Abstract
Kindlins are 4.1-ezrin-ridixin-moesin (FERM) domain containing proteins. There are three kindlins in mammals, which share high sequence identity. Kindlin-1 is expressed primarily in epithelial cells, kindlin-2 is widely distributed and is particularly abundant in adherent cells, and kindlin-3 is expressed primarily in hematopoietic cells. These distributions are not exclusive; some cells express multiple kindlins, and transformed cells often exhibit aberrant expression, both in the isoforms and the levels of kindlins. Great interest in the kindlins has emerged from the recognition that they play major roles in controlling integrin function. In vitro studies, in vivo studies of mice deficient in kindlins, and studies of patients with genetic deficiencies of kindlins have clearly established that they regulate the capacity of integrins to mediate their functions. Kindlins are adaptor proteins; their function emanates from their interaction with binding partners, including the cytoplasmic tails of integrins and components of the actin cytoskeleton. The purpose of this review is to provide a brief overview of kindlin structure and function, a consideration of their binding partners, and then to focus on the relationship of each kindlin family member with cancer. In view of many correlations of kindlin expression levels and neoplasia and the known association of integrins with tumor progression and metastasis, we consider whether regulation of kindlins or their function would be attractive targets for treatment of cancer.
Keywords: Kindlins, integrins, cancer, FERMT genes, cancer therapy
1. Introduction
Since the term “integrins” was coined more than 3 decades ago to designate a broadly distributed family of cell-surface adhesion receptors, the contributions of each of the 24 integrin family members in numerous physiological and pathological processes has remained a dominant theme in cell biology research. Indeed, it has now been broadly established that integrins have functions extending well beyond their primary roles in cell adhesion and migration; their contributions to bidirectional signaling, proliferation, gene regulation and cellular entry of pathogens have all been extensively documented. Research on integrins has extended from very basic investigations of their ligand binding repertoires and their three-dimensional structures to the clinical relevance of their antagonism as potential therapies. Indeed, clinical trials that led to approvals of several integrin directed drugs that have been used to treat patients with a variety of disorders, including thrombosis, cancer, ulcerative colitis and multiple sclerosis. Despite the breadth and depth of these studies, unanticipated findings regarding integrins and their functions have continued to emerge. Such a finding was the implication of kindlins into the integrin field starting about 10 years ago, but these cytosolic proteins are now accepted as pivotal regulators of integrin function1-7. A burgeoning subcontext of the relationship between kindlins and integrins is how their intercalation impacts cancer. Within the last 5 years, more than 70 publications have linked kindlins, integrins and cancer. In this review, we provide a brief synopsis of kindlin structure-function relationship, consider the latest findings on the role of kindlins in cancer, and then speculate as to the potential to target kindlins to inhibit cancer initiation, progression, metastasis and chemoresistance.
2. The kindlins
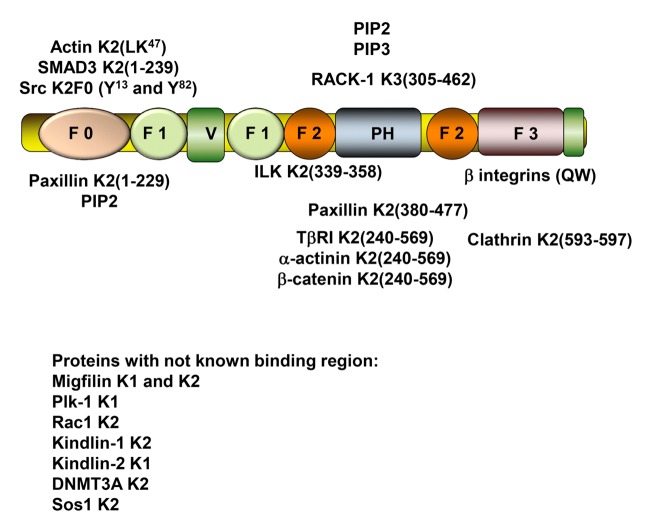
The prototypical structure of a kindlin is shown inFigure 1. Kindlins belong to the 4.1- ezrin-ridixin-moesin (FERM) domain containing protein family. Kindlins contain F1, F2 and F3 subdomains that typify FERM family members, and these subdomains are preceded by an N-terminal F0 subdomain in kindlins. A distinctive feature of kindlins is the insertion of a pleckstrin homology (PH) subdomain into the F2 subdomain (reviewed in 4). There are three kindlin family members in mammals, KINDLIN-1 (FERMT1; chromosome 20p12.3), KINDLIN-2 (FERMT2; chromosome 14q22.1) and KINDLIN-3 (FERMT3; chromosome 11q13.1). The three kindlins are highly homologous, sharing ~60% amino acid sequence identity1. Of the genomes analyzed, all metazoans, but no premetazoans, have at least one kindlin gene. Kindlin-2 is likely to have preserved the ancestral features of the kindlin family, and kindin-1 and kindlin-3 arose from duplications of the kindlin-2 gene. The ancestral kindlin itself appears to have evolved from duplication of the FERM domain in the N-terminal region of talin, and the two proteins share an overlapping function in integrin activation8,9. In humans, mutations leading to deficiencies of kindlin-1 cause Kindler Syndrome, which manifests with symptoms of skin fragility, blister formation, cutaneous atrophy, poikiloderma, and photosensitivity. Intestinal defects also occur frequently10-12. Mice deficient in kindlin-1 show similar phenotypes but the intestinal defects result in death shortly after birth13. Mutations in the kindlin-3 gene cause leukocyte adhesion deficiency type III (LAD III), which is characterized by high susceptibility to infections, spontaneous and episodic bleedings, and osteopetrosis14-17. These symptoms also occur in mice lacking kindlin-3 and result in mice that are only viable for a short time postnatally18. No deficiencies of kindlin-2 in humans have been reported to date, but disruption of kindlin-2 in mice results in embryonic lethality19. Mice with partial deficiency of kindlin-2, Kindlin-2+/-mice, exhibit no overt phenotype but display abnormal response in angiogenesis20, hemostasis21 and intracellular actin organization22. The three kindlins exhibit differences in their expression profiles: kindlin-1 is expressed mainly in epithelial cells; kindlin-2 is broadly expressed and is plentiful in endothelial cells, smooth muscle cells and fibroblasts23; and expression of kindlin-3 is restricted primarily to hematopoietic cells although it is also expressed in endothelial cells24. Several recent studies have, however, showed that aberrant expression of the kindlins occurs in several human cancers.
Figure 1. Binding partners of kindlins identified to date.
The numbers refer to the amino acid residues of the binding regions within the indicated kindlin.
K1 (kindlin-1); K2 (kindlin-2); K3 (kindlin-3); ILK (integrin-linked kinase); RACK1 (receptor for activated C kinase 1); TβRI (TGF-β receptor I kinase); Plk-1 (polo-like kinase 1); DNMT3A-DNA (DNA (cytosine-5) methyltransferase 3A); Sos-1 (Son of sevenless homolog 1).
3. Kindlins as adaptor proteins
Kindlins are adaptor proteins. They lack intrinsic enzymatic activity but rather bind multiple effectors and thereby can build large multimolecular and multifunctional complexes. The binding sites for several kindlin binding partners have been positioned within the organization of the prototypic kindlin inFigure 1. Phospholipid binding sites exist in the F025,26, F127,28 and PH subdomains29,30. These interactions may target kindlins to membranes and optimize their orientation to execute other kindlin-dependent functions such as integrin activation. F0 also harbors binding sites for actin22; F2 contains the ILK binding site31,32; and, in addition to its phospholipid binding properties, the PH subdomain also contains a paxillin binding site33; and the F3 subdomain contains a clathrin21 and the primary integrin binding site (e.g.34). However, the primary function of kindlins, the capacity to support integrin activation, requires all subdomains of the kindlin35. The location of these binding sites has usually been established for one kindlin and may extrapolate to the other kindlin family members based on homology. Interactions of kindlins with ADAP36, RACK137, scr38 and β-catenin39 also have been demonstrated. Some interactions may influence the function of an individual kindlin selectively as described in chapter 4. For example, ADAP can bind to both kindlin-2 and kindlin-3, but ADAP is restricted to hematopoietic cells36, where kindlin-3 exerts its major functions. Post-translational modifications of kindlins also occur, may be selective to specific kindlins and may influence the function of the modified kindlin38,40.
4. Functions of kindlins
Integrin-dependent functions: The most studied function of kindlins revolves around their role in integrin activation. Integrins can alter their affinity/avidity for their cognate ligands, a transition that is usually induced by stimulation of the integrin-bearing cell with agonists. Agonists may include G protein–coupled receptor ligands, growth factors, cytokines and shear stress (e.g. 41-44). Activation is particularly important for integrin-mediated responses of circulating blood cells, such as the adhesion of leukocytes to vascular cells45,46, of leukocytes to other blood cells46-48, or platelets to one another49. These responses do not occur in patients lacking kindlin-3; the integrin β1, β2 or β3 subclasses on hematopoietic cells do not undergo activation16. Integrins on adherent cells can also undergo activation although the changes are not as dramatic. Such integrin activation depends on inside-out signaling, which is a consequence of the binding of talin and kindlin to the cytoplasmic domain of integrins7,50. The detailed mechanisms of integrin activation have been the subject of reviews6,51,52 and are very dependent on the definition of “activation”. Is activation defined on a structural basis as straightening of the integrin legs from a bent to an extended conformation and/or opening of the headpiece, or is it the acquisition of functionally productive ligand binding53. Ligand binding and integrin clustering induce inside-out signaling. Frequently elicited consequences of outside-in signaling include cell spreading, changes in cell shape and gene expression. Kindlins are integrally involved in generating outside-in signals, which depends upon direct or indirect interactions with elements of actin cytoskeleton and the reorganization of focal adhesions, multimolecular signaling hubs within the cell.
Integrin-independent functions of kindlins: In a limited number of studies, functions have been assigned to kindlins that appear to be independent of their integrin binding activity. The integrin binding site of kindlins resides in their F3 (PTB-like) subdomain. Central to this binding function is a particular QW motif, Q614W615 in kindlin-2, and mutation of these residues to alanines markedly diminishes integrin binding activity. Using such mutant kindlins, the interaction of kindlin-2 with β-catenin to regulate Wnt signaling39 and kindlin-2 with clathrin to regulate cell surface expression of catabolic enzymes in endothelial cells54 have been identified as integrin-independent functions of kindlins. These mutations also demonstrated an integrin-independent role for kindlin-1 in Wnt signaling55. This strategy presumes that integrin binding to kindlins is completely disabled by the QW mutation. Clearly these mutations markedly reduce but may not completely disable integrin binding34.
5. Association of kindlins with cancer
The intimate interrelationship between integrins and cancer pathology has inevitably led to consideration of the role of kindlins in cancer. These efforts have identified associations of all three kindlin isoforms with cancers of many different tissues. In some cases, affected tissues is consistent with the distribution of the kindlin isoforms but expression levels are altered relative to the kindlin levels in the corresponding normal tissue (e.g., kindlin-1 and skin cancer55); whereas, in other cases, the particular kindlin is expressed at an unusual cell type (e.g. kindlin-3 sin breast cancer56).
5.1. Kindlin-1 and Cancer
One of the earliest evidences implicating kindlin-1 in cancer came from measurements of its mRNA expression levels. These levels were elevated in 60% of lung and 70% of colon cancers57. Kindlin-1 was also found to be associated with the pathology of glioma58. Kindlin-1 mRNA also was highly expressed in the pancreatic cancer cell lines and pancreatic cancer tissue59. Kindlin-1 protein was detected in the cytoplasm and membrane of the pancreatic cancer cells while normal ductal epithelial cells and stromal cells showed no expression. Sin and colleagues60 reported a role of kindlin-1 in the metastasis of tumors from various organs to the lungs and found that kindlin-1 expression correlated with a poor prognosis in both breast and lung adenocarcinoma60.
Despite these associations of kindlin-1 with cancer from many different organs, most studies documented aberrant kindlin-1 expression levels in cancers of epithelial origin59-65, consistent with its primary epithelial localization. In patients lacking kindlin-1 “Kindler Syndrome patients”, there is a suggestion of an increased risk of squamous cell carcinomas66-71; however, the rarity of the Kindler Syndrome precludes broad generalizations. Since kindlin-1 deficiency is lethal in mice due to intestinal manifestations. Rognoni and colleagues55 generated mice deficient in kindlin-1 in keratinocytes. These mice do exhibit an increased incidence of skin tumors that formed primarily as trichofolliculoma-like lesions and basal cell carcinomas, distinct from the tumors that were noted in patients with Kindler Syndrome55. Mechanistically, several interesting linkages have been uncovered between kindlin-1 and TGFβ activation, which exerts many opposing effects on the multiple steps associated with cancer progression and metastasis72. Gene expression microarray studies comparing the RNA profiles of TGFβ1-treated mammary epithelial cells with non-treated cells show that kindlin-1 is a TGFβ1 inducible gene73. Increase in kindlin-1 expression resulting from TGFβ1 treatment enhanced cell spreading and induced actin rearrangement, events correlated with the epithelial to mesenchymal transition (EMT), an important step in carcinogenesis73. TGF-β activation can be mediated by integrin αVβ6 and kindlin-1 can activate this integrin. Thus, an amplification loop may exist in which TGF-β enhances kindlin-1 synthesis and kindlin-1 enhances activation of TGF-β via αVβ6. High kindlin-1 levels have also been associated with high TGFβ-1 signaling in metastatic breast cancers60, and suppression of kindlin-1 in breast cancer cells significantly inhibited tumor growth and lung metastasis in an orthotopic mouse model. However, suppression of kindlin-1 in Kindler Syndrome patients may enhance cancer risk and, therefore, precludes broad generalizations.
5.2 Kindlin-2 and Cancer
Kindlin-2 expression has been found to be dysregulated in several cancer types: prostate74-77 breast64,78-80, lung65,81, colorectal cancer82, pancreas83,84 ovarian85,86, esophageal squamous cell carcinoma87-89, liver90, brain91, gastric cancer92,93, bladder94, and acute myeloid leukemia95. Given the association of kindlin-2 with several cancers of different origins, one can predict an important role that kindlin-2 may play in cancer pathogenesis. Kindlin-2 regulates tumor progression and metastasis by modulating several signaling pathways that are known to be critical for the regulation of cancer cell survival, proliferation, migration, invasion and metastasis. In fact, kindlin-2 has been associated with almost every hallmark of cancer75. In prostate cancer, kindlin-2 was found to promote the survival of prostate cancer cells by activating the nuclear factor kappa B (NFκB) survival pathway74. The invasive potential of prostate cancer cells was also activated as a result of the NFκB-mediated upregulation of matrix metalloproteinases expression and activity74. A positive feedback loop between kindlin-2 and TGF-β was identified to play a key role in promoting the progression and metastasis of pancreatic cancer84, which is characterized by its aggressiveness and the lack of effective therapies. While kindlin-2 expression levels were markedly elevated by Transforming Growth Factor 1 (TGF-β1) treatment, kindlin-2, in turn, activated the expression of TGF-β receptor I, a major component of TGF-β signaling84. Epithelial to mesenchymal transition, another significant hallmark of cancer, was found to be affected in esophageal squamous cell carcinoma as a result of dysregulation of kindlin-287. Zhang and colleagues87 described how the loss of miR-200b, a well-established regulator of EMT, enhances invasion of esophageal squamous cell carcinoma cells by activating the Kindlin-2/integrin β1/AKT signaling pathway. Conversely, overexpression of miR-200b in these cells inhibited the integrin β1-AKT signaling by specific targeting of kindlin-2, which in turn suppressed invasion of ESCC cells87. Epidermal growth factor receptor (EGFR) is a known activator of cell proliferation, migration and tumor invasion in several cancers, including the one originating from the breast. A recent study by Guo et al.80 established a critical link between kindlin-2 and EGFR, where a physical interaction between the two was found to be necessary for the stabilization of EGFR and subsequent activation of the migration and invasion of breast cancer cells. Finally, several studies, including ours75, established a critical function of kindlin-2 to the modulation of chemoresistance, yet another major hallmark of cancer72. The Zhan lab91 described how kindlin-2 modulates the cisplatin-induced apoptosis and cell death of human glioma cells by regulating the AKT/JNK and AKT/p38 signaling pathways while the Zhang lab77 found kindlin-2 to activate the cisplatin-mediated cell death of prostate cancer cells through the regulation of the Bcl-xL cell death pathway. Our study has also established a major role for kindlin-2 in the regulation of the chemotherapy-induced cell death and apoptosis of metastatic castration-resistant prostate cancer, for which effective treatments have yet to be developed75. Loss of expression of kindlin-2 in prostate cancer cell lines significantly enhanced the sensitivity of these cells to docetaxel-induced apoptosis and cell death. Mechanistically, we found miR-138 to specifically target and inhibit kindlin-2 in prostate cancer cell lines, which resulted in dysregulation of the kindlin-2/β1-integrin pathway, thereby identifying a novel miR-138/kindlin-2/β1-integrin signaling axis that is critical for the modulation of sensitivity to chemotherapeutics. Therefore, targeted inhibition of kindlin-2 could be combined with chemotherapy to develop an effective treatment for prostate cancer75.
5.3 Kindlin-3 and cancer
Although Kindlin-3 is mainly expressed in the hematopoietic system, there are surprisingly only limited reports about its involvement in blood cell cancers. Kindlin-3 was found to be associated with the pathology of chronic myeloid leukemia96 and acute myeloid leukemia95. Qu and colleagues96 showed that kindlin-3 may regulate the proliferation of human chronic myeloid leukemia K562 cells through the regulation of c-Myc protein expression and controlled the tumor growth of these cells in a xenograft model96. In another study, Wu and colleagues reported that levels of kindlin-3 increased in patients with acute myeloid leukemia after complete remission95. Recent studies have identified kindlin-3 in solid tumors but its role as a tumor promoter or tumor suppressor is controversial. Our published study has identified kindlin-3 as a promoter of breast cancer progression and metastasis56. Kindlin-3 was over-expressed in both breast cancer cell lines and primary tumors, which is consistent with several Oncomine (www.oncomine.com) datasets, where kindlin-3 was in the top 3% of gene products elevated in breast cancer (p< 10-12)56. In vitro analyses determined that kindlin-3 stimulates breast cancer migration and invasion, and in vivo studies in mice showed that kindlin-3 stimulated tumor progression and metastasis. This function was traced to induction of tumor angiogenesis as a result of enhanced VEGF secretion and macrophage recruitment, downstream of Twist, which also activates the EMT program56. Nevertheless, a recent study by Djaafri and colleagues97 concluded that kindlin-3 has tumor suppressor function. The Djaafri study also included in vitro and in vivo supporting data and even included the MDA-MB-231 breast cancer cell line used by Sossey-Alaoui et al56. The basis for these differences is not clear although Djaafri et al. concluded kindlin-3 was a tumor promoter in K562 leukemic cells.
6. Kindlins as a cancer therapeutic target
Two independent studies have shown that reduction in kindlin-2 levels, either with siRNA that targets kindlin mRNA directly or by overexpression of miRNAs that reduce kindlin expression, sensitizes tumor cell lines to chemotherapeutic agents such as docetaxel75 and cisplatin77,91. Knockdown of kindlin-2 would also blunt certain properties of tumor cells that are associated with cancer progression, such as angiogenesis, invasion, recruitment of tumor promoting macrophages and formation of invadopodia. Rather than at the gene expression level, it may be feasible to selectively inhibit selective functions of a specific kindlin family member. For example, most of the functions of kindlin in tumor cell biology revolve around their interaction with the cytoplasmic tails of integrins. Specific short peptide sequences within integrin β tails have been located that are necessary for kindlins to exert their integrin regulatory activity35. Peptides or peptidomimetics of these sequences that block kindlin binding to integrins could be delivered into cells and blunt responses. These sequences could be tailored to block interaction of kindlins with specific integrins, or individual kindlins with specific integrins. However, knocking down kindlins may not be the panacea for cancer treatment. In a recent publication, Rognoni et al.55 provided a comprehensive and valuable list of the reports in which kindlin levels were altered in tumors. In most of the listed studies, the levels of the kindlin under investigation were increased, but in six of the 20 reports, reduced levels of kindlin were associated with cancer or a transformed cell phenotype. As noted above, the absence of kindlin-1 in Kindler Syndrome patients appears to be associated with an increase in cancer. Thus, just as there is a TGF-β paradox, there may be a “kindlin paradox” and the value of a kindlin targeted therapy may need to be context specific. Despite this uncertainty, the relationship between kindlins and cancer remains an important interrelationship to dissect, and manipulation of kindlin functions and/or levels may provide insights into tumorogenesis and may ultimately offer a therapeutic strategy.
KEY POINTS
◊ Kindlins are FERM domain adaptor proteins
◊ Kindlins are critical regulators of integrin function
◊ Expression of each of the three kindlins is altered in many different human cancers
◊ Kindlins can influence the growth and metastatic properties of cancer cells in in vivo models and modulate sensitivities to chemotherapies
Acknowledgments
This work was supported in part by NIH grants HL096062 and HL073311.
Footnotes
Conflict of interests: The authors have no conflicts of interest to disclose.
4.1-ezrin-ridixin-moesin (FERM); leukocyte adhesion deficiency type III (LAD III); Epithelial to mesenchymal transition (EMT); Transforming growth factor-beta (TGF-b); Nuclear factor kappa B (NFkB); Epidermal growth factor receptor (EGFR)
DISCOVERIES is a peer-reviewed, open access, online, multidisciplinary and integrative journal, publishing high impact and innovative manuscripts from all areas related to MEDICINE, BIOLOGY and CHEMISTRY
References
- 1.Kindlins: essential regulators of integrin signalling and cell-matrix adhesion. Larjava Hannu, Plow Edward F, Wu Chuanyue. EMBO reports. 2008;9(12):1203–8. doi: 10.1038/embor.2008.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kindling the flame of integrin activation and function with kindlins. Plow Edward F, Qin Jun, Byzova Tatiana. Current opinion in hematology. 2009;16(5):323–8. doi: 10.1097/MOH.0b013e32832ea389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The Kindlin protein family: new members to the club of focal adhesion proteins. Meves Alexander, Stremmel Christopher, Gottschalk Kay, Fässler Reinhard. Trends in cell biology. 2009;19(10):504–13. doi: 10.1016/j.tcb.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 4.Kindlins in FERM adhesion. Malinin Nikolay L, Plow Edward F, Byzova Tatiana V. Blood. 2010;115(20):4011–7. doi: 10.1182/blood-2009-10-239269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kindlin: helper, co-activator, or booster of talin in integrin activation? Ye Feng, Petrich Brian G. Current opinion in hematology. 2011;18(5):356–60. doi: 10.1097/MOH.0b013e3283497f09. [DOI] [PubMed] [Google Scholar]
- 6.The final steps of integrin activation: the end game. Shattil Sanford J, Kim Chungho, Ginsberg Mark H. Nature reviews. Molecular cell biology. 2010;11(4):288–300. doi: 10.1038/nrm2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.SnapShot: talin and the modular nature of the integrin adhesome. Ye Feng, Lagarrigue Frederic, Ginsberg Mark H. Cell. 2014;156(6):1340–1340.e1. doi: 10.1016/j.cell.2014.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phylogenetic analysis of kindlins suggests subfunctionalization of an ancestral unduplicated kindlin into three paralogs in vertebrates. Khan Ammad Aslam, Janke Axel, Shimokawa Takashi, Zhang Hongquan. Evolutionary bioinformatics online. 2011;7:7–19. doi: 10.4137/EBO.S6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emergence and subsequent functional specialization of kindlins during evolution of cell adhesiveness. Meller Julia, Rogozin Igor B, Poliakov Eugenia, Meller Nahum, Bedanov-Pack Mark, Plow Edward F, Qin Jun, Podrez Eugene A, Byzova Tatiana V. Molecular biology of the cell. 2015;26(4):786–96. doi: 10.1091/mbc.E14-08-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loss of kindlin-1, a human homolog of the Caenorhabditis elegans actin-extracellular-matrix linker protein UNC-112, causes Kindler syndrome. Siegel Dawn H, Ashton Gabrielle H S, Penagos Homero G, Lee James V, Feiler Heidi S, Wilhelmsen Kirk C, South Andrew P, Smith Frances J D, Prescott Alan R, Wessagowit Vesarat, Oyama Noritaka, Akiyama Masashi, Al Aboud Daifullah, Al Aboud Khalid, Al Githami Ahmad, Al Hawsawi Khalid, Al Ismaily Abla, Al-Suwaid Raouf, Atherton David J, Caputo Ruggero, Fine Jo-David, Frieden Ilona J, Fuchs Elaine, Haber Richard M, Harada Takashi, Kitajima Yasuo, Mallory Susan B, Ogawa Hideoki, Sahin Sedef, Shimizu Hiroshi, Suga Yasushi, Tadini Gianluca, Tsuchiya Kikuo, Wiebe Colin B, Wojnarowska Fenella, Zaghloul Adel B, Hamada Takahiro, Mallipeddi Rajeev, Eady Robin A J, McLean W H Irwin, McGrath John A, Epstein Ervin H. American journal of human genetics. 2003;73(1):174–87. doi: 10.1086/376609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kindler surprise: mutations in a novel actin-associated protein cause Kindler syndrome. White Sharon J, McLean W H Irwin. Journal of dermatological science. 2005;38(3):169–75. doi: 10.1016/j.jdermsci.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 12.Kindler syndrome in mice and men. Duperret Elizabeth K, Ridky Todd W. Cancer biology & therapy. 2014;15(9):1113–6. doi: 10.4161/cbt.29482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loss of Kindlin-1 causes skin atrophy and lethal neonatal intestinal epithelial dysfunction. Ussar Siegfried, Moser Markus, Widmaier Moritz, Rognoni Emanuel, Harrer Christian, Genzel-Boroviczeny Orsolya, Fässler Reinhard. PLoS genetics. 2008;4(12):e1000289. doi: 10.1371/journal.pgen.1000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kindlin-3: a new gene involved in the pathogenesis of LAD-III. Mory Adi, Feigelson Sara W, Yarali Nese, Kilic Sara S, Bayhan Gulsum I, Gershoni-Baruch Ruth, Etzioni Amos, Alon Ronen. Blood. 2008;112(6):2591. doi: 10.1182/blood-2008-06-163162. [DOI] [PubMed] [Google Scholar]
- 15.LAD-1/variant syndrome is caused by mutations in FERMT3. Kuijpers Taco W, van de Vijver Edith, Weterman Marian A J, de Boer Martin, Tool Anton T J, van den Berg Timo K, Moser Markus, Jakobs Marja E, Seeger Karl, Sanal Ozden, Unal Sule, Cetin Mualla, Roos Dirk, Verhoeven Arthur J, Baas Frank. Blood. 2009;113(19):4740–6. doi: 10.1182/blood-2008-10-182154. [DOI] [PubMed] [Google Scholar]
- 16.A point mutation in KINDLIN3 ablates activation of three integrin subfamilies in humans. Malinin Nikolay L, Zhang Li, Choi Jeongsuk, Ciocea Alieta, Razorenova Olga, Ma Yan-Qing, Podrez Eugene A, Tosi Michael, Lennon Donald P, Caplan Arnold I, Shurin Susan B, Plow Edward F, Byzova Tatiana V. Nature medicine. 2009;15(3):313–8. doi: 10.1038/nm.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leukocyte adhesion deficiency-III is caused by mutations in KINDLIN3 affecting integrin activation. Svensson Lena, Howarth Kimberley, McDowall Alison, Patzak Irene, Evans Rachel, Ussar Siegfried, Moser Markus, Metin Ayse, Fried Mike, Tomlinson Ian, Hogg Nancy. Nature medicine. 2009;15(3):306–12. doi: 10.1038/nm.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kindlin-3 is essential for integrin activation and platelet aggregation. Moser Markus, Nieswandt Bernhard, Ussar Siegfried, Pozgajova Miroslava, Fässler Reinhard. Nature medicine. 2008;14(3):325–30. doi: 10.1038/nm1722. [DOI] [PubMed] [Google Scholar]
- 19.Kindlin-2 is an essential component of intercalated discs and is required for vertebrate cardiac structure and function. Dowling James J, Gibbs Elizabeth, Russell Mark, Goldman Daniel, Minarcik Jeremy, Golden Jeffrey A, Feldman Eva L. Circulation research. 2008;102(4):423–31. doi: 10.1161/CIRCRESAHA.107.161489. [DOI] [PubMed] [Google Scholar]
- 20.The integrin coactivator kindlin-2 plays a critical role in angiogenesis in mice and zebrafish. Pluskota Elzbieta, Dowling James J, Gordon Natalie, Golden Jeffrey A, Szpak Dorota, West Xiaoxia Z, Nestor Carla, Ma Yan-Qing, Bialkowska Katarzyna, Byzova Tatiana, Plow Edward F. Blood. 2011;117(18):4978–87. doi: 10.1182/blood-2010-11-321182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kindlin-2 regulates hemostasis by controlling endothelial cell-surface expression of ADP/AMP catabolic enzymes via a clathrin-dependent mechanism. Pluskota Elzbieta, Ma Yi, Bledzka Kamila M, Bialkowska Katarzyna, Soloviev Dmitry A, Szpak Dorota, Podrez Eugene A, Fox Paul L, Hazen Stanley L, Dowling James J, Ma Yan-Qing, Plow Edward F. Blood. 2013;122(14):2491–9. doi: 10.1182/blood-2013-04-497669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kindlin-2 directly binds actin and regulates integrin outside-in signaling. Bledzka Kamila, Bialkowska Katarzyna, Sossey-Alaoui Khalid, Vaynberg Julia, Pluskota Elzbieta, Qin Jun, Plow Edward F. The Journal of cell biology. 2016;213(1):97–108. doi: 10.1083/jcb.201501006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The Kindlins: subcellular localization and expression during murine development. Ussar Siegfried, Wang Hao-Ven, Linder Stefan, Fässler Reinhard, Moser Markus. Experimental cell research. 2006;312(16):3142–51. doi: 10.1016/j.yexcr.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 24.The integrin co-activator Kindlin-3 is expressed and functional in a non-hematopoietic cell, the endothelial cell. Bialkowska Katarzyna, Ma Yan-Qing, Bledzka Kamila, Sossey-Alaoui Khalid, Izem Lahoucine, Zhang Xiaoxia, Malinin Nikolay, Qin Jun, Byzova Tatiana, Plow Edward F. The Journal of biological chemistry. 2010;285(24):18640–9. doi: 10.1074/jbc.M109.085746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The structure of the N-terminus of kindlin-1: a domain important for alphaiibbeta3 integrin activation. Goult Benjamin T, Bouaouina Mohamed, Harburger David S, Bate Neil, Patel Bipin, Anthis Nicholas J, Campbell Iain D, Calderwood David A, Barsukov Igor L, Roberts Gordon C, Critchley David R. Journal of molecular biology. 2009;394(5):944–56. doi: 10.1016/j.jmb.2009.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Membrane binding of the N-terminal ubiquitin-like domain of kindlin-2 is crucial for its regulation of integrin activation. Perera H Dhanuja, Ma Yan-Qing, Yang Jun, Hirbawi Jamila, Plow Edward F, Qin Jun. Structure (London, England : 1993) 2011;19(11):1664–71. doi: 10.1016/j.str.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.A conserved lipid-binding loop in the kindlin FERM F1 domain is required for kindlin-mediated αIIbβ3 integrin coactivation. Bouaouina Mohamed, Goult Benjamin T, Huet-Calderwood Clotilde, Bate Neil, Brahme Nina N, Barsukov Igor L, Critchley David R, Calderwood David A. The Journal of biological chemistry. 2012;287(10):6979–90. doi: 10.1074/jbc.M111.330845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Increased expression of kindlin-2 is correlated with hematogenous metastasis and poor prognosis in patients with clear cell renal cell carcinoma. Yan Meisi, Zhang Lei, Wu Yiqi, Gao Lei, Yang Weiwei, Li Jing, Chen Yubing, Jin Xiaoming. FEBS open bio. 2016;6(7):660–5. doi: 10.1002/2211-5463.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kindlin-2 regulates podocyte adhesion and fibronectin matrix deposition through interactions with phosphoinositides and integrins. Qu Hong, Tu Yizeng, Shi Xiaohua, Larjava Hannu, Saleem Moin A, Shattil Sanford J, Fukuda Koichi, Qin Jun, Kretzler Matthias, Wu Chuanyue. Journal of cell science. 2011;124(Pt 6):879–91. doi: 10.1242/jcs.076976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Structural basis of phosphoinositide binding to kindlin-2 protein pleckstrin homology domain in regulating integrin activation. Liu Jianmin, Fukuda Koichi, Xu Zhen, Ma Yan-Qing, Hirbawi Jamila, Mao Xian, Wu Chuanyue, Plow Edward F, Qin Jun. The Journal of biological chemistry. 2011;286(50):43334–42. doi: 10.1074/jbc.M111.295352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Molecular basis of kindlin-2 binding to integrin-linked kinase pseudokinase for regulating cell adhesion. Fukuda Koichi, Bledzka Kamila, Yang Jun, Perera H Dhanuja, Plow Edward F, Qin Jun. The Journal of biological chemistry. 2014;289(41):28363–75. doi: 10.1074/jbc.M114.596692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Differences in binding to the ILK complex determines kindlin isoform adhesion localization and integrin activation. Huet-Calderwood Clotilde, Brahme Nina N, Kumar Nikit, Stiegler Amy L, Raghavan Srikala, Boggon Titus J, Calderwood David A. Journal of cell science. 2014;127(Pt 19):4308–21. doi: 10.1242/jcs.155879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kindlin-2 cooperates with talin to activate integrins and induces cell spreading by directly binding paxillin. Theodosiou Marina, Widmaier Moritz, Böttcher Ralph T, Rognoni Emanuel, Veelders Maik, Bharadwaj Mitasha, Lambacher Armin, Austen Katharina, Müller Daniel J, Zent Roy, Fässler Reinhard. eLife. 2016;5:e10130. doi: 10.7554/eLife.10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Direct interaction of kindlin-3 with integrin αIIbβ3 in platelets is required for supporting arterial thrombosis in mice. Xu Zhen, Chen Xue, Zhi Huiying, Gao Juan, Bialkowska Katarzyna, Byzova Tatiana V, Pluskota Elzbieta, White Gilbert C, Liu Junling, Plow Edward F, Ma Yan-Qing. Arteriosclerosis, thrombosis, and vascular biology. 2014;34(9):1961–7. doi: 10.1161/ATVBAHA.114.303851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Integrity of kindlin-2 FERM subdomains is required for supporting integrin activation. Xu Zhen, Gao Juan, Hong Jiang, Ma Yan-Qing. Biochemical and biophysical research communications. 2013;434(2):382–7. doi: 10.1016/j.bbrc.2013.03.086. [DOI] [PubMed] [Google Scholar]
- 36.ADAP interactions with talin and kindlin promote platelet integrin αIIbβ3 activation and stable fibrinogen binding. Kasirer-Friede Ana, Kang Jian, Kahner Bryan, Ye Feng, Ginsberg Mark H, Shattil Sanford J. Blood. 2014;123(20):3156–65. doi: 10.1182/blood-2013-08-520627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kindlin-3 mediates integrin αLβ2 outside-in signaling, and it interacts with scaffold protein receptor for activated-C kinase 1 (RACK1). Feng Chen, Li Yan-Feng, Yau Yin-Hoe, Lee Hui-Shan, Tang Xiao-Yan, Xue Zhi-Hong, Zhou Yi-Chao, Lim Wei-Min, Cornvik Tobias C, Ruedl Christiane, Shochat Susana G, Tan Suet-Mien. The Journal of biological chemistry. 2012;287(14):10714–26. doi: 10.1074/jbc.M111.299594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kindlin-2 phosphorylation by Src at Y193 enhances Src activity and is involved in Migfilin recruitment to the focal adhesions. Liu Zhaoli, Lu Danyu, Wang Xiang, Wan Junhu, Liu Chang, Zhang Hongquan. FEBS letters. 2015;589(15):2001–10. doi: 10.1016/j.febslet.2015.05.038. [DOI] [PubMed] [Google Scholar]
- 39.Kindlin 2 forms a transcriptional complex with β-catenin and TCF4 to enhance Wnt signalling. Yu Yu, Wu Junzhou, Wang Yunling, Zhao Ting, Ma Bo, Liu Yuqing, Fang Weigang, Zhu Wei-Guo, Zhang Hongquan. EMBO reports. 2012;13(8):750-758. doi: 10.1038/embor.2012.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Site-specific phosphorylation of kindlin-3 protein regulates its capacity to control cellular responses mediated by integrin αIIbβ3. Bialkowska Katarzyna, Byzova Tatiana V, Plow Edward F. The Journal of biological chemistry. 2015;290(10):6226–42. doi: 10.1074/jbc.M114.634436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thrombin signalling and protease-activated receptors. Coughlin S R. Nature. 2000;407(6801):258–64. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- 42.Role of ADP receptor P2Y(12) in platelet adhesion and thrombus formation in flowing blood. Remijn Jasper A, Wu Ya-Ping, Jeninga Ellen H, IJsseldijk Martin J W, van Willigen Gijsbert, de Groot Philip G, Sixma Jan J, Nurden Alan T, Nurden Paquita. Arteriosclerosis, thrombosis, and vascular biology. 2002;22(4):686–91. doi: 10.1161/01.atv.0000012805.49079.23. [DOI] [PubMed] [Google Scholar]
- 43.A mechanism for modulation of cellular responses to VEGF: activation of the integrins. Byzova T V, Goldman C K, Pampori N, Thomas K A, Bett A, Shattil S J, Plow E F. Molecular cell. 2000;6(4):851–60. [PubMed] [Google Scholar]
- 44.Integrins in mechanotransduction. Katsumi Akira, Orr A Wayne, Tzima Eleni, Schwartz Martin Alexander. The Journal of biological chemistry. 2004;279(13):12001–4. doi: 10.1074/jbc.R300038200. [DOI] [PubMed] [Google Scholar]
- 45.Leukocyte-endothelial adhesion molecules. Carlos T M, Harlan J M. Blood. 1994;84(7):2068–101. [PubMed] [Google Scholar]
- 46.The dynamic regulation of integrin adhesiveness. Diamond M S, Springer T A. Current biology : CB. 1994;4(6):506–17. doi: 10.1016/s0960-9822(00)00111-1. [DOI] [PubMed] [Google Scholar]
- 47.Neutrophil rolling, arrest, and transmigration across activated, surface-adherent platelets via sequential action of P-selectin and the beta 2-integrin CD11b/CD18. Diacovo T G, Roth S J, Buccola J M, Bainton D F, Springer T A. Blood. 1996;88(1):146–57. [PubMed] [Google Scholar]
- 48.Targeting platelet-leukocyte interactions: identification of the integrin Mac-1 binding site for the platelet counter receptor glycoprotein Ibalpha. Ehlers Raila, Ustinov Valentin, Chen Zhiping, Zhang Xiaobin, Rao Ravi, Luscinskas F William, Lopez Jose, Plow Edward, Simon Daniel I. The Journal of experimental medicine. 2003;198(7):1077–88. doi: 10.1084/jem.20022181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.The fibrinogen-dependent pathway of platelet aggregation. Marguerie G A, Plow E F. Annals of the New York Academy of Sciences. 1983;408:556–66. doi: 10.1111/j.1749-6632.1983.tb23272.x. [DOI] [PubMed] [Google Scholar]
- 50.Spatial coordination of kindlin-2 with talin head domain in interaction with integrin β cytoplasmic tails. Bledzka Kamila, Liu Jianmin, Xu Zhen, Perera H Dhanuja, Yadav Satya P, Bialkowska Katarzyna, Qin Jun, Ma Yan-Qing, Plow Edward F. The Journal of biological chemistry. 2012;287(29):24585–94. doi: 10.1074/jbc.M111.336743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Regulation of integrin affinity on cell surfaces. Schürpf Thomas, Springer Timothy A. The EMBO journal. 2011;30(23):4712–27. doi: 10.1038/emboj.2011.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.The kindlins at a glance. Karaköse Esra, Schiller Herbert B, Fässler Reinhard. Journal of cell science. 2010;123(Pt 14):2353–6. doi: 10.1242/jcs.064600. [DOI] [PubMed] [Google Scholar]
- 53.Integrin function in vascular biology: a view from 2013. Plow Edward F, Meller Julia, Byzova Tatiana V. Current opinion in hematology. 2014;21(3):241–7. doi: 10.1097/MOH.0000000000000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kindlin-2 regulates hemostasis by controlling endothelial cell-surface expression of ADP/AMP catabolic enzymes via a clathrin-dependent mechanism. Pluskota E, Ma Y, Bledzka KM, Bialkowska K, Soloviev DA, Szpak D. Blood. 2013;122(14):2491–2499. doi: 10.1182/blood-2013-04-497669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kindlin-1 controls Wnt and TGF-β availability to regulate cutaneous stem cell proliferation. Rognoni Emanuel, Widmaier Moritz, Jakobson Madis, Ruppert Raphael, Ussar Siegfried, Katsougkri Despoina, Böttcher Ralph T, Lai-Cheong Joey E, Rifkin Daniel B, McGrath John A, Fässler Reinhard. Nature medicine. 2014;20(4):350–9. doi: 10.1038/nm.3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kindlin-3 enhances breast cancer progression and metastasis by activating Twist-mediated angiogenesis. Sossey-Alaoui Khalid, Pluskota Elzbieta, Davuluri Gangarao, Bialkowska Katarzyna, Das Mitali, Szpak Dorota, Lindner Daniel J, Downs-Kelly Erinn, Thompson Cheryl L, Plow Edward F. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2014;28(5):2260–71. doi: 10.1096/fj.13-244004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.URP1: a member of a novel family of PH and FERM domain-containing membrane-associated proteins is significantly over-expressed in lung and colon carcinomas. Weinstein Edward J, Bourner Maureen, Head Richard, Zakeri Hamideh, Bauer Christopher, Mazzarella Richard. Biochimica et biophysica acta. 2003;1637(3):207–16. doi: 10.1016/s0925-4439(03)00035-8. [DOI] [PubMed] [Google Scholar]
- 58.Inhibition of collagen XVI expression reduces glioma cell invasiveness. Bauer Richard, Ratzinger Sabine, Wales Lynn, Bosserhoff Anja, Senner Volker, Grifka Joachim, Grässel Susanne. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2011;27(3-4):217–26. doi: 10.1159/000327947. [DOI] [PubMed] [Google Scholar]
- 59.Kindlin-1 expression is involved in migration and invasion of pancreatic cancer. Mahawithitwong Prawej, Ohuchida Kenoki, Ikenaga Naoki, Fujita Hayato, Zhao Ming, Kozono Shingo, Shindo Koji, Ohtsuka Takao, Aishima Shinichi, Mizumoto Kazuhiro, Tanaka Masao. International journal of oncology. 2013;42(4):1360–6. doi: 10.3892/ijo.2013.1838. [DOI] [PubMed] [Google Scholar]
- 60.Role of the focal adhesion protein kindlin-1 in breast cancer growth and lung metastasis. Sin Soraya, Bonin Florian, Petit Valérie, Meseure Didier, Lallemand François, Bièche Ivan, Bellahcène Akeila, Castronovo Vincent, de Wever Olivier, Gespach Christian, Lidereau Rosette, Driouch Keltouma. Journal of the National Cancer Institute. 2011;103(17):1323–37. doi: 10.1093/jnci/djr290. [DOI] [PubMed] [Google Scholar]
- 61.Expression of Kindlin-1 in human hepatocellular carcinoma and its prognostic significance. Ma Hua-Xing, Shu Qing-Hua, Pan Jing-Jing, Liu Dong, Xu Ge-Liang, Li Jian-Sheng, Ma Jin-Liang, Jia Wei-Dong, Yv Ji-Hai, Ge Yong-Sheng. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2015;36(6):4235–41. doi: 10.1007/s13277-015-3060-8. [DOI] [PubMed] [Google Scholar]
- 62.Collagen XVI induces expression of MMP9 via modulation of AP-1 transcription factors and facilitates invasion of oral squamous cell carcinoma. Bedal Konstanze B, Grässel Susanne, Oefner Peter J, Reinders Joerg, Reichert Torsten E, Bauer Richard. PloS one. 2014;9(1):e86777. doi: 10.1371/journal.pone.0086777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cutaneous and laryngeal squamous cell carcinoma in mixed epidermolysis bullosa, kindler syndrome. Mizutani Hiromi, Masuda Koji, Nakamura Naomi, Takenaka Hideya, Tsuruta Daisuke, Katoh Norito. Case reports in dermatology. 2012;4(2):133–8. doi: 10.1159/000339619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Opposite role of Kindlin-1 and Kindlin-2 in lung cancers. Zhan Jun, Zhu Xiang, Guo Yongqing, Wang Yunling, Wang Yuxiang, Qiang Guangliang, Niu Miaomiao, Hu Jinxia, Du Juan, Li Zhilun, Cui Jia, Ma Bo, Fang Weigang, Zhang Hongquan. PloS one. 2012;7(11):e50313. doi: 10.1371/journal.pone.0050313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kindlin-2 is expressed in malignant mesothelioma and is required for tumor cell adhesion and migration. An Zhengwen, Dobra Katalin, Lock John G, Strömblad Staffan, Hjerpe Anders, Zhang Hongquan. International journal of cancer. 2010;127(9):1999–2008. doi: 10.1002/ijc.25223. [DOI] [PubMed] [Google Scholar]
- 66.Kindler syndrome. Ashton G H S. Clinical and experimental dermatology. 2004;29(2):116–21. doi: 10.1111/j.1365-2230.2004.01465.x. [DOI] [PubMed] [Google Scholar]
- 67.Unusual molecular findings in Kindler syndrome. Arita K, Wessagowit V, Inamadar A C, Palit A, Fassihi H, Lai-Cheong J E, Pourreyron C, South A P, McGrath J A. The British journal of dermatology. 2007;157(6):1252–6. doi: 10.1111/j.1365-2133.2007.08159.x. [DOI] [PubMed] [Google Scholar]
- 68.Kindler syndrome: a focal adhesion genodermatosis. Lai-Cheong J E, Tanaka A, Hawche G, Emanuel P, Maari C, Taskesen M, Akdeniz S, Liu L, McGrath J A. The British journal of dermatology. 2009;160(2):233–42. doi: 10.1111/j.1365-2133.2008.08976.x. [DOI] [PubMed] [Google Scholar]
- 69.Aggressive squamous cell carcinoma in Kindler syndrome. Emanuel Patrick O, Rudikoff Donald, Phelps Robert G. Skinmed. 2006;5(6):305–7. doi: 10.1111/j.1540-9740.2006.05369.x. [DOI] [PubMed] [Google Scholar]
- 70.Kindlin-1 Is required for RhoGTPase-mediated lamellipodia formation in keratinocytes. Has Cristina, Herz Corinna, Zimina Elena, Qu Hai-Yan, He Yinghong, Zhang Zhi-Gang, Wen Ting-Ting, Gache Yannick, Aumailley Monique, Bruckner-Tuderman Leena. The American journal of pathology. 2009;175(4):1442–52. doi: 10.2353/ajpath.2009.090203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kindler syndrome complicated by squamous cell carcinoma of the hard palate: successful treatment with high-dose radiation therapy and granulocyte-macrophage colony-stimulating factor. Lotem M, Raben M, Zeltser R, Landau M, Sela M, Wygoda M, Tochner Z A. The British journal of dermatology. 2001;144(6):1284–6. doi: 10.1046/j.1365-2133.2001.04262.x. [DOI] [PubMed] [Google Scholar]
- 72.Hallmarks of cancer: the next generation. Hanahan Douglas, Weinberg Robert A. Cell. 2011;144(5):646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 73.The Kindler syndrome protein is regulated by transforming growth factor-beta and involved in integrin-mediated adhesion. Kloeker Susanne, Major Michael B, Calderwood David A, Ginsberg Mark H, Jones David A, Beckerle Mary C. The Journal of biological chemistry. 2004;279(8):6824–33. doi: 10.1074/jbc.M307978200. [DOI] [PubMed] [Google Scholar]
- 74.Kindlin-2 promotes invasiveness of prostate cancer cells via NF-κB-dependent upregulation of matrix metalloproteinases. Yang Jia-rong, Pan Tie-jun, Yang Hui, Wang Tao, Liu Wei, Liu Bo, Qian Wei-hong. Gene. 2016;576(1 Pt 3):571–6. doi: 10.1016/j.gene.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 75.miR-138-Mediated Regulation of KINDLIN-2 Expression Modulates Sensitivity to Chemotherapeutics. Sossey-Alaoui Khalid, Plow Edward F. Molecular cancer research : MCR. 2016;14(2):228–38. doi: 10.1158/1541-7786.MCR-15-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.A feedback regulation between Kindlin-2 and GLI1 in prostate cancer cells. Gao Jianchao, Khan Ammad Aslam, Shimokawa Takashi, Zhan Jun, Strömblad Staffan, Fang Weigang, Zhang Hongquan. FEBS letters. 2013;587(6):631–8. doi: 10.1016/j.febslet.2012.12.028. [DOI] [PubMed] [Google Scholar]
- 77.Kindlin-2 controls sensitivity of prostate cancer cells to cisplatin-induced cell death. Gong Xiaowei, An Zhengwen, Wang Yunling, Guan Lizhao, Fang Weigang, Strömblad Staffan, Jiang Yong, Zhang Hongquan. Cancer letters. 2010;299(1):54–62. doi: 10.1016/j.canlet.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 78.Kindlin 2 promotes breast cancer invasion via epigenetic silencing of the microRNA200 gene family. Yu Yu, Wu Junzhou, Guan Lizhao, Qi Lihua, Tang Yan, Ma Bo, Zhan Jun, Wang Yunling, Fang Weigang, Zhang Hongquan. International journal of cancer. 2013;133(6):1368–79. doi: 10.1002/ijc.28151. [DOI] [PubMed] [Google Scholar]
- 79.Kindlin-2 promotes genome instability in breast cancer cells. Zhao Ting, Guan Lizhao, Yu Yu, Pei Xuelian, Zhan Jun, Han Ling, Tang Yan, Li Feng, Fang Weigang, Zhang Hongquan. Cancer letters. 2013;330(2):208–16. doi: 10.1016/j.canlet.2012.11.043. [DOI] [PubMed] [Google Scholar]
- 80.Kindlin-2 interacts with and stabilizes EGFR and is required for EGF-induced breast cancer cell migration. Guo Baohui, Gao Jianchao, Zhan Jun, Zhang Hongquan. Cancer letters. 2015;361(2):271–81. doi: 10.1016/j.canlet.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 81.The novel focal adhesion gene kindlin-2 promotes the invasion of gastric cancer cells mediated by tumor-associated macrophages. Shen Zhanlong, Ye Yingjiang, Kauttu Tuuli, Seppänen Hanna, Vainionpää Sanna, Wang Shan, Mustonen Harri, Puolakkainen Pauli. Oncology reports. 2013;29(2):791–7. doi: 10.3892/or.2012.2137. [DOI] [PubMed] [Google Scholar]
- 82.Kindlin-2 inhibited the growth and migration of colorectal cancer cells. Ren Yuanyuan, Jin Hongsong, Xue Zhidong, Xu Qianlang, Wang Songhua, Zhao Guojun, Huang Junxing, Huang He. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2015;36(6):4107–14. doi: 10.1007/s13277-015-3044-8. [DOI] [PubMed] [Google Scholar]
- 83.Kindlin-2 expression in peritumoral stroma is associated with poor prognosis in pancreatic ductal adenocarcinoma. Mahawithitwong Prawej, Ohuchida Kenoki, Ikenaga Naoki, Fujita Hayato, Zhao Ming, Kozono Shingo, Shindo Koji, Ohtsuka Takao, Mizumoto Kazuhiro, Tanaka Masao. Pancreas. 2013;42(4):663–9. doi: 10.1097/MPA.0b013e318279bd66. [DOI] [PubMed] [Google Scholar]
- 84.Kindlin-2 induced by TGF-β signaling promotes pancreatic ductal adenocarcinoma progression through downregulation of transcriptional factor HOXB9. Zhan Jun, Song Jiagui, Wang Peng, Chi Xiaochun, Wang Yunling, Guo Yongqing, Fang Weigang, Zhang Hongquan. Cancer letters. 2015;361(1):75–85. doi: 10.1016/j.canlet.2015.02.039. [DOI] [PubMed] [Google Scholar]
- 85.Kindlin-2 inhibits serous epithelial ovarian cancer peritoneal dissemination and predicts patient outcomes. Ren Caixia, Du Juan, Xi Chenguang, Yu Yu, Hu Ajin, Zhan Jun, Guo Hongyan, Fang Weigang, Liu Congrong, Zhang Hongquan. Biochemical and biophysical research communications. 2014;446(1):187–94. doi: 10.1016/j.bbrc.2014.02.087. [DOI] [PubMed] [Google Scholar]
- 86.Increased expression of kindlin 2 in luteinized granulosa cells correlates with androgen receptor level in patients with polycystic ovary syndrome having hyperandrogenemia. Yang Mei, Du Juan, Lu Danyu, Ren Caixia, Shen Huan, Qiao Jie, Chen Xi, Zhang Hongquan. Reproductive sciences (Thousand Oaks, Calif.) 2014;21(6):696–703. doi: 10.1177/1933719113512536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Loss of miR-200b promotes invasion via activating the Kindlin-2/integrin β1/AKT pathway in esophageal squamous cell carcinoma: An E-cadherin-independent mechanism. Zhang Hai-Feng, Alshareef Abdulraheem, Wu Chengsheng, Li Shang, Jiao Ji-Wei, Cao Hui-Hui, Lai Raymond, Xu Li-Yan, Li En-Min. Oncotarget. 2015;6(30):28949–60. doi: 10.18632/oncotarget.5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.A three-protein signature and clinical outcome in esophageal squamous cell carcinoma. Cao Hui-Hui, Zhang Shi-Yi, Shen Jin-Hui, Wu Zhi-Yong, Wu Jian-Yi, Wang Shao-Hong, Li En-Min, Xu Li-Yan. Oncotarget. 2015;6(7):5435–48. doi: 10.18632/oncotarget.3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.miR-200b suppresses invasiveness and modulates the cytoskeletal and adhesive machinery in esophageal squamous cell carcinoma cells via targeting Kindlin-2. Zhang Hai-Feng, Zhang Kai, Liao Lian-Di, Li Li-Yan, Du Ze-Peng, Wu Bing-Li, Wu Jian-Yi, Xu Xiu-E, Zeng Fa-Min, Chen Bo, Cao Hui-Hui, Zhu Meng-Xiao, Dai Li-Hua, Long Lin, Wu Zhi-Yong, Lai Raymond, Xu Li-Yan, Li En-Min. Carcinogenesis. 2014;35(2):292–301. doi: 10.1093/carcin/bgt320. [DOI] [PubMed] [Google Scholar]
- 90.Kindlin-2: a novel prognostic biomarker for patients with hepatocellular carcinoma. Ge Yong-Sheng, Liu Dong, Jia Wei-Dong, Li Jian-Sheng, Ma Jin-Liang, Yu Ji-Hai, Xu Ge-Liang. Pathology, research and practice. 2015;211(3):198–202. doi: 10.1016/j.prp.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 91.Mig-2 attenuates cisplatin-induced apoptosis of human glioma cells in vitro through AKT/JNK and AKT/p38 signaling pathways. Ou Yun-wei, Zhao Zi-tong, Wu Chuan-yue, Xu Bai-nan, Song Yong-mei, Zhan Qi-min. Acta pharmacologica Sinica. 2014;35(9):1199–206. doi: 10.1038/aps.2014.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Novel focal adhesion protein kindlin-2 promotes the invasion of gastric cancer cells through phosphorylation of integrin β1 and β3. Shen Zhanlong, Ye Yingjiang, Kauttu Tuuli, Seppänen Hanna, Vainionpää Sanna, Wang Shan, Mustonen Harri, Puolakkainen Pauli. Journal of surgical oncology. 2013;108(2):106–12. doi: 10.1002/jso.23353. [DOI] [PubMed] [Google Scholar]
- 93.Kindlin-2: a novel adhesion protein related to tumor invasion, lymph node metastasis, and patient outcome in gastric cancer. Shen Zhanlong, Ye Yingjiang, Dong Lingyi, Vainionpää Sanna, Mustonen Harri, Puolakkainen Pauli, Wang Shan. American journal of surgery. 2012;203(2):222–9. doi: 10.1016/j.amjsurg.2011.06.050. [DOI] [PubMed] [Google Scholar]
- 94.Kindlin-2 expression in arsenite- and cadmium-transformed bladder cancer cell lines and in archival specimens of human bladder cancer. Talaat Sherine, Somji Seema, Toni Conrad, Garrett Scott H, Zhou Xu Dong, Sens Mary Ann, Sens Donald A. Urology. 2011;77(6):1507.e1–7. doi: 10.1016/j.urology.2011.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.[Expression of Kindlins and angiopoietins in acute myeloid leukemia]. Wu Wen-Bin, Zhang Qing, Li Yan, Shan Shi-Long, Li Xiao-Yan, Tian Zheng, Tang Ke-Jing, Wang Min, Rao Qing, Mi Ying-Chang. Zhongguo shi yan xue ye xue za zhi. 2012;20(1):7–11. [PubMed] [Google Scholar]
- 96.Kindlin-3 interacts with the ribosome and regulates c-Myc expression required for proliferation of chronic myeloid leukemia cells. Qu Jing, Ero Rya, Feng Chen, Ong Li-Teng, Tan Hui-Foon, Lee Hui-Shan, Ismail Muhammad H B, Bu Wen-Ting, Nama Srikanth, Sampath Prabha, Gao Yong-Gui, Tan Suet-Mien. Scientific reports. 2015;5:18491. doi: 10.1038/srep18491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.A novel tumor suppressor function of Kindlin-3 in solid cancer. Djaafri Ibtissem, Khayati Farah, Menashi Suzanne, Tost Jorg, Podgorniak Marie-Pierre, Sadoux Aurelie, Daunay Antoine, Teixeira Luis, Soulier Jean, Idbaih Ahmed, Setterblad Niclas, Fauvel Françoise, Calvo Fabien, Janin Anne, Lebbé Celeste, Mourah Samia. Oncotarget. 2014;5(19):8970–85. doi: 10.18632/oncotarget.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]