Abstract
Background
Re-irradiation (re-RT) of the thorax is challenging due to the impact of prior therapies on normal tissues, and there are few reports of definitive re-RT. The treatment toxicities and efficacy of re-RT are not well known. The aim of the present study was to assess the safety and efficacy of definitive re-RT of the thorax.
Methods
Patients who were treated with thoracic re-RT between March 2007 and December 2014 were retrospectively analyzed. Primary and re-irradiation plans were required to have an overlap of dose distributions for the 80 % isodose level. All doses were recalculated to an equivalent dose of 2 Gy per fraction (EQD2). When possible, analysis of dose accumulation was carried out using the medical image merge (MIM) (®) software program (version 6.5, MIM Software Inc., Cleveland, OH). Administration dosages for organs at risk were defined.
Results
Fourteen (67 %) and seven (33 %) patients with non-small cell carcinoma (NSCLC) and small cell carcinoma (SCLC), respectively, were identified. The patients’ median age was 72 (range 53–85) years. Fifteen patients (71 %) had “proximal” tumors, defined as tumors at the distal 2 cm of the trachea, carina, and main bronchi. The median interval from initial RT to re-RT was 26.8 (range 11.4–92.3) months. Re-RT was delivered by X-ray beam and proton beam therapy in 20 (95 %) patients and 1 (5 %) patient, respectively. The median radiation dose of re-RT was 60 (range 54–87.5) Gy10 and 50 (range 50.0–87.5) Gy10 for patients with NSCLC and SCLC, respectively. Grade 3 acute radiation pneumonitis occurred in only one patient. There were no other serious complications. The median follow-up time was 22.1 (range 2.3–56.4) months. The median local progression-free survival time (LPFS) and overall survival time (OS) were 12.9 (95 % confidence interval (CI): 8.9–27.9) months and 31.4 (95 % CI: 16.9–45.9) months, respectively. Patients receiving ≥ 60 Gy10 at re-RT had longer LPFS (p = 0.04).
Conclusions
Good safety with longer OS than in previous reports was demonstrated. Re-RT seems to be a promising treatment option. Further study to define the risk-benefit ratios is necessary.
Keywords: Lung cancer, Recurrence tumor, Re-irradiation
Background
Lung cancer remains one of the most prevalent and deadliest malignancies worldwide, accounting for around 1.8 million deaths each year [1]. External beam radiation therapy (RT) plays an important role in curative management strategies for both small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC) [2–6]. In NSCLC patients treated with concurrent chemoradiation to the thorax, 5-year rates of locoregional recurrence approach 30 % [7]. In SCLC patients, locoregional recurrence occurred in 36 % of patients [8]. Disease recurrence is still the dominant cause of death after initial treatment.
Second-line chemotherapy is usually selected even when no distant metastasis is observed. For patients with NSCLC and SCLC, progression-free survival (PFS) is 2–8.5 months and 3.3–3.7 months, and median overall survival (OS) is 5.6–11.5 months and 5.5–6.4 months, respectively [9–17].
Re-irradiation (re-RT) has not been considered first-choice, because high-dose re-RT might cause severe radiation injury. Since Green et al reported the efficacy and safety of re-RT as definitive therapy for intrathoracic disease [18], several studies of re-RT have been reported. However, there are few reports of re-RT with curative intent [19–32]. Additionally, there are few detailed reports about the total dose of initial RT and re-RT for each organ at risk [23–28]. Furthermore, due to the characteristics of recurrent disease, long-term observation of treatment results is difficult. The efficacy and safety of re-RT are unclear. Thus, our institutional experience was retrospectively analyzed to investigate the clinical outcomes, including survival and toxicities.
Methods
This study was approved by the institutional review board of Shizuoka Cancer Center. Medical records were retrospectively obtained. Patients who were registered in the Radiology Information System in Shizuoka Cancer Center between September 2002 and December 2014 were identified. Patients who fulfilled the following criteria were included: (1) the location of the primary tumor was “lung” or “trachea”; (2) recurrences were confirmed by evidence seen on computerized tomography (CT) and/or positron emission tomography (PET) showing the reappearance or enlargement of the tumor shadow within the previous irradiated volume; (3) the patient received external beam radiotherapy to the chest at least twice; (4) the patient was administered at least 45 Gy each time; (5) there was overlap of the two separate dose distributions for the 80 % dose level each time; and (6) there was no evidence of active metastasis at re-RT.
Records were reviewed for patient and disease characteristics, prior fractionated radiotherapy dosimetric parameters, re-RT dosimetric parameters, toxicities, treatment response, local progression-free survival (LPFS), PFS, and OS. Tumors were re-staged using the Union for International Cancer Control TNM Classification of Malignant Tumors 7th edition (UICC 7th). Previous radiotherapy and re-RT variables reviewed included dates of therapy, prescription dose, graphic dose distributions, planning target volume (PTV) size, and tumor location. The treated part was defined as follows: “proximal” was defined as the distal 2 cm of the trachea, the carina, and the right and left main bronchi. Analysis of dose accumulation was carried out after rigid registration using the medical image merge (MIM) (®) software program (version 6.5, MIM Software Inc., Cleveland, OH), if data were available. All doses were recalculated to an equivalent dose of 2 Gy per fraction (EQD2) with the formula: d*n*((d + α/β)/(2 + α/β)), with d the dose per fraction (Gy) and n the number of fractions. For tumor dose and acute side effects, an α/β value of 10 Gy was used (Gy10), and for late effects, an α/β value of 3 Gy was used (Gy3) [28]. The administration dosage for organs at risk was defined according to the Radiation Therapy Oncology Group (RTOG) contouring atlas of lung (https://www.rtog.org/CoreLab/ContouringAtlases/LungAtlas.aspx). The severities of acute (within 30 days of ending radiation) and late toxicities were evaluated according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. Treatment response was evaluated according to the response evaluation criteria in solid tumors (RECIST) version 1.1. OS was defined as the interval between the index date and the date of death or censored on the last date of confirmed survival, whichever came first. LPFS and PFS were calculated from the index date to the earliest date of local failure and systemic failure or death, whichever came first. Local failure was defined as recurrence within the re-RT site. The index date was the first day of re-RT. The survival curves were calculated using the Kaplan-Meier method, and the differences between the curves were analyzed using the log-rank test. A univariate significance level of P < 0.05 was used for identifying predictors.
Results
Forty-two patients were found to have received re-RT within the previous irradiation volume. Twenty-one patients were excluded; the reasons for exclusion are shown in Fig. 1. Finally, 14 patients (67 %) with NSCLC and seven patients (33 %) with SCLC who received re-RT were identified. Informed consent was obtained from all patients at the time of re-RT.
Fig. 1.
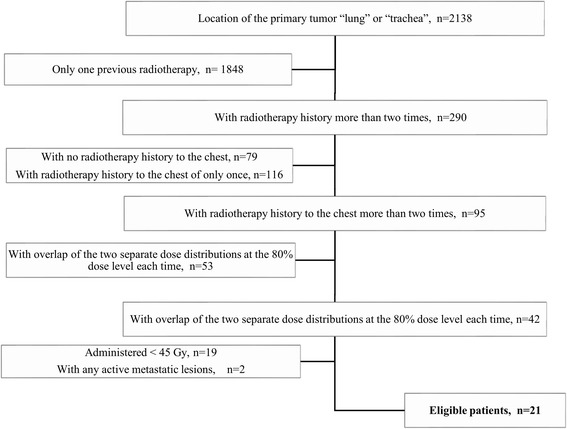
Patient selection and exclusion criteria
Patient and disease characteristics at re-RT are shown in Table 1. The median age was 72 years (range 53–85 years). All patients except one had Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0–1. The median interval between the end of initial RT and recurrence was 26.0 months (range 7.0–87.8 months). Recurrence was documented pathologically in only two cases (11 %), and their results were the same as the initial pathology. The majority of tumor recurrence (85 %) occurred at the original tumor site. Fifteen patients (71 %) had proximal recurrent tumor. One patient had stage IV disease at initial RT. He had surgery as initial treatment. He then developed solitary brain metastasis and local tumor recurrence. For both lesions, local radiotherapy appeared to offer the possibility of radical cure.
Table 1.
Patient and disease characteristics at the time of re-irradiation
| Sex, n (%) | ||
| Male | 15 | (71) |
| Female | 6 | (29) |
| Median age at re-RT, years (range) | 72 | (53–85) |
| ECOG PS, n (%) | ||
| 0–1 | 20 | (95) |
| 2 | 1 | (5) |
| Histology at primary RT, n (%) | ||
| NSCLC | 14 | (67) |
| Squamous cell carcinoma | 6 | |
| Adenocarcinoma | 5 | |
| Others | 3 | |
| SCLC | 7 | (33) |
| Clinical Stage at primary RT, n (%) | ||
| IA / B | 4 | (19) |
| IIA / B | 4 | (19) |
| IIIA/ B | 12 | (57) |
| IV | 1 | (5) |
| Pattern of recurrence, n (%) | ||
| Primary tumor | 15 | (71) |
| Initially existent metastatic LN (± primary tumor) | 3 | (14) |
| Intrapulmonary new lesion | 1 | (5) |
| Regional LN new lesion | 1 | (5) |
| Both intrapulmonary and regional LN new region | 1 | (5) |
| Irradiation tumor location, n (%) | ||
| Proximal | 15 | (71) |
| Peripheral | 6 | (29) |
re-RT re-irradiation, PS performance status, primary RT primary radiotherapy, NSCLC Non small cell lung cancer, SCLC Small cell lung cancer, LN lymph node
Initial and re-RT treatment details are shown in Table 2. The median interval from initial RT to re-RT was 26.8 months (range 11.4–92.3 months). At the time of initial RT, the median radiation dose was 60 Gy10 (range 51.8–87.5 Gy10) for NSCLC patients, while each SCLC patient received 43.1 Gy10. Radiotherapy was delivered by X-ray beam therapy in 20 patients (95 %). Only one (5 %) patient with stage IA (UICC 7th) NSCLC refused surgery and was treated using proton beam therapy. He had proximal tumor and chronic heart failure. Proton beam therapy had the advantage of avoiding organs at risk, including the heart. In addition, avoiding organs at risk led to an increased prescription dose. Concurrent chemotherapy was delivered in 16 (76 %) patients. At the time of re-RT, the median radiation dose was 60 Gy10 (range 54–87.5 Gy10) for NSCLC patients and 50 Gy10 (range 50–87.5 Gy10) for SCLC patients. Radiotherapy was delivered by X-ray beam therapy in 19 patients (90 %) and by proton beam therapy in 2 patients (10 %). Only one patient (5 %) was administered concurrent chemotherapy. The median PTV volume was 57.4 cc (range 7–601.7 cc). Prior radiotherapy and re-RT details are listed in Table 2.
Table 2.
Overview of the initial RT and re-RT details
| Median interval to re-RT, months (range) | 26.8 (11.4–92.3) | |||
|---|---|---|---|---|
| Initial radiation | Re-irradiation | |||
| Median RT dose, Gy10 (range) | ||||
| NSCLC | 60 | (51.8–87.5) | 60 | (54–87.5) |
| SCLC | 43.1 | all patients | 50 | (50–87.5) |
| Radiation technique, n (%) | ||||
| Conventional fractionation | 10 | (48) | 13 | (62) |
| Hyperfractionation | 8 | (38) | 0 | (0) |
| Hypofractionation | 3 | (14) | 8 | (38) |
| Radiation delivery, n (%) | ||||
| Photon therapy | 20 | (95) | 19 | (90) |
| Proton therapy | 1 | (5) | 2 | (10) |
| Concurrent chemotherapy, n (%) | ||||
| Yes | 16 | (76) | 1 | (5) |
| No | 5 | (24) | 20 | (95) |
| Median PTV, cc (range) | 57.4 | (7–601.7) | ||
RT radiotherapy, NSCLC Non small cell lung cancer, SCLC Small cell lung cancer, PTV planning target volume
Dose distributions of initial RT and re-RT were accumulated for 13 patients with NSCLC and 5 patients with SCLC. The median cumulative maximum dose of the lung was 130 Gy3. The median percentage of total lung volume receiving a cumulative dose of 20 Gy3 (V20) was 17 % (range 5–34 %). The mean total lung dose was 12 Gy3. The median cumulative dosage that was irradiated to a 1 cc volume and the mean irradiated volume of the organs at risk are shown in Table 3. Four patients received a cumulative dosage of over 50 Gy3 to a 1 cc volume.
Table 3.
Dose distribution of initial RT and re-RT
| Initial | Re-irradiation | Total | |
|---|---|---|---|
| Lung dose volume (%) | |||
| V5 | |||
| Ipsilateral lung | 43 | 32 | 55 |
| Contralateral lung | 6 | 0.5 | 11 |
| Total lung | 22 | 16 | 30 |
| V20 | |||
| Ipsilateral lung | 32 | 15 | 38 |
| Contralateral lung | 2 | 0 | 3 |
| Total lung | 14 | 7 | 17 |
| Mean dose, Gy3 | |||
| Ipsilateral lung | 17 | 10 | 25 |
| Contralateral lung | 1 | 1 | 3 |
| Total lung | 8 | 5 | 12 |
| Heart | 3 | 7 | 5 |
| Total median dose, Gy3 | D1cc | D10cc | |
| Spinal cord | 32 | 25 | |
| Esophagus | 42 | 33 | |
| Aorta | 87 | 65 | |
| Pulmonary artery | 93 | 61 | |
| Heart | 49 | 46 | |
| Trachea | 87 | 41 | |
| Skin | 59 | 36 | |
| Total mean volume,cc | |||
| Spinal cord ≥40 Gy3 | 0.3 | ||
| Spinal cord ≥50 Gy3 | 0 | ||
| Heart ≥70 Gy3 | 0 | ||
| Esophagus ≥70 Gy3 | 5 | ||
| Aorta ≥70 Gy3 | 7 | ||
| Pulmonary artery ≥70 Gy3 | 4 |
V5 (20) The percentage of lung receiving 5 (20) Gy3 or more, OAR organ at risk, D1 (10) cc the dosage irradiated to a 1 (10) cc volume
The most frequent acute toxicity reported was Grade 1–2 radiation dermatitis. No ≥ Grade 3 acute and late toxicities were observed, except in one patient with grade 3 acute pneumonitis that was suspected to be radiotherapy-induced (radiation pneumonitis) (Table 4). Grade 1–2 late pulmonary toxicity was observed in 9 patients (52 %), but there were no serious complications.
Table 4.
Toxicity
| Grade 1-2 | Grade 3 | Grade 4 | |
|---|---|---|---|
| Acute toxicities, n | |||
| Radiation dermatitis | 9 | 0 | 0 |
| Esophagitis | 4 | 0 | 0 |
| Pneumonitis | 3 | 1 | 0 |
| Anorexia | 3 | 0 | 0 |
| Malaise | 2 | - | - |
| Late toxicities, n | |||
| Pneumonitis | 9 | 0 | 0 |
| Chest wall pain | 1 | 0 | - |
| Pruritus | 1 | 0 | - |
At the time of analysis, 6 patients were alive. The median follow-up time was 22.1 months (range 2.3–56.4 months). The responses to re-RT were complete response (CR) in 4 patients (19 %) and partial response (PR) in 5 patients (24 %). Disease progression after re-RT was observed in 14 patients (67 %). The first failure after re-RT occurred in the field of re-RT in 10 patients (71 %), including 2 patients who had both in and out of field recurrence. Chemotherapy after disease progression was administered to 5 patients (24 %). Among them, three patients had NSCLC, and two patients had SCLC. The median PFS was 10.9 months (95 % confidence interval (CI): 6.8–27.9 months). The median LPFS was 12.9 months (95 % CI: 8.9–27.9 months). The 1-year and 2-year LPFS rates were 57 % and 34 %, respectively (Fig. 2). The median OS was 31.4 months (95 % CI: 16.9–45.9 months). The 1-year and 2-year OS rates were 76 % and 64 %, respectively (Fig. 3).
Fig. 2.
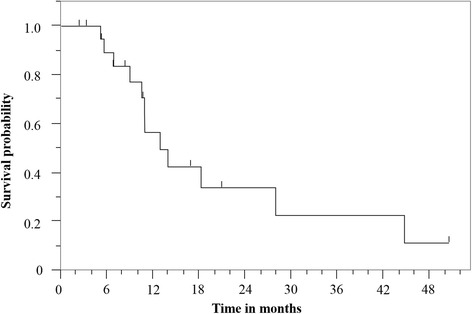
Kaplan-Meier curve of local progression-free survival for all patients
Fig. 3.
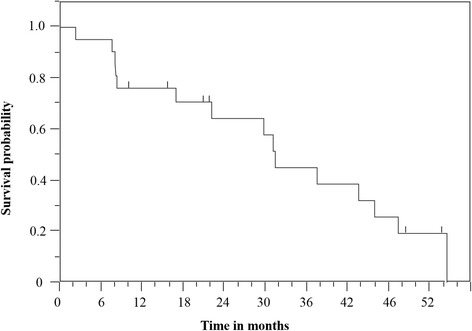
Kaplan-Meier curve of overall survival for all patients
On univariate analysis, PTV < 75 cc was associated with longer OS (p = 0.03), and patients receiving ≥ 60 Gy10 at re-RT had longer LPFS (p = 0.04). There were no significant differences in OS and LPFS with any other factors. The median OS with or without subsequent chemotherapy was 37.4 months and 31.4 months, respectively; there was no significant difference (P = 0.57) (Table 5).
Table 5.
Univariate analysis: Impact on survival after re-RT
| Variable | Number | Median LPFS |
P value | Median OS |
P value |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 15 | 12.9 | 0.86 | 31.4 | 0.12 |
| Female | 6 | 12.4 | 43.0 | ||
| Age, years | |||||
| ≥ 75 | 9 | 13.9 | 0.96 | 37.3 | 0.97 |
| < 75 | 12 | 10.9 | 31.4 | ||
| Interval between end of initial RT and recurrence, months | |||||
| ≥ 24 | 11 | 13.8 | 0.66 | 31.4 | 0.48 |
| < 24 | 10 | 10.9 | 31.0 | ||
| Interval between initial RT and re-RT, months | |||||
| ≥ 24 | 11 | 13.8 | 0.66 | 31.4 | 0.48 |
| < 24 | 10 | 10.9 | 31.0 | ||
| Re-RT dose, Gy10 | |||||
| ≥ 60 | 16 | 18.2 | 0.04 | 37.4 | 0.41 |
| < 60 | 5 | 6.8 | 22.1 | ||
| PTV size, cc | |||||
| > 75 | 8 | 12.9 | 0.78 | 16.9 | 0.03 |
| ≤ 75 | 13 | 13.8 | 43.5 | ||
| Pathology | |||||
| NSCLC | 14 | 13.9 | 0.88 | 31.4 | 0.56 |
| SCLC | 7 | 10.9 | 37.5 | ||
| Location | |||||
| Proximal | 15 | 2.8 | 0.62 | 22.1 | 0.99 |
| Peripheral | 6 | 18.2 | 37.4 | ||
| Further chemotherapy after re-RT | |||||
| Yes | 5 | 37.4 | 0.57 | ||
| No | 9 | 31.4 |
RT radiotherapy, re-RT re-irradiation, NSCLC Non small cell lung cancer, SCLC Small cell lung cancer, PTV planning target volume; Bold values represent statistically significant factors
Discussion
The outcomes of 21 patients given re-RT for recurrent tumors of the thorax were reviewed. There was some therapeutic efficacy with a median follow-up time of 22.1 months and median OS of 31.4 months. To the best of our knowledge, this study had longer follow-up and the best survival outcomes compared with any previous reports regarding re-RT for recurrent lung cancer. In the present study, PTV volume was significantly associated with OS. Reyngold et al. also reported that PTV volume <75 cc was significantly associated with longer OS [29]. The present patients had a generally small PTV. As size increases, the tumor becomes more radiation-resistant due to the presence of hypoxic cells. The generally smaller PTV might have had an impact on both LPFS and OS. It was not possible to identify any other factors significantly associated with OS. However, the good OS might be explained by patient selection. In our institution, we discuss the indications for treatment within a cancer board consisting of thoracic surgeons, thoracic physicians, radiation oncologists, and radiologists. All patients had good ECOG PS of 0-1. The median interval between initial RT and re-RT was 25 months, and all patients except one had an interval over 1 year. Despite a median interval of over 2 years, the majority of tumor recurrence occurred at the original tumor site, with no widening to new regional lymph nodes. This suggests the possibility that the improvement of OS reflected tumors with slow growth and spread. Small PTV volume [29] and long treatment interval [30, 31] are each reported as factors significantly associated with longer OS. This supports the present considerations. Finally, when the present OS is compared with previous reports, with advancing diagnostic technology, it is possible that patients with minute metastases were excluded. In contrast, the median LPFS was not as good as OS. An explanation for this is that the median OS in previous reports was generally poor. There is a possibility that they could not observe recurrence over a sufficient time period in the previous reports. The present result may also reflect longer follow-up. Thus, this does not imply that treatment efficacy was poor. However, receiving a dose over 60 Gy10 was significantly associated with longer LPFS. In addition, De Bari et al. also reported that outcome results of re-RT with stereotactic ablative radiotherapy appear superior to those achievable with conventional radiotherapy and/or chemoradiotherapy [22]. Dose escalation needs to be considered.
Grade 3 acute radiation pneumonitis occurred in only one patient. There were no other serious complications. The present results suggest the safety of re-RT over a long period. In addition, it is important that tumor location be reviewed when we think about the safety of radiotherapy to the lung. It has been proposed that the risk of irradiation is greater to centrally located lung tumors even in primary treatment [33]. In the situation of re-RT, this risk may increase. In fact, Trovo et al., Kilburn et al., and Paulen et al. reported fatal toxicity of re-RT for centrally located tumors [22–24, 32]. The present study included centrally located tumors in two-thirds of patients. Despite this, excellent safety was seen with long-term observation. The explanations for the good safety may be as follows. First, the patients had a long interval between primary RT and re-RT. This may contribute to organ repair [34]. Next, the present patients had a generally small PTV volume, which contributed to small irradiation field size. Third, the proportion of patients receiving 2 Gy per fraction was high. This contributed to lower summed EQD2. However, De Ruysscher et al. reported that the incidence of severe lung toxic effects did not differ greatly in patients treated with conventional 3D conformal radiotherapy compared with patients treated with SABR [20]. One of the reasons for this is that the choice of the treatment in studies that they reviewed may have depended on tumor location. Finally, priority was given to avoiding including the spinal cord and esophagus in the irradiation field.
Although patient selection may be required, good OS and safety of re-RT to the thorax were reported. This is beneficial in offering a treatment option to patients with recurrent thoracic tumors. A future problem is to define the risk-benefit ratios with dose escalation.
The present study has several limitations. First, this study was a single-institution, retrospective study. Second, re-RT was not compared with other treatment options for patients with locally recurrent tumor. It is unclear whether re-RT is the optimal treatment for these patients. Third, the number of patients was small, which may explain why statistical significance was not reached. Finally, interval time contributing to the effect of dosage was not considered when the prior and re-RT doses were estimated. When we use the therapeutic procedure in patients with a shorter interval between primary RT and re-RT, attention should be paid to treatment safety, since this has not been established for such patients.
Conclusions
Re-RT to the thorax for recurrent tumor seems to be a promising and safe treatment option even in patients with proximal tumor. However, local control was not satisfactory. With carefully selected indications, further study is needed to define the optimal treatment dose.
Abbreviations
CI, confidence interval; CR, complete response; CT, computerized tomography; CTCAE, common terminology criteria for adverse event; ECOG, eastern cooperative oncology group; EQD2, equivalent dose of 2 Gy per fraction; LN, lymph node; LPFS, local progression-free survival; NSCLC, non small cell lung cancer; OS, overall survival; PET, positron emission tomography; PFS, progression-free survival; PR, partial response; PTV, planning target volume; RECIST, response evaluation criteria in solid tumors; re-RT, re-irradiation; RT, radiotherapy; RTOG, radiation therapy oncology group; SABR, stereotactic ablative radiation therapy; SCLC, small cell lung cancer; UICC 7th, the union for international cancer control TNM classification of malignant tumors 7th edition; V20, the median percentages of the lungs receiving a cumulative dose of 20 Gy3
Acknowledgements
This study was supported by Haruo Yamashita1, Masahiro Konno1, and Tatsuya Segawa1, who assisted with data collection.
1Radiation and Proton Therapy Center, Shizuoka Cancer Center, 1007 Shimonagakubo, Nagaizumi-cho, Sunto-gun, Shizuoka 411-8777, Japan
Funding
No sources of funding for the research reported.
Authors’ contributions
KS participated in the design of the study and collected and assembled the data, performed the statistical analysis, and drafted the manuscript. HH conceived of the study, participated in its design and coordination, and helped draft the manuscript. TN helped draft the manuscript. HA, HO, TO, SN, NT, TT revised the manuscript and made substantial contributions to the interpretation of the results. All authors read and approved the final manuscript.
Authors’ information
Kiyomi Sumita: Resident, Radiation and Proton Therapy Center, Shizuoka Cancer Center, and graduate student, Department of Radiology, Kansai Medical University. M.D.
Hideyuki Harada: Chief, Radiation and Proton Therapy Center, Shizuoka Cancer Center. M.D., Ph.D.
Hirofumi Asakura: Chief, Radiation and Proton Therapy Center, Shizuoka Cancer Center. M.D., Ph.D.
Hirofumi Ogawa: Senior Staff, Radiation and Proton Therapy Center, Shizuoka Cancer Center. M.D.
Tsuyoshi Onoe: Senior Staff, Radiation and Proton Therapy Center, Shizuoka Cancer Center. M.D., Ph.D.
Shigeyuki Murayama: Chief, Radiation and Proton Therapy Center, Shizuoka Cancer Center. M.D., Ph.D.
Satoaki Nakamura: Associate professor, Department of Radiology, Kansai Medical University. M.D., Ph.D.
Noboru Tanigawa: Professor and Chairman, Department of Radiology, Kansai Medical University. M.D., Ph.D.
Toshiaki Takahashi: Chief, Division of Thoracic Oncology Center. M.D., Ph.D.
Tetsuo Nishimura1. Manager, Radiation and Proton Therapy Center, Shizuoka Cancer Center. M.D, Ph.D.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
This study was approved by the institutional review board of Shizuoka Cancer Center.
Contributor Information
Kiyomi Sumita, Email: sumitkiy@hirakata.kmu.ac.jp.
Hideyuki Harada, Email: h.harada@scchr.jp.
Hirofumi Asakura, Email: h.asakura@scchr.jp.
Hirofumi Ogawa, Email: h.ogawa@scchr.jp.
Tsuyoshi Onoe, Email: t.onoe@scchr.jp.
Shigeyuki Murayama, Email: s.murayama@scchr.jp.
Satoaki Nakamura, Email: satoaki@nakamura.pro.
Noboru Tanigawa, Email: tanigano@hirakata.kmu.ac.jp.
Toshiaki Takahashi, Email: t.takahashi@scchr.jp.
Tetsuo Nishimura, Email: t.nishimura@scchr.jp.
References
- 1.Agency for Research on Cancer, Worlf Health. [http://globocan.iarc.fr/pages/fact_sheets_cancer.aspx]. Accessed 6 Apr 2016.
- 2.Pritchard RS, Anthony SP. Chemotherapy plus radiotherapy compared with radiotherapy alone in the treatment of locally advanced, unresectable, non-small-cell lung cancer. A meta-analysis. Ann Intern Med. 1996;125:723–729. doi: 10.7326/0003-4819-125-9-199611010-00003. [DOI] [PubMed] [Google Scholar]
- 3.Marino P, Preatoni A, Cantoni A. Randomized trials of radiotherapy alone versus combined chemotherapy and radiotherapy in stages IIIa and IIIb nonsmall cell lung cancer. A meta-analysis. Cancer. 1995;76:593–601. doi: 10.1002/1097-0142(19950815)76:4<593::AID-CNCR2820760409>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 4.Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. Non-small Cell Lung Cancer Collaborative Group. Bmj. 1995;311:899–909 [PMC free article] [PubMed]
- 5.Pignon JP, Arriagada R, Ihde DC, Johnson DH, Perry MC, Souhami RL, Brodin O, Joss RA, Kies MS, Lebeau B, et al. A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N Engl J Med. 1992;327:1618–1624. doi: 10.1056/NEJM199212033272302. [DOI] [PubMed] [Google Scholar]
- 6.Warde P, Payne D. Does thoracic irradiation improve survival and local control in limited-stage small-cell carcinoma of the lung? A meta-analysis. J Clin Oncol. 1992;10:890–895. doi: 10.1200/JCO.1992.10.6.890. [DOI] [PubMed] [Google Scholar]
- 7.Auperin A, Le Pechoux C, Rolland E, Curran WJ, Furuse K, Fournel P, Belderbos J, Clamon G, Ulutin HC, Paulus R, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:2181–2190. doi: 10.1200/JCO.2009.26.2543. [DOI] [PubMed] [Google Scholar]
- 8.Turrisi AT, 3rd, Kim K, Blum R, Sause WT, Livingston RB, Komaki R, Wagner H, Aisner S, Johnson DH. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med. 1999;340:265–271. doi: 10.1056/NEJM199901283400403. [DOI] [PubMed] [Google Scholar]
- 9.Shepherd FA, Dancey J, Ramlau R, Mattson K, Gralla R, O’Rourke M, Levitan N, Gressot L, Vincent M, Burkes R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18:2095–2103. doi: 10.1200/JCO.2000.18.10.2095. [DOI] [PubMed] [Google Scholar]
- 10.Fossella FV, DeVore R, Kerr RN, Crawford J, Natale RR, Dunphy F, Kalman L, Miller V, Lee JS, Moore M, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol. 2000;18:2354–2362. doi: 10.1200/JCO.2000.18.12.2354. [DOI] [PubMed] [Google Scholar]
- 11.Hanna N, Shepherd FA, Fossella FV, Pereira JR, De Marinis F, von Pawel J, Gatzemeier U, Tsao TC, Pless M, Muller T, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–1597. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 12.Thatcher N, Chang A, Parikh P, Rodrigues Pereira J, Ciuleanu T, von Pawel J, Thongprasert S, Tan EH, Pemberton K, Archer V, Carroll K. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer) Lancet. 2005;366:1527–1537. doi: 10.1016/S0140-6736(05)67625-8. [DOI] [PubMed] [Google Scholar]
- 13.Maruyama R, Nishiwaki Y, Tamura T, Yamamoto N, Tsuboi M, Nakagawa K, Shinkai T, Negoro S, Imamura F, Eguchi K, et al. Phase III study, V-15-32, of gefitinib versus docetaxel in previously treated Japanese patients with non-small-cell lung cancer. J Clin Oncol. 2008;26:4244–4252. doi: 10.1200/JCO.2007.15.0185. [DOI] [PubMed] [Google Scholar]
- 14.Kim ES, Hirsh V, Mok T, Socinski MA, Gervais R, Wu YL, Li LY, Watkins CL, Sellers MV, Lowe ES, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet. 2008;372:1809–1818. doi: 10.1016/S0140-6736(08)61758-4. [DOI] [PubMed] [Google Scholar]
- 15.O’Brien ME, Ciuleanu TE, Tsekov H, Shparyk Y, Cucevia B, Juhasz G, Thatcher N, Ross GA, Dane GC, Crofts T. Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. J Clin Oncol. 2006;24:5441–5447. doi: 10.1200/JCO.2006.06.5821. [DOI] [PubMed] [Google Scholar]
- 16.von Pawel J, Schiller JH, Shepherd FA, Fields SZ, Kleisbauer JP, Chrysson NG, Stewart DJ, Clark PI, Palmer MC, Depierre A, et al. Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol. 1999;17:658–667. doi: 10.1200/JCO.1999.17.2.658. [DOI] [PubMed] [Google Scholar]
- 17.Eckardt JR, von Pawel J, Pujol JL, Papai Z, Quoix E, Ardizzoni A, Poulin R, Preston AJ, Dane G, Ross G. Phase III study of oral compared with intravenous topotecan as second-line therapy in small-cell lung cancer. J Clin Oncol. 2007;25:2086–2092. doi: 10.1200/JCO.2006.08.3998. [DOI] [PubMed] [Google Scholar]
- 18.Green N, Melbye RW. Lung cancer: retreatment of local recurrence after definitive irradiation. Cancer. 1982;49:865–868. doi: 10.1002/1097-0142(19820301)49:5<865::AID-CNCR2820490507>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 19.Jeremic B, Videtic GM. Chest reirradiation with external beam radiotherapy for locally recurrent non-small-cell lung cancer: a review. Int J Radiat Oncol Biol Phys. 2011;80:969–977. doi: 10.1016/j.ijrobp.2011.01.069. [DOI] [PubMed] [Google Scholar]
- 20.De Ruysscher D, Faivre-Finn C, Le Pechoux C, Peeters S, Belderbos J. High-dose re-irradiation following radical radiotherapy for non-small-cell lung cancer. Lancet Oncol. 2014;15:e620–624. doi: 10.1016/S1470-2045(14)70345-6. [DOI] [PubMed] [Google Scholar]
- 21.Drodge CS, Ghosh S, Fairchild A. Thoracic reirradiation for lung cancer: a literature review and practical guide. Ann Palliat Med. 2014;3:75–91. doi: 10.3978/j.issn.2224-5820.2014.03.04. [DOI] [PubMed] [Google Scholar]
- 22.De Bari B, Filippi AR, Mazzola R, Bonomo P, Trovo M, Livi L, Alongi F. Available evidence on re-irradiation with stereotactic ablative radiotherapy following high-dose previous thoracic radiotherapy for lung malignancies. Cancer Treat Rev. 2015;41:511–518. doi: 10.1016/j.ctrv.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Kilburn JM, Kuremsky JG, Blackstock AW, Munley MT, Kearns WT, Hinson WH, Lovato JF, Miller AA, Petty WJ, Urbanic JJ. Thoracic re-irradiation using stereotactic body radiotherapy (SBRT) techniques as first or second course of treatment. Radiother Oncol. 2014;110:505–510. doi: 10.1016/j.radonc.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trovo M, Minatel E, Durofil E, Polesel J, Avanzo M, Baresic T, Bearz A, Del Conte A, Franchin G, Gobitti C, et al. Stereotactic body radiation therapy for re-irradiation of persistent or recurrent non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2014;88:1114–1119. doi: 10.1016/j.ijrobp.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 25.Kelly P, Balter PA, Rebueno N, Sharp HJ, Liao Z, Komaki R, Chang JY. Stereotactic body radiation therapy for patients with lung cancer previously treated with thoracic radiation. Int J Radiat Oncol Biol Phys. 2010;78:1387–1393. doi: 10.1016/j.ijrobp.2009.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu H, Zhang X, Vinogradskiy YY, Swisher SG, Komaki R, Chang JY. Predicting radiation pneumonitis after stereotactic ablative radiation therapy in patients previously treated with conventional thoracic radiation therapy. Int J Radiat Oncol Biol Phys. 2012;84:1017–1023. doi: 10.1016/j.ijrobp.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McAvoy SA, Ciura KT, Rineer JM, Allen PK, Liao Z, Chang JY, Palmer MB, Cox JD, Komaki R, Gomez DR. Feasibility of proton beam therapy for reirradiation of locoregionally recurrent non-small cell lung cancer. Radiother Oncol. 2013;109:38–44. doi: 10.1016/j.radonc.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 28.Meijneke TR, Petit SF, Wentzler D, Hoogeman M, Nuyttens JJ. Reirradiation and stereotactic radiotherapy for tumors in the lung: dose summation and toxicity. Radiother Oncol. 2013;107:423–427. doi: 10.1016/j.radonc.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 29.Reyngold M, Wu AJ, McLane A, Zhang Z, Hsu M, Stein NF, Zhou Y, Ho AY, Rosenzweig KE, Yorke ED, Rimner A. Toxicity and outcomes of thoracic re-irradiation using stereotactic body radiation therapy (SBRT) Radiat Oncol. 2013;8:99. doi: 10.1186/1748-717X-8-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tada T, Fukuda H, Matsui K, Hirashima T, Hosono M, Takada Y, Inoue Y. Non-small-cell lung cancer: reirradiation for loco-regional relapse previously treated with radiation therapy. Int J Clin Oncol. 2005;10:247–250. doi: 10.1007/s10147-005-0501-1. [DOI] [PubMed] [Google Scholar]
- 31.Trakul N, Harris JP, Le QT, Hara WY, Maxim PG, Loo BW, Jr, Diehn M. Stereotactic ablative radiotherapy for reirradiation of locally recurrent lung tumors. J Thorac Oncol. 2012;7:1462–1465. doi: 10.1097/JTO.0b013e31825f22ce. [DOI] [PubMed] [Google Scholar]
- 32.Peulen H, Karlsson K, Lindberg K, Tullgren O, Baumann P, Lax I, Lewensohn R, Wersall P. Toxicity after reirradiation of pulmonary tumours with stereotactic body radiotherapy. Radiother Oncol. 2011;101:260–6. [DOI] [PubMed]
- 33.Timmerman R, McGarry R, Yiannoutsos C, Papiez L, Tudor K, DeLuca J, Ewing M, Abdulrahman R, DesRosiers C, Williams M, Fletcher J. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24:4833–9. [DOI] [PubMed]
- 34.Halperin EC, et al. Perez & Brady’s Principlesand Practice of Radiation Oncology. sixth ed. Philadelphia: Lippincott Williams &Wilkins; 2013.


