Abstract
BACKGROUND
BRAF V600 mutations occur in various nonmelanoma cancers. We undertook a histology-independent phase 2 “basket” study of vemurafenib in BRAF V600 mutation–positive nonmelanoma cancers.
METHODS
We enrolled patients in six prespecified cancer cohorts; patients with all other tumor types were enrolled in a seventh cohort. A total of 122 patients with BRAF V600 mutation–positive cancer were treated, including 27 patients with colorectal cancer who received vemurafenib and cetuximab. The primary end point was the response rate; secondary end points included progression-free and overall survival.
RESULTS
In the cohort with non–small-cell lung cancer, the response rate was 42% (95% confidence interval [CI], 20 to 67) and median progression-free survival was 7.3 months (95% CI, 3.5 to 10.8). In the cohort with Erdheim–Chester disease or Langerhans’-cell histiocytosis, the response rate was 43% (95% CI, 18 to 71); the median treatment duration was 5.9 months (range, 0.6 to 18.6), and no patients had disease progression during therapy. There were anecdotal responses among patients with pleomorphic xanthoastrocytoma, anaplastic thyroid cancer, cholangiocarcinoma, salivary-duct cancer, ovarian cancer, and clear-cell sarcoma and among patients with colorectal cancer who received vemurafenib and cetuximab. Safety was similar to that in prior studies of vemurafenib for melanoma.
CONCLUSIONS
BRAF V600 appears to be a targetable oncogene in some, but not all, nonmelanoma cancers. Preliminary vemurafenib activity was observed in non–small-cell lung cancer and in Erdheim–Chester disease and Langerhans’-cell histiocytosis. The histologic context is an important determinant of response in BRAF V600–mutated cancers. (Funded by F. Hoffmann–La Roche/Genentech; ClinicalTrials.gov number, NCT01524978.)
BRAF V600 mutations occur in approximately 50% of cutaneous melanomas and result in constitutive activation of downstream signaling through the mitogen-activated protein kinase (MAPK) pathway.1,2 Vemurafenib (Zelboraf, F. Hoffmann–La Roche/Genentech) is a selective oral inhibitor of the BRAF V600 kinase and is associated with a response rate of approximately 50% and improved survival among patients with BRAF V600E mutation–positive metastatic melanoma.3
Efforts by the Cancer Genome Atlas4 and other initiatives to characterize the genetic landscape of most tumor types have identified BRAF V600 mutations in nonmelanoma cancers, including colorectal cancer,5,6 non–small-cell lung cancer,7 papillary thyroid cancer,8 diffuse gliomas,9 cholangiocarcinoma,10 hairy-cell leukemia,11 multiple myeloma,12 Langerhans’-cell histiocytosis,13 and Erdheim–Chester disease.14,15 In some of these cancers, the BRAF V600 mutation is associated with an aggressive disease phenotype and shortened disease-free and overall survival.16,17 In more than half of nonmelanoma cancer types in which BRAF V600 mutations have been identified, the incidence of mutations is less than 5%.
The efficacy of vemurafenib in these non-melanoma cancers has not been systematically explored, despite its considerable therapeutic potential. The large number of tumor types involved, the low frequency of BRAF V600 mutations, and the rarity of some of the cancers make disease-specific studies difficult to conduct. To address this important unmet clinical need, we designed a histology-independent, flexible, early phase 2 “basket” study of vemurafenib in patients with nonmelanoma cancers harboring BRAF V600 mutations. This study design allows for the enrollment of patients with various types of cancer. Our study included six cohorts of patients with prespecified cancers (non–small-cell lung cancer, ovarian cancer, colorectal cancer, cholangiocarcinoma, breast cancer, and multiple myeloma), as well as a seventh (all-others) cohort, which permitted enrollment of patients with any other BRAF V600 mutation–positive cancer. The protocol allowed for additional tumor-specific cohorts to be analyzed if a sufficient number of patients were enrolled in the all-others cohort. Patients with papillary thyroid cancer and those with hairy-cell leukemia were excluded because the overall incidence of BRAF V600 mutations in these cancers is high enough to warrant disease-specific studies. The goal of this study was to identify promising signals of activity in individual tumor types that could then be definitively explored. Here, we report the preliminary clinical efficacy of vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations.
METHODS
PATIENTS
From April 11, 2012, through June 10, 2014, we enrolled 122 patients with BRAF V600–mutated cancers from 23 centers worldwide. All patients enrolled at the time of the June 10, 2014, cutoff date were included in the analysis. BRAF V600 mutations were identified by means of mutational analysis assays routinely performed at each participating site. Additional key eligibility criteria were measurable disease, according to Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1,18 and an Eastern Cooperative Oncology Group (ECOG) performance-status score of 0 to 2 (on a 5-point scale, with larger numbers indicating greater disability). Patients previously treated with a BRAF or MEK inhibitor were ineligible, as were patients with melanoma, papillary thyroid cancer, leukemia, or lymphoma. Additional eligibility criteria are provided in the protocol, available with the full text of this article at NEJM.org. Written informed consent was obtained from all study participants.
STUDY DESIGN AND TREATMENT
The primary objective of the study was to evaluate the efficacy of vemurafenib in patients with BRAF V600 mutation–positive cancers. Efficacy was evaluated on the basis of the response rate at week 8, as assessed by the site investigators according to RECIST, version 1.1,18 or the criteria of the International Myeloma Working Group (IMWG).19 Secondary objectives included assessments of the best overall response, clinical benefit rate (defined as the overall proportion of patients with a complete or partial response or stable disease), duration of response, progression-free survival, overall survival, and safety. Because this was a signal-generating study in a heterogeneous population of patients with advanced cancer, a control group was not used.
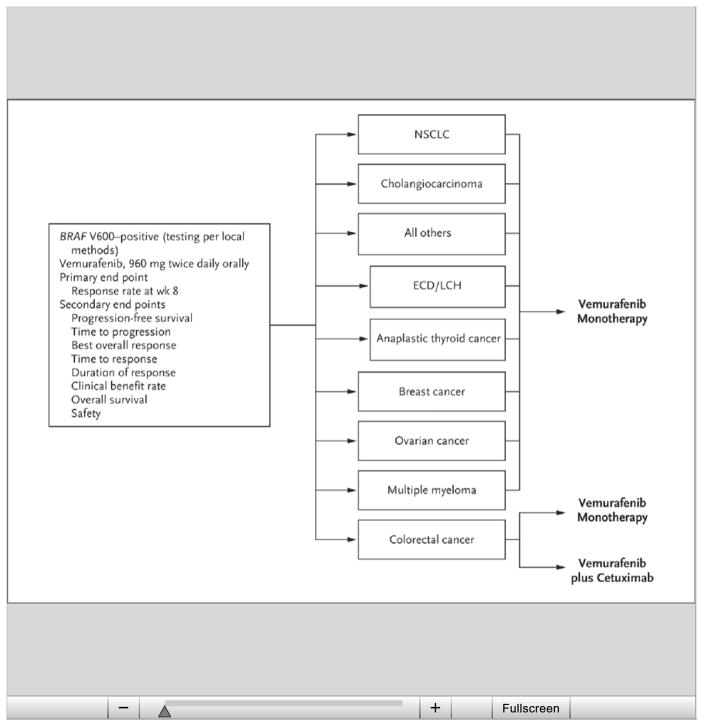
The study schema is shown in Figure 1. On the basis of the number of participants enrolled, two tumor-specific cohorts were added to the original six: patients with Erdheim–Chester disease or Langerhans’-cell histiocytosis and those with anaplastic thyroid cancer. The breast cancer cohort was closed owing to insufficient accrual, and the single patient with breast cancer was included in the all-others cohort. Similarly, enrollment in the multiple myeloma and ovarian cancer cohorts was not sufficient for the stage 1 analysis in our two-stage analytic plan (see the Statistical Analysis section below), and the patients in these cohorts were therefore included in the all-others cohort.
Figure 1. Study Design and Cohorts.
The all-others cohort included cervical cancer, brain tumors, head and neck cancer, esophageal and gastric cancers, pancreatic cancer, sarcoma, and carcinoma of unknown primary type. The breast cancer cohort was closed because of insufficient accrual; the single patient with breast cancer was included in the all-others cohort for the purposes of this report. The ovarian cancer and multiple myeloma cohorts did not have sufficient numbers of patients for a stage 1 analysis and therefore did not undergo formal analysis. Preliminary efficacy results for these cohorts are included with the results for the all-others cohort for the purposes of this report. ECD/LCH denotes Erdheim–Chester disease or Langerhans’-cell histiocytosis, and NSCLC non–small-cell lung cancer.
Vemurafenib alone had insufficient activity in patients with BRAF V600–mutated colorectal cancer, confirming the findings from a phase 1 study.20 Emerging laboratory data suggest that resistance in colorectal cancer might be mediated through feedback activation of epidermal growth factor receptor (EGFR) signaling.21,22 To evaluate this hypothesis, we amended the study protocol to include an assessment of the safety and efficacy of vemurafenib combined with cetuximab, an anti-EGFR antibody. A standard 3+3 dose-escalation design (three to six patients per cohort and up to three dose levels tested) was used to establish the recommended (maximum tolerated) combination dose. An additional cohort of patients with colorectal cancer received vemurafenib and cetuximab at the recommended combination dose (960 mg of vemurafenib administered orally twice daily and an intravenous loading dose of 400 mg of cetuximab per square meter of body-surface area, followed by a weekly intravenous dose of 250 mg per square meter), and the safety and efficacy were assessed. All other patients received vemurafenib alone at an oral dose of 960 mg twice daily.
ASSESSMENTS
Tumor assessments were performed by means of computed tomographic or magnetic resonance imaging of the chest, abdomen, and pelvis at baseline and then every 8 weeks until disease progression, death, or withdrawal from the study (see Fig. S1 in the Supplementary Appendix, available at NEJM.org). Patients with multiple myeloma were evaluated according to the IMWG criteria (Table S1 in the Supplementary Appendix).19 Information on survival and new anticancer therapy was documented every 3 months in the follow-up period. Adverse events were graded by the site investigators, according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 4.0,23 until day 28 after discontinuation of the study treatment.
STUDY OVERSIGHT
The study was designed by the steering committee in collaboration with the team from F. Hoffmann–La Roche (the sponsor) and was conducted in accordance with the provisions of the Declaration of Helsinki and Good Clinical Practice guidelines. The institutional review board or human research ethics committee at each participating center approved the protocol. Data were collected at each site and monitored by the sponsor. The first and last authors wrote the first draft of the manuscript with support from representatives of the sponsor. Editorial assistance that did not involve writing was provided by Apothecom and was funded by the sponsor. All the authors vouch for the accuracy and completeness of the reported data and for the fidelity of the study to the protocol and approved the decision to submit the manuscript for publication.
STATISTICAL ANALYSIS
An adaptive Simon two-stage design24 was used for all tumor-specific cohorts in order to minimize the number of patients treated if vemurafenib was deemed ineffective for a specific tumor type. The primary efficacy end point was the response rate at week 8. All response rates are reported here with 95% confidence intervals. Waterfall plots were used to illustrate maximum tumor shrinkage during the study, as compared with baseline. Kaplan–Meier methods were used to estimate progression-free and overall survival. No adjustments were made for multiple hypothesis testing that could result in false positive findings.
In this study, a response rate of 15% at week 8 was considered to be low, a response rate of 45% was considered to be high, and a response rate of 35% was considered to be low but still desirable and indicative of efficacy. Assuming response rates as specified in the hypothesis testing, a power of 80% for a high response rate and 70% for the low but still desirable response rate, and a two-sided alpha level of 0.1, we calculated that the number of patients required in each cohort would be 7, 13, or 19, depending on the results obtained. The study would be analyzed for efficacy at stage 1, at stage 2, and 9 months after the last patient was enrolled. Because the study remains open, available stage 1 and 2 results are presented, in addition to preliminary safety and efficacy results for all patients enrolled at the time of the cutoff date. Final response rates and time-to-event analyses might change with additional follow-up.
RESULTS
PATIENTS
The characteristics of the 122 enrolled patients who received at least one dose of vemurafenib are shown in Table 1. Additional disease-specific characteristics are shown in Table S2 in the Supplementary Appendix. The most common disease types were colorectal cancer (37 patients), non–small-cell lung cancer (20), Erdheim–Chester disease or Langerhans’-cell histiocytosis (18), primary brain tumors (13), cholangiocarcinoma (8), anaplastic thyroid cancer (7), and multiple myeloma (5). Eighty-nine percent of patients had received at least one previous line of therapy. Ninety-five patients received vemurafenib mono-therapy, and 27 patients with colorectal carcinoma received vemurafenib and cetuximab combination therapy.
Table 1.
Demographic and Clinical Characteristics of the Patients at Baseline, According to Cohort.*
| Characteristic | NSCLC (N = 20) | Colorectal Cancer | Multiple Myeloma (N = 5) | Cholangiocarcinoma (N = 8) | ECD or LCH (N = 18) | Anaplastic Thyroid Cancer (N = 7) | Other† (N = 27) | |
|---|---|---|---|---|---|---|---|---|
| Received Vemurafenib (N = 10) | Received Vemurafenib + Cetuximab (N = 27) | |||||||
| Sex — no. (%) | ||||||||
|
| ||||||||
| Male | 14 (70) | 5 (50) | 10 (37) | 4 (80) | 3 (38) | 7 (39) | 4 (57) | 9 (33) |
|
| ||||||||
| Female | 6 (30) | 5 (50) | 17 (63) | 1 (20) | 5 (62) | 11 (61) | 3 (43) | 18 (67) |
|
| ||||||||
| Yr of age — median (range) | 61 (48–83) | 59 (49–64) | 63 (45–81) | 64 (58–68) | 53 (37–66) | 64 (35–83) | 65 (55–81) | 55 (18–77) |
|
| ||||||||
| ECOG performance status† | ||||||||
|
| ||||||||
| 0 or 1 | 16 (80) | 10 (100) | 25 (93) | 4 (80) | 7 (88) | 15 (83) | 4 (57) | 22 (81) |
|
| ||||||||
| ≥2 | 4 (20) | 0 | 2 (7) | 1 (20) | 1 (12) | 3 (17) | 3 (43) | 5 (19) |
|
| ||||||||
| Prior systemic therapies — no. (%)‡ | ||||||||
|
| ||||||||
| Any | 19 (95) | 10 (100) | 27 (100) | 5 (100) | 8 (100) | 11 (61) | 7 (100) | 21 (78) |
|
| ||||||||
| None | 1 (5) | 0 | 0 | 0 | 0 | 7 (39) | 0 | 6 (22) |
|
| ||||||||
| 1 | 10 (50) | 1 (10) | 5 (19) | 0 | 2 (25) | 2 (11) | 5 (71) | 6 (22) |
|
| ||||||||
| 2 | 4 (20) | 2 (20) | 11 (41) | 2 (40) | 1 (12) | 7 (39) | 1 (14) | 5 (19) |
|
| ||||||||
| ≥3 | 5 (25) | 7 (70) | 11 (41) | 3 (60) | 5 (62) | 2 (11) | 1 (14) | 10 (37) |
|
| ||||||||
| Prior radiation — no. (%) | 4 (20) | 4 (40) | 6 (22) | 2 (40) | 3 (38) | 0 | 6 (86) | 18 (67) |
|
| ||||||||
| BRAF V600 mutation — no. (%) | ||||||||
|
| ||||||||
| V600E | 18 (90) | 8 (80) | 24 (89) | 5 (100) | 7 (88) | 17 (94) | 7 (100) | 25 (93) |
|
| ||||||||
| V600G | 1 (5) | 0 | 0 | 0 | 0 | 0 | 0 | 2 (7) |
|
| ||||||||
| V600 unknown | 1 (5) | 2 (20) | 3 (11) | 0 | 1 (12) | 1 (6) | 0 | 0 |
ECD denotes Erdheim–Chester disease, ECOG Eastern Cooperative Oncology Group, LCH Langerhans’-cell histiocytosis, and NSCLC non–small-cell lung cancer.
Tumor types in this cohort included breast cancer, cervical cancer, brain tumors, head and neck cancer, esophageal and gastric cancers, pancreatic cancer, sarcoma, ovarian cancer, and tumors of unknown type.
Prior systemic therapies were determined on the basis of a manual review of cohort listings.
EFFICACY
Clinical activity, including partial or complete response and tumor regression (Table 2 and Fig. 2 and 3, and Table S3 and Fig. S2 and S3 in the Supplementary Appendix), and prolonged disease stabilization (Fig. S4 in the Supplementary Appendix) were observed in several tumor types, including non–small-cell lung cancer (8 partial responses in the cohort of 20 patients), Erdheim–Chester disease or Langerhans’-cell histiocytosis (1 complete and 5 partial responses in the cohort of 18 patients), anaplastic thyroid cancer (1 complete and 1 partial response in the cohort of 7 patients), cholangiocarcinoma (1 partial response in the cohort of 8 patients), with anecdotal responses in patients with salivary-duct cancer, clear-cell sarcoma, low-grade serous ovarian cancer, glioblastoma, anaplastic ependymoma, pancreatic cancer, and carcinoma of unknown primary type.
Table 2.
Preliminary Best Response According to Cohort.*
| Variable | NSCLC (N = 20) | Colorectal Cancer | Cholangiocarcinoma (N = 8) | ECD or LCH (N = 18) | Anaplastic Thyroid Cancer (N = 7) | |
|---|---|---|---|---|---|---|
| Vemurafenib (N = 10) | Vemurafenib + Cetuximab (N = 27) | |||||
| Patients with ≥1 postbaseline assessment — no. | 19 | 10 | 26 | 8 | 14 | 7 |
|
| ||||||
| Complete response — no. (%) | 0 | 0 | 0 | 0 | 1 (7) | 1 (14) |
|
| ||||||
| Partial response — no. (%) | 8 (42) | 0 | 1 (4) | 1 (12) | 5 (36) | 1 (14) |
|
| ||||||
| Stable disease — no. (%) | 8 (42) | 5 (50) | 18 (69) | 4 (50) | 8 (57) | 0 |
|
| ||||||
| Progressive disease — no. (%) | 2 (11) | 5 (50) | 7 (27) | 3 (38) | 0 | 4 (57) |
|
| ||||||
| Missing data — no. (%)† | 1 (5) | 0 | 0 | 0 | 0 | 1 (14) |
|
| ||||||
| Overall response — no. (%) [95% CI] | 8 (42) [20–67] | 0 | 1 (4) [<1–20] | 1 (12) [<1–53] | 6 (43) [18–71] | 2 (29) [4–71] |
The denominator for patients with a complete or partial response, stable disease, or progressive disease is the number of patients with a postbaseline assessment or early withdrawal. Of the 19 patients in the NSCLC cohort, 1 patient withdrew before the assessment of response but was included in the denominator for the efficacy assessment (as having had no response).
All patients with missing data withdrew early.
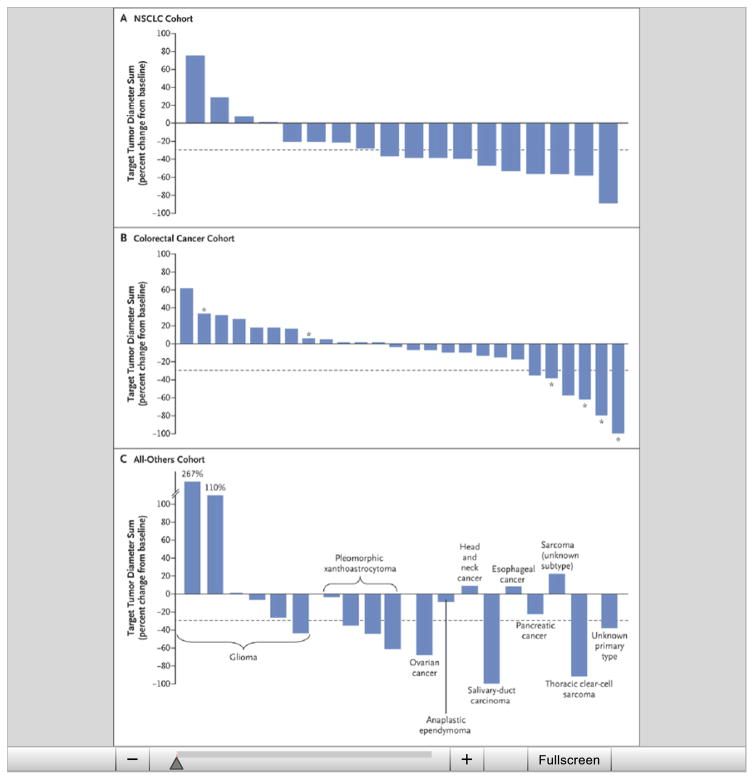
Figure 2. Maximum Percent Change from Baseline in the Sum of the Diameters of Target Lesions.
The change from baseline in the target lesion diameter is shown for patients who had measurable disease at baseline according to Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1, and who underwent at least one post-treatment evaluation; dashed lines indicate −30% change, the minimum necessary to qualify for partial response according to RECIST. Data are shown for 18 patients in the NSCLC cohort (Panel A), 26 patients in the colorectal cancer cohort who were treated with vemurafenib plus cetuximab (Panel B), and patients in the all-others cohort (i.e., patients with tumor types that were not prespecified) plus 1 patient with low-grade serous ovarian cancer (Panel C). The tumor types in the all-others cohort included gliomas, head and neck cancer, pancreatic cancer, pleomorphic xantho-astrocytoma, esophageal and gastric cancers, sarcoma, and carcinoma of unknown primary type. Five patients (1 in the NSCLC cohort and 4 in the all-others cohort) died before evaluation. Asterisks indicate patients in the dose-escalation stage (dose levels 1 and 2).
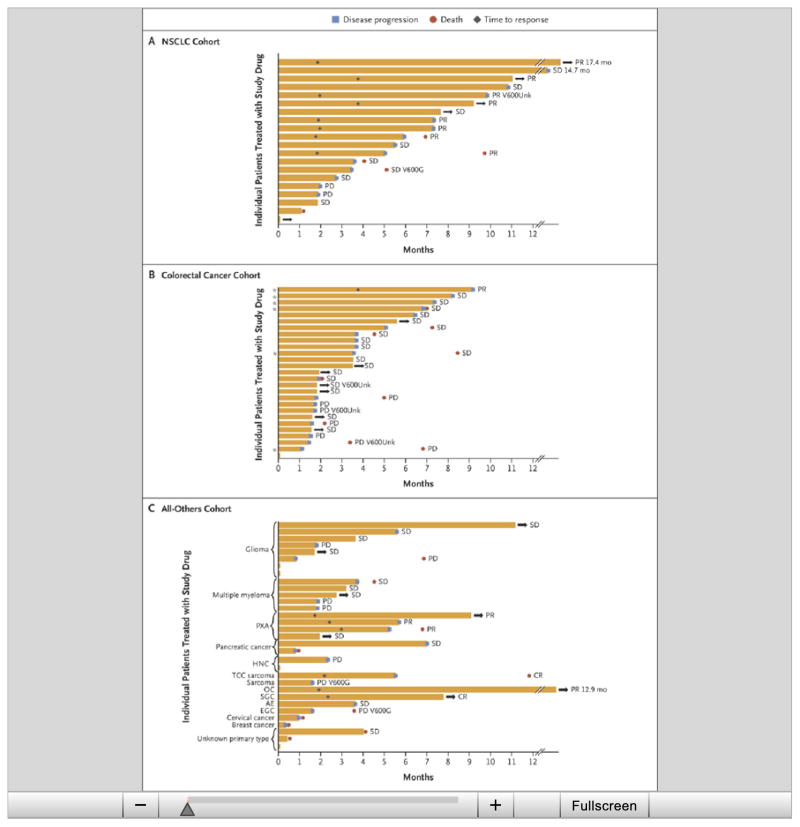
Figure 3. Time to Events in Individual Patients and According to the Best Overall Response.
The time to events is shown for patients who had measurable disease at baseline according to RECIST. Results are shown for 20 patients in the NSCLC cohort (Panel A), 27 patients in the colorectal cancer cohort who received vemurafenib plus cetuximab (Panel B), and patients in the all-others cohort plus 1 patient with low-grade serous ovarian cancer and 5 patients with multiple myeloma (Panel C). The bar length represents the duration of progression-free survival. Arrows indicate patients who were still receiving the study drug, and asterisks patients in the dose-escalation stage (dose levels 1 and 2). AE denotes anaplastic ependymoma, CR complete response, EGC esophageal and gastric cancer, HNC head and neck cancer, OC low-grade serous ovarian cancer, PR partial response, PXA pleomorphic xanthoastrocytoma, SD stable disease, SGC salivary-gland carcinoma, TCC thoracic clear-cell, V600G BRAF V600G mutation, and V600Unk unknown BRAF V600 mutation.
Among the 19 patients with non–small-cell lung cancer who underwent at least one assessment after baseline or withdrew early, the objective response rate was 42% (95% confidence interval [CI], 20 to 67). Tumor regression was observed in the majority of the patients (14 of 19). The median progression-free survival was 7.3 months (95% CI, 3.5 to 10.8). The 12-month rate of progression-free survival was 23% (95% CI, 6 to 46). The median overall survival has not yet been reached; the preliminary 12-month overall survival rate was 66% (95% CI, 36 to 85). Data from 15 of the 20 patients in the cohort were censored for overall survival estimates. At the time of the cutoff date, 5 patients were still receiving therapy.
In the cohort of patients with Erdheim–Chester disease or Langerhans’-cell histiocytosis, 14 patients could be evaluated for a response at the time of analysis. In total, 6 of the 14 patients had a response (1 complete and 5 partial responses), for a response rate of 43% (95% CI, 18 to 71). Disease regression was observed in the majority of patients (12 of 14), and improvement in disease-related symptoms was observed across all degrees of tumor regression. With a median treatment duration of 5.9 months (range, 0.6 to 18.6), no patients had progressive disease while receiving treatment. Four patients discontinued treatment because of adverse events. One of these patients had disease progression during the follow-up period. Median progression-free and overall survival had not been reached at the time of the analysis. The preliminary 12-month progression-free survival rate was 91% (95% CI, 51 to 99), and the 12-month overall survival rate was 100%. Since data were censored for high proportions of the patients (for 17 of the 18 patients in the analysis of progression-free survival and for all 18 patients in the analysis of overall survival), these data are not mature.
In the cohort of patients with colorectal cancer who received vemurafenib monotherapy, no responses were observed, and the median progression-free survival and overall survival were 4.5 months (95% CI, 1.0 to 5.5) and 9.3 months (95% CI, 5.6 to not reached), respectively. In the cohort of patients with colorectal cancer who received vemurafenib and cetuximab, one response was observed; however, approximately half the patients had tumor regression that did not meet the standard criteria for a partial response. Median progression-free survival and overall survival for patients receiving combination therapy were 3.7 months (95% CI, 1.8 to 5.1) and 7.1 months (95% CI, 4.4 to not reached), respectively. Patients in both colorectal cancer cohorts were heavily pretreated, with a median of two lines of previous therapy (range, one to six).
Three of four patients with anaplastic pleomorphic xanthoastrocytoma had partial responses. Responses were also observed in patients with the following tumor types: anaplastic thyroid cancer (two patients), cholangiocarcinoma (one patient), salivary-duct cancer (one patient), soft-tissue sarcoma (one patient), and ovarian cancer (one patient). In three of these patients (one each with anaplastic thyroid cancer, cholangiocarcinoma, and ovarian cancer), the responses have persisted for more than 12 months. Additional tumor regression that did not meet criteria for a response was observed in three patients with glioblastoma and one patient each with anaplastic ependymoma, pancreatic cancer, and carcinoma of unknown primary type. No patients with multiple myeloma have had a response to date.
SAFETY
Common adverse events reported for 20% or more of the patients and adverse events of special interest overall and for specific cohorts are shown in Tables S4 and S5, respectively, in the Supplementary Appendix. Overall, safety data for vemurafenib monotherapy were similar to data from previous studies of vemurafenib in cutaneous melanoma, although samples sizes within individual cohorts were too small to allow a definitive comparison.25 The most common adverse events among all patients receiving vemurafenib monotherapy were rash (68% of patients), fatigue (56%), and arthralgia (40%).
DISCUSSION
Results from this histology-independent, bio-marker-selected, early phase 2 basket study show modest antitumor activity in cancers that sporadically express the BRAF V600 mutation. A growing number of agents have been approved for use in biomarker-positive cancers, including human epidermal growth factor receptor 2–positive breast cancer26 and gastric cancer,27 EGFR-mutated lung cancer,28 ALK-translocated lung cancer,29 and KIT (CD117)–positive gastrointestinal stromal tumor.30 Systematic profiling efforts have shown that many approved and investigational biomarkers are present in various tumor types, although often at low frequencies.4 Basket studies may permit the detection of early signals of activity across multiple tumor types simultaneously while also allowing for the possibility that tumor lineage might influence drug sensitivity. The flexible biostatistical design of this study, including the addition of a cohort of patients with any tumor types that were not prespecified, facilitated identification of modest activity in certain orphan cancers.
Our goal was to identify promising signals of activity in individual tumor types that could be pursued in subsequent studies with statistically robust efficacy end points or through protocol amendment and expanded enrollment in the current study. We found that vemurafenib had preliminary efficacy in BRAF V600 mutation–positive non–small-cell lung cancer, Erdheim–Chester disease, and Langerhans’-cell histiocytosis. In the cohort of patients with non–small-cell lung cancer, 90% of whom had received prior platinum-based chemotherapy, the response rate was 42% (95% CI, 20 to 67). This rate compares favorably with the 7% response rate reported for standard second-line docetaxel in molecularly unselected patients.31,32 In patients with Erdheim–Chester disease or Langerhans’-cell histiocytosis, which are closely related orphan diseases with no approved therapies for adults, the response rate was 43% (95% CI, 18 to 71), and none of the patients had disease progression while receiving therapy, despite a median treatment duration of 5.9 months. These data, which reinforce and extend findings from a recent case series study of off-label vemurafenib treatment in Erdheim–Chester disease,33 suggest that the effect of BRAF inhibition on the natural history of Erdheim–Chester disease and Langerhans’-cell histiocytosis may be clinically significant.
The basket study design is noteworthy because it allows for the possibility that different tumor types with the same molecular biomarker might differ in their sensitivity to therapy targeted at that biomarker. The absence of responses in the cohort of patients with colorectal cancer who received vemurafenib monotherapy underscores this possibility. Although the precision of the response-rate estimates reported here is limited by small sample sizes, the rates in several cohorts, including the anaplastic thyroid cancer and cholangiocarcinoma cohorts, appear to be lower than the rate reported for cutaneous melanoma. These data show that BRAF V600–mutated tumor types do not respond uniformly to BRAF-targeted therapy. An important implication is that conventional tumor nosology based on organ site (with molecular subtypes) cannot be entirely replaced by molecular nosology (e.g., BRAF-mutated cancers). The adaptive nature of basket studies allows investigators to perform rapid assessments of novel therapeutic approaches based on important laboratory discoveries. In the case of colorectal cancers, we amended the study to evaluate the safety and efficacy of combined treatment with vemurafenib and cetuximab because of initial disappointment with the results of vemurafenib monotherapy. Although the clinical activity of this combination was modest, outcomes might have been influenced by the high proportion of patients who had previously received treatment with anti-EGFR antibody (44%, 12 of 27 patients). Given the highly aggressive and chemotherapy-resistant nature of BRAF V600–mutated colorectal cancers, strategies using dual EGFR and BRAF inhibition deserve further evaluation.
One challenge in interpreting the results of basket studies is drawing inferences from small numbers of patients. Although partial responses and tumor regression were observed in patients with anaplastic thyroid cancer, cholangiocarcinoma, anaplastic pleomorphic xanthoastrocytoma, high-grade gliomas, salivary-duct cancer, low-grade serous ovarian cancer, clear-cell sarcoma, carcinoma of unknown primary type, and pancreatic cancer, the largest of these subgroups consisted of only eight patients. In several cases, only a single patient with the tumor type was treated. Although these responses are noteworthy because of the limited therapeutic options available for many of these cancers, they must be interpreted with caution. In the absence of more definitive data, which might not be forthcoming for many diseases owing to logistical impediments, these data present a challenge to clinicians who want to make treatment decisions on the basis of tumor genomic profiling.
In conclusion, we found that the BRAF V600 mutation is a targetable oncogene in some, but not all, cancer types. Histology-independent, biomarker-selected basket studies are feasible and can serve as a tool for developing molecularly targeted cancer therapy. Confirmation of promising activity identified in basket studies will often necessitate additional studies.
Supplementary Material
Acknowledgments
Supported by F. Hoffmann–La Roche/Genentech.
We thank Lada Mitchell, Ph.D., and Philipp Schlatter, Ph.D., employees of F. Hoffmann–La Roche, for their assistance in study design.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 2.Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135–47. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- 3.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–16. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinstein JN, Collisson EA, Mills GB, et al. The Cancer Genome Atlas Pan-Cancer Analysis Project. Nat Genet. 2013;45:1113–20. doi: 10.1038/ng.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Cutsem E, Köhne CH, Láng I, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29:2011–9. doi: 10.1200/JCO.2010.33.5091. [DOI] [PubMed] [Google Scholar]
- 6.De Roock W, Claes B, Bernasconi D, et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol. 2010;11:753–62. doi: 10.1016/S1470-2045(10)70130-3. [DOI] [PubMed] [Google Scholar]
- 7.Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014;311:1998–2006. doi: 10.1001/jama.2014.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kimura ET, Nikiforova MN, Zhu Z, Knauf JA, Nikiforov YE, Fagin JA. High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res. 2003;63:1454–7. [PubMed] [Google Scholar]
- 9.Brennan CW, Verhaak RG, McKenna A, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155:462–77. doi: 10.1016/j.cell.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goeppert B, Frauenschuh L, Renner M, et al. BRAF V600E-specific immuno-histochemistry reveals low mutation rates in biliary tract cancer and restriction to intrahepatic cholangiocarcinoma. Mod Pathol. 2014;27:1028–34. doi: 10.1038/modpathol.2013.206. [DOI] [PubMed] [Google Scholar]
- 11.Tiacci E, Trifonov V, Schiavoni G, et al. BR AF mutations in hairy-cell leukemia. N Engl J Med. 2011;364:2305–15. doi: 10.1056/NEJMoa1014209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lohr JG, Stojanov P, Carter SL, et al. Widespread genetic heterogeneity in multiple myeloma: implications for targeted therapy. Cancer Cell. 2014;25:91–101. doi: 10.1016/j.ccr.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Badalian-Very G, Vergilio JA, Degar BA, et al. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood. 2010;116:1919–23. doi: 10.1182/blood-2010-04-279083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haroche J, Charlotte F, Arnaud L, et al. High prevalence of BRAF V600E mutations in Erdheim-Chester disease but not in other non-Langerhans cell histiocytoses. Blood. 2012;120:2700–3. doi: 10.1182/blood-2012-05-430140. [DOI] [PubMed] [Google Scholar]
- 15.Diamond EL, Dagna L, Hyman DM, et al. Consensus guidelines for the diagnosis and clinical management of Erdheim-Chester disease. Blood. 2014;124:483–92. doi: 10.1182/blood-2014-03-561381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tol J, Nagtegaal ID, Punt CJA. BRAF mutation in metastatic colorectal cancer. N Engl J Med. 2009;361:98–9. doi: 10.1056/NEJMc0904160. [DOI] [PubMed] [Google Scholar]
- 17.Marchetti A, Felicioni L, Malatesta S, et al. Clinical features and outcome of patients with non-small-cell lung cancer harboring BRAF mutations. J Clin Oncol. 2011;29:3574–9. doi: 10.1200/JCO.2011.35.9638. [DOI] [PubMed] [Google Scholar]
- 18.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 19.Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–73. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 20.Kopetz S, Desai J, Chan E, et al. PLX4032 in metastatic colorectal cancer patients with mutant BRAF tumors. J Clin Oncol. 2010;28(Suppl:15s) abstract. [Google Scholar]
- 21.Prahallad A, Sun C, Huang S, et al. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature. 2012;483:100–3. doi: 10.1038/nature10868. [DOI] [PubMed] [Google Scholar]
- 22.Corcoran RB, Ebi H, Turke AB, et al. EGFR-mediated re-activation of MAPK signaling contributes to insensitivity of BRAF mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer Discov. 2012;2:227–35. doi: 10.1158/2159-8290.CD-11-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE), version 4.0. Bethesda, MD: National Cancer Institute; 2009. NIH publication no. 09-7473. ( http://evs.nci.nih.gov/ftp1/CTCAE/About.html) [Google Scholar]
- 24.Lin Y, Shih WJ. Adaptive two-stage designs for single-arm phase IIA cancer clinical trials. Biometrics. 2004;60:482–90. doi: 10.1111/j.0006-341X.2004.00193.x. [DOI] [PubMed] [Google Scholar]
- 25.Zelboraf (vemurafenib) prescribing information. South San Francisco, CA: Genentech; 2014. ( http://www.gene.com/download/pdf/zelboraf_prescribing.pdf) [Google Scholar]
- 26.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for meta-static breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 27.Bang Y-J, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–97. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 28.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–46. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 29.Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385–94. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 30.Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–80. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 31.Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18:2095–103. doi: 10.1200/JCO.2000.18.10.2095. [DOI] [PubMed] [Google Scholar]
- 32.Fossella FV, DeVore R, Kerr RN, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. J Clin Oncol. 2000;18:2354–62. doi: 10.1200/JCO.2000.18.12.2354. [DOI] [PubMed] [Google Scholar]
- 33.Haroche J, Cohen-Aubart F, Emile J-F, et al. Dramatic efficacy of vemurafenib in both multisystemic and refractory Erdheim-Chester disease and Langerhans cell histiocytosis harboring the BRAF V600E mutation. Blood. 2013;121:1495–500. doi: 10.1182/blood-2012-07-446286. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.