Abstract
Background
The detrimental effects of chronic heavy alcohol use on the cardiovascular system are well established and broadly appreciated. Integrated cardiovascular response to an acute dose of alcohol has been less studied. This study examined the early effects of an acute dose of alcohol on the cardiovascular system, with particular emphasis on system variability and sensitivity. The goal was to begin to understand how acute alcohol disrupts dynamic cardiovascular regulatory processes prior to the development of cardiovascular disease.
Methods
Healthy participants (N = 72, age 21 to 29) were randomly assigned to an alcohol, placebo, or no-alcohol control beverage condition. Beat-to-beat heart rate (HR) and blood pressure (BP) were assessed during a low-demand cognitive task prior to and following beverage consumption. Between-group differences in neurocardiac response to an alcohol challenge (blood alcohol concentration ~ 0.06 mg/dl) were tested.
Results
The alcohol beverage group showed higher average HR, lower average stroke volume, lower HR variability and BP variability, and increased vascular tone baroreflex sensitivity after alcohol consumption. No changes were observed in the placebo group, but the control group showed slightly elevated average HR and BP after beverage consumption, possibly due to juice content. At the level of the individual, an active alcohol dose appeared to disrupt the typically tight coupling between cardiovascular processes.
Conclusions
A dose of alcohol quickly invoked multiple cardiovascular responses, possibly as an adaptive reaction to the acute pharmacological challenge. Future studies should assess how exposure to alcohol acutely disrupts or dissociates typically integrated neurocardiac functions.
Keywords: Intoxication, Heart Rate Variability, Blood Pressure, Vascular Tone, Baroreflex
THE LONG-term, detrimental effects of chronic heavy alcohol use on cardiovascular function are well established and broadly appreciated (e.g., American Heart Association, 2015; Corrao et al., 2004; National Institute on Alcohol Abuse and Alcoholism, 2015; Rehm et al., 2010). These chronic effects arise over time as the result of repeated bouts of acute intoxication and withdrawal, but each drinking episode is also acutely linked to its own complex time-and dose-dependent neural, muscular, and biochemical alterations within the cardiovascular system (Kahkonen et al., 2011; Kawano, 2010). Initially, these acute cardiovascular alterations are transient (Kawano, 2010), and system redundancies can effectively compensate for disruptions to any one given element (Joyner and Pedersen, 2011; Quinn and Kohl, 2012). Over time, however, these alterations can persist and become clinically evident as one of several diagnosable chronic cardiovascular diseases that are highly comorbid with alcohol dependence (Corrao et al., 2004; Rehm et al., 2010). In this study, we examined the early effects of an oral dose of alcohol on the cardiovascular system, with particular emphasis on changes in system variability and sensitivity, in a sample of healthy, young adult drinkers.
Interest in biological variability has exploded over the past decade, with significant literature now focusing on neural (Wang, 2010) and cardiovascular (Lehrer and Eddie, 2013; Rickards and Tzeng, 2014) oscillations. Greater variability in biological functions is generally thought to demonstrate more intact regulatory systems that are appropriately detecting and dynamically responding to perturbation (Kitano, 2004; Lehrer and Eddie, 2013; Thayer and Lane, 2000). In other words, more variability often implies a high degree of flexibility in a well-functioning biological system (Tapanainen et al., 2002; Vaschillo et al., 2011).
Heart rate variability (HRV; i.e., changes in the time intervals between consecutive heart beats) is perhaps the best known and most easily measured index of physiological variability. HRV reflects the continual, bidirectional communication between the heart and brain, which allows the individual to precisely adapt to changes in his/her internal milieu and external environment by integrating neural and cardiovascular reactions. In the resting state, it is a robust biomarker of health (Kemp and Quintana, 2013; Thayer et al., 2010). Following oral alcohol consumption, resting state HRV is reduced (reviewed in Romanowicz et al., 2011), as is HRV reactivity to emotionally valenced visual cues (Vaschillo et al., 2008).
The cardiovascular system, however, does not adapt solely by modulating HRV. Rather, it can flexibly respond to challenge by changing stroke volume, vascular tone, and blood pressure (BP) variability in addition to or in lieu of changing HRV (Liu et al., 2004; Ma and Zhang, 2006; Rickards and Tzeng, 2014; Vaschillo et al., 2012). System redundancy ensures that the cardiovascular system can buffer itself against internal (e.g., cognitive emotional) and external (e.g., pharmacological) challenges, and thus protect the brain from stroke and the heart from myocardial infarction. These system elements (i.e., heart rate [HR], vascular tone) may work independently or in tandem to generate fine-grained, real-time adjustments to optimize cardiovascular responding. Importantly, variability in each of these processes is linked through the baroreflex system, which is comprised of multiple parallel, closed-loop branches that control BP through changes in HR (i.e., the HR baroreflex loop), stroke volume (i.e., the stroke volume baroreflex loop), and vascular tone (i.e., the vascular tone baroreflex loop) (Casadei et al., 1992; Eckberg and Sleight, 1992; Vaschillo et al., 2012; Yambe et al., 2009). In addition, the baroreflex controls sympathetic nervous system activity. For example, the baroreflex decreases muscle sympathetic nerve activity when BP increases, and increases it when BP decreases (Hart et al., 2009). Under normal conditions, the baroreflex coordinates cardiovascular processes. When challenged by alcohol, however, it is unclear whether cardiovascular processes remain coordinated and/or whether the sensitivity of the baroreflex system to detect changes is compromised.
This study sought to provide a first step in characterizing system-wide cardiovascular disruption by examining the effects of acute alcohol intoxication on average HR, stroke volume, vascular tone, and BP, variability in each of these cardiovascular processes, and sensitivity in the HR, stroke volume, and vascular tone baroreflex branches. The effects of acute alcohol on the cardiovascular system were assessed on the ascending limb of the blood alcohol curve, a period of maximal alcohol absorption that is associated with stimulant/euphoric subjective experiences. Prior studies suggest that cardiac responses to alcohol are more tightly linked to the stimulant, compared to sedative effects of alcohol (Brunelle et al., 2007).
Our general hypothesis was that the reactions of individual cardiovascular parameters and baroreflex feedback loops to acute alcohol intoxication would be disparate but coordinated, such that they maintain general cardiovascular homeostasis. For average cardiovascular responses, based on prior studies using oral alcohol administration protocols and similar assessment time points, we hypothesized an increase in HR (Conrod et al., 2001), a decrease in stroke volume (Morvai et al., 1988), and vasodilation (Agewall et al., 2000). For variability, we predicted global suppression based on the premise that greater variability implies greater adaptability and better health (Lehrer and Eddie, 2013; Thayer and Lane, 2000; Vaschillo et al., 2011). Previous oral alcohol administration studies have observed a reduction in HRV following alcohol administration (Vaschillo et al., 2008). To our knowledge, no one has assessed whether stroke volume variability or vascular tone variability is disrupted during alcohol intoxication. Finally, oral alcohol administration has been shown to reduce HR baroreflex sensitivity (Fazio et al., 2001), and likewise it is possible that, due to its pharmacological actions on the nitric oxide pathway (Bau et al., 2005) and smooth muscle (Goslawski et al., 2013), alcohol would also reduce sensitivity in the vascular tone and stroke volume baroreflex branches. Thus, we tentatively hypothesized that alcohol would reduce baroreflex sensitivity across all baroreflex branches.
MATERIALS AND METHODS
Participants
Seventy-two participants were recruited via university/community bulletin boards and newspapers to take part in a single session cue reactivity study aimed at characterizing how alcohol changes cardiovascular functions in a restful state and in response to emotional and appetitive picture cues. This study addresses the first of these questions. Participants averaged 21.7 years of age (SD = 0.9); 44% were female. Because the study included the administration of an alcohol beverage, individuals were excluded if they were under 21 years of age, reported consuming less than 4 drinks (3 drinks for women) twice per month in the past year, or were more than 20% overweight or underweight from the ideal for gender, height, and body frame based on the Metropolitan Life Height–Weight Table (Metropolitan Life Insurance Company, 1983). Additional exclusion criteria included regular (greater than monthly) drug use, self-report of current learning disability, lifetime history of bipolar disorder or psychosis diagnosis, substance use treatment in the past year, biological mother heavy substance use during pregnancy, and for women, pregnancy. Eligibility was ascertained initially during a telephone screening interview.
Procedures
This study was approved by the university’s Institutional Review Board for the Protection of Human Subjects Involved in Research. All participants provided written informed consent and were compensated $50 for their time. Each participant completed a ~3-hour laboratory session (between 10:00 AM and 4:00 PM to minimize biological circadian variations). They were asked to refrain from alcohol or other drug use (except caffeine and cigarettes) for 24 hours before the laboratory session.
Upon arrival in the laboratory, weight, height, pregnancy status, and a zero blood alcohol concentration (BAC) were confirmed and participants were randomly assigned to the alcohol, placebo, or no-alcohol beverage group, as described below. A battery of questionnaires was completed. Participants were then seated in a comfortable chair 2.5 m in front of a large computer screen in a sound attenuated, dimly lit room. Dermal electrocardiogram (ECG) electrodes were placed ventrolateral to both deltoid muscles and above the left ankle. BP was measured using a sensor placed around the participant’s right middle finger between the first and third phalanges. ECG and beat-to-beat BP data were continuously recorded at a sample rate of 2000 Hz using a Power-lab Acquisition system (ADInstruments, Colorado Springs, CO) and a Finometer MIDI (Finapres, Amsterdam, the Netherlands), respectively. To ensure the quality and integrity of the ECG data during data collection, a brief pre-experimental assessment was performed and participants with abnormal ECGs were excluded prior to the initiation of the study; in this study, 3 were excluded prior to physiological recording for an abnormal ECG.
Physiological recording began with participants performing a standardized low cognitive demand “vanilla” task (Jennings et al., 1992) for 5 minutes. The participant viewed colored rectangles presented sequentially (1 rectangle every 10 seconds) on a computer screen and silently counted the number of blue rectangles. Compared with an unstructured resting baseline, this task produces HRV values with better between- and within-subject stability and generalizability across sessions (Jennings et al., 1992).
All participants consumed a volume-controlled beverage based on group assignment. Participants in the alcohol group (n = 24) were told that they would receive some amount of alcohol and were given mixer (orange, cranberry, and lime juice) with an active ethanol (EtOH) dose to achieve a target BAC of ~80 mg/dl, calculated based on body weight (0.90 ml/kg for men, 0.78 ml/kg for women), in a ratio of 4 parts mixer to 1 part alcohol (95% EtOH) (Bates and Martin, 1997). Participants in the placebo group (n = 24) were told that they would be given some amount of alcohol and received mixer with a physiologically inactive dose of alcohol (100 µl EtOH float per each cup) and other olfactory cues. The no-alcohol control group (n = 24) were told that they would not be given alcohol and received 100% mixer. Each beverage was divided into 3 equal drinks, and participants were instructed to consume each beverage evenly over a 5-minute period (total drinking time =15 minutes).
The vanilla task then was performed for a second time when alcohol group participants’ BAC reached ~60 mg/dl on the ascending limb of the blood alcohol curve (average: 10.6 minutes, SD = 15.2), or after 10 minutes for placebo and control participants. Physiological recordings continued during several picture cue presentation tasks, but BAC was not measured during these tasks. The current study focused on acute intoxication and resting state cardiovascular activity. Participants in the alcohol beverage group remained in the laboratory until their BAC returned to zero.
Measures
Participants’ sociodemographic information (sex, age, education, race) as well as quantity and frequency of alcohol and frequency of other drug use in the past 30 days, past year, and over the lifetime was assessed with self-report questionnaires. Family history of alcoholism status was ascertained using a standardized family history interview (Rice et al., 1995) and considered positive when a first-degree relative met criteria for alcohol dependence. Alcohol-related problems were assessed using the 25-item Alcohol Dependence Scale (Skinner and Horn, 1984) and the 18-item Rutgers Alcohol Problem Index (White and Labouvie, 2000) with 2 additional items added to gauge drunk driving and regretted sexual situations. Depression and anxiety symptoms were measured using the Beck Depression Inventory II (Beck, 1996) and Beck Anxiety Inventory (Beck and Steer, 1993), respectively. Table 1 shows that groups were not statistically different in terms of demographics, family history of alcohol dependence, alcohol use, and mood.
Table 1.
Participant Characteristics
| Control (n = 24) |
Placebo (n = 24) |
Alcohol (n = 24) |
|
|---|---|---|---|
| Sex (% female) | 46% | 50% | 50% |
| Agea | 21.7 (0.9) | 21.6 (0.9) | 21.8 (1.1) |
| Years of educationa | 15.5 (0.8) | 15.5 (1.0) | 15.2 (2.1) |
| Racea | |||
| White | 64% | 55% | 57% |
| Black/African American | 14% | 9% | 9% |
| Asian | 14% | 23% | 22% |
| Other | 9% | 14% | 13% |
| Family history of alcoholism | 13% | 13% | 17% |
| # Drinking occasions in past 30 daysb |
5.8 (5.4) | 6.8 (4.8) | 5.0 (4.7) |
| Typical # drinks per occasion in past 30 daysb |
4.5 (1.8) | 5.2 (2.0) | 4.91.8 |
| Alcohol Dependence Scaleb | 5.3 (3.2) | 5.8 (2.3) | 5.9 (3.3) |
| Rutgers Alcohol Problem Indexb | 6.3 (5.9) | 8.3 (6.8) | 6.5 (5.0) |
| Beck Anxiety Inventoryb | 4.4 (4.9) | 3.1 (3.2) | 2.9 (2.7) |
| Beck Depression Inventoryb | 5.2 (5.4) | 3.9 (3.5) | 3.6 (3.8) |
Demographic data were missing for 5 individuals.
Assessed over past 30 days.
Data are presented as mean ± standard deviation (in parentheses) or percent endorsing item
Continuous sequences of heart beat-to-beat intervals (RRI) were recorded during the pre-drinking and post-drinking vanilla tasks. RRI data were exported to WinCPRS software (Absolute Aliens Oy, Turku, Finland) for analysis. No recording occurred during the 15-minute beverage consumption phase that intervened between the 2 tasks. For assessment of HR dynamics, average HR across each task, time-domain HRV indices (standard deviation of normal-to-normal beats [SDNN], root of the mean squared differences of successive intervals [RMSSD], and percent of the number of pairs of adjacent normal-to-normal intervals differing by more than 50 ms [pNN50]) and frequency domain indices (low frequency [0.04 to 0.15 Hz] and high frequency [0.15 to 0.4 Hz]) were calculated. Frequency domain indices were computed from spectral analysis after cubic interpolation of the nonequidistant waveform and 4 Hz resampling. For stroke volume means and variability, the Model-Flow methodology for cardiac output measurement (Bogert and van Lieshout, 2005) was used. To evaluate vascular tone, pulse transit time was used as a proxy measure and measured for each heart beat as the time interval between the R-spike of the ECG waveform and the peak of the corresponding finger pulse. Higher pulse transit time corresponds to lower vascular tone (Bogert and van Lieshout, 2005). Measures of BP were derived from the vascular pressure waveform. Systolic BP represents arterial pressure during the systolic contraction of the heart. Mean arterial pressure reflects organ perfusion and was estimated as (systolic BP + 2X diastolic BP)/3.
HR, stroke volume, and vascular tone baroreflex sensitivities were calculated with cross-spectral analysis. Transfer functions were calculated with systolic BP as the input and RRI, stroke volume, or pulse transit time as the output. Sensitivity was estimated as the average power of the transfer function in the low frequency range where coherence between systolic BP (input) and the output was > 0.5 (Cooke et al., 1999). Baroreflex sensitivities evaluate the magnitude of change in RRI, stroke volume, and pulse transit time to a 1 mmHg change in systolic BP (Maestri et al., 1998; Pitzalis et al., 1998; Vaschillo et al., 2002, 2012).
Analysis
Testing for multivariate outliers using Mahalanobis distance (de Maesschalck et al., 2000) with criterion p < 0.001 identified no outliers. A mixed linear model (SAS 9.3; SAS Institute, Inc., Carey, NC) was tested for each cardiovascular index. Significant main and interaction effects were followed by post hoc tests of within-subjects effects for 3 a priori selected tests (i.e., pre-compared to post-beverage values in the alcohol, placebo, and control groups). A Bonferroni correction was used to mitigate alpha inflation by setting post hoc alpha levels at p < 0.017. Scatterplots were created to assess the relationships between pre- to post-beverage change in cardiovascular parameters at the level of the individual. Effect size calculations were performed using Cohen’s drm (for repeated measures designs) as suggested by Lakens (2013).
To investigate whether alcohol group participants’ time to achieve 0.06% BAC was related to their physiological responses to the alcohol dose, correlations between time to achieve a 0.06% BAC and post-drinking physiological indices were examined. No significant associations were found (all p > 0.05).
RESULTS
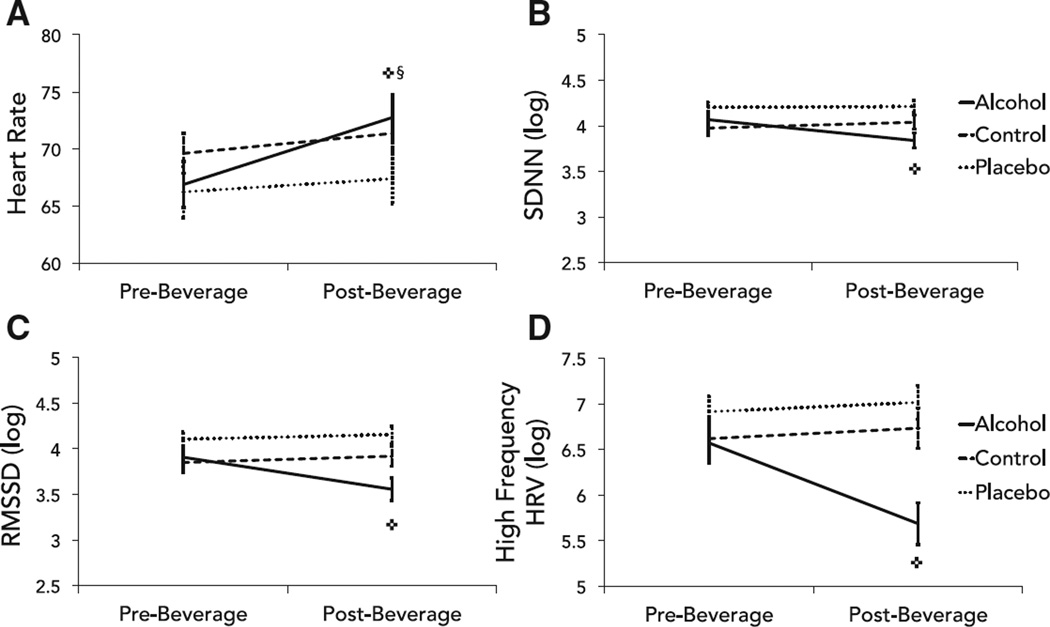
As predicted, average HR significantly increased and multiple indices of HRV significantly decreased following oral alcohol consumption (Fig. 1). Unexpectedly, average HR also increased in the control group. There was a main effect of time (but not beverage group) on mean HR, and a significant group × time interaction (Table 2). Least squares means comparisons indicated that HR increased significantly from the pre- to post-drinking task in the alcohol group (medium effect size) and control group (very small effect size), but not placebo group. For HRV indices, there were significant main effects of time, group, and significant group × time interactions for pNN50, SDNN, RMSSD, and high frequency HRV (Table 3). Least squares means comparisons indicated a significant reduction in these HRV indices in the alcohol group following beverage administration, but not in the control or placebo groups. There were no significant main or interaction effects for low frequency HRV.
Fig. 1.
Differences in (A) mean heart rate and heart rate variability between groups. Heart rate variability was measured as (B) the standard deviation of all normal-to-normal intervals (SDNN), (C) the root mean square of successive differences (RMSSD), and (D) high frequency heart rate variability (HRV). Error bars represent standard error; ✜change in alcohol group, p < 0.01; §change in control group, p < 0.01.
Table 2.
Average Cardiovascular Activity Before and After Beverage Consumption and Significance of Differences Between Reactions to Juice (Control), an Inactive Dose of Alcohol (Placebo), or an Active Dose of Alcohol (Alcohol)
| Control | Placebo | Alcohol | Main Effect Time F |
Group × Time Interaction F |
|
|---|---|---|---|---|---|
| Heart rate (beats per minute) | |||||
| Pre-beverage | 69.6 ± 8.4 | 66.2 ±11.0 | 66.9 ± 9.9 | 51.24* | 11.21* |
| Post-beverage | 71.4 ± 8.4 | 67.4 ± 10.6 | 72.8 ± 9.8 | ||
| Change | 1.8** (d = 0.17) | 1.2 (d = 0.11) | 5.9** (d = 0.60) | ||
| Stroke volume (ml) | |||||
| Pre-beverage | 70.5 ± 12.2 | 72.2 ± 17.7 | 69.7 ± 15.7 | 2.66 | 5.22* |
| Post-beverage | 69.6 ± 13.3 | 73.3 ± 16.4 | 63.1 ± 9.9 | ||
| Change | −0.9 (d = 0.07) | 1.1 (d = 0.06) | −6.6** (d = 0.45) | ||
| Pulse transit time (ms) | |||||
| Pre-beverage | 283.8 ± 19.0 | 285.7 ± 26.3 | 289.9 ± 26.9 | 35.02* | 2.36 |
| Post-beverage | 275.1 ± 13.4 | 279.0 ± 23.0 | 286.4 ± 22.1 | ||
| Change | −8.7 (d = 0.47) | −6.7 (d = 0.27) | −3.5 (d = 0.14) | ||
| Systolic blood pressure (mmHg) | |||||
| Pre-beverage | 117.0 ± 13.1 | 126.2 ± 19.2 | 116.9 ± 13.5 | 7.01* | 9.54* |
| Post-beverage | 125.9 ± 13.2 | 130.7 ± 18.2 | 113.2 ± 12.6 | ||
| Change | 8.9** (d = 0.68) | 4.5 (d = 0.24) | −3.7 (d = 0.29) | ||
| Mean arterial blood pressure (mmHg) | |||||
| Pre-beverage | 79.8 ± 10.1 | 83.5 ± 13.9 | 80.1 ± 8.7 | 13.21* | 5.89* |
| Post-beverage | 85.4 ± 9.6 | 85.7 ± 12.9 | 79.9 ± 9.7 | ||
| Change | 5.6** (d = 0.57) | 2.2 (d = 0.17) | −0.2 (d = 0.03) |
Statistical analyses performed using log-transformed variables, except heart rate.
F significant at p < 0.05;
pairwise comparisons showed significant changes pre- to post-beverage consumption, p < 0.01.
All indices presented as mean ± standard deviation. Cohen’s d was computed to estimate effect sizes.
Table 3.
Variability of Cardiovascular Functions Before and After Beverage Consumption and Significance of Differences Between Groups in Response to Beverage Consumption
| Control | Placebo | Alcohol | Main Effect Time F |
Group × Time Interaction F |
|
|---|---|---|---|---|---|
| Heart rate variability (HRV) | |||||
| pNN50 (%) | |||||
| Pre-beverage | 31.5 ± 23.9 | 41.9 ± 18.2 | 34.0 ± 23.2 | 7.33* | 14.89* |
| Post-beverage | 32.3 ± 24.4 | 45.5 ± 18.9 | 21.4 ± 19.9 | ||
| Change | 0.8 (d = 0.03) | 3.6 (d = 0.19) | −12.6** (d = 0.58) | ||
| SDNN (ms) | |||||
| Pre-beverage | 57.2 ± 23.1 | 69.1 ± 17.8 | 63.8 ± 27.5 | 6.40* | 11.61* |
| Post-beverage | 61.5 ± 27.3 | 70.5 ± 20.2 | 50.6 ± 20.2 | ||
| Change | 4.3 (d = 0.17) | 1.4 (d = 0.07) | −13.2** (d = 0.55) | ||
| RMSSD (ms) | |||||
| Pre-beverage | 54.3 ± 31.7 | 65.6 ± 26.5 | 59.0 ± 34.8 | 11.44* | 20.19* |
| Post-beverage | 58.6 ± 38.5 | 69.7 ± 26.8 | 41.5 ± 24.2 | ||
| Change | 4.3 (d = 0.12) | 4.1 (d = 0.15) | −17.5** (d = 0.42) | ||
| High frequency HRV (ms2) | |||||
| Pre-beverage | 1279.0 ± 1460.3 | 1424.4 ± 1658.9 | 1256.8 ± 1363.5 | 14.66* | 23.09* |
| Post-beverage | 1485.8 ± 2006.1 | 1556.4 ± 1381.6 | 522.4 ± 625.0 | ||
| Change | 206.8 (d = 0.12) | 132.0 (d = 0.09) | −734.4** (d = 0.70) | ||
| Low frequency HRV (ms2) | |||||
| Pre-beverage | 994.2 ± 864.7 | 1575.4 ± 1110.4 | 1424.4 ± 1379.4 | 2.44 | 1.68 |
| Post-beverage | 879.6 ± 566.0 | 1469.4 ± 1447.7 | 995.0 ± 970.1 | ||
| Change | −114.6 (d = 0.15) | −106.0 (d = 0.08) | −429.4 (d = 0.35) | ||
| Stroke volume variability (ml) | |||||
| Pre-beverage | 5.9 ±1.8 | 6.6 ± 2.1 | 6.0 ± 2.2 | 0.59 | 2.18 |
| Post-beverage | 6.3 ± 2.6 | 6.7 ±1.9 | 4.9 ±1.4 | ||
| Change | 0.4 (d = 0.16) | 0.1 (d = 0.04) | −1.1 (d = 0.53) | ||
| Pulse transit time variability (ms) | |||||
| Pre-beverage | 5.6 ±1.2 | 5.9 ±1.9 | 5.9 ±1.6 | 8.93* | 0.60 |
| Post-beverage | 5.4 ±1.1 | 5.4 ±1.4 | 5.3 ±1.0 | ||
| Change | −0.2 (d = 0.14) | −0.5 (d = 0.31) | −0.5 (d = 0.45) | ||
| Systolic blood pressure variability (mmHg) | |||||
| Pre-beverage | 5.8 ± 2.0 | 6.3 ±1.8 | 6.1 ± 1.5 | 7.27* | 5.29* |
| Post-beverage | 5.6 ±1.9 | 6.4 ± 2.0 | 4.9 ±1.4 | ||
| Change | −0.2 (d = 0.14) | 0.1 (d = 0.06) | −1.2** (d = 0.86) |
SDNN, standard deviation of normal-to-normal intervals; RMSSD, square root of the mean squared differences of successive normal-to-normal intervals.
F significantat p < 0.05;
pairwise comparisons showed significant changes pre- to post-beverage consumption, p < 0.01; d reflects Cohen’s d effect size for repeated measures.
All indices presented as mean ± standard deviation; all analyses were performed on log-transformed variables, except for pNN50 (percent of adjacent normal-to-normal interval pairs differing by >50 ms).
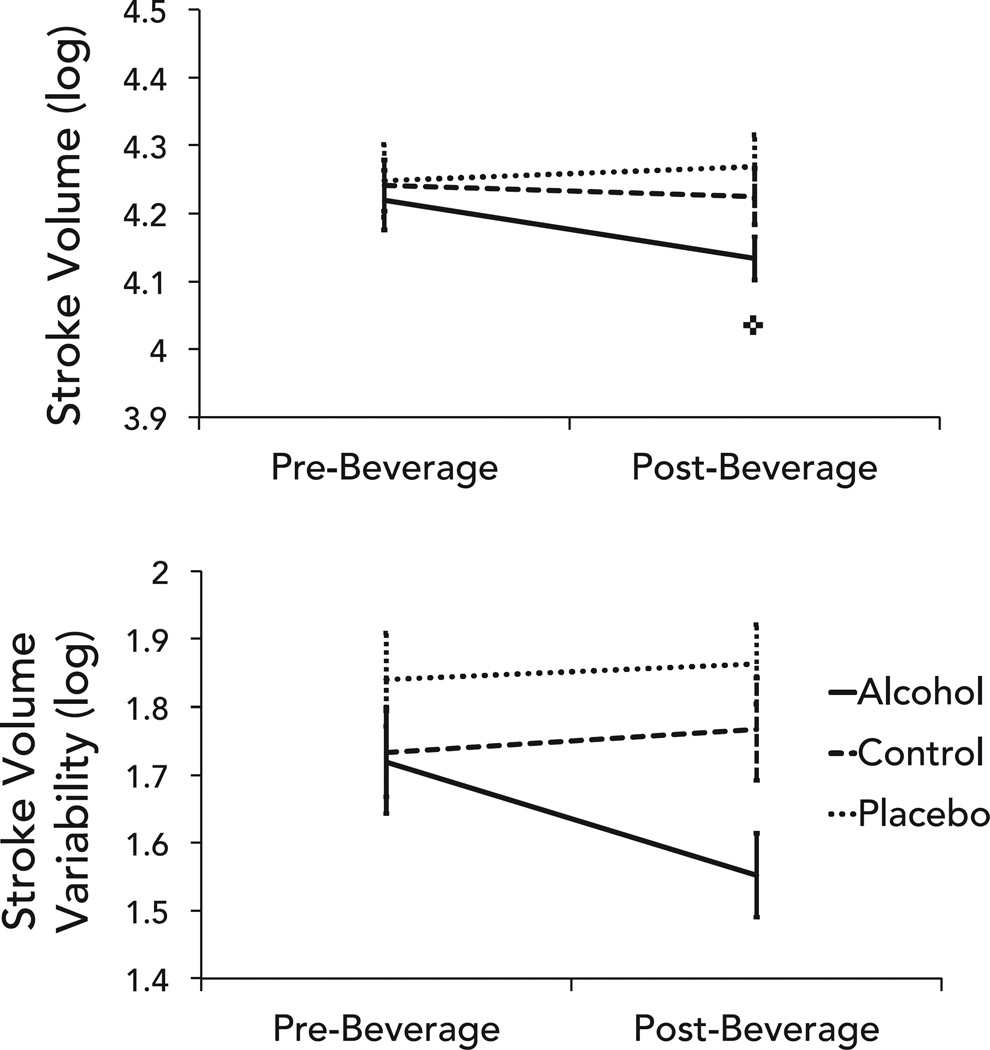
There was a significant group × time interaction for mean stroke volume with a significant reduction in the alcohol group, but no corresponding changes in the control or placebo groups (Fig. 2, Table 2). There was a significant main effect of group on stroke volume variability as measured by stroke volume deviation, but the post hoc analyses revealed no significant differences. There were no time or interaction effects (Table 3).
Fig. 2.
Differences in mean stroke volume (top) and stroke volume variability (bottom) between groups pre- and post-beverage consumption. Error bars represent standard error; ✜change in alcohol group, p < 0.01.
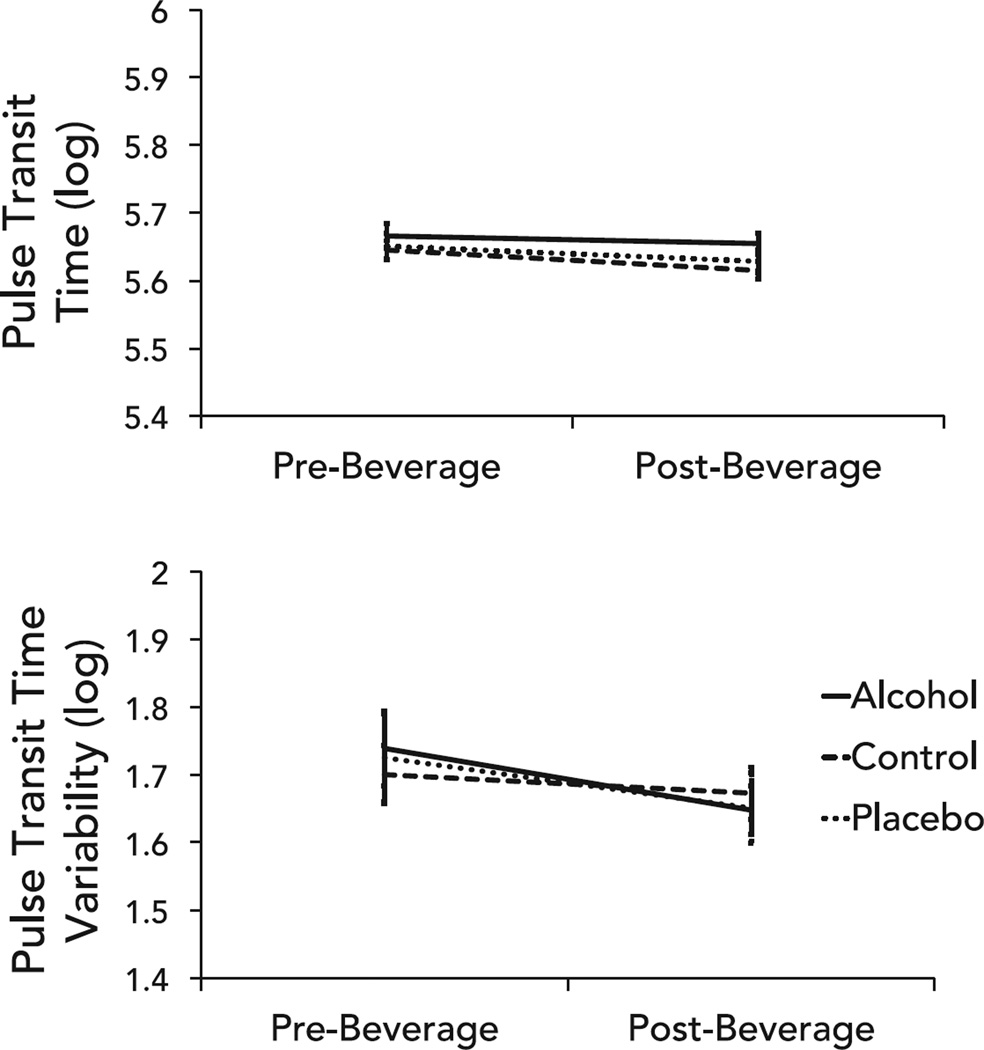
There was a significant main effect of time with no significant interactions for both mean (Table 2) and variability (Table 3) indices of vascular tone. Mean pulse transit time increased and the standard deviation of pulse transit time decreased consistently across groups (Fig. 3).
Fig. 3.
Differences in vascular tone represented by mean pulse transit time (top) and pulse transit time variability (bottom) between groups from pre- and post-beverage consumption. Error bars represent standard error.
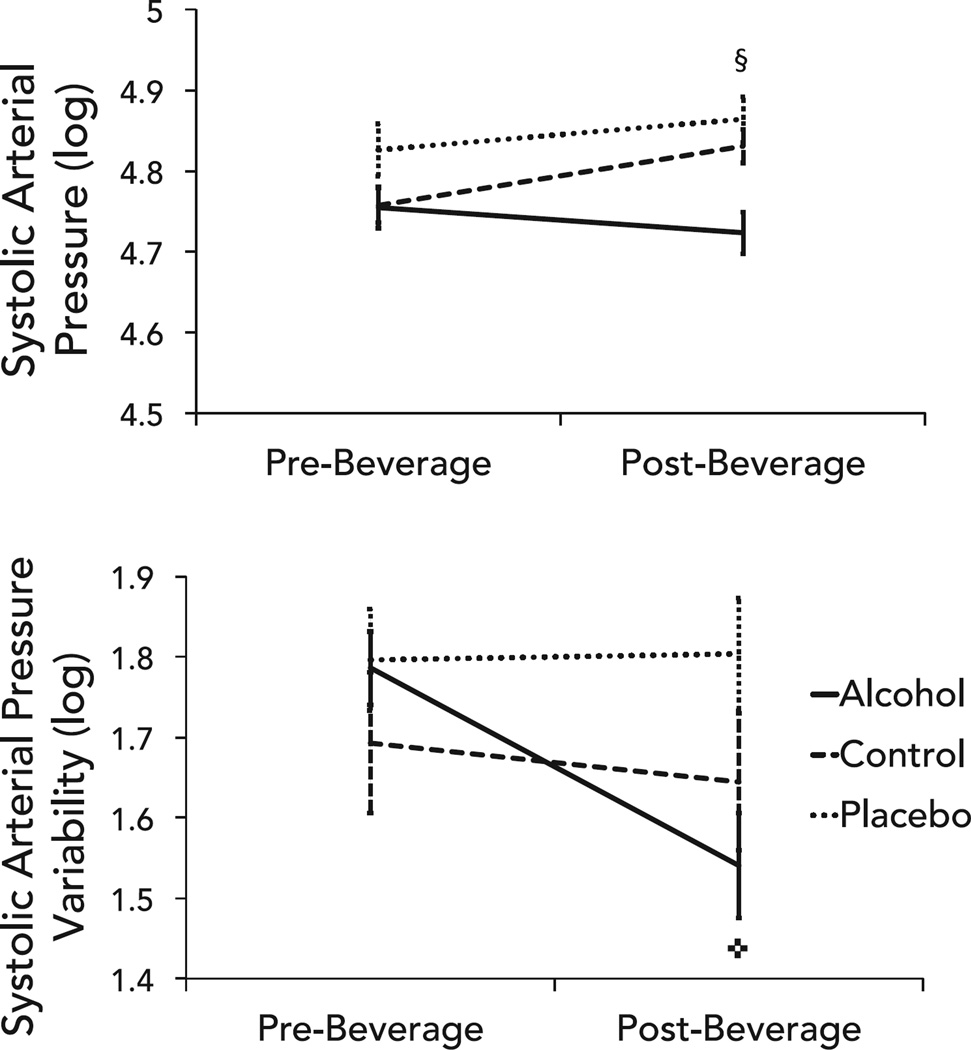
Alcohol did not alter average systolic or mean arterial pressure but significantly reduced systolic BP variability (Fig. 4). There were significant group, time, and group × time interactions for average systolic and mean BP. Least squares means comparisons indicated that only changes in the control group were significant (Table 2). Specifically, the control group showed a medium effect size increase in BP from pre- to post-drinking. There was a significant main effect of time and group × time interaction for the standard deviation of systolic BP (Table 3). As hypothesized, BP variability significantly decreased from pre- to post-drinking in the alcohol group; no pre- to post-beverage changes in BP variability were observed in the placebo or control groups.
Fig. 4.
Differences in mean systolic blood pressure (top) and systolic blood pressure variability (bottom) between groups pre- and post-beverage consumption. Error bars represent standard error; ✜change in alcohol group, p < 0.01; §change in control group, p < 0.01.
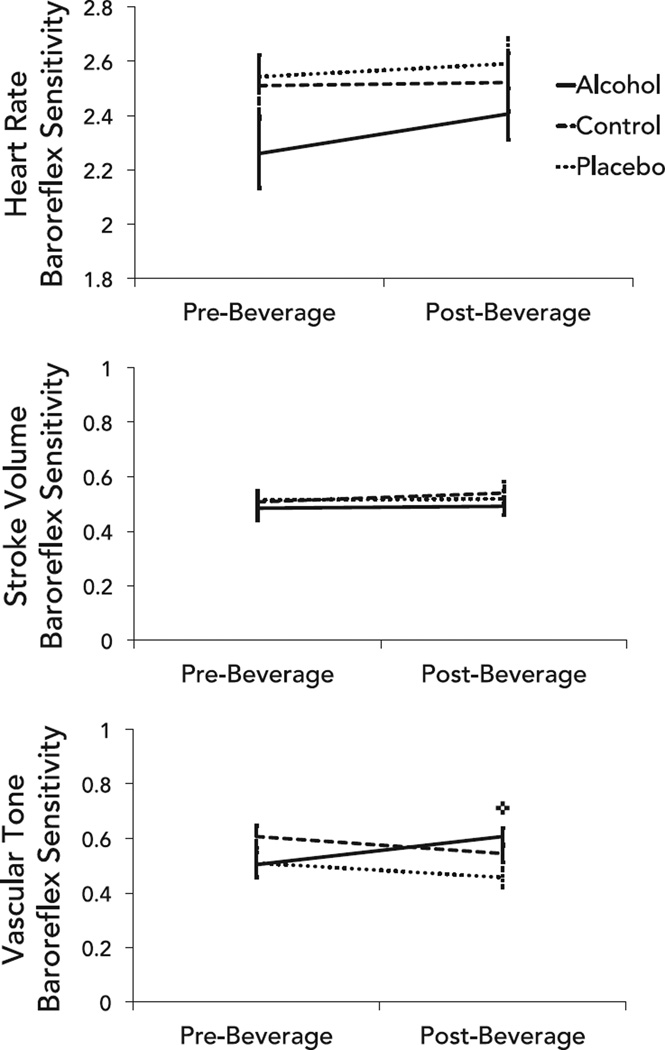
Alcohol intoxication did not affect HR and stroke volume baroreflex sensitivities, but did increase vascular tone baroreflex sensitivity (Fig. 5). That is, the sensitivity of neither the HR baroreflex nor the stroke volume baroreflex changed as a function of beverage group or time (Table 4), whereas a significant group × time interaction (Table 4) showed that vascular tone baroreflex sensitivity increased from pre- to post-drinking in the alcohol group, but not placebo or control groups.
Fig. 5.
Differences in baroreflex sensitivity pre- and post-beverage consumption in the heart rate (top), stroke volume (middle), and vascular tone (bottom) baroreflex branches. Error bars represent standard error; ✜change in alcohol group, p < 0.01.
Table 4.
Sensitivities of Cardiovascular Functions Before and After Beverage Consumption and Significance of Differences Between Alcohol, Placebo, and Control Groups in Response to Beverage Consumption
| Control | Placebo | Alcohol | Main Effect Time F |
Group × Time Interaction F |
|
|---|---|---|---|---|---|
| Heart rate baroreflex sensitivitya | |||||
| Pre-beverage | 14.0 ± 7.2 | 13.2 ± 3.9 | 11.7 ± 8.7 | 0.77 | 0.44 |
| Post-beverage | 14.8 ± 12.7 | 14.8 ± 7.0 | 12.3 ± 6.4 | ||
| Change | 0.4 (d = 0.07) | −1.6 (d = 0.26) | −0.6 (d = 0.09) | ||
| Stroke volume baroreflex sensitivitya | |||||
| Pre-beverage | 0.70 ± 0.4 | 0.69 ± 0.3 | 0.66 ± 0.4 | 0.39 | 0.12 |
| Post-beverage | 0.75 ± 0.4 | 0.70 ± 0.3 | 0.65 ± 0.3 | ||
| Change | 0.05 (d = 0.15) | 0.01 (d = 0.00) | −0.01 (d = 0.03) | ||
| Vascular tone baroreflex sensitivitya | |||||
| Pre-beverage | 0.86 ± 0.4 | 0.70 ± 0.4 | 0.70 ± 0.4 | 0.16 | 8.88* |
| Post-beverage | 0.74 ± 0.3 | 0.60 ± 0.3 | 0.90 ± 0.3 | ||
| Change | −0.12 (d = 0.34) | −0.10 (d = 0.28) | 0.20** (d = 0.45) |
Baroreflex sensitivity is the change in heart rate, stroke volume, and pulse transit time in response to 1 mmHg change in systolic blood pressure.
F significant at p < 0.05;
pairwise comparisons showed significant changes pre- to post-beverage consumption, p < 0.01; d represents Cohen’s d effect size for repeated measures.
All indices presented as mean with ± standard deviation; all statistical analyses were performed on log-transformed variables.
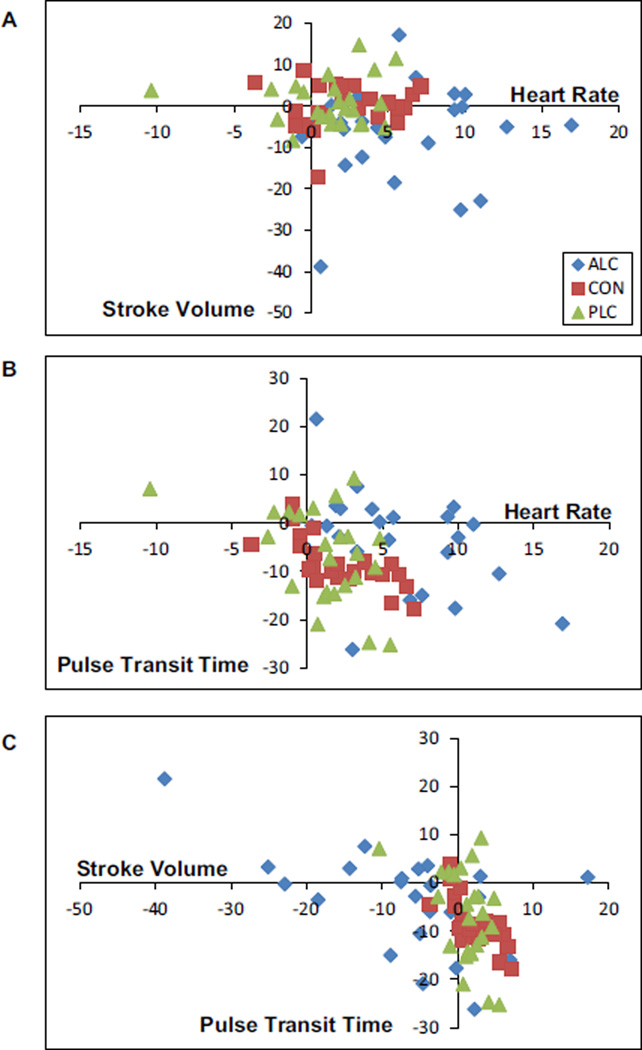
To explore whether the relationship between cardiovascular processes is disrupted by acute alcohol intoxication, individual-level changes (pre- to post-beverage consumption) in average HR, stroke volume, and vascular tone were plotted. Figure 6 graphically depicts how an active dose of alcohol alters cardiovascular functioning. Specifically, in Fig. 6A, HR and stroke volume changes were tightly clustered among individuals in the control (red squares) and placebo (green triangles) groups. Change in average stroke volume among these individuals was typically below (+/−) 10 ml (+/−) and changes in HR were typically positive but < 6 beats per minute. Participants who received an active alcohol beverage (blue diamonds), on the other hand, showed less aggregated and often more dramatic changes. Moreover, the relationship of stroke volume change to HR change was altered in some individuals: Some showed dramatic changes in HR with changes in stroke volume akin to individuals in the control and placebo groups; others showed large changes in both HR and stroke volume. This same pattern of exaggerated, uncoupled, and sometimes opposite cardiovascular relationships following alcohol consumption also was seen when changes in vascular tone (measured as changes in pulse transit time) were plotted against HR (Fig. 6B) and stroke volume (Fig. 6C).
Fig. 6.
Graphical depiction of the relationships between pre- to post-beverage changes in heart rate and stroke volume (A), heart rate and pulse transit time (B), and stroke volume and pulse transit time (C) in each participant.
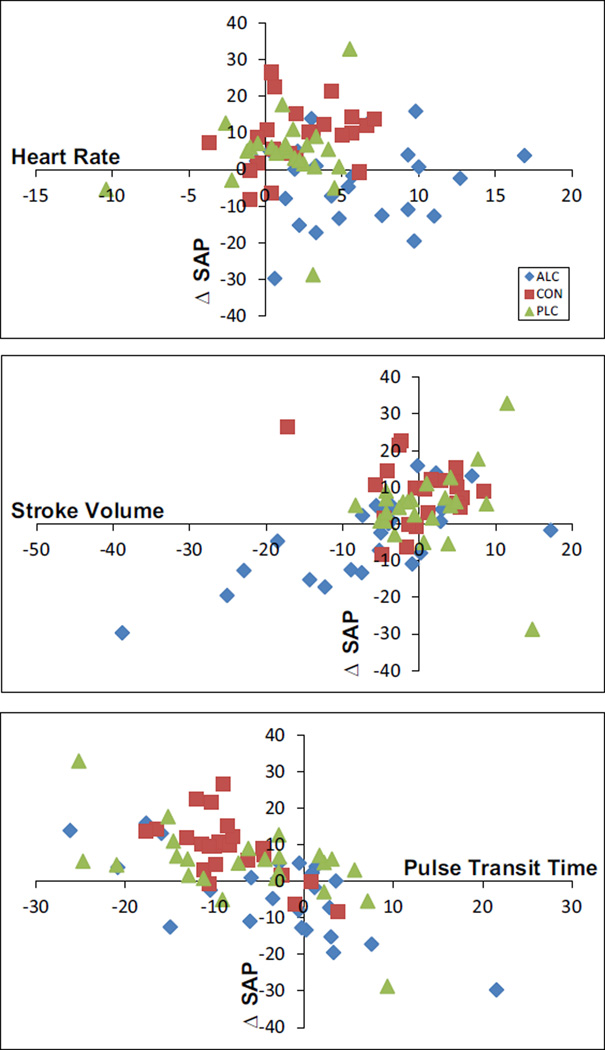
The relationship between changes in average systolic arterial pressure also was assessed in comparison with changes in average HR, stroke volume, and pulse transit time (Fig. 7). Alcohol consumption again appeared to result in exaggerated and less well-regulated shifts in cardiovascular functioning. Individuals in the control and placebo groups showed coupling of HR changes with systolic pressure changes (top panel) as expected based on the HR baroreflex branch function. BP and HR changes in individuals in the alcohol group, however, appeared uncoupled, with many individuals showing reduced BP even when HR was increased. In the middle panel, individuals in the control and placebo groups typically showed elevations in stroke volume and BP after beverage consumption, whereas the typical response among individuals in the alcohol group was for dramatic reductions in stroke volume with concurrent reductions in BP.
Fig. 7.
Graphical depiction of the relationship between pre- to post-beverage changes in systolic blood pressure (ΔSAP) to changes heart rate (top), stroke volume (middle), and pulse transit time (bottom) is shown.
DISCUSSION
Acute alcohol effects on HR have received more research attention relative to other cardiovascular processes due to their purported relation to subjective stimulant effects of alcohol on the ascending limb of the blood alcohol curve (e.g., Hendler et al., 2013). In this study, we examined how an articulated system of cardiovascular processes—including HR, stroke volume, vascular tone, and BP—changes early in the blood alcohol curve, when alcohol absorption and stimulant/euphoric subjective experiences are maximal. The goal was to understand how alcohol disrupts dynamic cardiovascular regulatory processes in young adults prior to the development of cardiovascular problems. The results suggest a complex interplay of acute alcohol effects on the heart and vessels that can be characterized as real-time changes in averages and variabilities of multiple cardiovascular functions and the sensitivity of 1 branch of the baroreflex. The results also provide preliminary support for the idea that each cardiovascular process demonstrates a unique reaction and that the coordinated links between processes typically observed in healthy people may become uncoupled to some extent during the ascending limb of the blood alcohol curve.
Consistent with several other oral alcohol administration studies (e.g., Brunelle et al., 2007; Spaak et al., 2008), we observed increased HR during intoxication. This elevation in average HR early in the ascending blood alcohol curve was observed with a concurrent reduction in average stroke volume, but no significant change in systolic and mean BP. This pattern of results suggests that, in young individuals without alcohol dependence, the neurally mediated increase in HR that is often noted in the literature may co-occur with an attenuation of the muscular pumping action of the heart (Morvai et al., 1988), which together allow BP to remain stable. Elevated BP is often observed in heavy users of alcohol (Puddey and Beilin, 2006), possibly suggesting that one means by which this happens is a breakdown in the adaptive coordination of these cardiac functions. Figure 6 is consistent with this hypothesis; many individuals who drank alcohol showed large, atypically coupled cardiovascular responses. The wide range of individual differences in HR/ stroke volume changes after alcohol consumption warrants further examination, perhaps especially among individuals with different alcohol use behaviors.
An elevated HR can suggest that neural innervation to the heart favors stimulation, through enhanced sympathetic and/or reduced parasympathetic activity. The present study found reductions in multiple HRV indices that are linked to vagal nerve traffic (Fig. 1), supporting the idea of reduced parasympathetic activity during the ascending limb of the blood alcohol curve. This is consistent with prior studies (Gonzalez Gonzalez et al., 1992; Koskinen et al., 1994; Spaak et al., 2010) that have demonstrated that alcohol acutely disrupts vagal activity. The reduction observed in low frequency HRV, which is thought to reflect multiple cardiac control mechanisms in addition to parasympathetic input, was not statistically significant.
There was a statistically significant, albeit modest reduction in average pulse transit time across all groups from pre- to post-beverage assessment, suggesting that some vasoconstriction occurred. Alcohol intoxication has been associated with an acute vasodilatory response after a minimum of 30 minutes (Agewall et al., 2000); however, Newlin (1985, 1986) showed that, upon ingestion of alcohol, pulse transit time in young male drinkers increased (i.e., vasodilation), but this dilation initially served only to offset the vasoconstriction observed in anticipation of alcohol. The small average reductions in pulse transit time observed in the present sample of young adult drinkers may be due to assessment early in the blood alcohol curve when the compensatory conditioned response (vasoconstriction) to alcohol is present, but the unconditioned, pharmacological actions of alcohol on vessels (vasodilation) have not yet been initiated. However, most individuals in all groups (including those in the control group who had no expectation of alcohol consumption) showed some degree of vasoconstriction (Fig. 6). Interestingly, this vascular change was very differently linked to HR (Fig. 6B) and stroke volume (Fig. 6C) in the alcohol group compared to control and placebo groups. For example, vasoconstriction is typically associated with an increase in preload, and thus in stroke volume; however, for many individuals in the alcohol group, this relationship was not seen. In Fig. 7, whereas nearly all control participants and many placebo participants showed the expected coupling of vasoconstriction with higher BP, participants who consumed alcohol tended to show reduced BP, independent of changes in blood vessels.
Systolic BP did not significantly change in the alcohol or placebo groups, but increased in the control group. The placebo group, however, did show a nonsignificant, small effect size increase in BP, whereas the alcohol group showed a nonsignificant, small effect size decrease. At the level of the individual (Fig. 7), BP is clearly elevated in the majority of control and placebo group participants, possibly due to consumption of juice (i.e., blood volume or blood sugar changes). Alcohol group participants, on the other hand, showed a wide range of BP reactions to alcohol with most demonstrating a reduction in systolic pressure. Pulse transit time and BP are 2 measures of vascular dynamics and would be expected to be positively correlated. Thus, an elevation of BP in conjunction with vasoconstriction (Fig. 7, bottom panel) is internally consistent. The lack of BP change seen after consuming a beverage containing alcohol is consistent with the hypothesis that alcohol exerts various, often countervailing actions on the heart and vessels (as well as on other organ and system processes) that, only when considered together, can explain changes in BP. HRV and systolic BP variability were significantly reduced in the alcohol group. Stroke volume variability and vascular tone variability trended in same direction (Figs 3 and 4), although the changes did not reach statistical significance. Thus, even very early in the blood alcohol curve, there is a loss of variability in the cardiovascular system, particularly in HR and systolic pressure. This points to a reduction in adaptive modulation of the cardiovascular system, which is likely to have both physiological and psychological consequences. That is, the heart and brain are believed to use variability in HR, stroke volume, pulse transit time, and BP to relay information about the internal and external environment and ensure an integrated and adaptive mind–body responses to daily, as well as extreme, cognitive and emotional challenges (Porges, 2009; Soroko and Trubachev, 2010; Vasilevskii et al., 1993).
We also hypothesized that acute alcohol would decrease the sensitivity of the 3 parallel baroreflex feedback loops. This global hypothesis was not supported, rather a more nuanced pattern of cardiovascular changes was evident. HR baroreflex sensitivity, for example, did not significantly change during the early portion of the ascending limb of the blood alcohol curve. Some prior evidence suggests that HR baroreflex sensitivity is dampened by acute administration of alcohol in healthy adults at 40 minutes (Fazio et al., 2001) and 1 hour (Koskinen et al., 1994) post-consumption. The present results suggest that earlier in the course of intoxication (~10 minutes), beat-to-beat intervals in HR begin to become less dynamic (i.e., reduced HRV), but remain tightly linked to BP dynamics.
On the other hand, vascular tone variability remained stable, whereas vascular tone baroreflex sensitivity was increased by alcohol. This implies that vascular tone retained its full dynamic range, but reacted more strongly to a 1-unit change in BP after, compared to before, alcohol was consumed. This finding may suggest hypersensitivity of the vasculature during early alcohol absorption, a stronger link between pressure and flow, and/or the activation of the sympathetic nervous system, which is a crucial regulator of this branch of the baroreflex (Eichmann and Brunet, 2014). This latter explanation would suggest that early in the ascending limb of the blood alcohol curve, drinking alcohol serves to both reduce parasympathetic activity and increase sympathetic activity, creating 2 parallel pathways of system loading. It may also suggest that the vascular tone baroreflex branch is the first branch to detect the presence of alcohol in the system and is preemptively activated to offset the overall suppressive effects of alcohol on the cardiovascular system following oral consumption. Future studies, such as those assessing muscle sympathetic nerve activity during intoxication, may help clarify this hypothesis.
Together these results suggest that shortly after consumption of alcohol, multiple interrelated changes occur to the cardiovascular system. The averaged group changes were often antagonistic (e.g., elevated HR but lower stroke volume), which may serve to stabilize BP. The characterization of individual-level cardiovascular responses to alcohol (Figs 6 and 7), however, suggest that early in the ascending limb of the blood alcohol curve, cardiovascular processes may become uncoupled or underregulated. A critical next step is to characterize the time course of these acute cardiovascular disruptions and examine how uncoupling of these processes participates in changes across the developmental trajectories of alcohol use behaviors. It is possible that, over time, with repeated bouts of intoxication, the ability of the cardiovascular system to stabilize BP becomes increasingly compromised and that the resultant dysregulation could influence automatic visceral reactions to internal and external cues that promote continued drinking.
Several caveats should be considered. First, the sample size was relatively small. Second, although the experimental design was aimed at reducing differences in alcohol absorption, individual differences in absorption times were observed, as is common in oral alcohol administration studies. Third, we did not measure changes in blood glucose from drinking juice-based beverages, but juice volume was adjusted based on body weight and thus we expect juice-related changes to be similar across the young, average weight, and healthy participants in this study. Fourth, we also cannot rule out the possibility that changes in chemoreflex sensitivity may have influenced cardiovascular reactions to alcohol. Fifth, the study design was not able to differentiate the extent to which changes in HR, stroke volume, vascular tone, and BP were peripherally (each process separately) and centrally (in a coordinated fashion) mediated. Each individual process appeared to be influenced in unique ways by alcohol, but all showed evidence for exaggerated responding compared to control and placebo beverage response. There are clearly demonstrated direct pharmacological effects of alcohol on the heart muscle and vessel walls, which implies that alcohol would exert independent effects on functions, such as HR and pulse transit time. Via the direct effects of alcohol on vessel walls, alcohol also likely disrupts the functionality of the baroreceptors, which, in turn, could result in a coordinated reaction across the 3 parallel baroreflex branches. Further, alcohol directly impacts neurons, neurotransmission, and neuronal functioning, which could alter efferent firing patterns via the vagus and sympathetic nerves that could differentially impact the heart and vessels based on their innervations. Some evidence of compensatory conditioned reactions was also noted, suggesting that the expectation of alcohol was, to some degree, sufficient to induce cardiovascular reactions in social drinkers. Sixth, this study assessed resting state cardiovascular reactions at only 2 time points. This was due to the larger study design that included a cue reactivity paradigm that began immediately after the 5-minute post-beverage assessment described here. Prior studies have assessed individual cardiovascular reactions to acute alcohol at multiple time points and found time-dependent changes (e.g., Gonzalez Gonzalez et al., 1992), namely reductions in high frequency HRV as early as 5 minutes and elevations in low frequency HRV starting at ~20 to 30 minutes after alcohol ingestion. No study, to the best of our knowledge, has assessed the full spectrum of cardiovascular reactions to alcohol throughout the blood alcohol curve.
In conclusion, it is well established that long-term heavy use of alcohol has pervasive physiological consequences and that the repeated intoxication/withdrawal cycles associated with alcohol use taxes physiological systems from ingestion through complete elimination. The cardiovascular system is not spared from these effects and, as such, cardiovascular disease factors prominently in alcohol-related deaths (Corrao et al., 2004). However, little is known about the developmental progression of alcohol effects on the cardiovascular system, which begin as transient disruptions and compensatory responses in young, healthy drinkers and then in some chronic heavy drinkers progress to frank disease states. The present study serves as an initial step toward understanding this progression by suggesting that alcohol intoxication invokes multiple, often antagonistic cardiovascular responses in the healthy, adaptive system that may serve to stabilize BP and cardiovascular integrity. Future studies should focus on the uncoupling in these response patterns across the blood alcohol curve and as alcohol use disorders develop.
Acknowledgments
This study was funded with the support of K01AA017473, K02AA00325, K24AA021778, R21AA020367, and HHSN275201000003C.
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
- Agewall S, Wright S, Doughty RN, Whalley GA, Duxbury M, Sharpe N. Does a glass of red wine improve endothelial function? Eur Heart J. 2000;21:74–78. doi: 10.1053/euhj.1999.1759. [DOI] [PubMed] [Google Scholar]
- American Heart Association. [Accessed November 13, 2015];Alcohol and heart health. 2015 Available at: http://www.heart.org/HEARTORG/GettingHealthy/NutritionCenter/HealthyEating/Alcohol-and-Heart-Health_UCM_305173_Article.jsp-.VkUey4TGNYw.
- Bates ME, Martin CS. Immediate, quantitative estimation of blood alcohol concentration from saliva. J Stud Alcohol. 1997;58:531–538. doi: 10.15288/jsa.1997.58.531. [DOI] [PubMed] [Google Scholar]
- Bau PFD, Bau CHD, Naujorks AA, Rosito GA. Early and late effects of alcohol ingestion on blood pressure and endothelial function. Alcohol. 2005;37:53–58. doi: 10.1016/j.alcohol.2005.10.034. [DOI] [PubMed] [Google Scholar]
- Beck AT. Beck Depression Inventory-II (BDI-II). The Psychological Corporation. San Antonio, TX: Harcourt, Brace and Company; 1996. [Google Scholar]
- Beck AT, Steer RA. Beck Anxiety Inventory Manual. San Antonio, TX: Harcourt Brace Jovanovich & Company; 1993. [Google Scholar]
- Bogert LWJ, van Lieshout JJ. Non-invasive pulsatile arterial pressure and stroke volume changes from the human finger. Exp Physiol. 2005;90:437–446. doi: 10.1113/expphysiol.2005.030262. [DOI] [PubMed] [Google Scholar]
- Brunelle C, Barrett SP, Pihl RO. Relationship between the cardiac response to acute intoxication and alcohol-induced subjective effects throughout the blood alcohol concentration curve. Hum Psychopharmacol. 2007;22:437–443. doi: 10.1002/hup.866. [DOI] [PubMed] [Google Scholar]
- Casadei B, Meyer TE, Coats AJS, Conway J, Sleight P. Baroreflex control of stroke volume in man: an effect mediated by the vagus. J Physiol. 1992;448:539–550. doi: 10.1113/jphysiol.1992.sp019056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrod PJ, Peterson JB, Pihl RO. Reliability and validity of alcohol-induced heart rate increase as a measure of sensitivity to the stimulant properties of alcohol. Psychopharmacology. 2001;157:20–30. doi: 10.1007/s002130100741. [DOI] [PubMed] [Google Scholar]
- Cooke WH, Hoag JB, Crossman AA, Kuusela TA, Tahvanainen KUO, Eckberg DL. Human responses to upright tilt: a window on central autonomic integration. J Physiol. 1999;517:617–628. doi: 10.1111/j.1469-7793.1999.0617t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrao G, Bagnardi V, Zambon A, La Vecchia C. A meta-analysis of alcohol consumption and the risk of 15 diseases. Prev Med. 2004;38:613–619. doi: 10.1016/j.ypmed.2003.11.027. [DOI] [PubMed] [Google Scholar]
- Eckberg DL, Sleight P. Human Baroreflexes in Health and Disease. Oxford, UK: Clarendon Press; 1992. [Google Scholar]
- Eichmann A, Brunet I. Arterial innervation in development and disease. Sci Transl Med. 2014;6:252ps9. doi: 10.1126/scitranslmed.3008910. [DOI] [PubMed] [Google Scholar]
- Fazio M, Bardelli M, Macaluso L, Fiammengo F, Mattei PL, Bossi M, Fabris B, Fischetti F, Pascazio L, Candido R, Carretta R. Mechanics of the carotid artery wall and baroreflex sensitivity after acute ethanol administration in young healthy volunteers. Clin Sci. 2001;101:253–260. [PubMed] [Google Scholar]
- Gonzalez Gonzalez J, Mendez Llorens A, Mendez Novoa A, Cordero Valeriano JJ. Effect of acute alcohol ingestion on short-term heart rate fluctuations. J Stud Alcohol Drugs. 1992;53:86–90. doi: 10.15288/jsa.1992.53.86. [DOI] [PubMed] [Google Scholar]
- Goslawski M, Piano MR, Bian J-T, Church EC, Szczurek M, Phillips SA. Binge drinking impairs vascular function in young adults. J Am Coll Cardiol. 2013;62:201–207. doi: 10.1016/j.jacc.2013.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart EC, Joyner MJ, Wallin BG, Karlsson T, Curry TB, Charkoudian N. Baroreflex control of muscle sympathetic nerve activity: a nonpharmacological measure of baroreflex sensitivity. Am J Physiol Heart Circ Physiol. 2009;298:H816–H822. doi: 10.1152/ajpheart.00924.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendler RA, Ramchandani VA, Gilman J, Hommer DW. Stimulant and sedative effects of alcohol. Curr Top Behav Neurosci. 2013;13:489–509. doi: 10.1007/7854_2011_135. [DOI] [PubMed] [Google Scholar]
- Jennings JR, Kamarck T, Stewart C, Eddy M, Johnson P. Alternate cardiovascular baseline assessment techniques: vanilla or resting baseline. Psychophysiology. 1992;29:742–750. doi: 10.1111/j.1469-8986.1992.tb02052.x. [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Pedersen BK. Ten questions about systems biology. J Physiol. 2011;589(Pt 5):1017–1030. doi: 10.1113/jphysiol.2010.201509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahkonen S, Zvartau E, Lipsanen J, Bondarenko B. Effects of alcohol withdrawal on cardiovascular system. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:550–553. doi: 10.1016/j.pnpbp.2010.12.015. [DOI] [PubMed] [Google Scholar]
- Kawano Y. Physio-pathological effects of alcohol on the cardiovascular system: its role in hypertension and cardiovascular disease. Hypertens Res. 2010;33:181–191. doi: 10.1038/hr.2009.226. [DOI] [PubMed] [Google Scholar]
- Kemp AH, Quintana DS. The relationship between mental and physical health: insights from the study of heart rate variability. Int J Psychophysiol. 2013;89:288–296. doi: 10.1016/j.ijpsycho.2013.06.018. [DOI] [PubMed] [Google Scholar]
- Kitano H. Biological robustness. Nat Rev Genet. 2004;5:826–837. doi: 10.1038/nrg1471. [DOI] [PubMed] [Google Scholar]
- Koskinen P, Virolainen J, Kupari M. Acute alcohol intake decreases short-term heart rate variability in healthy subjects. Clin Sci. 1994;87:225–230. doi: 10.1042/cs0870225. [DOI] [PubMed] [Google Scholar]
- Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer fort-tests and ANOVAs. Front Psychol. 2013;4:863. doi: 10.3389/fpsyg.2013.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer P, Eddie D. Dynamic processes in regulation and some implications for biofeedback and biobehavioral interventions. Appl Psychophysiol Biofeedback. 2013;38:143–155. doi: 10.1007/s10484-013-9217-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Yambe T, Sasada H, Nanka S, Tanaka A, Nagatomi R, Nitta S. Comparison of heart rate variability and stroke volume variability. Auton Neurosci. 2004;116:69–75. doi: 10.1016/j.autneu.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Ma HT, Zhang YT. Spectral analysis of pulse transit time variability and its coherence with other cardiovascular variabilities; Paper presented at the Annual International Conference of the IEEE Engineering in Medicine & Biology Society; New York, NY. 2006. Aug-Sep. [DOI] [PubMed] [Google Scholar]
- de Maesschalck R, Jouan-Rimbaud D, Massart DL. The Mahalanobis distance. Chemometr Intell Lab Syst. 2000;50:1–18. [Google Scholar]
- Maestri R, Pinna GD, Mortara A, La Rovere MT, Tavazzi L. Assessing baroreflex sensitivity in post-myocardial infarction patients: comparison of spectral and phenylephrine techniques. J Am Coll Cardiol. 1998;31:344–351. doi: 10.1016/s0735-1097(97)00499-3. [DOI] [PubMed] [Google Scholar]
- Metropolitan Life Insurance Company. Metropolitan height and weight tables. Stat Bull Metrop Life Found. 1983;64:3–9. [PubMed] [Google Scholar]
- Morvai V, Nadhazi Z, Molnar GY, Ungvary GY, Folly G. Acute effects of low doses of alcohol on the cardiovascular system in young men. Acta Med Hung. 1988;45:339–348. [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. [Accessed November 13, 2015];Alcohol’s effects on the body. 2015 Available at: http://www.niaaa.nih.gov/alcohol-health/alcohols-effects-body.
- Newlin DB. Human conditioned compensatory response to alcohol cues: initial evidence. Alcohol. 1985;2:507–509. doi: 10.1016/0741-8329(85)90124-7. [DOI] [PubMed] [Google Scholar]
- Newlin DB. Conditioned compensatory response to alcohol placebo in humans. Psychopharmacology. 1986;88:247–251. doi: 10.1007/BF00652249. [DOI] [PubMed] [Google Scholar]
- Pitzalis MV, Mastropasqua F, Passantino A, Massari F, Ligurgo L, Forleo C, Balducci C, Lombardi F, Rizzon P. Comparison between noninvasive indices of baroreceptor sensitivity and the phenylephrine method in post-myocardial infarction patients. Circulation. 1998;97:1362–1367. doi: 10.1161/01.cir.97.14.1362. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal theory: new insights into adaptive reactions of the autonomic nervous system. Cleve Clin J Med. 2009;76(Suppl 2):S86–S90. doi: 10.3949/ccjm.76.s2.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puddey IB, Beilin LJ. Alcohol is bad for blood pressure. Clin Exp Pharmacol Physiol. 2006;33:847–852. doi: 10.1111/j.1440-1681.2006.04452.x. [DOI] [PubMed] [Google Scholar]
- Quinn TA, Kohl P. Mechano-sensitivity of cardiac pacemaker function: pathophysiological relevance, experimental implications, and conceptual integration with other mechanisms of rhythmicity. Prog Biophys Mol Biol. 2012;110:257–268. doi: 10.1016/j.pbiomolbio.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm J, Baliunas D, Borges GL, Graham K, Irving H, Kehoe T, Parry CD, Patra J, Popova S, Poznyak V, Roerecke M, Room R, Samokhvalov AV, Taylor B. The relation between different dimensions of alcohol consumption and burden of disease: an overview. Addiction. 2010;105:817–843. doi: 10.1111/j.1360-0443.2010.02899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Hes-selbrock VM, Nurnberger JI, Jr, Schuckit MA, Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcohol Clin Exp Res. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Rickards CA, Tzeng YC. Arterial pressure and cerebral blood flow variability: friend or foe? A review. Front Physiol. 2014;5:120. doi: 10.3389/fphys.2014.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanowicz M, Schmidt JE, Bostwick JM, Mrazek DA, Karpyak VM. Changes in heart rate variability associated with acute alcohol consumption: current knowledge and implications for practice and research. Alcohol Clin Exp Res. 2011;35:1092–1105. doi: 10.1111/j.1530-0277.2011.01442.x. [DOI] [PubMed] [Google Scholar]
- Skinner HA, Horn JI. Alcohol Dependence Scale: User’s Guide. Toronto, ON, Canada: Addiction Research Foundation; 1984. [Google Scholar]
- Soroko SI, Trubachev VV. Neurophysiological and Psychophysiological Bases of Adaptive Biocontrol. Russia: Politekhnika-Servis, St. Petersburg; 2010. [Google Scholar]
- Spaak J, Merlocco AC, Soleas GJ, Tomlinson G, Morris BL, Picton P, Notarius CF, Chan CT, Floras JS. Dose-related effects of red wine and alcohol on hemodynamics, sympathetic nerve activity, and arterial diameter. Am J Physiol Heart Circ Physiol. 2008;294:H605–H612. doi: 10.1152/ajpheart.01162.2007. [DOI] [PubMed] [Google Scholar]
- Spaak J, Tomlinson G, McGowan CL, Soleas GJ, Morris BL, Picton P, Notarius CF, Chan CT, Floras JS. Dose-related effects of red wine and alcohol on heart rate variability. Am J Physiol Heart Circ Physiol. 2010;298:H2226–H2231. doi: 10.1152/ajpheart.00700.2009. [DOI] [PubMed] [Google Scholar]
- Tapanainen JM, Thomsen PEB, Kober L, Torp-Pedersen C, Makikallio TH, Still AM, Lindgren KS, Huikuri HV. Fractal analysis of heart rate variability and mortality after an acute myocardial infarction. Am J Cardiol. 2002;90:347–352. doi: 10.1016/s0002-9149(02)02488-8. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. J Affect Disord. 2000;61:201–216. doi: 10.1016/s0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Yamamoto SS, Brosschot JF. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol. 2010;141:122–131. doi: 10.1016/j.ijcard.2009.09.543. [DOI] [PubMed] [Google Scholar]
- Vaschillo EG, Bates ME, Vaschillo B, Lehrer P, Udo T, Mun EY, Ray S. Heart rate variability response to alcohol, placebo, and emotional picture cue challenges: effects of 0.1-Hz stimulation. Psychophysiology. 2008;45:847–858. doi: 10.1111/j.1469-8986.2008.00673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaschillo EG, Lehrer P, Rishe N, Konstantinov M. Heart rate variability biofeedback as a method for assessing baroreflex function: a preliminary study of resonance in the cardiovascular system. Appl Psychophysiol Biofeedback. 2002;27:1–27. doi: 10.1023/a:1014587304314. [DOI] [PubMed] [Google Scholar]
- Vaschillo EG, Vaschillo B, Buckman JF, Pandina RJ, Bates ME. The investigation and clinical significance of resonance in the heart rate and vascular tone baroreflexes, in. In: Fred A, Filipe J, Gamboa H, editors. Biomedical Engineering Systems and Technologies: Communications in Computer and Information Science. Vol. 127. Heidelberg, Germany: Springer; 2011. pp. 224–237. [Google Scholar]
- Vaschillo EG, Vaschillo B, Buckman JF, Pandina RJ, Bates ME. Measurement of vascular tone and stroke volume baroreflex gain. Psychophysiology. 2012;49:193–197. doi: 10.1111/j.1469-8986.2011.01305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilevskii NN, Sidorov YA, Suvorov NB. Role of biorhythmological processes in mechanisms of adaptation and correction of regulatory dysfunctions. Hum Physiol. 1993;19:50–54. [PubMed] [Google Scholar]
- Wang XJ. Neurophysiological and computational principles of cortical rhythms in cognition. Physiol Rev. 2010;90:1195–1268. doi: 10.1152/physrev.00035.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White HR, Labouvie EW. Longitudinal trends in problem drinking as measured by the Rutgers Alcohol Problem Index. Alcohol Clin Exp Res. 2000;24:76A. [Google Scholar]
- Yambe T, Imachi K, Shiraishi Y, Yamaguchi T, Shibata M, Kameyama T, Yoshizawa M, Sugita N. Baroreflex sensitivity of an arterial wall during rotary blood pump assistance. Artif Organs. 2009;33:767–770. doi: 10.1111/j.1525-1594.2009.00864.x. [DOI] [PubMed] [Google Scholar]