Abstract
Iniparib is an investigational agent with antitumor activity of controversial mechanism of action. Two previous trials in advanced triple-negative breast cancer (TNBC) in combination with gemcitabine and carboplatin showed some evidence of efficacy that was not confirmed. This phase II randomized neoadjuvant study was designed to explore its activity and tolerability with weekly paclitaxel (PTX) as neoadjuvant treatment in TNBC patients. 141 patients with Stage II–IIIA TNBC were randomly assigned to receive PTX (80 mg/m2, d1; n = 47) alone or in combination with iniparib, either once-weekly (PWI) (11.2 mg/kg, d1; n = 46) or twice-weekly (PTI) (5.6 mg/kg, d1, 4; n = 48) for 12 weeks. Primary endpoint was pathologic complete response (pCR) in the breast. pCR rate was similar among the three arms (21, 22, and 19 % for PTX, PWI, and PTI, respectively). Secondary efficacy endpoints were comparable: pCR in breast and axilla (21, 17, and 19 %); best overall response in the breast (60, 61, and 63 %); and breast conservation rate (53, 54, and 50 %). Slightly more patients in the PTI arm presented grade 3/4 neutropenia (4, 0, and 10 %). Grade 1/2 (28, 22, and 29 %), but no grade 3/4 neuropathy, was observed. There were no differences in serious adverse events and treatment-emergent adverse events leading to treatment discontinuation among the three arms. Addition of iniparib to weekly PTX did not add relevant antitumor activity or toxicity. These results do not support further evaluation of the combination of iniparib at these doses plus paclitaxel in early TNBC.
Keywords: Breast cancer, Iniparib, Paclitaxel, Triple-negative breast cancer, Neoadjuvant chemotherapy
Introduction
Triple-negative breast cancer (TNBC) is a heterogeneous group of breast cancer diseases defined by the lack of expression of estrogen (ER), progesterone (PgR), and HER2 receptors and accounts for up to 20 % of all invasive breast tumors [1]. Because of its particular aggressiveness, higher incidence among younger patients, and the lack of targeted agents to treat it, systemic therapy is restricted to cytotoxic agents, which rarely offer clinical benefit in advanced disease. Conversely, in early stages, preoperative chemotherapy attains high rates of pathological complete response (pCR), which is associated with longer disease-free survival (DFS), suggesting it may serve as a strong surrogate marker of long-term outcome [2].
Phenotypically, TNBC has many similarities to BRCA1-associated breast cancer, suggesting dysfunctional DNA repair mechanisms. Selective synthetic lethality with Poly (ADP-ribose) polymerase (PARP) inhibitors has been observed in tumors lacking homologous recombination proteins such as BRCA1 o BRCA2 [1]. Iniparib (4-iodo-3-nitrobenzamide, SAR240550, BSI-201) is an antitumor agent that evaluated in clinical trials both as a single agent and in combination with chemotherapy for the treatment of advanced solid tumors. Iniparib was originally investigated as a poly (ADP-ribose) polymerase (PARP) inhibitor; however, subsequent preclinical studies showed that iniparib does not possess characteristics typical of the PARP inhibitors [3, 4]. Sanofi terminated the development of iniparib in 2013.
An early phase II randomized trial of iniparib in combination with carboplatin and gemcitabine in metastatic TNBC patients reported statistically significant improvement in response rate, progression-free survival (PFS), and overall survival (OS) versus chemotherapy alone [5]. Nevertheless, a phase III trial in the same patient population failed to confirm these efficacy results, although a potential PFS and OS benefit was observed for patients in second and third line of treatment [6]. Simultaneously to this pivotal phase III trial, our neoadjuvant study aimed to explore the clinical activity and tolerability of two iniparib schedules with weekly paclitaxel as preoperative therapy in early TNBC.
Patients and methods
Patients
Women age ≥18 years with histologically confirmed Stage II–IIIA invasive breast cancer eligible for definitive surgery and hormone receptor-negative [ER and PgR negative defined as <10 % tumor staining by immunohistochemistry (IHC) or Allred <5] and HER2-negative (IHC: 0 or 1?; or IHC: 2? and FISH-negative) centrally confirmed status, were included. BRCA1/2 testing was not mandatory. The supplementary material provides further details.
Study design
The SOLTI NeoPARP trial is a randomized multicenter open-label phase II study. From September 2010 to October 2011, 145 patients were enrolled in 22 sites in Spain, France, and Germany. Patients were stratified by clinical nodal status (present vs. not) and tumor size (< or ≥ 5 cm). The trial included 3 parallel treatment groups randomly assigned in a 1:1:1 ratio: paclitaxel alone (PTX) (80 mg/m2, d1; n = 47) or in combination with iniparib, on either a once-weekly (PWI) (11.2 mg/kg, d1; n = 46) or twice-weekly (PTI) (5.6 mg/kg, d1, 4; n = 48) schedule. The study treatment was given for a maximum of 12 administrations over a maximum treatment period of 15 weeks. No crossover was planned. Patients who completed study treatment, withdrew consent, or experienced intolerable toxicity or disease progression underwent surgery according to local practices.
After definitive surgery, adjuvant treatment was administered as per investigator’s preference. The cut-off date for the primary analysis was 30 days after the date of last patient surgery.
The protocol was approved by the Ethics Committees from all participating institutions and Health Authorities in the three countries. The study was conducted in accordance with Good Clinical Practice principles, the Declaration of Helsinki, and all local regulations. All patients provided written informed consent.
Statistics are detailed in the supplementary material.
Assessments
The primary objective of the study was pCR rate in the breast, defined as the complete absence of invasive carcinoma on histological examination at the time of definitive surgery by an independent, blinded central pathology review in each arm of treatment. The secondary objectives were pCR rate in the breast and axilla, objective response rate (ORR) by imaging per RECIST criteria, breast conservation rate, DFS, and OS in each treatment arm, and safety profiles of each treatment.
The Intent-to-treat (ITT) population, comprising all randomized patients, was used for the efficacy analysis. The evaluable patients (EP) population for pathologic response includes all randomized and treated patients with central or local tumor pathology assessment after definitive surgery, including patients who prematurely discontinued treatment due to lack of efficacy as non-responders. This population was used for the supportive efficacy analysis on pCR. The safety population is the subset of randomized patients who received at least one (even incomplete) part of the study treatments. All analyses were based on the treatment actually received by each patient.
Tumor assessments were performed by clinical exam, mammogram, and breast ultrasound or MRI at baseline, at week 6 on-treatment, and prior to definitive surgery. Safety assessments were performed weekly and included adverse event (AE) monitoring, hematology and biochemistry, vital signs, and physical examination. AEs were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 3.0.
Results
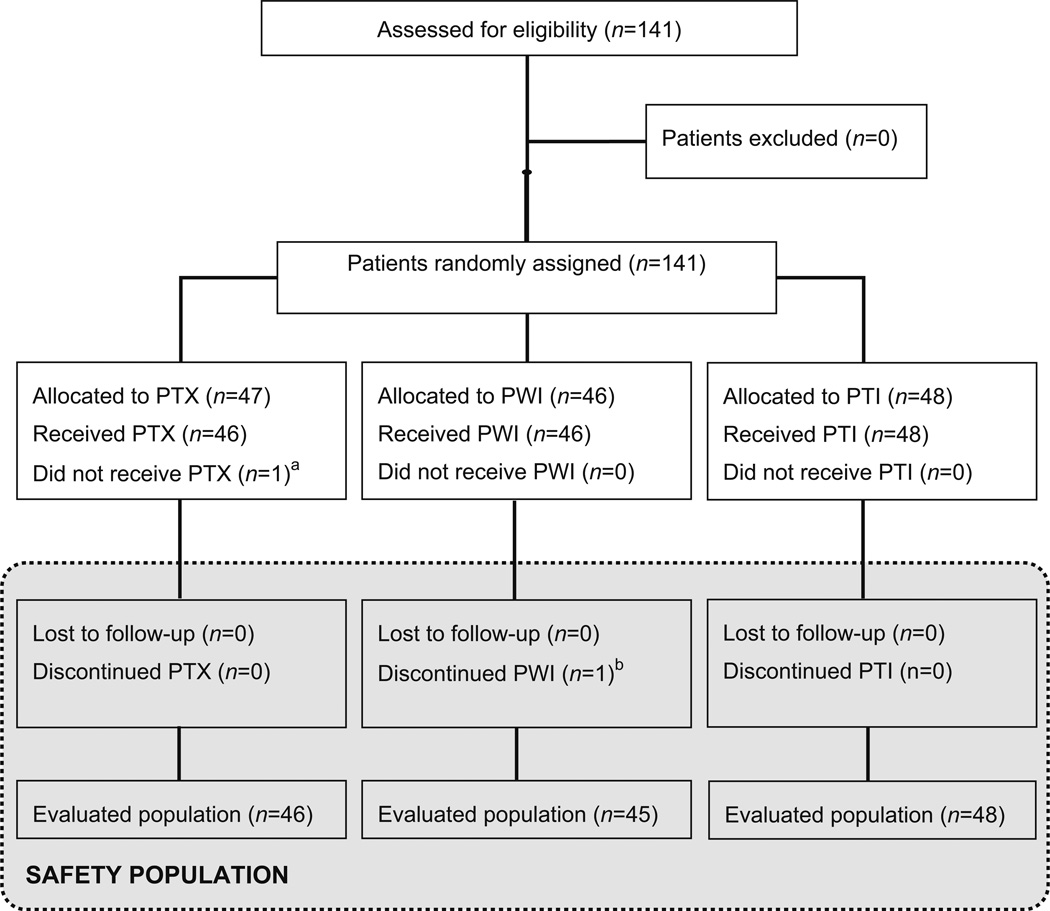
The trial enrolled 141 patients: 47 were randomized to the PTX arm, 46 patients to the PWI, and 48 patients to the PTI. The safety population comprised 140 patients, as 1 patient was randomized but not treated due to investigator’s decision. The evaluable population included 139 patients, as 1 patient discontinued treatment at Cycle 3 due to a contralateral breast cancer present at baseline discovered retrospectively (Fig. 1).
Fig. 1.
Patient disposition (CONSORT diagram). 141 patients with Stage II–IIIA TNBC were randomly assigned to receive PTX (80 mg/m2, d 1; n = 47) alone or in combination with iniparib, on either a once-weekly (PWI) (11.2 mg/kg, d1; n = 46) or twice-weekly (PTI) (5.6 mg/kg, d1, 4; n = 48) schedule for 12 weeks. a Patient was not treated due to investigator’s decision. b Patient discontinued treatment at cycle 3 due to a contralateral breast cancer present at baseline discovered retrospectively
Overall, demographic and tumor characteristics were well balanced among the 3 treatment arms (Table 1). Fewer patients were premenopausal in the PTX arm (43 %) compared to the PWI (59 %) and the PTI (54 %) arms. Most patients had Stage II tumors, and approximately 12 % had Stage IIIA, with approximately 58 % of patients being classified as node-negative at baseline in each of the 3 arms. All patients had centrally confirmed ER/PgR and HER2-negative status, with >85 % being ER and PgR ≤1 %.
Table 1.
Patient and tumor characteristics in the ITT population
| PTX (n = 47) | PWI (n = 46) | PTI (n = 48) | |
|---|---|---|---|
| Age (years) | |||
| Median | 50 | 49 | 49 |
| Min:Max | 29:73 | 27:78 | 30:75 |
| ECOG performance status [n (%)] | |||
| 0 | 44 (94 %) | 44 (96 %) | 46 (96 %) |
| 1 | 3 (6 %) | 2 (4 %) | 2 (4 %) |
| Menopausal status [n (%)] | |||
| Premenopausal | 20 (43 %) | 27 (59 %) | 26 (54 %) |
| Postmenopausal | 27 (57 %) | 19 (41 %) | 22 (46 %) |
| Stage at initial diagnosis [n (%)] | |||
| IIA | 26 (55 %) | 24 (52 %) | 25 (52 %) |
| IIB | 15 (32 %) | 15 (33 %) | 17 (35 %) |
| IIIA | 6 (13 %) | 5 (11 %) | 6 (13 %) |
| IIIB | 0 | 1 (2 %) | 0 |
| IIIC | 0 | 1 (2 %) | 0 |
| Axillary nodal involvement | |||
| No | 27 (57 %) | 26 (57 %) | 28 (58 %) |
| Yes | 20 (43 %) | 20 (44 %) | 20 (42 %) |
| Tumor size | |||
| ≤5 cm | 42 (89 %) | 42 (91 %) | 42 (88 %) |
| >5 cm | 5 (11 %) | 4 (9 %) | 6 (13 %) |
| ER/PgR/HER2 statusa,b [n (%)] | |||
| Triple negative | 47 (100 %) | 46 (100 %) | 48 (100 %) |
| ER [n (%)] | |||
| <10 % | 47 (100 %) | 46 (100 %) | 48 (100 %) |
| ≤1 % | 46 (98 %) | 39 (85 %) | 42 (88 %) |
ER- and PR-negative <10 % IHC or <10 fmol/mg protein by biochemistry
HER2 negative non-overexpressing by IHC (negative) or IHC (equivocal) and fluorescence in situ hybridization (FISH) negative
More than 80 % of the patients completed the planned 12 cycles of treatment in each arm. Two (4 %) patients in the PTX, 4 (9 %) patients in the PWI arm, and 7 (15 %) patients in the PTI arm discontinued study treatment due to objective disease progression demonstrated by imaging. Additionally, 4 (9 %) patients in the PTX arm discontinued treatment due to lack of efficacy as per investigator’s decision in the absence of imaging. In the PWI arm, 1 patient received 11 cycles due to an administrative error. In the PTI arm, 1 patient’s treatment was stopped at Cycle 9 as per investigator’s decision due to radiological complete response.
Efficacy
There were no differences among the 3 arms in terms of centrally reviewed pCR rate in the breast; PTX: 10 (21 %) patients, PWI: 10 (22 %) patients, and PTI: 9 (19 %) patients (Table 2). 27 patients presented pCR in both breast and axilla; PTX: 10 (21 %) patients, PWI: 8 (17 %) patients, PTI: 9 (19 %) patients. The ORR per RECIST was similar in the three arms (PTX: 60 %, PWI: 61 %, PTI: 63 %). Finally, breast conservation was achieved in 53, 54, and 50 % of patients in the PTX, PWI, and PTI arms, respectively (Table 3).
Table 2.
Centralized pathological complete response (pCR) in the breast in the ITT population
| PTX (n = 47) | PTW (n = 46) | PTI (n = 48) | |
|---|---|---|---|
| pCR in breast [n (%)] | |||
| Yes | 10 (21 %) | 10 (22 %) | 9 (19 %) |
| No | 36 (77 %) | 35 (76 %) | 39 (81 %) |
| Not evaluable | 1 (2 %) | 1 (2 %) | 0 |
| pCR rate | 10 (21 %) | 10 (22 %) | 9 (19 %) |
| 95 % CI | (11–36 %) | (11–36 %) | (9–33 %) |
ITT intent-to-treat, CI confidence interval
Table 3.
Secondary efficacy objectives in the ITT population
| PTX (n = 47) | PTW (n = 46) | PTI (n = 48) | |
|---|---|---|---|
| pCR in breast and axilla | 10 (21 %) | 8 (17 %) | 9 (19 %) |
| 95 % CI | (11–36 %) | (8–31 %) | (9–33 %) |
| Best overall response | |||
| Complete response | 3 (6 %) | 0 | 2 (4 %) |
| Partial response | 25 (53 %) | 28 (61 %) | 28 (58 %) |
| Stable disease | 15 (32 %) | 15 (33 %) | 12 (25 %) |
| Progressive disease | 2 (4 %) | 2 (4 %) | 6 (13 %) |
| Not evaluable | 2 (4 %) | 1 (2 %) | 0 |
| Overall response rate | 28 (60 %) | 28 (61 %) | 30 (63 %) |
| 95 % CI | (44–74 %) | (45–75 %) | (47–76 %) |
| Breast conservation rate | 25 (53 %) | 25 (54 %) | 24 (50 %) |
| 95 % CI | (38–68 %) | (39–69 %) | (35– 65 %) |
ITT intent-to-treat, CI confidence interval
Safety
There was no difference in drug exposure among the 3 treatment arms with >80 % of the patients completing 12 cycles of study treatment. The mean relative dose intensity (RDI) of PTX was ≥99 % in the three arms. There was no noticeable difference in the mean RDI for iniparib between the two iniparib-containing arms (RDI > 98 %) (Supplementary Table S1). Incidences of serious adverse events (SAEs) and treatment-emergent adverse events (TEAEs) leading to treatment discontinuation were comparable (Supplementary Tables S2 and S3). The incidences of TEAEs of all grades were similar in the 3 arms, including that of sensory neuropathy (Table 4, Supplementary Tables S4 and S5). The only remarkable finding was the excess of Grade 3/4 neutropenia (including 1 patient with Grade 3 neutropenic infection) and the numerically greater usage of G-CSF in the PTI arm. Nevertheless, the number of patients with all-grade neutropenia as a laboratory parameter was similar among the 3 arms (Supplementary Table S6).
Table 4.
Summary of more frequent treatment-emergent adverse events occurring in at least 10 % of the patients in the safety population
| PT n (%) | PTX (n = 46) | PWI (n = 46) | PTI (n = 48) | |||
|---|---|---|---|---|---|---|
| All grades | Grade 3–4 | All grades | Grade 3–4 | All grades | Grade 3–4 | |
| Any TEAE | 43 (94 %) | 5 (11 %) | 45 (98 %) | 5 (11 %) | 47 (98 %) | 16 (33 %) |
| Nasopharyngitis | 8 (17 %) | 0 | 7 (15 %) | 0 | 6 (13 %) | 0 |
| Neutropenia | 2 (4 %) | 2 (4 %) | 3 (7 %) | 0 | 5 (10 %) | 5 (10 %) |
| Peripheral sensory neuropathy | 13 (28 %) | 0 | 10 (22 %) | 0 | 14 (29 %) | 0 |
| Paresthesia | 8 (17 %) | 0 | 5 (11 %) | 0 | 4 (8 %) | 0 |
| Conjunctivitis | 3 (7 %) | 0 | 2 (4 %) | 0 | 5 (10 %) | |
| Vertigo | 1 (2 %) | 0 | 0 | 0 | 5 (10 %) | 0 |
| Diarrhea | 8 (17 %) | 0 | 18 (39 %) | 1 (2 %) | 14 (29 %) | 0 |
| Nausea | 4 (9 %) | 0 | 7 (15 %) | 0 | 11 (23 %) | 0 |
| Stomatitis | 4 (9 %) | 0 | 6 (13 %) | 0 | 8 (17 %) | 0 |
| Constipation | 5 (11 %) | 0 | 7 (15 %) | 0 | 5 (10 %) | 0 |
| Alopecia | 34 (74 %) | NA | 28 (61 %) | NA | 33 (69 %) | NA |
| Rash | 7 (15 %) | 0 | 3 (7 %) | 0 | 6 (13 %) | 0 |
| Myalgia | 6 (13 %) | 0 | 5 (11 %) | 0 | 3 (6 %) | 0 |
| Arthralgia | 6 (13 %) | 0 | 5 (11 %) | 0 | 1 (2.1 %) | 0 |
| Asthenia | 26 (57 %) | 0 | 21 (46 %) | 0 | 24 (50 %) | 0 |
| Fatigue | 8 (17 %) | 0 | 6 (13 %) | 0 | 6 (13 %) | 0 |
| Peripheral edema | 4 (9 %) | 0 | 1 (2 %) | 0 | 5 (10 %) | 0 |
Only rows with frequency of at least 5 % in at least one column are shown
Discussion
The NeoPARP study explored the addition of 2 different doses and schedules of iniparib in combination with weekly paclitaxel as primary systemic therapy for women with newly diagnosed stage II–IIIA TNBC. None of the experimental schedules improved the pCR rate in the breast, nor were there any meaningful improvements in any of the secondary efficacy endpoints relative to the PTX control arm. The in-breast pCR rates as assessed by central blinded review were 21, 22, and 19 % for the PTX, PWI, and PTI arms, respectively. Secondary objectives, such as pCR rates in breast and axilla by local review and ORR were very similar between arms, as well. Overall, our results show that there is a lack of efficacy in the addition of weekly or twice-weekly iniparib to weekly paclitaxel in this early TNBC population.
Iniparib was originally considered an irreversible inhibitor of PARP1, an enzyme that plays a critical role in the repair of single-stranded DNA breaks [7]. The biological rationale for combining a PARP inhibitor with DNA-damaging agents such as chemotherapy in TNBC is based on the fact that BRCA1 dysfunctions are common in TNBC patients [1, 8, 9]. However, while this trial was underway, two groups demonstrated that iniparib does not act as a PARP1 inhibitor at pharmacological concentrations [3, 4]. Prior to these findings, a phase II randomized study of iniparib in combination with carboplatin and gemcitabine had reported improved ORR, PFS, and OS in metastatic TNBC patients [5]. Nevertheless, a phase III trial with similar treatment regimens, failed to confirm this benefit. Interestingly, an exploratory analysis suggested a possible PFS and OS advantage with iniparib for those patients in the second- and third-line treatment settings [6].
The NeoPARP study was the first trial to investigate the potential synergism of iniparib with a non-platinum cytotoxic. Weekly paclitaxel was deemed a rational chemotherapeutic partner as it is a well-established first-line therapy for advanced disease and widely used in early-stage regimens [9–11]. Despite not achieving any of its prespecified objectives, the NeoPARP study design was innovative and provides methodological and clinical information that may prove useful for the efficacy assessment of anticancer agents in the neoadjuvant setting and, in particular, TNBC. It is worth considering that the timely evaluation of new compounds and their potential biomarkers via proof-of-concept Phase II neoadjuvant trials may prevent embarking in large and costly late-stage studies based on insufficient evidence.
Efficacy assumptions in the NeoPARP study were based on data reported by Green et al. [12], which compared 12 doses of weekly paclitaxel versus 4 cycles of the same drug administered every 3 weeks in patients with stage I–IIIB breast cancer, followed by 4 cycles of FAC. The primary endpoint, pCR in breast and axilla, was 28 % in the weekly paclitaxel arm. In another Phase III study that compared capecitabine plus docetaxel followed by fluorouracil, epirubicin, and cyclophosphamide (FEC) or weekly paclitaxel followed by FEC, pCR rate in the weekly paclitaxel arms was only 16 % [13]. In our trial, pCR was approximately 20 % in each arm. Although this trial comparison suggests that addition of FAC or FEC does not significantly enhance pCR to weekly paclitaxel, it is important to remark that our trial enrolled only TNBC who are considered more chemosensitive than other breast cancer subtypes included as well in the other trials. Given that iniparib, at the doses and schedules used in our trial, virtually lacked anticancer activity, one can safely assume that the rate of pCR that we report here represents weekly paclitaxel activity alone. This is, to our knowledge, the largest cohort of patients treated with this regimen in the neoadjuvant setting. Our data indicate that paclitaxel alone leads to very low in breast pCR rates in patients with early-stage TNBC and may be useful for future studies that investigate other agents in combination with paclitaxel in this population.
In terms of the primary endpoint, pCR in breast, and not in both breast and axilla, was the preferred choice, despite the knowledge that the latter is a stronger predictor of DFS and OS [2]. This was decided due to the impossibility to harmonize axillary evaluation at baseline and at the time of surgery in a multicenter trial. Indeed, patients underwent different types of axillary procedures [sentinel lymph node biopsy (SLNB) and/or lymphadenectomy] at different timings (baseline and/or surgery) resulting in an incomplete availability of material for central review. Our trial is, therefore, a good example of this methodological dilemma for neoadjuvant research. Rapid changes in axillary management, lack of standardized procedures, and the increasing usage of molecular techniques for SLNB evaluation are challenges for harmonizing axillar evaluation. The heterogeneity in axillary evaluation presented in this trial demonstrates the potential difficulties to use pCR as surrogated endpoint for accelerated approval of new breast cancer drugs as recommended by the FDA [14].
Finally, in regards to safety, as iniparib has been tested almost exclusively in combination regimens and in patients with metastatic cancer, its AE profile had not been well defined. This study provided a unique opportunity to observe it in patients with virtually no cancer symptoms. Moreover, the mild toxicity of weekly paclitaxel, the use of a control arm with weekly paclitaxel only, and the 2 doses of iniparib offered a good scenario for an adequate safety comparison. In fact, an increased incidence and severity of AEs were not observed, bringing up the question of whether the optimal dose and schedule of iniparib has been adequately defined during Phase I, which could partly explain the failure to demonstrate any impactful efficacy benefit in later-stage clinical trials.
In conclusion, the results from this study do not support further evaluation of iniparib at 5.6 mg/kg twice-weekly or 11.2 mg/kg weekly, in combination with paclitaxel in early TNBC. Further research utilizing tumor samples collected in the context of this trial may help shed some light into the profiles of those tumors sensitive to neoadjuvant paclitaxel.
Supplementary Material
Acknowledgments
Funding This work was fully supported by Sanofi.
Employment: EJC (Sanofi), IGR (Sanofi); Remuneration: ALC (Roche, Lilly, Pfizer, Novartis), J. Balmaña (AstraZeneca), HR (Roche, Pierre Fabre, AstraZeneca), SGS (Roche, GlaxoSmithKline, Novartis, Teva, Eisai, Celgene); Stock ownership: ALC (MEDSIR ARO), EJC (Sanofi), IGR (Sanofi); Consultant/Advisory Role: ALC (Roche, AstraZeneca, Pfizer), CV (Bristol-Myers Squibb, GlaxoSmithKline, Roche, Novartis), SD (Novartis, Pfizer, Roche), J. Balmaña (AstraZeneca), SGS (AstraZeneca), JG (Novartis), NH (AstraZeneca); Funding: ALC (MEDSIR ARO, Celgene, Roche), SD (Novartis, Pfizer, Roche, Puma, Amgen, AstraZeneca), J. Balmaña (AstraZeneca, Pharmamar), HR (Roche, Amgen, Pierre Fabre), NH (Biomarin), NRR (Roche, Bristol-Myers Squibb, Janssen).
Footnotes
Presented at the American Society of Clinical Oncology Annual Meeting, June 1–5, 2012, Chicago, IL.
Clinical trial number: This trial was registered in clinicaltrials.gov (NCT01204125).
Electronic supplementary material The online version of this article (doi:10.1007/s10549-015-3616-8) contains supplementary material, which is available to authorized users.
Compliance with ethical standards
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent Informed consent was obtained from all individual participants included in the study.
Conflict of Interest
All remaining authors have declared no conflicts of interest.
References
- 1.Cleere DW. Triple-negative breast cancer: a clinical update. Commun Oncol. 2010;7:203–211. [Google Scholar]
- 2.Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 3.Patel AG, De Lorenzo SB, Flatten KS, et al. Failure of iniparib to inhibit poly(ADP-Ribose) polymerase in vitro. Clin Cancer Res. 2012;18:1655–1662. doi: 10.1158/1078-0432.CCR-11-2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu X, Shi Y, Maag DX, et al. Iniparib nonselectively modifies cysteine-containing proteins in tumor cells and is not a bona fide PARP inhibitor. Clin Cancer Res. 2012;18:510–523. doi: 10.1158/1078-0432.CCR-11-1973. [DOI] [PubMed] [Google Scholar]
- 5.O’Shaughnessy J, Osborne C, Pippen JE, et al. Iniparib plus chemotherapy in metastatic triple-negative breast cancer. N Engl J Med. 2011;364:205–214. doi: 10.1056/NEJMoa1011418. [DOI] [PubMed] [Google Scholar]
- 6.O’Shaughnessy J, Schwartzberg L, Danso MA, et al. Phase III study of iniparib plus gemcitabine and carboplatin versus gemcitabine and carboplatin in patients with metastatic triple-negative breast cancer. J Clin Oncol. 2014;32:3840–3847. doi: 10.1200/JCO.2014.55.2984. [DOI] [PubMed] [Google Scholar]
- 7.Ossovskaya V, Li L, Broude E, et al. Abstract #5552: BSI-201 enhances the activity of multiple classes of cytotoxic agents and irradiation in triple negative breast cancer. Cancer Res. 2009;69:5552. [Google Scholar]
- 8.Patel AG, Sarkaria JN, Kaufmann SH. Nonhomologous end joining drives poly(ADP-ribose) polymerase (PARP) inhibitor lethality in homologous recombination-deficient cells. Proc Natl Acad Sci USA. 2011;108:3406–3411. doi: 10.1073/pnas.1013715108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ossovskaya V, Wang Y, Budoff A, et al. Exploring molecular pathways of triple-negative breast cancer. Genes Cancer. 2011;2:870–879. doi: 10.1177/1947601911432496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esteva FJ, Franco SX, Hagan MK, et al. An open-label safety study of lapatinib plus trastuzumab plus paclitaxel in first-line HER2-positive metastatic breast cancer. Oncologist. 2013;18:661–666. doi: 10.1634/theoncologist.2012-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rugo HS, Campone M, Amadori D, et al. A randomized, phase II, three-arm study of two schedules of ixabepilone or paclitaxel plus bevacizumab as first-line therapy for metastatic breast cancer. Breast Cancer Res Treat. 2013;139:411–419. doi: 10.1007/s10549-013-2552-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green MC, Buzdar AU, Smith T, et al. Weekly paclitaxel improves pathologic complete remission in operable breast cancer when compared with paclitaxel once every 3 weeks. J Clin Oncol. 2005;23:5983–5992. doi: 10.1200/JCO.2005.06.232. [DOI] [PubMed] [Google Scholar]
- 13.Kelly CM, Green MC, Broglio K, et al. Phase III trial evaluating weekly paclitaxel versus docetaxel in combination with capecitabine in operable breast cancer. J Clin Oncol. 2012;30:930–935. doi: 10.1200/JCO.2011.36.2079. [DOI] [PubMed] [Google Scholar]
- 14.U. S. Department of Health and Human Services, Center for Drug Evaluation and Research US. Guidance for industry: pathologic complete response in neoadjuvant treatment of high-risk early-stage breast cancer—Use as an endpoint to support accelerated approval. 2014 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.