Abstract
The tissue inhibitor of metalloproteinase-1 (TIMP-1) inhibits the extracellular matrix–degrading activity of several matrix metalloproteinases, thereby regulating cancer cell invasion and metastasis. Studies describing the expression pattern and cellular localization of TIMP-1 in gastric cancer are, however, highly discordant. We addressed these inconsistencies by performing immunohistochemistry and in situ hybridization analyses in a set of 49 gastric cancer lesions to reexamine the TIMP-1 localization. In addition, we correlated these findings to clinicopathological parameters. We show that strong expression of TIMP-1 protein and mRNA was observed in a subpopulation of stromal fibroblast-like cells at the periphery of the cancer lesions. In a few cases, a small fraction of cancer cells showed weak expression of TIMP-1 protein and mRNA. The stromal TIMP-1-expressing cells were mainly tumor-associated myofibroblasts. In the normal-appearing mucosa, scattered TIMP-1 protein was only found in chromogranin A positive cells. TIMP-1-positive myofibroblasts at the invasive front of the tumors were more frequently seen in intestinal than in diffuse histological subtype cases (p=0.009). A significant trend to a higher number of cases showing TIMP-1 staining in myofibroblasts with increasing tumor, node, metastasis (TNM) stage was also revealed (p=0.041). In conclusion, tumor-associated myofibroblasts are the main source of increased TIMP-1 expression in gastric cancer.
Keywords: gastric cancer, immunohistochemistry, in situ hybridization, invasion, myofibroblasts, neuroendocrine cells, TIMP-1
Introduction
A crucial step for cancer cell invasion and metastasis is the breakdown of the extracellular matrix (ECM), which is mediated by proteolytic enzymes such as the matrix metalloproteinases (MMPs).1,2 The ECM-degrading activity of MMPs is regulated in situ by a few endogenous inhibitors; tissue inhibitor of metalloproteinase-1 (TIMP-1) is one among them.3 The mRNA and protein levels of this MMP inhibitor are substantially increased in neoplasia, including colorectal cancer.4,5 In contrast to its expected anti-invasive activity, high levels of TIMP-1 in plasma are associated with poor prognosis in colorectal and breast cancers,6,7 suggesting that TIMP-1 may have additional biological activities. In line with this, TIMP-1 has been implicated in stimulation of cell growth, inhibition of apoptosis, and induction of angiogenesis.5,8,9
A few studies describing the localization and expression pattern of TIMP-1 in gastric cancer have been published; however, the results of these are highly discordant. In two studies, for example, it was found that TIMP-1 mRNA and protein are predominantly expressed by stromal cells located in the periphery of the tumors, but they did not characterize the TIMP-1-expressing cell type(s).10,11 Others, in contrast, point to the cancer cells as the main contributors to the TIMP-1 expression in gastric cancer.12–14 More recently, a study disclosed that, in addition to cancer cells, infiltrating inflammatory cells make a substantial contribution to the TIMP-1 expression in gastric cancer.15 Most of these localization studies have correlated TIMP-1 expression in different cell subpopulations with clinicopathological parameters such as tumor size, depth of invasion, lymph node and distant metastasis, tumor stage, and patient survival.11,13–15 The veracity of these statistical associations, however, may be compromised considering the above-mentioned discrepancies in the description of the TIMP-1 expression pattern. It is, therefore, evident that a reexamination of the expression pattern and cellular localization of TIMP-1 in gastric cancer is highly needed.
In the present study, we have revisited the findings of older studies in gastric cancer to clarify the expression pattern and cellular localization of TIMP-1 in gastric cancer. By using immunohistochemistry and in situ hybridization, we here demonstrate that TIMP-1 is mainly expressed by tumor-associated myofibroblasts (also known as cancer-associated fibroblasts [CAFs]) located in the stromal compartment at the periphery of invasive gastric adenocarcinomas of both intestinal and diffuse subtypes. We did, however, also find TIMP-1 expression in a very minor fraction of endothelial cells and macrophages at the periphery of the cancer as well as in few cancer cells. In addition, we examined the correlation between TIMP-1 expression and clinicopathological parameters and found that TIMP-1 expression is higher in the intestinal subtype compared with that in the diffuse subtype and that its elevated expression levels correlate with increasing tumor, node, metastasis (TNM) stage.
Materials and Methods
Tissue Samples
Formalin-fixed and paraffin-embedded (FFPE) gastric cancer tissue samples were obtained postoperatively from 49 gastric cancer cases from Haukeland University Hospital, Bergen, Norway. The histopathological classification was given according to the Laurén Classification System. Thus, of the 49 gastric cancer cases, 25 were classified as intestinal subtype and 24 as diffuse subtype. According to the criteria of Union for International Cancer Control, the 49 cases were staged as follows: five (10%) stage I, 10 (20%) stage II, 15 (31%) stage III, and 19 (39%) stage IV (Table 1). The tissue samples were procured from the Tissue Bank of the Department of Pathology of the Haukeland University Hospital, Bergen, Norway, and the Ethical and Research Committee of the Haukeland University Hospital approved their use (REK 053228). The present study was performed in accordance with the Helsinki Declaration.
Table 1.
TIMP-1 Immunoreactivity in Gastric Cancer According to Histological Subtype and TNM Stage.
| Parameter | Total | Intestinal | Diffuse |
|---|---|---|---|
| Total number of cases | 49 | 25 | 24 |
| Cases according to TNM stage | |||
| I | 5 | 2 | 3 |
| II | 10 | 5 | 5 |
| III | 15 | 6 | 9 |
| IV | 19 | 12 | 7 |
| Cases according to TIMP-1-positive cell type | |||
| Myofibroblasts, tumor invasion front | 35 | 22 | 13 |
| Myofibroblasts, tumor central areas | 22 | 13 | 9 |
| Myofibroblasts, tumor luminal side | 16 | 10 | 6 |
| Cancer cells | 9 | 4 | 5 |
| Neuroendocrine cells | 39 | 20 | 19 |
| Cases with TIMP-1-positive myofibroblasts tumor invasion front according to TNM stage | |||
| I | 2 | 1 | 1 |
| II | 6 | 4 | 2 |
| III | 11 | 6 | 5 |
| IV | 16 | 11 | 5 |
Abbreviation: TIMP-1, tissue inhibitor of metalloproteinase-1; TNM, tumor, node, metastasis.
Antibodies
Monoclonal mouse antihuman TIMP-1 antibody (clone VT7) was purchased from Dako (Glostrup, Denmark) and has been described previously.5,16,17 Monoclonal antibodies (mAbs) against pan cytokeratin (pan-CK; clone AE1/AE3) were used for the detection of cancer cells, anti-CD68 mAb (clone PG-M1) for detection of macrophages, anti-α-smooth muscle actin (α-SMA) mAb (clone 1A4) for detection of myofibroblasts, anti-CD34 mAb (clone QBEnd 10) for detection of endothelial cells, anti-chromogranin A mAb (clone DAK-A3) for detection of neuroendocrine cells, as well as EnVision horseradish-peroxidase-conjugated polymer antimouse IgG (code K4001) and the EnVision G|2 Doublestain System (code K5361), which were all purchased from Dako. As subtype control, an mAb directed against trinitrophenyl (TNP) hapten (IgG1) was used and previously described.18
Immunoperoxidase Staining
Three-micrometer-thick FFPE tissue sections were deparaffinized with xylene and hydrated in gradual series of ethanol–water dilutions. For all tissue sections, heat-induced epitope retrieval in a T/T Micromed microwave processor (Milestone, Sorisole, Italy) was performed at 98°C for 10 min (TIMP-1, α-SMA, CD34, and TNP) or 15 min (CD68 and chromogranin A) in Tris-EGTA (TEG) buffer (pH 9.0; 10-mM Tris, 0.5-mM EGTA). Endogenous peroxidase activity was blocked by incubation in 1% hydrogen peroxide solution for 15 min and, thereafter, washed in Tris-buffered saline (TBS-T; 50-mM Tris-HCL, 150-mM NaCl, 0.5% Triton X-100, pH 7.6), and then manually mounted on Shandon racks with immunostaining cover plates (Thermo Shandon, Pittsburgh, PA). The primary antibodies were diluted in Antibody Diluent with background-reducing components (code S3022; Dako), applied to the sections, and incubated overnight at 4°C at the following dilutions: TIMP-1 mAb: 0.23 µg/ml, α-SMA: 0.4 µg/ml, CD34: 0.13 µg/ml, CD68: 0.3 µg/ml, pan-CK: 0.36 µg/ml, and chromogranin A: 2.1 µg/ml. Subsequently, the primary antibodies were detected with EnVision horseradish-peroxidase-conjugated polymer antimouse IgG for 45 min. Each incubation step was followed by washes in TBS-T. The reactions were visualized by incubating the sections with NovaRed for 9 min (Vector Laboratories, Burlingame, CA) and counterstained with Mayer’s hematoxylin for 30 s and, thereafter, dehydrated in ethanol and mounted with Pertex (Histolab Products, Västra Frölunda, Sweden).
Negative Controls
Negative staining controls included (1) substitution of the monoclonal anti-TIMP-1 with an antibody of irrelevant specificity (anti-TNP mAb), incubated at the same concentration to that used for the specific primary antibody, and (2) preincubation of the mAb against TIMP-1 with a 10-fold molar excess of purified recombinant soluble human TIMP-1 protein (kind gift from professor Gillian Murphy, University of Cambridge, UK) for 2 hr at room temperature before application to the tissue sections.
Double-Staining Immunohistochemistry
FFPE sections of 3 µm were double stained using antibodies against TIMP-1 and pan-CK, TIMP-1 and α-SMA, TIMP-1 and CD34, or TIMP-1 and CD68. Double stainings were done with the EnVision G|2 Doublestain System, following the manufacturer’s instructions with minor modifications. Antigen retrieval was performed by heat-induced epitope retrieval by 15 min in TEG buffer as described above. After pretreatment, the slides were mounted on Shandon racks with immunostaining cover plates (Thermo Shandon). The endogenous peroxidase activity was blocked by incubating with Dual Endogenous Enzyme Block reagent provided in the kit for 15 min (Dako). The TIMP-1 mAb was diluted in Antibody Diluent with background-reducing components (0.23 µg/ml), incubated for 2 hr at room temperature, detected with Polymer/HRP (45 min; Dako), and developed with diaminobenzidine (DAB) chromogene. Subsequently, Doublestain Block reagent was applied to the slides and incubated for 15 min. The second primary antibody was diluted in Antibody Diluent with background-reducing components (pan-CK: 0.36 µg/ml, α-SMA: 0.4 µg/ml, CD34: 0.13 µg/ml, or CD68: 0.3 µg/ml) and incubated overnight at 4°C. Rabbit/Mouse (LINK; Dako) reagent was subsequently applied to the slides and incubated for 30 min. The second primary antibody was detected with Polymer/AP reagent (45 min; Dako) and visualized with Permanent Red (Dako). The slides were counterstained with Mayer’s hematoxylin and were finally dehydrated in an oven at 60C for 1 hr before coverslips were mounted with Pertex (Histolab Products).
In Vitro Transcription and In Situ Hybridization
The generation of the antisense and sense TIMP-1 riboprobes, in vitro transcription, and in situ hybridization were performed as described previously.4,5,19 Briefly, antisense and sense TIMP-1 riboprobes were generated from PCR constructs f104, f105, and f106.4 The 35S-radioactivity concentration of the probes was adjusted by dilution in 10-mM 1,4-dithiothreitol (DTT) and deionized formamide to 500,000 cpm/µl. Under RNase-free conditions, 3-µm-thick tissue sections were deparaffinized, hydrated, and pretreated with TEG buffer for 10 min at 98°C. The sections were developed after exposure to autoradiographic emulsion (ILFORD Imaging UK Limited, Mobberley, UK) for 7 days at 4°C (some of the sections were in addition exposed for 15 days).5,19
Statistical Analysis
The chi-square test was done to evaluate the possible difference between intestinal and diffuse histological subtypes regarding the frequency of cases having TIMP-1-positive cells. The trend exact test was performed to determine whether the frequency of cases with TIMP-1-expressing cells varies according to the TNM stage. p values less than 0.05 were considered significant in all cases. Statistical calculations were done using SAS (version 9.1; SAS Institute, Cary, NC).
Results
Localization of TIMP-1 in Gastric Cancer
The localization of TIMP-1 protein was determined using immunohistochemistry in FFPE tissue sections from 49 gastric cancer cases. Overall, TIMP-1 immunoreactivity was observed in all 25 intestinal and 24 diffuse subtypes of gastric cancer (Fig. 1).
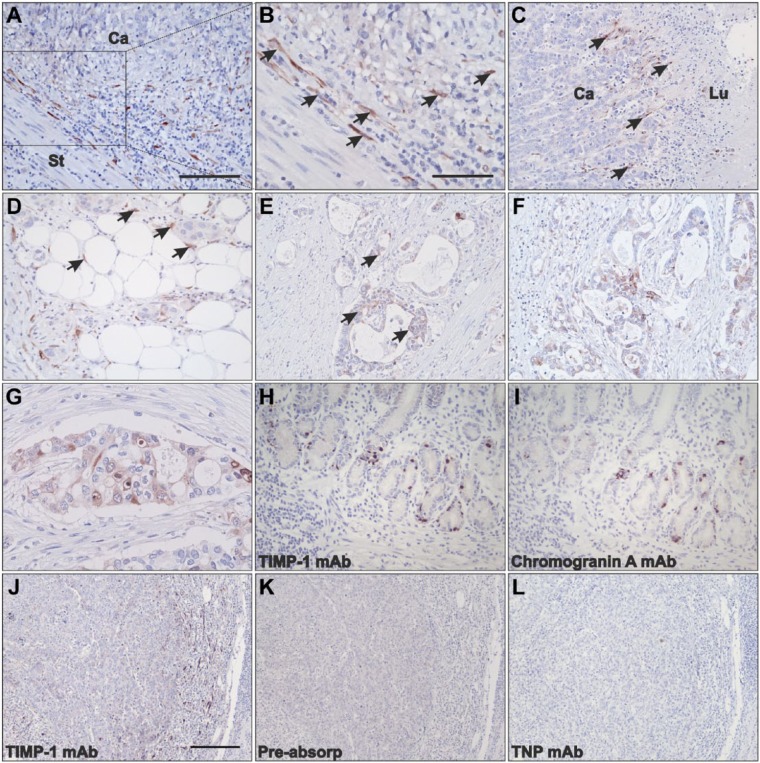
Figure 1.
Localization of TIMP-1 protein in gastric cancer tissue. Tissue sections from intestinal (A–C, E–G, J–l) and diffuse (D, H, I) subtypes were processed for immunoperoxidase staining with mAbs against TIMP-1 (A-H, J), chromogranin A (I), anti-TIMP-1 mAb preincubated with TIMP-1 recombinant protein (K), and an mAb against TNP (L). In intestinal subtype gastric cancer, TIMP-1 is focally upregulated in the stromal tissue at the invasive front (A, B, and J; Ca: cancer, St: stroma) and toward the luminal surface (C; Lu: lumen). TIMP-1 expression at the periphery of the tumors is mainly conferred to fibroblast-like cells (arrows in panels B and C). In diffuse subtype, TIMP-1 is also observed in stromal fibroblast-like cells surrounding cancer cell nests (arrows in panel D). Weak TIMP-1 staining is seen in cancer cells in a few cases of intestinal and diffuse subtypes (E–G). In nonneoplastic gastric mucosa, scattered TIMP-1-positive cells are observed (H) in similar location to those positive for chromogranin A staining on adjacent sections (I). TIMP-1 staining (J) is precluded in adjacent sections incubated with anti-TIMP-1 mAb preabsorbed with TIMP-1 recombinant protein (K) or TNP mAb (L). Scale bars: A, C, D, E, F = 100 µm; B, G = 50 µm; H, I = 100 µm; J–L = 200 µm. Abbreviations: TIMP-1, tissue inhibitor of metalloproteinase-1; mAb, monoclonal antibody; TNP, trinitrophenyl.
In 22 intestinal subtype cases, strong staining was predominantly observed in stromal fibroblast-like cells located at the invasive front of the malignant growth (Fig. 1A and B). TIMP-1 immunoreactivity was also pronounced in fibroblast-like cells located in the stromal compartment toward the luminal side of the gastric mucosa, in particular underneath ulcerative wounds in all 10 intestinal cases where this tissue structure was present (Fig. 1C). In the diffuse subtype of gastric cancer, stromal fibroblast-like cells surrounding small cancer cell “islands” and in the periphery toward the luminal side of the tumors showed intense TIMP-1 staining (13 and six cases, respectively; Fig. 1D). Scattered fibroblast-like cells located in the central areas of the tumor also showed TIMP-1-positive staining in 13 intestinal and nine diffuse gastric cancers (data not shown).
In addition, weak TIMP-1 staining was observed in a small subpopulation of gastric cancer cells in four cases of the intestinal subtype and five cases of the diffuse subtype (Fig. 1E–G). Intriguingly, we observed intense cytoplasmic TIMP-1 immunoreactivity in single cells scattered between epithelial cells, in particular at the bottom of the crypts, in normal-appearing gastric mucosa adjacent to the cancer (Fig. 1H). These cells have previously been reported as neuroendocrine cells.17 In fact, immunohistochemical staining of parallel tissue sections for chromogranin A, a marker of neuroendocrine cell differentiation, showed intense staining of cells at the bottom of the crypts, in a pattern similar to that observed for the TIMP-1 staining (Fig. 1I). No TIMP-1 staining was observed in either epithelial cells or the lamina propria in nonneoplastic mucosa (data not shown).
We also found TIMP-1 immunoreactivity in fibroblast-like cells surrounding small arteries distant to the cancer area in seven cases of the intestinal subtype and four cases of the diffuse subtype (data not shown). The number of cases with TIMP-1-positive staining at different locations and cell types is summarized in Table 1.
The specificity of the anti-TIMP-1 mAb was challenged by preincubation of the antibody with a 10-fold molar excess of recombinant human TIMP-1 protein before adding it to the tissue sections. As compared with tissue sections incubated with the anti-TIMP-1 mAb alone (Fig. 1J), no staining was observed after applying this mixture to adjacent tissue sections (Fig. 1K). We also substituted the anti-TIMP-1 mAb with anti-TNP, an mAb of irrelevant specificity. No specific staining was obtained with this mAb when incubated at similar concentration as the anti-TIMP-1 mAb (Fig 1L).
TIMP-1 mRNA Expression in Gastric Cancer
The expression of TIMP-1 was also analyzed in parallel on 19 representative tissue samples of gastric cancer (10 of the intestinal subtype and nine of the diffuse subtype) by in situ hybridization with an antisense probe specific for TIMP-1 to determine the site of synthesis. In accordance with the immunohistochemical analysis, TIMP-1 mRNA expression was observed in all samples of intestinal and diffuse subtypes (Fig. 2). In nine out of 10 cancer cases of the intestinal subtype, TIMP-1 mRNA signal was primarily seen in stromal fibroblast-like cells located at the peripheral invasion zone of the malignant lesion, in a similar pattern as found for TIMP-1 protein (Fig. 2A, D, and G). A few scattered fibroblast-like cells located in the central areas of the tumor also showed weak TIMP-1 mRNA signal in eight of the analyzed intestinal subtype cases (data not shown). In seven out of nine diffuse subtype cases, high TIMP-1 mRNA expression was mainly observed in fibroblast-like cells around small clusters of gastric cancer cells both at the peripheral invasive front and in central areas of the tumor (Fig. 2B and E), also in a pattern similar to that found for TIMP-1 protein.
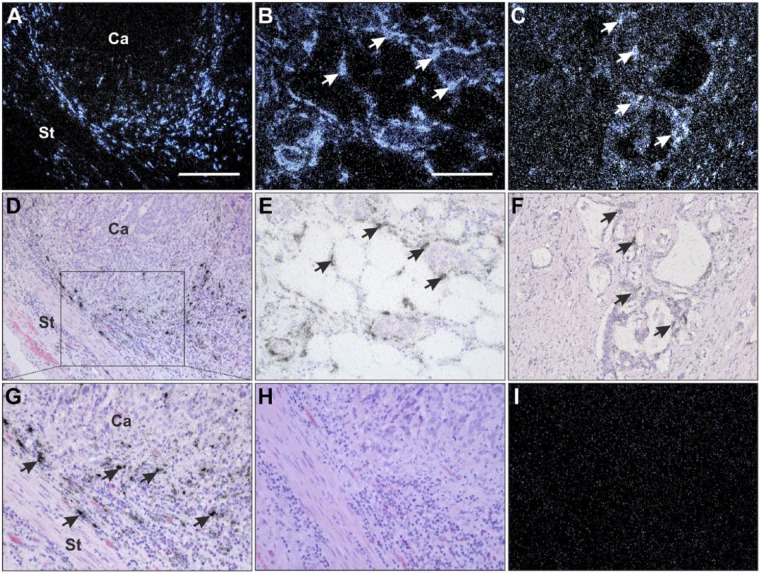
Figure 2.
Localization of TIMP-1 mRNA in gastric cancer. Tissue sections from intestinal (A, C, D, F–I) and diffuse (B and E) subtypes were processed for in situ hybridization using TIMP-1 mRNA antisense (A–G) and sense (H and I) probes. The TIMP-1 mRNA signal is visualized as white spots in dark-field illumination (A–C, I) and black silver grains in bright-field illumination (D–H). TIMP-1 mRNA is observed in the stromal compartment at the periphery of invasive cancer cells in both intestinal (A, D, G; Ca: cancer, St: stroma) and diffuse (B and E) subtype tumors. TIMP-1 in situ hybridization signal is primarily detected in spindle-shape cells surrounding cancer cells in both histological subtypes (arrows in panels B, E, and G). TIMP-1 mRNA is also detected in a minor fraction of cancer cells (arrows) in a few tumor lesions of both histological subtypes (C and F). No specific TIMP-1 mRNA signal is observed in tissue sections incubated with a TIMP-1 sense probe (H and I), compared with an adjacent section hybridized with TIMP-1 antisense probe (G). Scale bars: A–D = 200 µm; B, C, E–I = 100 µm. Abbreviation: TIMP-1, tissue inhibitor of metalloproteinase-1.
In both intestinal and diffuse cancer subtypes, strong TIMP-1 mRNA signal was seen in fibroblast-like cells located in the tumor stroma toward the gastric lumen (data not shown). In five intestinal subtype and three diffuse subtype gastric cancer cases, a few TIMP-1 mRNA expressing fibroblast-like cells were also observed around small arteries, not directly associated with the cancer area (data not shown).
In agreement with the results from the immunohistochemical analysis, weak TIMP-1 mRNA signal was seen in a small fraction of the cancer cells in four intestinal subtype and five diffuse subtype cases when the hybridized sections were exposed for 15 days (normally exposing time was only 7 days; Fig. 2C and F). Importantly, these are the same cases showing TIMP-1 immunoreactivity in cancer cells, strongly indicating that TIMP-1 is synthesized by these cancer cells. No TIMP-1 mRNA signal was seen in the normal-appearing gastric mucosa adjacent to the cancer growth (data not shown). Surprisingly, we did not find any TIMP-1 mRNA in the neuroendocrine cells scattered between epithelial cells at the bottom of the crypts, in normal-appearing gastric mucosa adjacent to the cancer. This is in contrast to the immunohistochemical analysis where these neuroendocrine cells showed TIMP-1 cytoplasmic staining.
As the negative control, adjacent sections from all the 19 cases were hybridized with a TIMP-1 sense probe, showing no specific signal (see Fig. 2H and I, as compared with antisense probe shown in Fig. 2G).
Characterization of TIMP-1-Expressing Cells in Gastric Cancer
As described above, both immunoperoxidase staining and in situ hybridization showed that TIMP-1 protein and mRNA are predominantly expressed by stromal fibroblast-like cells surrounding the cancer lesions. To determine the identity of these TIMP-1 expressing cells, we performed double staining for the simultaneous detection of TIMP-1 and any of the following antigens: α-SMA (myofibroblasts), cytokeratin (cancer and epithelial cells), CD34 (endothelial cells), and CD68 (monocytes/macrophages) on tissue sections from five representative cases. Double immunohistochemistry revealed that TIMP-1 is primarily expressed by α-SMA-positive stromal cells at the invasive front of the gastric cancers in all samples tested (Fig. 3A and D). As α-SMA is a marker for myofibroblasts,20 this indicates that the cellular source of TIMP-1 is tumor-associated myofibroblasts (also known as CAFs). TIMP-1 staining was also seen in α-SMA-positive stromal cells toward the luminal side of the gastric mucosa (data not shown). Not all α-SMA-positive cells expressed TIMP-1 but only a subpopulation of them. We also confirmed that some of the cancer cells at the invasive front were positive for TIMP-1 (Fig. 3C).
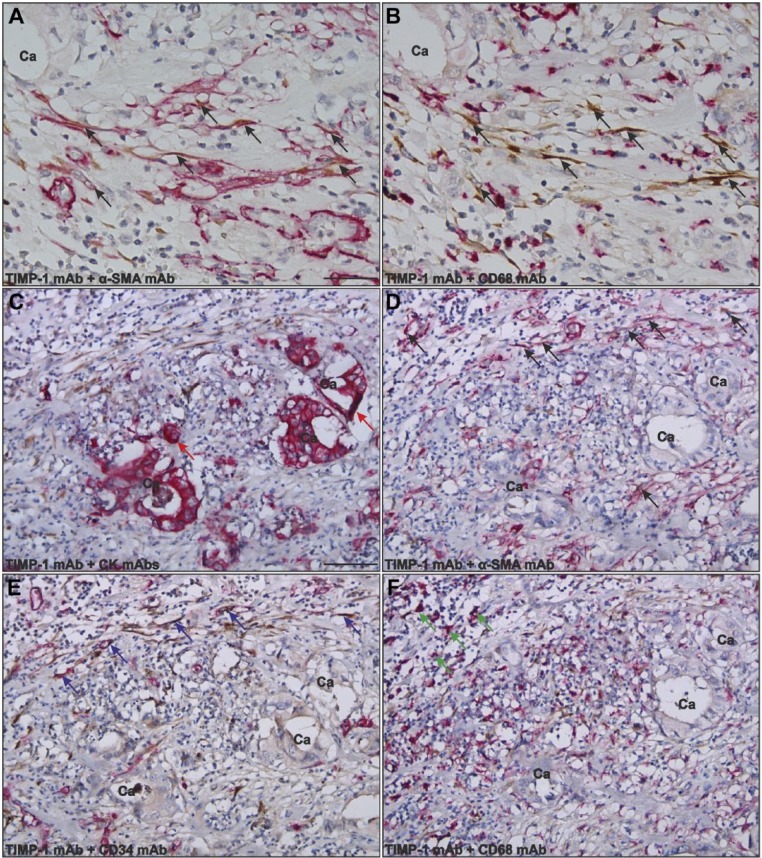
Figure 3.
Identification of TIMP-1-positive cells in gastric cancer. Adjacent paraffin-embedded tissue sections were processed for double immunohistochemistry for TIMP-1 and α-SMA (A, D), TIMP-1 and CK (C), TIMP-1 and CD34 (E), or TIMP-1 and CD68 (B, F) using the EnVision G|2 Double System kit from Dako. The TIMP-1 staining was visualized with DAB (brown) and the stainings for α-SMA, CD68, CK, and CD34 were visualized with Permanent Red (magenta). Numerous TIMP-1-positive cells were found at the periphery of the cancer (indicated with Ca) in all samples tested. The vast majority of the TIMP-1-positive cells (black arrows in panels A, B, D) are also positive for α-SMA (black arrows in A, D) but negative for CD68 (B), indicating that these cells are myofibroblasts (panels A and B represent adjacent sections). TIMP-1 positivity is also seen in a few cancer cells (red arrows in panel C), in CD34-positive endothelial cells (blue arrows in panel E), and in a few CD68-positive macrophages (green arrows in panel F). Scale bars: A, B = 30 µm; C–F = 120 µm. Abbreviations: TIMP-1, tissue inhibitor of metalloproteinase-1; α-SMA, α-smooth muscle actin; CK, cytokeratin; DAB, diaminobenzidine; mAb, monoclonal antibody.
TIMP-1 positivity was also seen in some CD34-positive endothelial cells found close to the tumor periphery (Fig 3E) as well as in vascular structures distant to the cancer (data not shown) and in very few CD68-positive macrophages also at the tumor periphery (Fig. 3F).
TIMP-1 Expression in Relation to Clinicopathological Aspects of the Gastric Cancer
In previous studies in gastric cancer, the intensity of the TIMP-1 immunostaining in stromal cells has been correlated with TNM stage, depth of tumor invasion, and patient survival.11 These observations prompted us to explore whether the expression of TIMP-1, as determined by immunohistochemistry, was correlated with clinicopathological aspects of the disease in our set of cases. Interestingly, we found that the number of cases showing TIMP-1 immunoreactivity in tumor-associated myofibroblasts at the invasive front of the tumors was significantly higher in the intestinal histological subtype than in the diffuse histological subtype of gastric cancer (88% and 54%, respectively; p=0.009, χ2 = 6.87, Table 1). Furthermore, we found a significant trend to a higher number of cases showing TIMP-1 staining in tumor-associated myofibroblasts with increasing TNM stage (trend exact test p=0.041, Table 1). The latter analysis was also done after stratifying the cases according to histological subtype, and similar trends were also suggested although not significant (not shown), probably due to the low number of cases within each histological subtype (Table 1).
Discussion
Despite that several studies have described the expression pattern and cellular localization of TIMP-1 in gastric cancer, the results of such reports are highly discordant.10–15 Considering that most of these studies have reported correlation between TIMP-1 expression and several clinicopathological parameters and that the TIMP-1 expression pattern has not been properly determined, compromising thus the veracity of these statistical associations, a reexamination of the expression pattern and cellular localization of TIMP-1 in gastric cancer is imperative. In this study, we have therefore performed thorough immunohistochemical and in situ hybridization analyses to provide an accurate characterization of the expression pattern and cellular localization of TIMP-1 in gastric cancer.
The present study shows that TIMP-1 is mainly located in a subpopulation of tumor-associated myofibroblasts (also known as CAFs), primarily in the invasion zone. In a few cases, however, TIMP-1 is located in a small fraction of cancer cells, similar to what has been reported in colorectal cancer.4,21 We also show that TIMP-1 is present in cells located in the vascular structure and in very few macrophages located peritumorally at the invasive front. The localization of TIMP-1 protein has been obtained using a thoroughly characterized mAb against human TIMP-1.5,16,17 The site of synthesis, that is the location of the TIMP-1 mRNA expression, has been determined using a specific antisense RNA probe derived from human TIMP-1 cDNA.4 In addition, we challenged the specificity of these reagents by including stringent negative controls such as preincubation of the anti-TIMP-1 mAb with recombinant TIMP-1 protein, the use of a subtype control mAb directed against an irrelevant antigen (TNP), and the in situ hybridization of adjacent tissue sections with a TIMP-1 sense probe, all of them showing no specific reactions. We, therefore, conclude that the observed TIMP-1 staining and mRNA signal represent genuine TIMP-1 expression.
The strongest contribution to the expression of TIMP-1 in our set of gastric cancer samples was from a subpopulation of tumor-associated myofibroblasts located in the periphery of the tumors. These are definitely newly formed accessory cells participating in the invasive process. The finding is consistent with studies by Holten-Andersen et al.4 and Illemann et al.5 in colorectal cancer. Two studies in gastric cancer reported that peritumoral stromal cells are the main contributors to the TIMP-1 protein and mRNA; however, no characterization of the cell type(s) expressing neither protein nor mRNA of this inhibitor was performed.10,11 In contrast, our results strongly disagree with studies in gastric cancer by Murray et al.,12 Zhang et al.,13 Seo et al.,14 and Mroczko et al.,15 who found TIMP-1 immunostaining exclusively or predominantly in the neoplastic cells. Two studies12,15 also detected TIMP-1 in macrophages, polymorphonuclear leukocytes, and lymphocytes. Intriguingly, none of these investigations reported TIMP-1 immunoreactivity in fibroblastic cells.
The discordances between our results and those of the above-mentioned immunohistochemical studies in gastric cancer are most likely explained by methodological differences. First, the anti-TIMP-1 mAbs employed are different, and no detailed information regarding the generation and characterization of those antibodies has been presented; therefore cross-reactivity problems due to low specificity or affinity cannot be totally excluded. Second, important information about the immunostaining procedure was not disclosed by some of the previous studies, specifically the buffer used for antigen retrieval and the temperature in which this step was done, the concentration of primary antibody, and the incubation time for the secondary antibody.12–15 Epitope unmasking is a crucial step in immunohistochemistry and must be carefully optimized because this strongly influences the intensity and specificity of the staining.17,22,23 Finally, for most of these previous investigations, the controls employed for testing the specificity of the TIMP-1 immunostaining reactions were not optimal.10–14 Despite all these methodological considerations, we should not disregard the possibility that ethnic differences in the patient populations may also contribute to the disparities between the previously published reports and between these and our study.
We found that most of the TIMP-1-expressing cells had a fibroblast-like morphology and coexpressed α-SMA, a molecule typically expressed by myofibroblasts adjacent to cancer cell nests.20 Hence, based on the spindle-shape morphology and their peritumoral localization, we concluded that these cells were indeed myofibroblasts. Studies in colorectal cancer and colorectal liver metastasis have also identified the stromal fibroblast-like cells expressing TIMP-1 in these lesions as myofibroblasts.4,5 Myofibroblasts (also known as CAFs) are important components of the tumor-reactive stroma that contribute actively to tumor progression.20 Myofibroblasts are among the most important sources of proteins involved in ECM remodeling, including collagen type IV, all the members of the plasminogen activation system (uPA, uPAR, and PAI-1), several MMPs (e.g., MMP2, MMP11, MMP13), and TIMP-1.4,5,23–28 Importantly, for several of these molecules, high expression has been correlated to poor prognosis in various types of cancer.6,26,28,29 A recent in vitro study showed that fibroblasts treated with TGFβ1 acquire a myofibroblast phenotype with increased α-SMA expression and substantial upregulation of TIMP-1 protein.30 Also, it has been shown that TIMP-1 is induced in human prostate stromal fibroblasts when treated with conditioned media from androgen-resistant prostate cancer cell lines, but the secreted factor(s) responsible for this effect were not identified.31 It is important to explore in vivo the potential correlation between the expression of TIMP-1 and cytokines or growth factors to elucidate the potential inducer(s) of this inhibitor in the local microenvironment of gastric tumors.
Despite the major contribution of myofibroblasts to the TIMP-1 expression in gastric cancer lesions, an intriguing observation made during our study was nonetheless the unexpected presence of TIMP-1 immunoreactivity in neuroendocrine cells, which are negative for TIMP-1 mRNA. Although strong TIMP-1 protein expression in neuroendocrine cells has been previously reported in the esophagus, stomach, small intestine, colon, and rectum,17 to our knowledge, TIMP-1 mRNA has not been detected. In vivo and in vitro studies in several other human normal and neoplastic cells of neuroendocrine origin have found expression of TIMP-1 at the protein and mRNA level.17,31,32 The function of TIMP-1 in neuroendocrine cells is unclear, but it has been suggested that TIMP-1 upregulation is an outcome of neuroendocrine cell differentiation.17,31,32 In fact, there is good correlation between TIMP-1 and chromogranin A levels in tumor tissue and blood of castration-resistant prostate cancer patients.31 It is unknown, however, when during neuroendocrine cell differentiation is TIMP-1 expression induced. Based on this evidence, it is tempting to speculate that the absence of TIMP-1 mRNA signal in neuroendocrine cells of the normal-appearing gastric mucosa may be connected to the downregulation of TIMP-1 transcription due to the state of differentiation of these cells.
A particularly interesting finding in the present study was the significant difference in the expression of TIMP-1 between histological subtypes of gastric cancer. Specifically, we found that the number of cases showing TIMP-1-positive staining in myofibroblasts located at the peripheral invasive front of the tumor was higher in the intestinal subtype than in the diffuse subtype. Previous investigations in gastric cancer have explored the link between TIMP-1 and the histological subtype by performing immunohistochemistry,11 RT-PCR,33,34 or ELISA35–37; however, none of them revealed a statistically significant correlation. Intestinal and diffuse subtypes of gastric cancer show very different histological features,38,39 as reflected at the molecular level.40,41 Our finding suggests that TIMP-1 is among the complex array of molecules that are differentially induced between the two histological subtypes of gastric cancer. Future studies should clarify the link between these two parameters and the biological relevance that this may have.
Another important finding in this study was the increment in the number of cases having TIMP-1-positive myofibroblasts in connection to TNM stage. This is consistent with previous studies reporting a significant correlation between TIMP-1 expression and pathological tumor stage at the mRNA33 and protein level in both tissue11 and serum.35–37,42 These and our findings, taken together, clearly indicate that in gastric cancer, the expression of TIMP-1 gets further upregulated as the disease progresses, which probably is a secondary event to the tumor disease. This phenomenon has also been observed in other malignant tumors of the gastrointestinal tract, including colorectal and esophageal cancers.4,6,43,44 According to this, high TIMP-1 levels may be an indication of more advanced and aggressive tumors, suggesting a potential use of TIMP-1 as a biomarker for assessing gastric cancer patient outcome. In colorectal, breast, lung, and prostate cancers, for example, TIMP-1 has convincingly shown prognostic utility, specifically in predicting patient survival.6,7,45–48 Also, in some of these cancer types, TIMP-1 has been suggested as a diagnostic and predictive marker.49,50 The link between TIMP-1 and patient outcome has also been suggested in gastric cancer by using several approaches, including RT-PCR, immunohistochemistry, and ELISA (both in tissue extracts and serum/plasma)11,35,37,42,51; however, most of these studies are relatively small and present some methodological deficiencies. Larger prospective trials are, therefore, needed not only to validate the prognostic utility of TIMP-1 but also to investigate the potential of this molecule as a diagnostic or predictive marker in gastric cancer.
In conclusion, this study adds relevant information with regard to the expression of TIMP-1 in gastric cancer. First, we disentangle the so far blurred expression pattern of TIMP-1 and show that this protein is primarily expressed by stromal myofibroblasts located in the periphery of cancer lesions, which possibly represents a secondary marker of invasive growth. Second, our study adds new information by revealing that TIMP-1 expression is dissimilar between histological subtypes of gastric cancer, being higher in the less malignant intestinal type than in the diffuse type. We also confirm previous reports indicating that TIMP-1 expression generally increases as the disease gets further advanced. The suggested properties of TIMP-1 as a proliferative, proangiogenic, and antiapoptotic molecule,5,8 in conjunction with its convincing association with poor patient outcome in several cancers, advocate for studying the relation of TIMP-1 with histological subtype and tumor stage in larger groups of gastric cancer patients. This is important to further evaluate the potential of TIMP-1 as a novel biomarker in clinical settings. Importantly, the present study underlines the relevance of a precise cellular localization of potential tumor markers with complex functions before drawing any solid conclusions on potential associations between those molecules and clinicopathological parameters.
Acknowledgments
The authors thank Lotte Frederiksen and Lene Kjær Callesen for excellent technical assistance.
Footnotes
Author Contributions: WA-A, ODL, and MI designed the study. KO, AS, and ODL obtained the formalin-fixed and paraffin-embedded (FFPE) tissue blocks, clinicopathological data, and ethical permission. WA-A and MI performed the experiments. WA-A, ODL, and MI performed histological (microscopic) analysis. IJC performed statistical analysis. WA-A, ODL, GH-H, MP, and MI drafted the manuscript and revised it critically for important intellectual content. All authors have read and approved the final manuscript.
Competing Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Danish Cancer Society (grant R2-A261-09-S2, to W.A.-A.), European Community’s Seventh Framework Program (FP7/2007–2011) under grant 201279 (to G.H-.H and M.I.), Axel Muusfeldts Fond (to W.A.-A.), and Krista og Viggo Petersens Fond (to W.A.-A.).
Literature Cited
- 1. Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–74. [DOI] [PubMed] [Google Scholar]
- 2. Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baker AH, Edwards DR, Murphy G. Metalloproteinase inhibitors: biological actions and therapeutic opportunities. J Cell Sci. 2002;115:3719–27. [DOI] [PubMed] [Google Scholar]
- 4. Holten-Andersen MN, Hansen U, Brünner N, Nielsen HJ, Illemann M, Nielsen BS. Localization of tissue inhibitor of metalloproteinases 1 (TIMP-1) in human colorectal adenoma and adenocarcinoma. Int J Cancer. 2005;113:198–206. [DOI] [PubMed] [Google Scholar]
- 5. Illemann M, Eefsen RH, Bird NC, Majeed A, Osterlind K, Laerum OD, Alpízar-Alpízar W, Lund IK, Høyer-Hansen G. Tissue inhibitor of matrix metalloproteinase-1 expression in colorectal cancer liver metastases is associated with vascular structures. Mol Carcinog. 2016;55:193–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Holten-Andersen MN, Stephens RW, Nielsen HJ, Murphy G, Christensen IJ, Stetler-Stevenson W, Brünner N. High preoperative plasma tissue inhibitor of metalloproteinase-1 levels are associated with short survival of patients with colorectal cancer. Clin Cancer Res. 2000;6:4292–99. [PubMed] [Google Scholar]
- 7. Schrohl AS, Holten-Andersen MN, Peters HA, Look MP, Meijer-van Gelder ME, Klijn JG, Brünner N, Foekens JA. Tumor tissue levels of tissue inhibitor of metalloproteinase-1 as a prognostic marker in primary breast cancer. Clin Cancer Res. 2004;10:2289–98. [DOI] [PubMed] [Google Scholar]
- 8. Stetler-Stevenson WG. Tissue inhibitors of metalloproteinases in cell signaling: metalloproteinase-independent biological activities. Sci Signal. 2008;1:re6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ries C. Cytokine functions of TIMP-1. Cell Mol Life Sci. 2014;71:659–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hong SI, Park IC, Hong WS, Son YS, Lee SH, Lee JI, Choi DW, Moon NM, Choe TB, Jang JJ. Overexpression of tissue inhibitors of metalloproteinase-1 and -2 in the stroma of gastric cancer. J Korean Med Sci. 1996;11:474–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Joo YE, Seo KS, Kim HS, Rew JS, Park CS, Kim SJ. Expression of tissue inhibitors of metalloproteinases (TIMPs) in gastric cancer. Dig Dis Sci. 2000;45:114–21. [DOI] [PubMed] [Google Scholar]
- 12. Murray GI, Duncan ME, Arbuckle E, Melvin WT, Fothergill JE. Matrix metalloproteinases and their inhibitors in gastric cancer. Gut. 1998;43:791–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang S, Li L, Lin JY, Lin H. Imbalance between expression of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in invasiveness and metastasis of human gastric carcinoma. World J Gastroenterol. 2003;9:899–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Seo YS, Park JJ, Kim JH, Kim JY, Yeon JE, Kim JS, Byun KS, Bak YT. Usefulness of MMP-9/TIMP-1 in predicting tumor recurrence in patients undergoing curative surgical resection for gastric carcinoma. Dig Dis Sci. 2007;52:753–9. [DOI] [PubMed] [Google Scholar]
- 15. Mroczko B, Lukaszewicz-Zajac M, Groblewska M, Czyzewska J, Gryko M, Guzińska-Ustymowicz K, Kemona A, Kedra B, Szmitkowski M. Expression of tissue inhibitors of metalloproteinase 1 (TIMP-1) in gastric cancer tissue. Folia Histochem Cytobiol. 2009;47:511–6. [DOI] [PubMed] [Google Scholar]
- 16. Møller Sørensen N, Dowell BL, Stewart KD, Jensen V, Larsen L, Lademann U, Murphy G, Nielsen HJ, Brünner N, Davis GJ. Establishment and characterization of 7 new monoclonal antibodies to tissue inhibitor of metalloproteinases-1. Tumour Biol. 2005;26:71–80. [DOI] [PubMed] [Google Scholar]
- 17. Sorensen IV, Fenger C, Winther H, Foged NT, Lademann U, Brünner N, Usher PA. Characterization of anti-TIMP-1 monoclonal antibodies for immunohistochemical localization in formalin-fixed, paraffin-embedded tissue. J Histochem Cytochem. 2006;54:1075–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shulman M, Wilde CD, Köhler G. A better cell line for making hybridomas secreting specific antibodies. Nature. 1978;276:269–70. [DOI] [PubMed] [Google Scholar]
- 19. Illemann M, Bird N, Majeed A, Laerum OD, Lund LR, Danø K, Nielsen BS. Two distinct expression patterns of urokinase, urokinase receptor and plasminogen activator inhibitor-1 in colon cancer liver metastases. Int J Cancer. 2009;124:1860–70. [DOI] [PubMed] [Google Scholar]
- 20. Desmoulière A, Guyot C, Gabbiani G. The stroma reaction myofibroblast: a key player in the control of tumor cell behavior. Int J Dev Biol. 2004;48:509–17. [DOI] [PubMed] [Google Scholar]
- 21. Newell KJ, Witty JP, Rodgers WH, Matrisian LM. Expression and localization of matrix-degrading metalloproteinases during colorectal tumorigenesis. Mol Carcinog. 1994;10:199–206. [DOI] [PubMed] [Google Scholar]
- 22. Nielsen BS, Sehested M, Duun S, Rank F, Timshel S, Rygaard J, Johnsen M, Danø K. Urokinase plasminogen activator is localized in stromal cells in ductal breast cancer. Lab Invest. 2001;81:1485–501. [DOI] [PubMed] [Google Scholar]
- 23. Alpízar-Alpízar W, Nielsen BS, Sierra R, Illemann M, Ramírez JA, Arias A, Durán S, Skarstein A, Ovrebo K, Lund LR, Laerum OD. Urokinase plasminogen activator receptor is expressed in invasive cells in gastric carcinomas from high- and low-risk countries. Int J Cancer. 2010;126:405–15. [DOI] [PubMed] [Google Scholar]
- 24. Nielsen BS, Rank F, López JM, Balbin M, Vizoso F, Lund LR, Danø K, López-Otín C. Collagenase-3 expression in breast myofibroblasts as a molecular marker of transition of ductal carcinoma in situ lesions to invasive ductal carcinomas. Cancer Res. 2001;61:7091–100. [PubMed] [Google Scholar]
- 25. Nielsen BS, Rank F, Illemann M, Lund LR, Danø K. Stromal cells associated with early invasive foci in human mammary ductal carcinoma in situ coexpress urokinase and urokinase receptor. Int J Cancer. 2007;120:2086–95. [DOI] [PubMed] [Google Scholar]
- 26. Lund IK, Illemann M, Thurison T, Christensen IJ, Høyer-Hansen G. uPAR as anti-cancer target: evaluation of biomarker potential, histological localization, and antibody-based therapy. Curr Drug Targets. 2011;12:1744–60. [DOI] [PubMed] [Google Scholar]
- 27. Lecomte J, Masset A, Blacher S, Maertens L, Gothot A, Delgaudine M, Bruyère F, Carnet O, Paupert J, Illemann M, Foidart JM, Lund IK, Høyer-Hansen G, Noel A. Bone marrow-derived myofibroblasts are the providers of pro-invasive matrix metalloproteinase 13 in primary tumor. Neoplasia. 2012;14:943–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rolff HC, Christensen IJ, Vainer B, Svendsen LB, Eefsen RL, Wilhelmsen M, Lund IK, Høyer-Hansen G, Nielsen HJ, Illemann M; Danish Collaborative Research Group on Colorectal Cancer. The prognostic and predictive value of soluble type IV collagen in colorectal cancer: a retrospective multicenter study. Clin Cancer Res. 2016;22:2427–34. [DOI] [PubMed] [Google Scholar]
- 29. Alpizar-Alpizar W, Christensen IJ, Santoni-Rugiu E, Skarstein A, Ovrebo K, Illemann M, Laerum OD. Urokinase plasminogen activator receptor on invasive cancer cells: a prognostic factor in distal gastric adenocarcinoma. Int J Cancer. 2012;131:E329–36. [DOI] [PubMed] [Google Scholar]
- 30. Webber JP, Spary LK, Sanders AJ, Chowdhury R, Jiang WG, Steadman R, Wymant J, Jones AT, Kynaston H, Mason MD, Tabi Z, Clayton A. Differentiation of tumour-promoting stromal myofibroblasts by cancer exosomes. Oncogene. 2015;34:290–302. [DOI] [PubMed] [Google Scholar]
- 31. Gong Y, Chippada-Venkata UD, Galsky MD, Huang J, Oh WK. Elevated circulating tissue inhibitor of metalloproteinase 1 (TIMP-1) levels are associated with neuroendocrine differentiation in castration resistant prostate cancer. Prostate. 2015;75:616–27. [DOI] [PubMed] [Google Scholar]
- 32. Tomita T. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in pituitary adenomas: possible markers of neuroendocrine cells. Endocr Pathol. 1997;8:305–13. [DOI] [PubMed] [Google Scholar]
- 33. Mimori K, Mori M, Shiraishi T, Fujie T, Baba K, Haraguchi M, Abe R, Ueo H, Akiyoshi T. Clinical significance of tissue inhibitor of metalloproteinase expression in gastric carcinoma. Br J Cancer. 1997;76:531–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang YY, Li L, Zhao ZS, Wang HJ. Clinical utility of measuring expression levels of KAP1, TIMP1 and STC2 in peripheral blood of patients with gastric cancer. World J Surg Oncol. 2013;11:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yoshikawa T, Tsuburaya A, Kobayashi O, Sairenji M, Miyagi Y. Protein levels of tissue inhibitor of metalloproteinase-1 in tumor extracts as a marker for prognosis and recurrence in patients with gastric cancer. Gastric Cancer. 2006;9:106–13. [DOI] [PubMed] [Google Scholar]
- 36. Wang CS, Wu TL, Tsao KC, Sun CF. Serum TIMP-1 in gastric cancer patients: a potential prognostic biomarker. Ann Clin Lab Sci. 2006;36:23–30. [PubMed] [Google Scholar]
- 37. Yoshikawa T, Cho H, Tsuburaya A, Kobayashi O. Impact of plasma tissue inhibitor of metalloproteinase-1 on long-term survival in patients with gastric cancer. Gastric Cancer. 2009;12:31–36. [DOI] [PubMed] [Google Scholar]
- 38. Catalano V, Labianca R, Beretta GD, Gatta G, de Braud F, Van Cutsem E. Gastric cancer. Crit Rev Oncol Hematol. 2005;54:209–41. [DOI] [PubMed] [Google Scholar]
- 39. Vauhkonen M, Vauhkonen H, Sipponen P. Pathology and molecular biology of gastric cancer. Best Pract Res Clin Gastroenterol. 2006;20:651–74. [DOI] [PubMed] [Google Scholar]
- 40. Nobili S, Bruno L, Landini I, Napoli C, Bechi P, Tonelli F, Rubio CA, Mini E, Nesi G. Genomic and genetic alterations influence the progression of gastric cancer. World J Gastroenterol. 2011;17:290–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yasui W, Sentani K, Sakamoto N, Anami K, Naito Y, Oue N. Molecular pathology of gastric cancer: research and practice. Pathol Res Pract. 2011;207:608–12. [DOI] [PubMed] [Google Scholar]
- 42. Kemik O, Kemik AS, Sumer A, Dulger AC, Adas M, Begenik H, Hasirci I, Yilmaz O, Purisa S, Kisli E, Tuzun S, Kotan C. Levels of matrix metalloproteinase-1 and tissue inhibitors of metalloproteinase-1 in gastric cancer. World J Gastroenterol. 2011;17:2109–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mori M, Mimori K, Sadanaga N, Inoue H, Tanaka Y, Mafune K, Ueo H, Barnard GF. Prognostic impact of tissue inhibitor of matrix metalloproteinase-1 in esophageal carcinoma. Int J Cancer. 2000;88:575–8. [DOI] [PubMed] [Google Scholar]
- 44. Holten-Andersen MN, Fenger C, Nielsen HJ, Rasmussen AS, Christensen IJ, Brünner N, Kronborg O. Plasma TIMP-1 in patients with colorectal adenomas: a prospective study. Eur J Cancer. 2004;40:2159–64. [DOI] [PubMed] [Google Scholar]
- 45. Gouyer V, Conti M, Devos P, Zerimech F, Copin MC, Créme E, Wurtz A, Porte H, Huet G. Tissue inhibitor of metalloproteinase 1 is an independent predictor of prognosis in patients with nonsmall cell lung carcinoma who undergo resection with curative intent. Cancer. 2005;103:1676–84. [DOI] [PubMed] [Google Scholar]
- 46. Kuvaja P, Talvensaari-Mattila A, Turpeenniemi-Hujanen T. High preoperative plasma TIMP-1 is prognostic for early relapse in primary breast carcinoma. Int J Cancer. 2008;123:846–51. [DOI] [PubMed] [Google Scholar]
- 47. Birgisson H, Nielsen HJ, Christensen IJ, Glimelius B, Brünner N. Preoperative plasma TIMP-1 is an independent prognostic indicator in patients with primary colorectal cancer: a prospective validation study. Eur J Cancer. 2010;46:3323–31. [DOI] [PubMed] [Google Scholar]
- 48. Oh WK, Vargas R, Jacobus S, Leitzel K, Regan MM, Hamer P, Pierce K, Brown-Shimer S, Carney W, Ali SM, Kantoff PW, Lipton A. Elevated plasma tissue inhibitor of metalloproteinase-1 levels predict decreased survival in castration-resistant prostate cancer patients. Cancer. 2011;117:517–25. [DOI] [PubMed] [Google Scholar]
- 49. Holten-Andersen MN, Christensen IJ, Nielsen HJ, Stephens RW, Jensen V, Nielsen OH, Sørensen S, Overgaard J, Lilja H, Harris A, Murphy G, Brünner N. Total levels of tissue inhibitor of metalloproteinases 1 in plasma yield high diagnostic sensitivity and specificity in patients with colon cancer. Clin Cancer Res. 2002;8:156–64. [PubMed] [Google Scholar]
- 50. Schrohl AS, Meijer-van Gelder ME, Holten-Andersen MN, Christensen IJ, Look MP, Mouridsen HT, Brünner N, Foekens JA. Primary tumor levels of tissue inhibitor of metalloproteinases-1 are predictive of resistance to chemotherapy in patients with metastatic breast cancer. Clin Cancer Res. 2006;12:7054–8. [DOI] [PubMed] [Google Scholar]
- 51. Grunnet M, Mau-Sorensen M, Brunner N. Tissue inhibitor of metalloproteinase 1 (TIMP-1) as a biomarker in gastric cancer: a review. Scand J Gastroenterol. 2013;48:899–905. [DOI] [PubMed] [Google Scholar]