]
- 24.Jungblut AD, Lovejoy C, Vincent WF. Global distribution of cyanobacterial ecotypes in the cold biosphere. ISME J. 2009;4:191–202. doi: 10.1038/ismej.2009.113. [DOI] [PubMed] [Google Scholar]
- 25.Chrismas NAM, Anesio AM, Sánchez-Baracaldo P. Multiple adaptations to polar and alpine environments within cyanobacteria: a phylogenomic and Bayesian approach. Front Microbiol. 2015;6:1070. doi: 10.3389/fmicb.2015.01070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Komárek J, Kaštovský J, Ventura S, Turicchia S, Šmarda J. The cyanobacterial genus Phormidesmis. Algol Stud. 2009;129:41–59. doi: 10.1127/1864-1318/2009/0129-0041. [DOI] [Google Scholar]
- 27.Zeng Y-X, Yan M, Yu Y, Li H-R, He J-F, Sun K, Zhang F. Diversity of bacteria in surface ice of Austre Lovénbreen glacier, Svalbard. Arch Microbiol. 2013;195:313–322. doi: 10.1007/s00203-013-0880-z. [DOI] [PubMed] [Google Scholar]
- 28.Bartrons M, Catalan J, Casamayor EO. High bacterial diversity in epilithic biofilms of oligotrophic Mountain Lakes. Microb Ecol. 2012;64:860–869. doi: 10.1007/s00248-012-0072-4. [DOI] [PubMed] [Google Scholar]
- 29.An LZ, Chen Y, Xiang S-R, Shang T-C, Tian L-D. Differences in community composition of bacteria in four glaciers in western China. Biogeosciences. 2010;7:1937–1952. doi: 10.5194/bg-7-1937-2010. [DOI] [Google Scholar]
- 30.Hodson A, Bøggild C, Hanna E, Huybrechts P, Langford H, Cameron K, Houldsworth A. The cryoconite ecosystem on the Greenland ice sheet. Ann Glaciol. 2010;51:123–129. doi: 10.3189/172756411795931985. [DOI] [Google Scholar]
- 31.Takeuchi N, Kohshima S, Seko K. Structure, formation, and darkening process of albedo-reducing material (cryoconite) on a Himalayan glacier: a granular algal mat growing on the glacier. Arct Antarct Alp Res. 2001;33:115–122. doi: 10.2307/1552211. [DOI] [Google Scholar]
- 32.Langford H, Hodson A, Banwart S, Boggild C. The microstructure and biogeochemistry of Arctic cryoconite granules. Ann Glaciol. 2010;51:87–94. doi: 10.3189/172756411795932083. [DOI] [Google Scholar]
- 33.Cook J, Edwards A, Hubbard A. Biocryomorphology: integrating microbial processes with ice surface hydrology, topography, and roughness. Front Earth Sci. 2015;3:78.
- 34.Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiology. 1979;111:1–61. doi: 10.1099/00221287-111-1-1. [DOI] [Google Scholar]
- 35.Xie M, Ren M, Yang C, Yi H, Li Z, Li T, Zhao J. Metagenomic analysis reveals symbiotic relationship among bacteria in microcystis-dominated community. Front Microbiol. 2016;7:56. doi: 10.3389/fmicb.2016.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hall EW, Kim S, Appadoo V, Zare RN. Lysis of a single cyanobacterium for whole genome amplification. Micromachines. 2013;4:321–332. doi: 10.3390/mi4030321. [DOI] [Google Scholar]
- 37.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, Pyshkin AV, Sirotkin AV, Vyahhi N, Tesler G, Alekseyev MA, Pevzner PA. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 2012;19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wick RR, Schultz MB, Zobel J, Holt KE. Bandage: interactive visualization of de novo genome assemblies. Bioinformatics. 2015;31:3350–3352. doi: 10.1093/bioinformatics/btv383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mulkidjanian AY, Koonin EV, Makarova KS, Mekhedov SL, Sorokin A, Wolf YI, Dufresne A, Partensky F, Burd H, Kaznadzey D, Haselkorn R, Galperin MY. The cyanobacterial genome core and the origin of photosynthesis. PNAS. 2006;103:13126–13131. doi: 10.1073/pnas.0605709103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–60. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Markowitz VM, Chen I-MA, Palaniappan K, Chu K, Szeto E, Grechkin Y, Ratner A, Jacob B, Huang J, Williams P, Huntemann M, Anderson I, Mavromatis K, Ivanova NN, Kyrpides NC. IMG: the integrated microbial genomes database and comparative analysis system. Nucl Acids Res. 2012;40:D115–D122. doi: 10.1093/nar/gkr1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Galardini M, Biondi EG, Bazzicalupo M, Mengoni A. CONTIGuator: a bacterial genomes finishing tool for structural insights on draft genomes. Source Code Biol Med. 2011;6:11. doi: 10.1186/1751-0473-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Waterhouse AM, Procter JB, Martin DMA, Clamp M, Barton GJ. Jalview Version 2—a multiple sequence alignment editor and analysis workbench. Bioinformatics. 2009;25:1189–1191. doi: 10.1093/bioinformatics/btp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJE. The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc. 2015;10:845–858. doi: 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krzywinski MI, Schein JE, Birol I, Connors J, Gascoyne R, Horsman D, Jones SJ, Marra MA. Circos: an information aesthetic for comparative genomics. Genome Res. 2009;19(9):1639–45. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rambaldi D, Ciccarelli FD. FancyGene: dynamic visualization of gene structures and protein domain architectures on genomic loci. Bioinformatics. 2009;25:2281–2282. doi: 10.1093/bioinformatics/btp381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Inkscape v0.91 http://inkscape.org/en/download Accessed 16 Sept 2015
- 49.The Joint Genome Institute Integrated Microbial Genomes database, http://img.jgi.doe.gov/cgi-bin/w/main.cgi. Accessed 18 May 2016
- 50.Sánchez-Baracaldo P. Origin of marine planktonic cyanobacteria. Sci Rep. 2015;5:17418. doi: 10.1038/srep17418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taton A, Grubisic S, Ertz D, Hodgson DA, Piccardi R, Biondi N, Tredici MR, Mainini M, Losi D, Marinelli F, Wilmotte A. Polyphasic study of antarctic cyanobacterial strains. J Phycol. 2006;42:1257–1270. doi: 10.1111/j.1529-8817.2006.00278.x. [DOI] [Google Scholar]
- 52.Liu K, Raghavan S, Nelesen S, Linder CR, Warnow T. Rapid and accurate large-scale coestimation of sequence alignments and phylogenetic trees. Science. 2009;324:1561–1564. doi: 10.1126/science.1171243. [DOI] [PubMed] [Google Scholar]
- 53.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucl Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Price MN, Dehal PS, Arkin AP. FastTree 2 – approximately maximum-likelihood trees for large alignments. PLoS ONE. 2010;5:e9490. doi: 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.FigTree v1.4.0 http://tree.bio.ed.ac.uk/software/figtree/ Accessed 5 Dec 2012
- 58.Ayala-del-Río HL, Chain PS, Grzymski JJ, Ponder MA, Ivanova N, Bergholz PW, Bartolo GD, Hauser L, Land M, Bakermans C, Rodrigues D, Klappenbach J, Zarka D, Larimer F, Richardson P, Murray A, Thomashow M, Tiedje JM. The genome sequence of Psychrobacter arcticus 273–4, a Psychroactive Siberian permafrost bacterium, reveals mechanisms for adaptation to low-temperature growth. Appl Environ Microbiol. 2010;76:2304–2312. doi: 10.1128/AEM.02101-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Methé BA, Nelson KE, Deming JW, Momen B, Melamud E, Zhang X, Moult J, Madupu R, Nelson WC, Dodson RJ, Brinkac LM, Daugherty SC, Durkin AS, DeBoy RT, Kolonay JF, Sullivan SA, Zhou L, Davidsen TM, Wu M, Huston AL, Lewis M, Weaver B, Weidman JF, Khouri H, Utterback TR, Feldblyum TV, Fraser CM. The psychrophilic lifestyle as revealed by the genome sequence of Colwellia psychrerythraea 34H through genomic and proteomic analyses. PNAS. 2005;102:10913–10918. doi: 10.1073/pnas.0504766102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.DasSarma S, Capes MD, Karan R, DasSarma P. Amino acid substitutions in cold-adapted proteins from Halorubrum lacusprofundi, an extremely halophilic microbe from Antarctica. PLoS ONE. 2013;8:e58587. doi: 10.1371/journal.pone.0058587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saunders NFW, Thomas T, Curmi PMG, Mattick JS, Kuczek E, Slade R, Davis J, Franzmann PD, Boone D, Rusterholtz K, Feldman R, Gates C, Bench S, Sowers K, Kadner K, Aerts A, Dehal P, Detter C, Glavina T, Lucas S, Richardson P, Larimer F, Hauser L, Land M, Cavicchioli R. Mechanisms of thermal adaptation revealed from the genomes of the antarctic archaea Methanogenium frigidum and Methanococcoides burtonii. Genome Res. 2003;13:1580–1588. doi: 10.1101/gr.1180903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aghajari N, Feller G, Gerday C, Haser R. Structures of the psychrophilic Alteromonas haloplanctis α-amylase give insights into cold adaptation at a molecular level. Structure. 1998;6:1503–1516. doi: 10.1016/S0969-2126(98)00149-X. [DOI] [PubMed] [Google Scholar]
- 63.Feller G, Arpigny JL, Narinx E, Gerday C. Molecular adaptations of enzymes from psychrophilic organisms. Comp Biochem Physiol A Mol Integr Physiol. 1997;118:495–499. doi: 10.1016/S0300-9629(97)00011-X. [DOI] [Google Scholar]
- 64.Russell NJ. Toward a molecular understanding of cold activity of enzymes from psychrophiles. Extremophiles. 2000;4:83–90. doi: 10.1007/s007920050141. [DOI] [PubMed] [Google Scholar]
- 65.Feller G, Zekhnini Z, Lamotte-Brasseur J, Gerday C. Enzymes from cold-adapted microorganisms - the Class C beta-lactamase from the antarctic psychrophile Psychrobacter Immobilis A5. Eur J Biochem. 1997;244:186–191. doi: 10.1111/j.1432-1033.1997.00186.x. [DOI] [PubMed] [Google Scholar]
- 66.Varin T, Lovejoy C, Jungblut AD, Vincent WF, Corbeil J. Metagenomic analysis of stress genes in microbial mat communities from Antarctica and the High Arctic. Appl Environ Microbiol. 2012;78:549–559. doi: 10.1128/AEM.06354-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stibal M, Tranter M. Laboratory investigation of inorganic carbon uptake by cryoconite debris from Werenskioldbreen, Svalbard. J Geophys Res. 2007;112:G04S33. doi: 10.1029/2007JG000429. [DOI] [Google Scholar]
- 68.Straus D, Walter W, Gross CA. DnaK, DnaJ, and GrpE heat shock proteins negatively regulate heat shock gene expression by controlling the synthesis and stability of sigma 32. Genes Dev. 1990;4:2202–2209. doi: 10.1101/gad.4.12a.2202. [DOI] [PubMed] [Google Scholar]
- 69.Lionard M, Péquin B, Lovejoy C, Vincent WF. Benthic cyanobacterial mats in the high arctic: multi-layer structure and fluorescence responses to osmotic stress. Front Microbiol. 2012;3:140. doi: 10.3389/fmicb.2012.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tang EPY, Tremblay R, Vincent WF. Cyanobacterial dominance of polar freshwater ecosystems: are high-latitude mat-formers adapted to low temperature? J Phycol. 1997;33:171–81. doi: 10.1111/j.0022-3646.1997.00171.x. [DOI] [Google Scholar]
- 71.Pereira SB, Mota R, Vieira CP, Vieira J, Tamagnini P. Phylum-wide analysis of genes/proteins related to the last steps of assembly and export of extracellular polymeric substances (EPS) in cyanobacteria. Sci Rep. 2015;5:14835. doi: 10.1038/srep14835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cuthbertson L, Mainprize IL, Naismith JH, Whitfield C. Pivotal roles of the outer membrane polysaccharide export and polysaccharide copolymerase protein families in export of extracellular polysaccharides in gram-negative bacteria. Microbiol Mol Biol Rev. 2009;73:155–177. doi: 10.1128/MMBR.00024-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kehr J-C, Dittmann E. Biosynthesis and function of extracellular glycans in cyanobacteria. Life. 2015;5:164–180. doi: 10.3390/life5010164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Madson M, Dunand C, Li X, Verma R, Vanzin GF, Caplan J, Shoue DA, Carpita NC, Reiter W-D. The MUR3 gene of Arabidopsis encodes a xyloglucan galactosyltransferase that is evolutionarily related to animal exostosins. Plant Cell. 2003;15:1662–1670. doi: 10.1105/tpc.009837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Römling U, Gomelsky M, Galperin MY. C-di-GMP: the dawning of a novel bacterial signalling system. Mol Microbiol. 2005;57:629–639. doi: 10.1111/j.1365-2958.2005.04697.x. [DOI] [PubMed] [Google Scholar]
- 76.Liang Z-X. The expanding roles of c-di-GMP in the biosynthesis of exopolysaccharides and secondary metabolites. Nat Prod Rep. 2015;32:663–683. doi: 10.1039/C4NP00086B. [DOI] [PubMed] [Google Scholar]
- 77.Hickman JW, Tifrea DF, Harwood CS. A chemosensory system that regulates biofilm formation through modulation of cyclic diguanylate levels. PNAS. 2005;102:14422–14427. doi: 10.1073/pnas.0507170102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.O’Connor JR, Kuwada NJ, Huangyutitham V, Wiggins PA, Harwood CS. Surface sensing and lateral subcellular localization of WspA, the receptor in a chemosensory-like system leading to c-di-GMP production. Mol Microbiol. 2012;86:720–729. doi: 10.1111/mmi.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Belas R. Biofilms, flagella, and mechanosensing of surfaces by bacteria. Trends Microbiol. 2014;22:517–527. doi: 10.1016/j.tim.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 80.Fiedler G, Arnold M, Hannus S, Maldener I. The DevBCA exporter is essential for envelope formation in heterocysts of the cyanobacterium Anabaena sp. strain PCC 7120. Mol Microbiol. 1998;27:1193–1202. doi: 10.1046/j.1365-2958.1998.00762.x. [DOI] [PubMed] [Google Scholar]
- 81.Staron P, Maldener I. All0809/8/7 is a DevBCA-like ABC-type efflux pump required for diazotrophic growth in Anabaena sp. PCC 7120. Microbiology. 2012;158:2537–2545. doi: 10.1099/mic.0.058909-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This Whole Genome Shotgun project has been deposited at DDBJ/ENA/GenBank under the accession LXYR00000000. The version described in this paper is version LXYR01000000. Genome sequence and annotation data are available at the JGI IMG/ER database and is available on GOLD under Analysis Project ID: Ga0078185. Phylogenetic data is deposited in the TreeBASE database and can be accessed at the following URL: http://purl.org/phylo/treebase/phylows/study/TB2:S19421.


