Abstract
Background and study aims
Radiofrequency ablation (RFA) is a new endoscopic palliation therapy for malignant biliary obstruction. The aim of this study was to compare the short-term effects of biliary drainage and adverse events of this technique with the standard of endoscopical treatment of hilar cholangiocarcinoma, photodynamic therapy (PDT).
Patients and methods
We retrospectively and since December 2012 prospectively investigated the efficacy and adverse events of RFA in patients with hilar cholangiocarcinoma in two tertiary referral centers between November 2011 and January 2013. The approach of the study was prospective, but because of the large amount of retrospectively included patients, the design of the study is overall retrospective. A group of 20 patients treated with PDT between April 2005 and May 2011 served as a historical control.
Results
Fourteen patients received 31 biliary RFAs and 20 patients received 36 PDTs. Within the RFA group, a significant decrease (p = 0.046) of the bilirubin level was seen 14 days after the first RFA (3.3 ± 3.9 (mg/dl) versus 2.3 ± 2.6 (mg/dl)). In the PDT group no significant decrease (p = 0.67) of the bilirubin level was obtained (4.1 ± 6.9 (mg/dl) versus 3.5 ± 5.3 (mg/dl)). In the PDT group (13/20, 65%) a significantly higher number of premature stent replacements (<3 months) after the first intervention was noticed in comparison with the RFA group (four of 14, 29%) (p < 0.01). Between the first and fifth procedure, post-interventional adverse events tend to occur more frequently in patients with PDT (eight of 20, 40%) than with RFA (three of 14, 21%) (p = 0.277).
Conclusions
Looking at the short-term effects, we conclude that RFA may present a therapeutic alternative to PDT for palliative treatment of malignant biliary obstruction because of its simple feasibility and moderate adverse event rate. To provide a definitive evaluation of the long-term effects and of overall median survival, a controlled trial with PDT must follow.
Keywords: Biliary cancer, radiofrequency ablation, endoscopy, biliary drainage, bile duct cancer, Klatskin tumor
Introduction
Only 20%–30% of patients presenting with symptoms caused by hilar cholangiocarcinomas (Klatskin tumors) can be cured by R0 resections.1 Therefore, in most patients with this type of bile duct cancer, only palliative concepts could be offered. Endoscopic transpapillary and/or percutaneous transhepatic biliary drainage offered the best survival in the past.2 In combination with sufficient drainage, reduction of tumor masses in the lumen of the bile duct was shown to prolong patient survival. Effectiveness of photodynamic therapy (PDT) was demonstrated in two randomized controlled and a few controlled studies.3–8 Today, endoscopic drainage in combination with intraluminal PDT is the best palliative concept that patients can be offered. Mean survival can be prolonged from six months to approximately 14 months (11–21 months according to the study) by adding PDT to sufficient endoscopic drainage therapy. A burden for the patients is the increased phototoxicity of the photosensitizer for three to four weeks.
Brachytherapy seems to be effective as shown in some smaller studies and in a retrospective analysis of data from the Surveillance, Epidemiology, and End Results (SEER) database.9,10 Because of the organizational challenge, it can be performed only in some centers.
Percutaneous radiofrequency ablation (RFA) is a well-established therapy for hepatocellular and intrahepatic cholangiocellular carcinoma.11–13 Recently, in two studies endoscopically applied RFA was presented for the treatment of malignant biliary obstruction.14,15 Both studies demonstrated immediate and 30-day safety and 30-, respectively, and 90-day biliary patency.
In this study, we retrospectively and since December 2012 prospectively investigated the drainage effects and adverse events of RFA in patients with hilar cholangiocarcinoma in two tertiary referral centers. A retrospectively analyzed group of 20 patients treated with PDT served as a historical control group. A main drawback of this study is the small number of patients included.
Patients and methods
All patients included in the RFA group of the study were admitted between November 2011 and January 2013 to Klinikum rechts der Isar, 2. Medical Department, Technische Universität Muenchen, and Academic Teaching Hospital Neuperlach, Department of Gastroenterology and Hepatology, Städtisches Klinikum München GmbH, Munich. A total of 14 patients with RFA therapy were included in this study. For every patient, palliative therapy due to unresectable bile duct cancer Bismuth IIIa–IV or unresectability due to liver metastases was concluded by the tumor board. Patients could choose either PDT or RFA because both local ablative therapies were available in both centers. Exclusion criteria were pregnancy, instability for endoscopy, uncorrected coagulopathy, and previous bleeding out of the bile duct. Data were collected detailing patient characteristics, hospital stays, and serial liver function tests to determine the presence of biliary obstruction or cholangitis before and after RFA. Adverse events like preliminary stent exchanges, cholangitis, liver abscess, and sepsis were noted. The study was approved by the local ethics committee and conducted in accordance with the Declaration of Helsinki (revised 2000). All patients in the study provided written consent. Institutional review board (IRB) approval was obtained in December 2012 (local ethics committee evaluation number: 5605/12), and after that date patients were included for the RFA study group from December 2012 to May 2013 prospectively. The study was retrospectively designed to include data of RFA treatments that have already been performed before IRB approval. RFA data were collected prospectively (six of 31 biliary RFAs, 19%) and were combined with retrospective data that were performed before IRB decision (25/31 biliary RFAs, 81%). The approach of the study was prospective but because of the large amount of retrospectively included patients, the design of the study is overall retrospective. Data for 20 patients treated by PDT were retrospectively collected between April 2005 and May 2011 at Klinikum rechts der Isar, Medical Department, Technische Universität Muenchen (Study Center 1). This study represents a case series with a comparison of two non-adjusted patient groups.
Intervention
Patients were under analgosedation by propofol. Endoscopic retrograde cholangiography (ERC) was performed with a standard videoduodenoscope Olympus TFJ 160-R (Olympus, Hamburg, Germany). The first ERC consisted of an endoscopic sphincterotomy (EPT), which was performed using a papillotome (Papillotomy knife Olympus, 4,5/6 Fr, KD-201Q-0730, Hamburg, Germany) that was introduced over a Terumo guide wire (Terumo Radiofocus Guide Wire M, RF-GS35263M,Terumo Corporation, Tokyo, Japan). If strictures were too straight to pass the RFA catheter (8 Fr), we performed dilation with a balloon and/or bougie.
RFA
The Habib EndoHPB (EMcision UK, London, United Kingdom) catheter has United States Food and Drug Administration and European Union European Conformity approval. It is a bipolar RFA probe that is 8 F (2.6 mm), 1.8-m long, and passes over 0.035-inch guide wires. The catheter has two ring electrodes 8 mm apart with the distal electrode 5 mm from the leading edge. The catheter was advanced over a wire under fluoroscopic guidance across the biliary stricture, and an ablation was conducted using a RITA 1500X RF generator (AngioDynamics, Latham, NY, USA) set at 400 kHz at 7 W for 90 s or an ERBE VIO 200 generator (ERBE Electromedicine GmbH) at 7 W for 90 s. Plastic stents 7.5 to 10 Fr were placed systematically after RFA. Our primary outcome measures were the safety and efficacy of RFA. For efficacy measures, data on stent patency after 14 days were collected. Immediate and 14-day adverse events and stent patency were also recorded.
PDT
The procedure was performed as described by Ortner et al.3 Shortly, patients treated with PDT received Photofrin at a dosage of 2 mg/kg body weight intravenously 48 hours before laser activation. Patients remained in a darkened room for three to four days after injection.
Adverse events
Bleeding was considered to be an adverse event if there was clinical evidence of bleeding and/or a decrease of hemoglobin concentration of at least 2 g per deciliter. Bleeding following the procedure was presumed to result from endoscopic intervention unless another cause was documented. Cholangitis was defined as post-procedural elevation of temperature more than 38.5℃ for more than 24 hours and/or an increase in infection parameters (leukocytes) of more than 20%, without evidence of other concomitant infections. Sepsis was defined in accordance with the International Sepsis Definitions Conference 2001.16 Perforation included retroperitoneal or bowel perforation, documented by any radiographic technique associated with clinical symptoms. Procedure-induced pancreatitis was defined as new abdominal pain in combination with serum amylase or lipase 24 hours raised after endoscopic intervention to more than three times the upper limit of normal levels, requiring an extended hospital stay of at least 48 hours. Adverse events were divided into minor and major adverse events.17
European Organisation for Research and Treatment of Cancer Quality of Life (QoL) Questionnaire-Core 30 (EORTC QLQ-C30) questionnaire
At Study Center 1 (seven patients), a retrospective and since December 2012 prospective analysis of the QoL within the RFA study group was determined by using the EORTC QLQ-C30 (Questionnaire Core 30; Aaronson et al. 1993).26 The EORTC QLQ-C30 questionnaire was used only for some of the patients (seven patients at Study Center 1) and was at the beginning of our study for all patients retrospectively. In the study process we evaluated the data for four of seven patients prospectively since December 2012. The evaluation of the EORTC QLQ-C30 contains a transformation of the values into a scale of 0–100. This is necessary to make the raw data comparable despite the different staggerings. First, for each scale the average value is formed, which is transformed afterward on a scale of 0–100. The values, which result after the transformation, are to be interpreted differently. High values regarding the function scales are to be rated as a high measure of operability. High values concerning the symptoms, however, speak for a strong development of these symptoms.
Study design
The design was a two-center (Klinikum rechts der Isar, 2. Medical Department, Technische Universität Muenchen (Study Center 1) and Academic Teaching Hospital Neuperlach, Department of Gastroenterology and Hepatology, Städtisches Klinikum München GmbH, Munich (Study Center 2)), retrospective and since December 2012 prospective study to demonstrate the safety, biliary patency, and adverse events of RFA therapy. Retrospectively, data from patients treated by PDT were analyzed. This study represents a case series with a comparison of two non-adjusted patient groups.
A main drawback of this study is the small number of patients included.
Statistical analysis
Results are expressed as the arithmetic mean ± SD. A statistical analysis was performed using the SPSS software (SPSS Inc, 21.0, Chicago, IL, USA). Categorical data were compared with the Chi-square test (C2). Ordinal data were indicated with the measures of central tendencies (mean value and standard variation). For the comparison of a parameter between patient groups (unconnected sample), the Student’s t-test was taken with normal distributed data. The Mann-Whitney U test (U test) was taken if in one or both groups no normal distribution was present. For the comparison of a parameter between patient groups (connected sample), the Student’s t test was taken with normal distributed data. The Wilcoxon test was used if in one or both groups no normal distribution was present. A p value < 0.05 were considered significant.
Results
Patient characteristics at study entry
RFA study group
Between November 2011 and January 2013, 14 patients (male to female: eight to six, 57/43%), mean age 73 ± 9 years (53–87 years) received 31 biliary RFA (Table 1). The basic illnesses of the treated patients included 11 Klatskin tumors (79%) (one Bismuth III A (7%), two Bismuth III b (14%), eight Bismuth IV (57%)), one distal extrahepatic (7%), one intrahepatic (7%) cholangiocarcinoma, and one metastatic neuroendocrine carcinoma with obstruction of the hepatic fork. Distant metastases were proven in five of 14 patients (36%) (hepatic (three), lymphatic metastases (two)). Three of 14 patients (21%) had received previous systemic chemotherapy (Gemcitabin mono; Adriamycin Cisplatin/Mitomycin) and four of 14 patients (29%) a concomitant chemotherapy. The first-line chemotherapy included a Gemcitabin/Cisplatin or, independent of the creatinine clearance, a Gemcitabin/Oxaliplatin-based protocol. Two of 14 patients (50%) were treated with a second-line therapy (FOLFIRI-protocol). One of 14 patients (7%) received a PDT eight months before the first RFA.
Table 1.
Patient characteristics of RFA and PDT study group at study entry.
| Clinical characteristics | RFA study group | PDT study group | p value | Significance |
|---|---|---|---|---|
| N | 14 | 20 | ||
| Male | 8 (57%) | 6 (30%) | 0.11 | n.s. |
| Female | 6 (43%) | 14 (70%) | 0.11 | n.s. |
| Mean age at onset of first intervention (years) | 73 ± 9 | 70 ± 12 | 0.22 | |
| Klatskin tumor | 11 (79%) | 15 (75%) | 0.45 | n.s. |
| Bismuth II | 0% | 1 (5%) | – | – |
| Bismuth III A | 1 (7%) | 0 | – | – |
| Bismuth III b | 2 (14%) | 1 (5%) | – | – |
| Bismuth IV | 8 (57%) | 13 (65%) | – | – |
| Distal extrahepatic cholangiocarcinoma | 1 (7%) | 1 (5%) | – | – |
| Intrahepatic cholangiocarcinoma | 1 (7%) | 3 (15%) | – | – |
| Other tumor entity | 1 (7%) | 1 (5%) | – | – |
| Tumor stage at initial diagnosis | ||||
| UICC IV | 13 (93%) | 20 (100%) | 0.23 | n.s. |
| Metastases | 5 (36%) | 8 (40%) | 0.8 | n.s. |
| Bilirubin levels [mg/dl] | 3.3 ± 3.9 | 4.1 ± 6.9 | 0.73 | n.s. |
| Mean Karnofsky score at first RFA/PDT | 64% or ECOG 2 | 63% or ECOG 1 | 0.09 | n.s. |
| Mean Karnofsky score at second RFA/PDT | 68% or ECOG 1 | 68% or ECOG 1 | 0.71 | n.s. |
| Mean Karnofsky score at third RFA/PDT | 64% or ECOG 2 | 55% or ECOG 2 | – | – |
| Mean Karnofsky score at fourth RFA/PDT | 63% or ECOG 2 | 45% or ECOG 3 | – | – |
| Stent diameter (7 Fr) at first intervention | 11 (78.6%) | 12 (60%) | 0.25 | n.s. |
–: Statistic tests were not used for the analysis because of too small patient numbers. RFA: radiofrequency ablation; PDT: photodynamic therapy; UICC: Union for International Cancer Control; ECOG: Eastern Cooperative Oncology Group.
PDT study group
Between April 2005 and May 2011, 20 patients (male to female: six to 14, 30/70%, mean age 70 ± 12 years (44–85 years)) received 36 PDT (Table 1). The basic illnesses of the treated patients included 15 Klatskin tumors (75%) (one Bismuth II (5%), one Bismuth III b (5%), 13 Bismuth IV (65%)), one distal extrahepatic (5%), and three intrahepatic (15%) cholangiocarcinoma. One patient with gallbladder cancer received PDT. Altogether, distant metastases have been proven in eight of 20 patients (40%) (hepatic filiae (six), lymphatic metastases (two)). The middle Karnofsky score at the time of the first PDT was 63% (Eastern Cooperative Oncology Group (ECOG) 1). Four of 20 (20%) patients received concomitant chemotherapy. The first-line chemotherapy included Gemcitabin-based chemotherapy (Gemcitabin/Oxaliplatin; Gemcitabin/Capecitabin).
Comparison of patient characteristics of both study groups
In relation to patient characteristics between the RFA and the historical PDT study group, no significant differences exist (Table 1).
Endoscopic interventions
Thirty-one biliary RFA (twice in seven patients (50%), three times in five patients (36%), four times in two patients (14%), five times in one patient (7%), six times in one patient (7%), and seven times in one patient (7%)) were performed via ERC. The mean interval from the initial diagnosis to the first RFA was 10 ± 9 months. The time interval between the following RFA lies between a minimum of two and a maximum of three months. The stent diameter at the time of the first intervention was 7 Fr (79%) in 11 patients and 10 Fr (7%) in one patient. One patient (7%) received a percutaneous biliary drain (PTCD) and one patient (7%) a metal stent at the time of initial diagnosis. Two further patients (14.3%) got a metal stent during the therapy process.
Twenty patients received 36 PDTs (twice in eight patients (40%), three times in five patients (25%), four times in two patients (10%), and five times in one patient (5%)). The first PDT was performed in a mean interval of 4 ± 3 months after the initial diagnosis. The time interval between the following interventions lies between a minimum of five and a maximum of 15 months. One patient (5%) received a metal stent at the time of initial diagnosis. The stent diameter at the time of the first intervention was 7 Fr in 12 patients (60%), 10 Fr in six patients (30%) and 11.5 Fr in one patient (5%). Six patients (30%) received a PTCD and two patients (10%) a metal stent during the therapy process.
Between both study groups there was no significant difference in relation to the frequency of a metal (p = 0.67) or a PTCD placement (p = 0.42). Three patients (21.4%) in the RFA group and three patients in the PDT study group (15%) had a metal stent; respectively, two patients (14.3%) versus six patients (30%) a PTCD in the observed time interval between the first and fifth intervention.
Drainage success (Tables 2 and 3; Figures 1–3)
Table 2.
Stent replacements (time interval between first and fifth intervention in each group).
| RFA study group | PDT study group | |
|---|---|---|
| Premature stent replacements after RFA/PDT (<3 months) (patients) | 5 (36%) | 16 (80%) |
| Once | 1 (20%) | 13 (81%) |
| Twice | 2 (40%) | 3 (19%) |
| Three times | 1 (20%) | – |
| Six times | 1 (20%) | – |
| Time interval of stent replacement (<3 months) after first RFA/PDT (months) | 1.4 ± 0.9 (n = 4) | 1.5 ± 0.6 (n = 13) |
| Time interval of stent replacement (<3 months) after second RFA/PDT (months) | 1.8 ± 0.5 (n = 5) | 1.3 ± 0.6 (n = 3) |
| Time interval of stent replacement (<3 months) after third RFA/PDT (months) | 1.7 ± 0.6 (n = 4) | 2 ± 0.0 (n = 1) |
RFA: radiofrequency ablation; PDT: photodynamic therapy.
Table 3.
Bilirubin levels before first and second RFA and after 14 days.
| RFA study group (patients) | Bilirubin level before first RFA (mg/dl) | Follow-up (14 days) | Bilirubin level before second RFA (mg/dl) | Follow-up (14 days) |
|---|---|---|---|---|
| 1 | 0.4 | a | 2.8 | 1.65 |
| 2 | 3.8 | 1.4 | 1.6 | 0.8 |
| 3 | 1.1 | a | 1.4 | 1.9 |
| 4 | 0.3 | 0.2 | 0.3 | 0.3 |
| 5 | 1.5 | 1.6 | – | – |
| 6 | 5.3 | 2.1 | – | – |
| 7 | 1.0 | 0.6 | – | – |
| 8 | 0.5 | 0.9 | 1.0 | 0.8 |
| 9 | 6.1 | 2.6 | – | – |
| 10 | 14.6 | 9.3 | – | – |
| 11 | 1.3 | 0.9 | – | – |
| 12 | 7.0 | 5.0 | – | – |
| 13 | 2.5 | 2.8 | – | – |
| 14 | 1.2 | 0.4 | 1.0 | 0.5 |
Values not available. RFA: radiofrequency ablation.
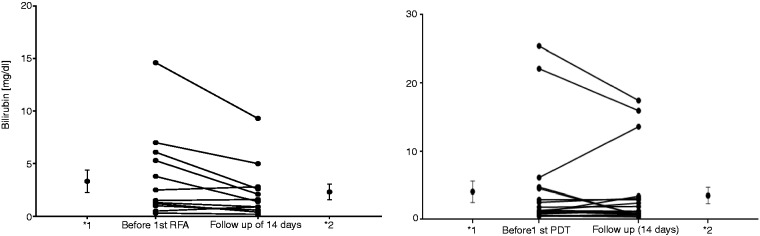
Figure 1.
Bilirubin levels before the first RFA/PDT and after 14 days in each case.
*Mean and error values (*1: before first RFA/PDT; 2* follow-up of 14 days). RFA: radiofrequency ablation; PDT: photodynamic therapy.
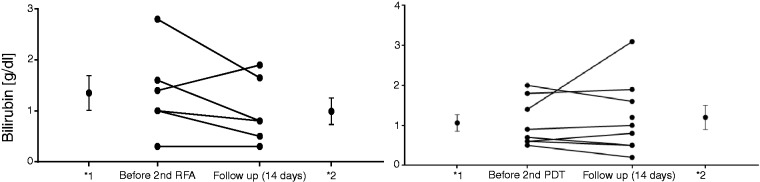
Figure 2.
Bilirubin levels before the second RFA/PDT and after 14 days in each case.
*Mean and error values (*1: before second RFA/PDT; 2* follow-up of 14 days). RFA: radiofrequency ablation; PDT: photodynamic therapy.
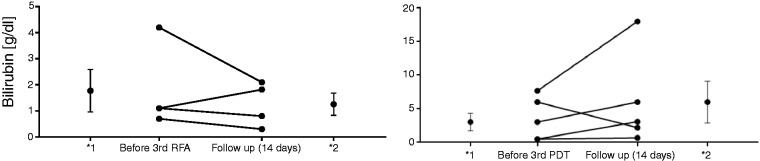
Figure 3.
Bilirubin levels before the third RFA/PDT and after 14 days in each case.
*Mean and error values (*1: before third RFA/PDT; 2* follow-up of 14 days). RFA: radiofrequency ablation; PDT: photodynamic therapy.
Within the RFA study group a significant reduction (p = 0.046) of the bilirubin level was seen in the interval of 14 days after the first RFA with a mean bilirubin value of 3.3 ± 3.9 (mg/dl) before versus 2.3 ± 2.6 (mg/dl) after RFA. In the PDT study group, no significant reduction of the bilirubin level was obtained with a middle value of 4.1 ± 6.9 (mg/dl) versus 3.5 ± 5.3 (mg/dl). For sequential interventions a slight reduction of the bilirubin level was seen in the RFA study group (second RFA: 1.4 ± 0.8 (mg/dl) versus 0.9 ± 0.6 (mg/dl); third RFA: 1.8 ± 1.6 (mg/dl) versus 1.4 ± 0.9 (mg/dl)) in comparison with the PDT group (second PDT: 1.1 ± 0.6 (mg/dl) versus 1.2 ± 0.9 (mg/dl); third PDT: 3.1 ± 3.5 (mg/dl) versus 6 ± 8.1 (mg/dl)) without achieving statistical significance in the respective group. Because of too small patient numbers in both study groups, no evaluation starting with the fourth intervention was performed. Comparing the extent of the reduction of the first RFA (1.1 ± 1.8 mg/dl) versus the first PDT (0.5 ± 3.2 mg/dl), a fairly minor advantage was received for RFA without reaching a significant difference (p = 0.636). This was also observed for subsequent interventions.
Procedure-related adverse events (Table 4)
Table 4.
Procedure-related adverse events (time interval between first and fifth intervention in each group).
| Adverse events | RFA study group | PDT study group |
|---|---|---|
| Cholangitis | 2 (14%) | 6 (30%) |
| Liver abscess | 1 (7%) | 1 (5%) |
| Sepsis | 1 (7%) | 1 (5%) |
| Bleeding | 0 | 0 |
| Perforation | 0 | 0 |
| Phototoxic reaction | – | 2 (10%) |
RFA: radiofrequency ablation; PDT: photodynamic therapy.
All RFA procedures were accomplished without any adverse events. Post-interventional in two of 14 cases (14%), cholangitis was observed. In two further cases (14%) post-interventionally, a liver abscess was determined. In one of these two cases this occurred with septic progression after the seventh RFA. Altogether, in two of four patients with proven post-interventional adverse events, a septic progression was observed. In particular, so-called “major adverse events” like bleeding, intensive care hospitalization, perforation, or death due to the intervention were not proven. Endoscopically reinterventions because of occluded stents were necessary in five of 13 patients (38%) with recurrent RFA in the interval. Post-interventional adverse events tended to occur more often in patients after photodynamic therapy (eight of 20 patients; 40%) than in the RFA group (three of 14 patients; 21%) for the time interval between the first and fifth intervention in each group (p = 0.277). In the PDT study group a post-interventional cholangitis was observed in six patients (30%), abscess in one patient (5%), sepsis in one patient (5%), and phototoxic reaction in two patients.
QoL of the RFA study group
Within the five functional scales, higher values were measured 14 days after the second and third application of RFA for physical operability, role function ability in everyday life, and for cognitive operability, for example (Table 5). This indicates that a higher level of functioning was observed. It is shown that patients tended to have less pain (27 ± 6 versus 28 ± 3) and lower loss of appetite (20 ± 10 versus 27 ± 15) after a second radiofrequency than before the intervention (Table 5). Similarly, symptom scales like diarrhea, sleep disturbances, obstipation, and financial burden tended to recover after the second RFA treatment. All symptom scales deteriorated after the third RFA application.
Table 5.
EORTC QLQ-C30 evaluation of RFA study group.
| Test 1 (before first RFA) | Test 2 (follow-up) | Test 3 (before second RFA) | Test 4 (follow-up) | Test 5 (before third RFA) | Test 6 (follow-up) | |
|---|---|---|---|---|---|---|
| Functional scales | ||||||
| Physical | 24 ± 6 | 24 ± 6 | 25 ± 7 | 28 ± 11 | 24 ± 8 | 29 ± 16 |
| Role | 24 ± 10 | 26 ± 8 | 20 ± 0,0 | 28 ± 10 | 25 ± 7 | 33 ± 11 |
| Emotional | 25 ± 5 | 25 ± 7 | 23 ± 7 | 24 ± 12 | 25 ± 4 | 31 ± 12 |
| Cognitive | 18 ± 8 | 18 ± 9 | 18 ± 8 | 22 ± 13 | 15 ± 7 | 25 ± 21 |
| Social | 28 ± 3 | 24 ± 9 | 27 ± 13 | 22 ± 8 | 20 ± 7 | 30 ± 14 |
| Global quality of life | 43 ± 13 | 35 ± 16 | 28 ± 10 | 28 ± 8 | 33 ± 4 | 28 ± 25 |
| Symptom scales | ||||||
| Fatigue | 27 ± 4 | 28 ± 7 | 28 ± 7 | 28 ± 7 | 28 ± 2 | 32 ± 12 |
| Nausea and vomiting | 13 ± 4 | 13 ± 4 | 13 ± 6 | 12 ± 3 | 15 ± 0 | 25 ± 14 |
| Pain | 22 ± 7 | 23 ± 5 | 28 ± 3 | 27 ± 6 | 15 ± 7 | 33 ± 4 |
| Dyspnea | 18 ± 10 | 20 ± 9 | 20 ± 10 | 20 ± 17 | 10 ± 0 | 25 ± 21 |
| Diarrhea | 12 ± 4 | 12 ± 4 | 13 ± 6 | 10 ± 0 | 15 ± 7 | 20 ± 14 |
| Sleep disturbance | 32 ± 8 | 33 ± 8 | 20 ± 10 | 17 ± 12 | 25 ± 21 | 30 ± 14 |
| Loss of appetite | 23 ± 10 | 23 ± 10 | 27 ± 15 | 20 ± 10 | 15 ± 7 | 25 ± 21 |
| Obstipation | 10 ± 0 | 17 ± 8 | 13 ± 6 | 10 ± 0 | 20 ± 14 | 25 ± 21 |
| Financial burden | 17 ± 8 | 18 ± 8 | 20 ± 14 | 16.6 ± 12 | 20 ± 14 | 25 ± 21 |
High values regarding the function scales are to be rated as a high measure of operability; small values concerning the symptoms, however, speak for a low development of these symptoms.
RFA: radiofrequency ablation; EORTC: European Organisation for Research and Treatment of Cancer; QLQ-C30: Quality of Life Questionnaire-Core 30.
Discussion
Local tumor reduction by PDT in combination with stent therapy is effective for reducing cholestasis. For patients with unresectable hilar cholangiocarcinoma, two randomized studies and a couple of controlled studies have shown a clear advantage in survival over stenting alone.18 Furthermore, some trials could demonstrate a gain in QoL or in performance status.3,5,7,8
Since the introduction of a novel bipolar catheter, RFA is available that could be introduced in the bile ducts during an endoscopic procedure.14 The ablation of the tumor by heat is technically easy to perform without any pretreatment. Although no sufficient data exist to prove the equivalence with photodynamic therapy, RFA is still widely used in many hospitals. Moreover, patients who are offered both methods are often inclined to prefer RFA because PDT introduces the burden of staying four to five days in the darkness for prevention of phototoxicity.19,20
Because there are no sufficient data on the efficacy of RFA published to date, we compared the data from our patients treated by RFA with historical data from a cohort of patients who underwent PDT. Patients from both study groups were shown to be comparable in their characteristics.
Both study groups benefited from consecutive drainage therapies and local tumor decrement, either by PDT or RFA. Short-term effects were noticed two weeks after each intervention. The first RFA procedures caused even a significant fall in the bilirubin level. Interestingly, a further decline in bilirubin levels continued until the second intervention. Subsequently, in the RFA group, the lowest bilirubin levels were measured at the end of the second intervention. It is interesting that before the third intervention (15 ± 11.4 months after study entry) cholestasis increased most probably due to the progression of tumor over the period. Similarly, the first PDT intervention caused a clear although not significant fall in cholestasis with a further decrease up to the start of the second session. After the second PDT, bilirubin levels stayed within the normal range and comparable with the RFA group raised over the period. The third PDT intervention (17.9 ± 12.9 months after initial diagnosis) failed to prevent a deterioration in cholestasis. An effect of chemotherapy on the improvement of cholestasis could be excluded because it was not performed during inpatient stay in both groups.
Furthermore, a premature stent replacement due to cholestasis or cholangitis by stent closure was necessary in 36% of individuals after RFA, and in 80% of individuals after PDT. Therefore, the time intervals between the interventions had to be shortened in these patients. The interval was 1.4 months in the subgroup of RFA patients and, similarly, 1.5 months in the subgroup of PDT patients with stent closure after the first intervention.
In these cases, an influence of accompanying chemotherapy could not be excluded on premature stent closure. In the RFA group, 57% of patients received a concomitant chemotherapy (CTx), whereas in the PDT group only 20% were treated with chemotherapy during this period. However, the effect of chemotherapy on stent closure is difficult to gauge: It could improve cholestasis by reducing the tumor burden or may cause cholangitis by immune suppression.21,22
So far it was shown in small studies that RFA is a procedure with a low to moderate adverse event rate. Minor adverse events were previously reported as pain (five of 20), asymptomatic biliary pancreatitis (one of 22 and one of 20), cholecystitis (two of 22 and one of 20) and cholangitis (five of 58). Major adverse events included cholangiosepsis (five of 58), hemobilia (three of 58), partial liver infarction (one of 58), gallbladder empyema (one of 58), hepatic coma (one of 58), and newly diagnosed left bundle branch block (one of 58).14,15,23 Tal et al. observed in their study with 12 patients in three cases hemobilia that occurred four to six weeks after RFA application.24
Even though in our study more procedures per patients (2.2) were applied than in previous studies (1.0–1.5), the adverse event rate was comparable: Two cases (two of 14) of cholangitis and two cases (two of 14) of post-interventional liver abscess were noted. Two of the four disorders progressed to sepsis, which could be treated by drainage therapy. We conclude that RFA may present a therapeutic alternative to PDT for palliative treatment of malignant biliary obstruction due to its simple feasibility and moderate adverse event rate. A limitation of this study is the small number of patients. Because of the small number of patients in the RFA group (14 patients) only a reduced evaluation is possible.
Similarly, in the historical group of PDT-treated patients, the adverse event rate was comparable with other studies. A median of 1.8 PDT per patient was applied, which is in the range (1.5–3) of previous studies.4 Cholangitis is a well-recognized adverse event in approximately 25% (0–56%) of all cases after PDT.4 In this study in six patients (30%), cholangitis was noticed after PDT. In one patient it progressed to a liver abscess (5%) and in one patient to sepsis (5%), which were treated by drainage therapy. In two patients (10%) a phototoxic reaction of the skin developed after PDT, which was treated by symptomatic therapy. In earlier studies, phototoxicity was reported in 14% (4%–33%) of all cases.4 In summary, in both study groups the adverse event rate is—in addition to the low phototoxicity rate in PDT patients—comparable, and excludes no treatment method.
Similar to this study, Strand et al. could show that RFA and PDT have generally comparable rates of adverse events contrasting both methods in a retrospective analysis.25 Placing metal stents in all patients after RFA they noticed significantly increased rates of occlusion and cholangits when compared with the PDT group. The authors speculated that significantly more follow-up ERCPs, reapplication of PDT, and exchanging of plastic stents in the PDT arm might be responsible for fewer occurrences of obstruction and cholangitis.25 This hypothesis is supported by this study in which no significant differences in adverse events were noticed between both methods. Here, type and size of stents, time intervals of scheduled stent replacements, and numbers of applications of PDT/RFA did not differ between the RFA and PDT arm.
To our knowledge this is the first study that investigated the QoL of patients with RFA treatment. It showed that RFA application basically had no negative effect on the QoL of the patients. To analyze the effect on daily living, we retrospectively and since December 2012 prospectively evaluated questionnaires for QoL before and after each RFA application. Function capacity and symptom severity were assessed by using a 10-point scale. Interestingly, it could be observed that after the second and third application of RFA, functional scales like physical, role, and cognitive improved slightly. Similarly, symptom scales like diarrhea, sleep disturbances, loss of appetite, obstipation, and financial burden tended to recover after the second RFA treatment. Adversely, all symptom scales deteriorated after the third RFA application—most probably because of the progression of the disease.
Limitations of this study are the small number of patients and the comparison of retrospectively collected RFA and PDT data. To overcome the weakness of the latter, we analyzed only those parameters that were suitable for comparison in a similar period: short-term effects of drainage therapy, the necessity of premature stent replacements, and adverse events after application of one of the procedures. In this regard, we tried to take possible confounders into consideration. Tumor stage and performance status at initial diagnosis, pretreatment bilirubin, diameter of stents, and number of procedures could be shown not to differ in both study groups. Regarding the drainage effects and the adverse events rates, the newly introduced RFA procedure appears not to be inferior to PDT therapy. Finally, of course no statements can be made by this study about the long-term effects like overall survival. A multicenter trial comparing both methods prospectively is urgently required.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
None declared.
References
- 1.Khan SA, Davidson BR, Goldin RD, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: An update. Gut 2012; 61: 1657–1669. [DOI] [PubMed] [Google Scholar]
- 2.Born P, Rösch T, Brühl K, et al. Long-term outcome in patients with advanced hilar bile duct tumors undergoing palliative endoscopic or percutaneous drainage. Z Gastroenterol 2000; 38: 483–489. [DOI] [PubMed] [Google Scholar]
- 3.Ortner ME, Caca K, Berr F, et al. Successful photodynamic therapy for nonresectable cholangiocarcinoma: A randomized prospective study. Gastroenterology 2003; 125: 1355–1363. [DOI] [PubMed] [Google Scholar]
- 4.Kahaleh M, Mishra R, Shami VM, et al. Unresectable cholangiocarcinoma: Comparison of survival in biliary stenting alone versus stenting with photodynamic therapy. Clin Gastroenterol Hepatol 2008; 6: 290–297. [DOI] [PubMed] [Google Scholar]
- 5.Witzigmann H, Berr F, Ringel U, et al. Surgical and palliative management and outcome in 184 patients with hilar cholangiocarcinoma: Palliative photodynamic therapy plus stenting is comparable to r1/r2 resection. Ann Surg 2006; 244: 230–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zoepf T, Jakobs R, Arnold JC, et al. Palliation of nonresectable bile duct cancer: Improved survival after photodynamic therapy. Am J Gastroenterol 2005; 100: 2426–2430. [DOI] [PubMed] [Google Scholar]
- 7.Shim CS, Cheon YK, Cha SW, et al. Prospective study of the effectiveness of percutaneous transhepatic photodynamic therapy for advanced bile duct cancer and the role of intraductal ultrasonography in response assessment. Endoscopy 2005; 37: 425–433. [DOI] [PubMed] [Google Scholar]
- 8.Wiedmann M, Berr F, Schiefke I, et al. Photodynamic therapy in patients with non-resectable hilar cholangiocarcinoma: 5-year follow-up of a prospective phase II study. Gastrointest Endosc 2004; 60: 68–75. [DOI] [PubMed] [Google Scholar]
- 9.Takamura A, Saito H, Kamada T, et al. Intraluminal low-dose-rate 192Ir brachytherapy combined with external beam radiotherapy and biliary stenting for unresectable extrahepatic bile duct carcinoma. Int J Radiat Oncol Biol Phys 2003; 57: 1357–1365. [DOI] [PubMed] [Google Scholar]
- 10.Shinohara ET, Guo M, Mitra N, et al. Brachytherapy in the treatment of cholangiocarcinoma. Int J Radiat Oncol Biol Phys 2010; 78: 722–728. [DOI] [PubMed] [Google Scholar]
- 11.Bruix J, Sherman M. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: An update. Hepatology 2011; 53: 1020–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu HX, Wang Y, Lu MD, et al. Percutaneous ultrasound-guided thermal ablation for intrahepatic cholangiocarcinoma. Br J Radiol 2012; 85: 1078–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim JH, Won HJ, Shin YM, et al. Radiofrequency ablation for the treatment of primary intrahepatic cholangiocarcinoma. AJR Am J Roentgenol 2011; 196: W205–W209. [DOI] [PubMed] [Google Scholar]
- 14.Steel AW, Postgate AJ, Khorsandi S, et al. Endoscopically applied radiofrequency ablation appears to be safe in the treatment of malignant biliary obstruction. Gastrointest Endosc 2011; 73: 149–153. [DOI] [PubMed] [Google Scholar]
- 15.Figueroa-Barojas P, Bakhru MR, Habib NA, et al. Safety and efficacy of radiofrequency ablation in the management of unresectable bile duct and pancreatic cancer: A novel palliation technique. J Oncol 2013; 2013: 910897–910897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med 2003; 29: 530–538. [DOI] [PubMed] [Google Scholar]
- 17.Weber A, Gaa J, Rosca B, et al. Complications of percutaneous transhepatic biliary drainage in patients with dilated and nondilated intrahepatic bile ducts. Eur J Radiol 2009; 72: 412–417. [DOI] [PubMed] [Google Scholar]
- 18.Lee TY, Cheon YK, Shim CS. Current status of photodynamic therapy for bile duct cancer. Clin Endosc 2013; 46: 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petersen BT, Chuttani R, Croffie J, et al. Photodynamic therapy for gastrointestinal disease. Gastrointest Endosc 2006; 63: 927–932. [DOI] [PubMed] [Google Scholar]
- 20.Soares KC, Kamel I, Cosgrove DP, et al. Hilar cholangiocarcinoma: Diagnosis, treatment options, and management. Hepatobiliary Surg Nutr 2014; 3: 18–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hezel AF, Zhu AX. Systemic therapy for biliary tract cancers. Oncologist 2008; 13: 415–423. [DOI] [PubMed] [Google Scholar]
- 22.Hong MJ, Cheon YK, Lee EJ, et al. Long-term outcome of photodynamic therapy with systemic chemotherapy compared to photodynamic therapy alone in patients with advanced hilar cholangiocarcinoma. Gut Liver 2014; 8: 318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dolak W, Schreiber F, Schwaighofer H, et al. Endoscopic radiofrequency ablation for malignant biliary obstruction: A nationwide retrospective study of 84 consecutive applications. Surg Endosc 2014; 28: 854–860. [DOI] [PubMed] [Google Scholar]
- 24.Tal AO, Vermehren J, Friedrich-Rust M, et al. Intraductal endoscopic radiofrequency ablation for the treatment of hilar non-resectable malignant bile duct obstruction. World J Gastrointest Endosc 2014; 6: 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strand DS, Cosgrove ND, Patrie JT, et al. RCP-directed radiofrequency ablation and photodynamic therapy are associated with comparable survival in the treatment of unresectable cholangiocarcinoma. Gastrointest Endosc 2014; 80: 794–804. [DOI] [PubMed] [Google Scholar]
- 26.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. Journal of the National Cancer Institute 1993; 85: 365–376. [DOI] [PubMed]