Abstract
Fistulas and fibrosis or strictures represent frequent complications in irritable bowel disease (IBD) patients. To date, treatment options for fistulas are limited and surgery is often required. Similarly, no preventive treatment for fibrosis and stricture formation has been established. Frequently, stricture formation and fibrosis precede fistula formation, indicating that both processes may be connected or interrelated. Knowledge about the pathology of both processes is limited. A crucial role for the epithelial-to-mesenchymal transition (EMT) in fistula development has been demonstrated. Of note, EMT also plays a major role in the pathogenesis of fibrosis in many organs, and most likely also plays that role in the intestine. In addition, aberrant matrix remodeling, as well as soluble factors such as tumor necrosis factor (TNF), interleukin 13 (IL-13) and tumor growth factor beta (TGFβ) were involved, both in the onset of the fistula and fibrosis formation. Both fistulas and fibrosis may occur due to deregulated wound healing mechanisms from chronic and severe intestinal inflammation; however, further research is required to obtain a better understanding of the complex pathophysiology of fistula and intestinal fibrosis formation, to allow the development of new and more effective preventive treatment options for those important disease complications.
Keywords: Epithelial-mesenchymal transition, fibrosis, fistula, interleukin 13, irritable bowel disease, metalloproteinase, tumor growth factor beta, wound healing
Introduction
Though most of inflammatory bowel disease (IBD) patients initially present with an inflammatory disease phenotype, about one-third of the patients already feature evidence of a strictured or penetrating intestinal complication at the time of diagnosis.1,2 Due to the longstanding and chronically relapsing nature of the disease, the initial inflammatory disease phenotype often changes towards a strictured and/or penetrating phenotype. This results in typical disease complications, such as the formation of stenosis or strictures, and/or fistulas.
About 70% of Crohn’s disease (CD) patients suffer from fistula or stenosis and the resulting intestinal obstruction during their lifetime; and almost two-thirds of them require surgery at least once within the 20 years following their initial diagnosis.3 Fistulas affect between 17–50% of CD patients during their disease course. The most common fistula subtype is the perianal fistula.4 While extensive disease at diagnosis is associated with the development of fistulas4, patients with ileitis alone and patients after laparotomy in combination with resection of the bowel do have a reduced risk for fistula occurrence.4,5
It is generally assumed that inflammation is a necessary trigger for both fibrosis and fistula formation. This has not formally been shown, as animal models such as mouse models only rarely and late may develop fibrosis, and usually there are no strictures of fistulas; however, the concept of an inflammatory trigger is generally accepted. For the further pathophysiological process of fibrosis or fistula formation, inflammation may only play a minor role. Anti-inflammatory treatment in IBD may not prevent fibrosis once excessive extracellular matrix (ECM) deposition has started, as outlined in a recent review by the members of the fourth scientific workshop of the ECCO (European Crohn's and Colitis Organisation).6,7 The pathophysiological mechanisms that are triggering and perpetuating fistula and/or fibrosis formation may be distinct from the ones regulating inflammation, especially chronic inflammation. This becomes important with respect to new therapeutic concepts such as SMAD7 antisense oligonucleotides that upregulate transforming growth factor beta (TGFβ) expression. A downregulation of the proinflammatory stimuli may well be associated with a pro-fibrotic effect. Therefore, caution should be applied when investigating the long-term effects of such a treatment.
Despite significant progress in the treatment of intestinal inflammation, the treatment and prevention of intestinal fibrosis and fistula formation is still in its infancy. We are only beginning to understand the pathophysiological mechanisms, which share some features, but of course also have distinct pathways and triggers. None of our recent therapeutic advances in IBD and none of the ones that so far can be foreseen in the next upcoming years will prevent, nor reverse, established fistulas or strictures with a satisfying success rate. This implies that (similar to what we have recently discussed for fibrosis)6 controlling inflammation may not affect the fistulas, once they have formed. The lack of a successful medical fistula treatment is partly due to the specific cellular and molecular pathways that lead to fistula formation. We need to understand further why epithelial-to-mesenchymal transition (EMT) occurs during fistula formation, and how it can be reverted. Thus, we have an urgent need for preclinical animal fistula models. Also, fistula drug development is hindered by the unpredictable evolution of fistulas, which may be complicated by an abscess at any time and by the recurrence of a fistular tract after cessation of treatment, making clinical trials long and expensive.
Similar to what has been discussed for fibrosis, it is necessary to view fistula formation as a pathological process that is distinct from inflammation, to finally be able to design and investigate specific and effective fistula-preventing or fistula-healing drugs. Understanding the pathways for fistula formation and comparing them to frequently coincident fibrosis formation will certainly help to achieve progress in this way.
Fistulas as a clinical problem
Fistulas represent a severe complication of CD and a still unresolved medical problem for treatment of CD patients. This is highlighted by the fact that fistula healing is hardly achievable and recurrences are frequent. Fistulas in CD patients often impair the quality of life because of the above mentioned limited treatment options. In population-based cohorts and meta-analyses the cumulative incidence of fistula formation varies largely between 17–50%.4,8–12 Schwartz et al.4 report a fistula occurrence of 35% over time in the Olmsted County cohort; with 54% of those being perianal, 24% being entero-enteric, 9% being rectovaginal and 13% involving other locations, such as entero-cutaneous, entero-vesical and intra-abdominal fistulas. One-third of the patients in this cohort had recurrent fistulas. Typical symptoms of perianal fistulas are persistent anal pain, painful defecation and purulent discharge.
Clinically, fistulas are classified as ‘simple fistulas’ (below the dentate line, single external opening, not painful, and with no evidence of rectovaginal fistula nor evidence of anorectal stricture) or ‘complex fistulas’ that are above the dentate line, have multiple openings, have the evidence of an abscess that is potentially associated with pain, and the presence of a rectovaginal fistula or anorectal stricture.13 Not surprisingly, the risk of perianal fistula development is higher in patients with colonic CD, in particular in those with rectal involvement.
In the Crohn’s disease activity index (CDAI), only 20 points are attributed to the presence of fistulas, regardless of the number and type, indicating that this index is not useful to characterize the burden caused by a fistulizing disease course. In addition, in many clinical trials currently under way and in recent years, the presence of a fistula was an exclusion criterion, limiting the evidence on potential upcoming therapies for fistula patients. Clinical trials of fistula therapies suffer from the lack of a reliable, reproducible and well-accepted index for fistulizing CD.
The placebo response to any fistula treatment is between 10% and 20%, which was confirmed in a meta-analysis by Pascua et al.14
Glucocorticoids may worsen fistula activity and increase the need for surgery and should subsequently be used with care in patients with fistulizing disease.15–17 This may indicate a fistulae-promoting effect of steroids that should be further investigated. Antibiotics such as metronidazole and ciprofloxacin are effective for the short-term management of fistulas and fistula-associated abscesses; however, the recurrence rate at withdrawal is high and complete healing is rarely achieved with antibiotics alone. Upon treatment with 6-mercaptopurine (6-MP), 31% of the fistulas present closed completely during the treatment, versus only 6% with placebo, in a clinical trial by Present et al.18 In the ACCENT 2 trial on fistulizing CD, out of 306 actively treated patients, 64% had an initial response with 50% or more of the fistulas closed.19 Of these initial responders, 36% showed healing after 52 weeks of treatment, compared with 19% of those treated with placebo.
In the CHARM trial on adalimumab, 113 patients also had perianal fistulas: 31% (21 of 70) of all randomized patients on active adalimumab maintenance treatment had complete fistula healing, compared with 13% (6 of 47) on placebo maintenance.20
Between 20–80% of CD patients with perianal fistulas will eventually require surgery and up to 30% of patients with complicated perianal CD may eventually require a permanent stoma.21,22
Fistula pathogenesis
A fistula represents a tract between two epithelial-lined surfaces. CD-associated fistulas occur in up to 50% of patients.23,24 The prevalence of perianal fistulas increases with disease duration and more distal localization of intestinal disease.23,25 Noteworthy is that in particular, perianal fistulas are not specific for CD, because they can also occur during infection, hidradenitis suppurativa and malignancy. Tuberculosis can histologically mimic CD, but is, at least in the Western world, much rarer than CD. Nevertheless, fistular perianal disease can also develop.26,27 Diagnosis of fistula is not based on histology, but rather on clinical assessment. Nowadays, classification systems of perianal disease do not require histology; however, histologic assessment can still be necessary in clinical practice, for example to confirm CD diagnosis and/or to exclude other underlying pathologies.28
The histologic features of CD fistulas are nonspecific. Often, the fistular tract may be identifiable microscopically and is lined by granulation tissue and/or ‘squamous’ epithelium. It is typically filled with debris, erythrocytes and acute inflammatory cells.23 Chronic inflammation and surrounding fibrosis are commonly observable, and granulomas may be detected in and around perianal fistulas. Fistulas may also occur before a patient gets diagnosed with CD. Fistulas probably arise as a chronic consequence of an acute inflammatory process with infection and suppuration.29 For example, a deep penetrating ulcer in the rectum or anus might fill with fecal material that is forced into the underlying tissue by luminal pressure. Anal gland or anal duct abscesses could also serve as a point of origin. The process of tissue destruction may be maintained by luminal antigens and bacteria.
Only a few studies have investigated the pathophysiology and histology of CD-associated fistulas. It has been demonstrated that intestinal and perianal fistulas from CD and non-CD patients feature flattened intestinal or narrow squamous epithelium in about 27–31% of cases. All of those investigated fistulas were surrounded by granulation tissue. Interestingly, in ‘non-epithelialized’ areas of the fistulas, there was a lining of myofibroblast-like cells (so-called ‘transitional cells’) that even form a new basement membrane (BM). Only CD fistulas, but not fistulas from otherwise healthy patients, exhibit areas with disordered myofibroblasts and fragmented BM. This suggests that mechanisms of fistula formation in CD differ from those in other diseases23; however, one might consider that medical treatment of CD also might affect histologic appearance.23
A further characteristic feature of CD fistulas is the presence of inflammatory cell populations in and around the fistulas. It was described that CD fistulas typically feature a central infiltration by CD45R0+ T cells, an underlying band of CD68+ macrophages, and a dense infiltrate of CD20+ B cells infiltrate into the outer wall. In contrast to the CD fistulas, control fistulas typically exhibit a dense macrophage infiltrate, and only few CD20+ B cells or CD45 R0+ T cells.23 In another study, accumulation of CD4 + CD161+ T cells with a Th17, Th17/Th1 and Th1 phenotype in CD perianal fistulas was demonstrated.30
Epithelial to mesenchymal transition
The general concept and possibly the driving force behind the development of CD-associated fistulas, as well as of intestinal fibrosis, is likely to be the so-called EMT. In general, EMT is a physiological process involved in embryogenesis, organ development, wound healing and tissue remodeling, but also plays a major role in pathological processes, such as tissue fibrosis and cancer progression.31,32 During the mechanism of EMT, epithelial cells lose essential epithelia-defining properties, including apico-basal polarity and epithelial-specific cell contacts; and gain qualities of mesenchymal cells (e.g. increased motility and cell spreading).31 Though EMT is an elementary process for several development steps in embryology, it also appears to be a central process in tumor development. EMT is characterized by downregulation of epithelia-specific proteins such as E-cadherin or claudin-4, accompanied by upregulation of mesenchymal proteins such as vimentin. This is partially due to coordinated regulation of a distinct set of transcription factors, such as SNAIL1, SLUG or TWIST.31
Recent studies demonstrate that EMT plays a critical role in the pathogenesis of CD-associated fistulas.23,33 CD fistula tracts are covered by intestinal epithelial cells (IEC), as well as by cells featuring mesenchymal-myofibroblast-like characteristics, the so-called ‘transitional cells’ (TC). These TC arise from highly polarized IEC by EMT, and express molecular markers that are typical for mesenchymal cells, such as vimentin and alpha smooth-muscle-actin (α-SMA), as well as for epithelial cells, like the cytokeratines (CKs) CK-8 and CK-20.33
Interestingly, such EMT-like events were also shown to occur in fibrotic regions of the intestine of ulcerative colitis (UC) patients. Here, fibroblast-like cells within fibrotic areas featured strongly express α-SMA and vimentin as markers of mesenchymal cells, but additionally revealed considerable staining with IEC markers such as CK-8, CK-20 and E-cadherin, indicating their epithelial origin. In these cells, there is also a nuclear localization of β-catenin and of the transcription factor SLUG, both being events that have been implicated in EMT development that can be detected. Furthermore, one can observe a strong expression of TGFβ, the most powerful driving force for EMT, in and around the CD fistula tracts, as well as of the UC fibrotic lesions.33,34 A further hint to the involvement of EMT in fistula pathogenesis is the detection of the SNAIL family transcription factors in and around CD-associated fistulas. While SNAIL1 is readily detected in the nuclei of TC lining the fistula tracts, SLUG can be detected in cells around the fistula tracts, but is almost absent in TC.35
Molecules of potential pathophysiological relevance during fistula formation
Evidence has been provided that IL-13 is strongly expressed in TC lining the fistula tract and to some lesser extent in fibrotic areas around the fistulas. Of note, TGFβ, which is strongly expressed around CD fistulae, induces the secretion of IL-13 from colonic lamina propria fibroblasts (CLPF) derived from patients with fistulizing disease, but not from non-IBD control patients nor CD patients without fistulas, suggesting a specific amplification loop.24,33 IL-13 also induces the expression of the EMT-associated transcription factor SLUG in an in vitro model of EMT using HT29 IEC spheroids, as well as of β6-integrin, a protein that is associated with cell invasiveness.24 Furthermore, tumor necrosis factor (TNF) and TNF-receptor 1 are also strongly expressed in CLPF lining CD-associated fistula tracts, and in surrounding fibrotic intestinal tissue, further supporting the ‘amplification loop’ theory.35 We demonstrated that TNF induces EMT and the expression of EMT-associated genes in HT29 spheroids.36 In addition, the Wnt-antagonist Dickkopf-homolog 1 (DKK-1) is expressed along fistula tracts in CD patients. The DKK-1 protein is induced by TGFβ and TNF, and limits TGFβ-induced IL-13 expression.37 Of note, also bacterial components seem to play a role for fistula development, since the bacterial wall component, muramyl-dipeptide (MDP), induces EMT in IEC, as well as the expression of fistula-associated molecules in IEC and fistula CLPF.36
In a previous study, a strong expression of matrix remodeling enzymes, matrix metalloproteinase (MMP)-3 and MMP-9 were observed in CD fistulae; while the expression of tissue inhibitors of metalloproteinases (TIMP)-1, TIMP-2 and TIMP-3 was low, compared with normal colon. This suggests that an altered MMP and TIMP balance might critically contribute to fistula formation, through enhanced ECM degradation.38 Of note in CD patients, fibrotic areas are often in close vicinity to fistulae and fistulae are almost always surrounded by fibrotic tissue. Interestingly, increased levels of IL-13 in the fibrotic intestine of CD patients are produced by a previously not described population of cells expressing high levels of IL-13Rα1 and IL-13 in the muscle layer of CD intestine.39 The phenotype of these cells (KIR+ CD45+ CD56+/− CD3− IL-13Rα1+) suggests that they belong to the spectrum of innate lymphoid cells (ILC). It has been shown that fibroblasts down-regulate MMP-2 as well as TNF-induced MMP-1 and MMP-9 in response to IL-13.39 All of those observations strongly suggest EMT-like processes in the pathogenesis of CD-associated fistulae (Figure 1).23,24,33,35–37 In addition, fistula-associated molecules seem to be associated with the development of so-called fistula-carcinomas in CD patients.40
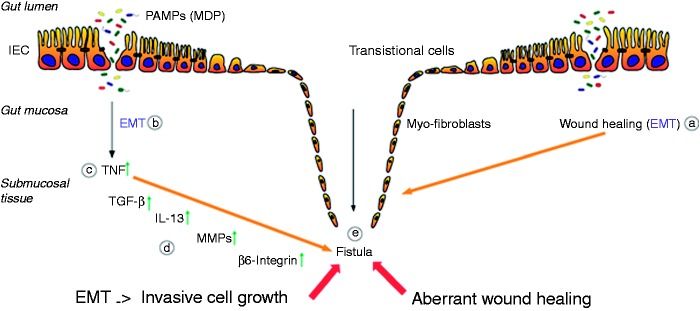
Figure 1.
Pathogenesis of Crohn’s disease-associated fistulae. Due to an epithelial barrier defect several PAMPs, e.g. MDP, are able to enter the gut mucosa. Both the process of wound repair (a) and the inflammatory response caused by PAMPs (b), induce the event of EMT. First, an increased expression of TNF is initiated (c), resulting in an upregulation of TGF-β production. This triggers the expression and secretion of IL-13 as well as of molecules associated with cell invasiveness, as β6-integrin (d). The enhanced activity of MMPs, as well as the upregulation of the protein expression, favors the transformation of the IECs towards the invasive myofibroblast forms, which finally results in the fistula formation (e).
EMP: epithelial-to-mesenchymal transition; IEC: intestinal epithelial cell; IL-13: interleukin 13; MDP: muramyl dipeptide; MMPs: membrane metalloproteinases; PAMPs: pathogen-associated molecular patterns; TGFβ: tumor growth factor beta; TNF: tumor necrosis factor
Wound healing
During IBD, severe mucosal tissue damage occurs that requires efficient wound healing mechanisms. This tissue damage is due to the effects of macrophages and neutrophils that induce local tissue damage. These cells secrete reactive oxygen radicals and tissue-degrading enzymes and release pro-inflammatory cytokines, chemotactic and cell-activating peptides.41 In cases of severe tissue damage, so-called myofibroblasts migrate to these areas with mucosal defects. These myofibroblasts then exhibit the ability to contract the wound area and to produce ECM, to limit the extent of tissue damage.41 In the setting of acute intestinal inflammation, a limited tissue damage occurs that finally results in a complete restitution of the damaged tissue. More severe acute or moderate chronic inflammation causes severe or chronic tissue degradation and damage. These events are normally followed by tissue repair of what might already cause fibrosis and scars. Severe acute and long-lasting chronic tissue damage can finally result in severe fibrosis, which is what promotes development of intestinal strictures and obstruction.41 In the pathogenesis of CD fistulas, one can observe a lowered migration of myofibroblasts, aberrant ECM production and, as a compensatory mechanism, the IECs invade the wounded area aiming to close the wound area. In contrast, in the development of intestinal fibrosis, one can observe increased proliferation and migration of myofibroblasts, as well as enhanced matrix synthesis.41
Comparison of fistula formation and intestinal fibrosis
On a molecular level, fibrosis is defined as the excessive accumulation of ECM that finally leads to organ dysfunction.31 The underlying key factors are chronic tissue damage in IBD patients due to chronic inflammation, aberrant wound healing and an expansion of mesenchymal cells (namely fibroblasts, myofibroblasts and smooth muscle cells).42 In general, fibroblasts are continuously producing a certain amount of ECM; however, in response to injury or inflammation, the mesenchymal cells rapidly proliferate, invade the affected sites from within and without the intestine following the chemical gradient of certain growth factors, and are finally activated by a mix of cytokines secreted from immune and non-immune cells.42 As a result, these mesenchymal cells produce excessive amounts of ECM, mainly collagens43; however, in both CD and UC, the expression and activity of MMP as well as of their inhibitors TIMP are elevated; this suggests that intestinal fibrosis in IBD is not only the result of excessive local ECM production, but rather of an imbalance in the regular tissue-remodeling processes.41 Interestingly, aberrant matrix remodeling and ECM production and turn-over seem to be characteristic features of both fistulas and fibrosis. This is of particular interest, since CD fistulas are mainly surrounded by fibrotic tissue: This might be explained by the body’s aim of wound healing around the fistula tract. As the fistula might result from defective wound healing mechanisms, the fistula-surrounding fibrosis might serve the aim of limiting tissue damage and fistula growth, representing a rescue mechanism of the intestine. This theory is further supported by the fact that fibroblasts derived from dense fibrosis tissue reveal stronger migratory potential than CLPF associated with fistula formation. Alternatively, to compensate for the disability of these cells to repair tissue defects, epithelial cells might be reprogrammed via EMT, allowing them to migrate to the affected spot, which finally results in fistula formation due to this mechanism running out of control.44
Several studies strongly suggest an involvement of cytokines, such as IL-13 and TNF, as well as of growth factors such as TGFβ, in the pathogenesis of intestinal fibrosis.41,42 Interestingly, TNF and IL-13, as well as their receptors, were demonstrated to be highly expressed in TC lining fistula tracts, supporting similar mechanisms for the development of fistulas and of intestinal fibrosis. This observation is further supported by the observation that EMT seems to be critical for fistula development and that hallmarks of EMT can also be detected in intestinal fibrosis.34,45 TGFβ as the main inducer of EMT is also highly detectable in fistulas, as well as in regions of fibrosis.33,46 Moreover, β-catenin was found to be less expressed in the membrane, but strongly in the nucleus, hinting at transcriptional activity in fibrotic areas, as well as in the fistula region; however, SLUG expression was also clearly observed in the nuclei of mesenchymal cells in fibrotic areas, whereas in TC of CD fistulas, only poor expression was detectable.34,35 Nevertheless, it must be mentioned that all the papers cited regarding the pathogenetic role for EMT in Crohn's intestinal fistulas and fibrosis are based only on descriptive results obtained by hematoxylin-eosin staining, immunohistochemistry and electron microscopy. Thus, due to the current lack of functional studies on this topic, the actual relevance for EMT in fistula and fibrosis pathogenesis in CD patients still needs to be further confirmed.
A case report for a fistula-associated anal adenocarcinoma was reported that showed remarkable staining of the SLUG transcription factor in TC lining the fistula tract; however, this could also be associated with the carcinoma originating from those cells.40 While in fibrosis development, IL-13 induces TGFβ secretion and this is opposite in fistula development: Here, fistula myofibroblasts secrete IL-13 in response to TGFβ24,46; however, recently conflicting results were shown concerning the influence of IL-13 in gut fibrosis in stricturing CD.47 On this basis, a possible pro-fibrogenic role of IL-13 in CD needs to be further investigated critically. Indeed, fistula formation and intestinal fibrosis reveal several similarities, but also some remarkable differences (Figure 2). This clearly requires further studies, for a better understanding of both pathologies (Table 1). The complexity of all implicated factors is also illustrated by the fact that IFNγ was able to induce fibroblast apoptosis together with TNF, in an in vitro model studying fibrosis.48,49 In contrast, TNF was reported to induce intestinal fibrosis by upregulating collagen accumulation, extending the inflammatory state.49
Figure 2.
Comparison of fistulae formation and intestinal fibrosis formation in Crohn’s disease. Besides some differences in the development of both disease complications, there are also many similarities (orange ellipse), especially in regard to the mechanism of EMT.
ECM: extracellular matrix; EMT: epithelial-to-mesenchymal transition; IL-13: interleukin 13; MMP: membrane metalloproteinase; TC: transitional cells; TGFβ: tissue growth factor beta; TIMP: tissue inhibitor of metalloproteinase; TNF: tumor necrosis factor
Table 1.
Summary of similarities and differences in the pathophysiology of fistula formation and fibrosis. Common and distinct mechanisms in the pathogenesis of CD-associated fistula and fibrosis development: After common initial events, which lead to the onset of EMT, various signaling pathways are induced, which finally result in the formation of either fistula or fibrosis.
| Fistula | Fibrosis | ||
|---|---|---|---|
| Similarities | >Triggered by chronic inflammation | ||
| >Resulting stimulation of TNF expression | |||
| >Onset of EMT | |||
| >Different signaling cascades lead to an imbalance of MMPs and TIMPs | |||
| >Invasiveness of myofibroblasts | |||
| Differences | Signaling cascade after TNF upregulation: | Signaling cascade after TNF upregulation: | |
| strong expression of >TGFβ > IL-13 > MMPs > SNAIL1 and β6-Integrin | Regulation: TGFβ also induces DKK-1, which limits IL-13 expression | strong expression of >IL-13 > TGFβ > MMPs > excessive ECM deposition | |
CD: Crohn’s disease; DKK1: Dickkopf homolog 1; ECM: extracellular matrix; EMT: epithelial-to-mesenchymal transition; IL-13: interleukin 13; MMP: membrane metalloproteinase; TIMP: tissue inhibitor of metalloproteinase; TGF: tissue growth factor; TNF: tumor necrosis factor
Conclusions
Wound healing represents a common and important event during acute and chronic intestinal inflammation. If wound healing mechanisms are defective, either fistulas or fibrosis and subsequent stenosis/strictures can occur. On a pathogenetic level, EMT seems to be a crucial mechanism for the development of both fistulas as well as fibrosis; however, the exact mechanisms for their development are not yet defined and further studies are needed. In particular, since to date treatment options for fistula therapy are limited and fistula repair therefore represents one of the big unmet goals in IBD therapy, further fistula research is clearly needed.50
Funding
This work was supported by the Swiss National Science Foundation (grant numbers 314730-146204 and CRSII3_154488/1 to author MS) and the Swiss Irritable Bowel Disease Cohort (grant number 3347CO-108792 to author GR).
Conflict of interest
The authors declare that there is no conflict of interest. The funding institutions had no role in the study design and data interpretation. The authors declare the following previous funders also had no influence: GR had consulted to Abbot, Abbvie, Augurix, Boehringer, Calypso, FALK, Ferring, Fisher, Genentech, Essex/MSD, Novartis, Pfizer, Phadia, Roche, UCB, Takeda, Tillots, Vifor, Vital Solutions and Zeller; GR had received speaker's honoraria from Astra Zeneca, Abbott, Abbvie, FALK, MSD, Phadia, Tillots, UCB, and Vifor; GR had received educational grants and research grants from Abbot, Abbvie, Ardeypharm, Augurix, Calypso, Essex/MSD, FALK, Flamentera, Novartis, Roche, Takeda, Tillots, UCB and Zeller. MS had received speaker's honoraria from FALK. RB had no potential conflict of interest to disclose.
References
- 1.Cosnes J, Cattan S, Blain A, et al. Long-term evolution of disease behavior of Crohn's disease. Inflamm Bowel Dis 2002; 8: 244–250. [DOI] [PubMed] [Google Scholar]
- 2.Peyrin-Biroulet L, Loftus EV, Jr., Colombel JF, et al. The natural history of adult Crohn's disease in population-based cohorts. Am J Gastroenterol 2010; 105: 289–297. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein CN, Loftus EV, Jr., Ng SC, et al. Hospitalisations and surgery in Crohn's disease. Gut 2012; 61: 622–629. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz DA, Loftus EV, Jr., Tremaine WJ, et al. The natural history of fistulizing Crohn's disease in Olmsted County, Minnesota. Gastroenterology 2002; 122: 875–880. [DOI] [PubMed] [Google Scholar]
- 5.Kruis W, Scheuchenstein AM, Scheurlen C, et al. [Risk factors for the development of fistulas in Crohn disease]. Z Gastroenterol 1989; 27: 313–316. [PubMed] [Google Scholar]
- 6.Latella G, Rogler G, Bamias G, et al. Results of the 4th scientific workshop of the ECCO (I): Pathophysiology of intestinal fibrosis in IBD. J Crohns Colitis 2014; 8: 1147–1165. [DOI] [PubMed] [Google Scholar]
- 7.Lawrance IC, Rogler G, Bamias G, et al. Cellular and molecular mediators of intestinal fibrosis. J Crohns Colitis. Epub ahead of print 2 November 2015, pii: j.crohns.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKee RF, Keenan RA. Perianal Crohn's disease: Is it all bad news? Dis Colon Rectum 1996; 39: 136–142. [DOI] [PubMed] [Google Scholar]
- 9.Van Dongen LM, Lubbers EJ. Perianal fistulas in patients with Crohn's disease. Arch Surg 1986; 121: 1187–1190. [DOI] [PubMed] [Google Scholar]
- 10.Judge TA, Lichtenstein GR. Treatment of fistulizing Crohn's disease. Gastroenterol Clin North Am 2004; 33: 421–454, xi-xii. [DOI] [PubMed] [Google Scholar]
- 11.Hellers G, Bergstrand O, Ewerth S, et al. Occurrence and outcome after primary treatment of anal fistulae in Crohn's disease. Gut 1980; 21: 525–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Solomon MJ. Fistulae and abscesses in symptomatic perianal Crohn's disease. Int J Colorectal Dis 1996; 11: 222–226. [DOI] [PubMed] [Google Scholar]
- 13.Sandborn WJ, Fazio VW, Feagan BG, et al. AGA technical review on perianal Crohn's disease. Gastroenterology 2003; 125: 1508–1530. [DOI] [PubMed] [Google Scholar]
- 14.Pascua M, Su C, Lewis JD, et al. Meta-analysis: Factors predicting post-operative recurrence with placebo therapy in patients with Crohn's disease. Aliment Pharmacol Ther 2008; 28: 545–556. [DOI] [PubMed] [Google Scholar]
- 15.Jones JH, Lennard-Jones JE. Corticosteroids and corticotrophin in the treatment of Crohn's disease. Gut 1966; 7: 181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sparberg M, Kirsner JB. Long-term corticosteroid therapy for regional enteritis: An analysis of 58 courses in 54 patients. Am J Dig Dis 1966; 11: 865–880. [DOI] [PubMed] [Google Scholar]
- 17.Malchow H, Ewe K, Brandes JW, et al. European Cooperative Crohn's Disease Study (ECCDS): Results of drug treatment. Gastroenterology 1984; 86: 249–266. [PubMed] [Google Scholar]
- 18.Present DH, Korelitz BI, Wisch N, et al. Treatment of Crohn's disease with 6-mercaptopurine. A long-term, randomized, double-blind study. N Engl J Med 1980; 302: 981–987. [DOI] [PubMed] [Google Scholar]
- 19.Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn's disease. N Engl J Med 2004; 350: 876–885. [DOI] [PubMed] [Google Scholar]
- 20.Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: The CHARM trial. Gastroenterology 2007; 132: 52–65. [DOI] [PubMed] [Google Scholar]
- 21.Mueller MH, Geis M, Glatzle J, et al. Risk of fecal diversion in complicated perianal Crohn's disease. J Gastrointest Surg 2007; 11: 529–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loffler T, Welsch T, Muhl S, et al. Long-term success rate after surgical treatment of anorectal and rectovaginal fistulas in Crohn's disease. Int J Colorectal Dis 2009; 24: 521–526. [DOI] [PubMed] [Google Scholar]
- 23.Bataille F, Klebl F, Rummele P, et al. Morphological characterisation of Crohn's disease fistulae. Gut 2004; 53: 1314–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scharl M, Frei S, Pesch T, et al. Interleukin-13 and transforming growth factor beta synergise in the pathogenesis of human intestinal fistulae. Gut 2013; 62: 63–72. [DOI] [PubMed] [Google Scholar]
- 25.Gecse KB, Bemelman W, Kamm MA, et al. A global consensus on the classification, diagnosis and multidisciplinary treatment of perianal fistulising Crohn's disease. Gut 2014; 63: 1381–1392. [DOI] [PubMed] [Google Scholar]
- 26.Yu H, Liu Y, Wang Y, et al. Clinical, endoscopic and histological differentiations between Crohn's disease and intestinal tuberculosis. Digestion 2012; 85: 202–209. [DOI] [PubMed] [Google Scholar]
- 27.Makharia GK, Srivastava S, Das P, et al. Clinical, endoscopic and histological differentiations between Crohn's disease and intestinal tuberculosis. Am J Gastroenterol 2010; 105: 642–651. [DOI] [PubMed] [Google Scholar]
- 28.De Zoeten EF, Pasternak BA, Mattei P, et al. Diagnosis and treatment of perianal Crohn’s disease: NASPGHAN clinical report and consensus statement. J Pediatr Gastroenterol Nutr 2013; 57: 401–412. [DOI] [PubMed] [Google Scholar]
- 29.Plesec TP, Owens SR. Inflammatory and neoplastic disorders of the anal canal. In: Odze RD, Goldblum JR. (eds). Surgical pathology of the GI tract, liver, biliary tract and pancreas, Philadelphia, PA: Saunders Elsevier, 2015, pp. 887–920. [Google Scholar]
- 30.Maggi L, Capone M, Giudici F, et al. CD4+CD161+ T lymphocytes infiltrate Crohn's disease-associated perianal fistulas and are reduced by anti-TNF-alpha local therapy. Int Arch Allergy Immunol 2013; 161: 81–86. [DOI] [PubMed] [Google Scholar]
- 31.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest 2009; 119: 1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest 2003; 112: 1776–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bataille F, Rohrmeier C, Bates R, et al. Evidence for a role of epithelial mesenchymal transition during pathogenesis of fistulae in Crohn's disease. Inflamm Bowel Dis 2008; 14: 1514–1527. [DOI] [PubMed] [Google Scholar]
- 34.Scharl M, et al. Hallmarks of epithelial to mesenchymal transition are detectable in Crohn's disease associated intestinal fibrosis. Clin Transl Med 2015; 4: 1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scharl M, et al. Potential role for SNAIL family transcription factors in the etiology of Crohn's disease-associated fistulae. Inflamm Bowel Dis 2011; 17: 1907–1916. [DOI] [PubMed] [Google Scholar]
- 36.Frei SM, et al. A role for tumor necrosis factor and bacterial antigens in the pathogenesis of Crohn's disease-associated fistulae. Inflamm Bowel Dis 2013; 19: 2878–2887. [DOI] [PubMed] [Google Scholar]
- 37.Frei SM, et al. The role for Dickkopf-homolog-1 in the pathogenesis of Crohn's disease-associated fistulae. PLoS One 2013; 8: e78882–e78882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kirkegaard T, et al. Expression and localisation of matrix metalloproteinases and their natural inhibitors in fistulae of patients with Crohn's disease. Gut 2004; 53: 701–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bailey JR, et al. IL-13 promotes collagen accumulation in Crohn's disease fibrosis by down-regulation of fibroblast MMP synthesis: A role for innate lymphoid cells? PLoS One 2012; 7: e52332–e52332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scharl M, et al. Epithelial-to-mesenchymal transition in a fistula-associated anal adenocarcinoma in a patient with long-standing Crohn's disease. Eur J Gastroenterol Hepatol 2014; 26: 114–118. [DOI] [PubMed] [Google Scholar]
- 41.Rieder F, et al. Wound healing and fibrosis in intestinal disease. Gut 2007; 56: 130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rieder F. The gut microbiome in intestinal fibrosis: Environmental protector or provocateur? Sci Transl Med 2013; 5: 190ps10–190ps10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burke JP, et al. Fibrogenesis in Crohn's disease. Am J Gastroenterol 2007; 102: 439–448. [DOI] [PubMed] [Google Scholar]
- 44.Meier JK, et al. Specific differences in migratory function of myofibroblasts isolated from Crohn's disease fistulae and strictures. Inflamm Bowel Dis 2011; 17: 202–212. [DOI] [PubMed] [Google Scholar]
- 45.Scharl M, Rogler G. Pathophysiology of fistula formation in Crohn's disease. World J Gastrointest Pathophysiol 2014; 5: 205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rieder F, Fiocchi C. Intestinal fibrosis in IBD: A dynamic, multifactorial process. Nat Rev Gastroenterol Hepatol 2009; 6: 228–235. [DOI] [PubMed] [Google Scholar]
- 47.Biancheri P, et al. Absence of a role for interleukin-13 in inflammatory bowel disease. Eur J Immunol 2014; 44: 370–385. [DOI] [PubMed] [Google Scholar]
- 48.Bettenworth D, Rieder F. Reversibility of stricturing Crohn's disease: Fact or fiction? Inflamm Bowel Dis 2015; 22: 241–247. DOI: 10.1097/MIB.0000000000000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Speca S, et al. Cellular and molecular mechanisms of intestinal fibrosis. World J Gastroenterol 2012; 18: 3635–3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Siegmund B, et al. Results of the Fifth Scientific Workshop of the ECCO (II): Pathophysiology of perianal fistulizing disease. J Crohns Colitis.. Epub ahead of print 17 December 2015, pii: jjv228. [DOI] [PMC free article] [PubMed] [Google Scholar]