Abstract
Crohn’s disease (CD) activity assessments are dominated by inflammatory changes without discrete measurement of the coexisting fibrotic contribution to total bowel damage. Intestinal fibrosis impacts the development of severe structural complications and the overall natural history of CD. Measuring intestinal fibrosis is challenging and existing methods of disease assessment are unable to reliably distinguish fibrosis from inflammation. Both the immediate clinical need to measure fibrosis for therapeutic decision-making and the near-future need for tools to assess pipeline anti-fibrotic medications highlight the demand for biomarkers of fibrosis in CD. Developing non-invasive technologies exploit changes in intestinal perfusion, mechanical properties, and macromolecular content to provide quantitative markers of fibrosis. In this review of existing and experimental technologies for imaging intestinal fibrosis, we discuss the expanding capabilities of quantitative MR and ultrasound imaging, encouraging developments in non-invasive elastography, and emerging novel methods including photoacoustic imaging.
Keywords: Crohn’s disease; intestinal strictures; intestinal fibrosis; disease activity; elasticity imaging; magnetic resonance enterography
Introduction
Crohn’s disease (CD) is classically considered an idiopathic illness of intermittent segmental intestinal inflammation. Transmural bowel wall injury is also impacted by wound healing mechanisms which result in the development of cumulative and irreversible intestinal fibrosis. Over time, inflammation and fibrosis together cause progressive bowel wall thickening, stricture development, and obstructing and penetrating complications.1 An argument could be made that intestinal fibrosis is most responsible for a complicated and disabling CD course, considering that despite the introduction of anti-tumor necrosis factor alpha (TNF) therapies to control inflammation, surgical rates have not changed in nearly 20 years in the USA.2 The complexity of CD management decisions are often related to our inability to determine the relative contribution of inflammation and fibrosis in a damaged bowel segment. The degree of intestinal fibrosis present underlies common clinical questions for individual patients: What is the appropriate therapeutic intensity at diagnosis? Is the current therapeutic regimen sufficient to prevent future structural complications? Will increasing therapeutic intensity in the setting of existing stricturing disease be sufficient to prevent surgery? This review will discuss existing, emerging, and experimental imaging methods to detect and measure intestinal fibrosis in CD.
The challenges of assessing intestinal fibrosis
Because of the inability of ileocolonoscopy with biopsy to interrogate transmural injury, cross-sectional imaging methods, including ultrasound (US), computed tomography enterography (CTE), and magnetic resonance enterography (MRE), have become essential companions to endoscopy.3,4 Excellent image-based disease activity assessments, including the magnetic resonance index of activity (MaRIA), are strongly correlated with endoscopic activity, but principally score inflammatory factors.5 Yet, the concept that a high or low degree of bowel wall contrast enhancement is characteristic of predominantly inflammatory or fibrotic intestinal disease has been questioned. Adler and colleagues, along with other groups, have showed that that the radiologist global impression of whether a stricture was “active” or “inactive” was not associated with the presence or degree of fibrosis.6,7 The Lémann index represents an international effort to more comprehensively describe total bowel damage accounting for stricturing and penetrating disease using imaging.8 However, obvious strictures represent late stage macroscopic events, when the window for medical intervention is nearing closure. Further, claims that bowel dilation is associated with the degree of intestinal fibrosis remain controversial.9 One could make a pragmatic argument that treating to an endpoint of objective resolution of inflammation to halt the progression of fibrosis is sufficient. However, emerging data raises the possibility that while inflammation may initiate fibrogenesis, for poorly understood reasons, fibrosis could auto-propagate even after inflammation is extinguished.10 Taken together, these points capture the need to objectively measure intestinal fibrosis. Given our inability to safely and routinely obtain full thickness intestinal samples, several methods are being explored to provide a “virtual biopsy,” relying on changes in tissue perfusion, mechanics, and macromolecular composition as surrogate biomarkers of fibrosis.
Perfusion as a surrogate for intestinal fibrosis
The neovascularization and changes in vascular auto-regulation observed in intestine affected by CD have been studied as methods to grade histologic inflammation.11 Fibrotic histologic changes are hypothesized to retard blood flow. Careful quantitation of perfusion dynamics using traditional MRE methods has yielded encouraging data supporting its use as a marker of fibrosis. Comparing MRE to graded histology in 41 resected specimens, Rimola and colleagues found that imaging findings of inflammation, including high T2 signal, mucosal hyper-enhancement, and the presence of ulcerations, were associated with the histologic grade of inflammation (p = 0.02, 0.03, <0.01, respectively) but not the grade of fibrosis (p = 0.57, 0.60, 0.85, respectively).12,13 Alternatively, delayed progressive enhancement over a 7-minute observation period separated low- and high-grade fibrosis (14% vs. 35% enhancement gain, p < 0.01) but not inflammation. The authors also show that using high T2 signal intensity to grade inflammation and delayed progressive enhancement to grade fibrosis can together coarsely classify intestine histology, moderately agreeing with paired tissue sample analysis (κ = 0.59, 95% CI 0.40–0.77).
An alternative method of assessing tissue inflammation, diffusion weighted imaging (DWI), has also been studied as a quantitative method of assessing inflammation and may provide a method to distinguish inflammation from fibrosis.14 In a small study of 28 CD patients undergoing elective surgical resection, the mean apparent diffusion coefficient (ADC) of fibrotic vs. non-fibrotic tissue was 2282 (1998–2772) and 1714 (1433–2311), respectively, p = 0.023.15 The linear correlation between ADC and the degree of inflammation by histology was not statistically significant (p = 0.090), though a trend of decreasing ADC with increasing inflammation score was observed. At this juncture it is unclear whether DWI can discriminate intestinal fibrosis from inflammation sufficiently enough to be clinically meaningful. The most notable limitation of the these and other studies investigating fibrosis biomarkers is that the requirement for full thickness histology limits subjects to those undergoing surgery for intractable disease. Study samples are therefore biased towards moderate to severe fibrosis and few specimens contain intestinal damage dominated by inflammation; only 3/41 subjects had grade 1 fibrosis in the Rimola et al. study. The lack of access to full thickness histology, over a spectrum of disease durations and severities, remains a key hurdle in intestinal fibrosis research.
Tissue perfusion characteristics can also be assessed by ultrasound. Contrast-enhanced ultrasound (CE-US) uses intravenously administered microbubbles which can be quantified and standardized using image analysis platforms (Figure 1).16 Readouts can provide not only peak contrast values, but also tissue perfusion kinetics; this is a more objective and quantitative method than traditional Doppler US (Figure 1). A 14 patient study of CE-US showed that delayed contrast wash-in (p = 0.02) and wash-out (p = 0.008) were associated with therapeutic non-response, though no full thickness histology was available.17 A 39 subject study where patients were dichotomized as being predominantly fibrotic (requiring elective surgery for refractory stricturing disease), or inflammatory (no strictures, but required steroid or anti-TNF), found those judged to be fibrotic had a slower rate of perfusion compared to the inflammatory group (22.6 vs. 45.3 mL/min per 100 mL, p = 0.003).18 The same group found that the ratio of intestinal tissue perfusion to bowel wall thickness (cutoff of 0.56 mL/min/mm) predicted surgical vs. medically managed disease with an AUROC of 0.92 and sensitivity and specificity 82% and 94%, respectively. These CE-US studies presented are subject to error stemming from using clinical decisions, outcomes, and unverified gold standards as surrogates of fibrosis. It is also possible that the imaging findings influenced clinical decision making, biasing the results toward reporting an inflated accuracy. Though longitudinal studies and more histopathology correlates are needed, tissue perfusion characteristics quantified by MRE and CE-US appear correlated with intestinal fibrosis.
Figure 1.
Contrast enhanced ultrasound (CE-US) has demonstrated some promise in providing better measurement of intestinal inflammation based on quantitation of perfusion characteristics. Perfusion kinetics, including inflow, outflow, and flow rate, can all be assessed within an area of interest. CE-US is being explored as a means to separate inflammation from fibrosis in Crohn’s disease.
Tissue elasticity as a surrogate for intestinal fibrosis
The excess deposition of extracellular matrix along with disorganized smooth muscle proliferation together contribute to changes in the mechanical properties of fibrostenotic intestinal damage. Direct measurement of intestine elasticity in animal models and ex-vivo human CD intestinal specimens have shown the degree of fibrosis is associated the degree of tissue stiffness.19 This correlation has drawn attention to the use of non-invasive stiffness measurement as a surrogate marker of fibrosis. Ultrasound strain imaging quantifies the ‘hardness’ or ‘softness’ of a tissue as a function of tissue compressibility. Using a two-dimensional speckle tracking technique for quantitation of tissue distensibility, non-invasive strain assessment strongly correlated with direct stiffness measurements of intestine.20 Using the TNBS rat model of intestinal injury, ultrasound strain imaging was able to distinguish inflamed from fibrotic-inflammatory tissue (2.07 vs. 1.10, p = 0.037) and also correlated with the presence of fibrosis in a small human pilot (ρ = 0.81, p = 0.008). An important limitation of this bowel elastography technique was that it does not produce real time results, requiring extensive post-study image processing.
New approaches to elastography are being investigated to determine their ability to measure intestinal fibrosis and predict therapeutic responsiveness in CD. In a small study of 10 CD patients undergoing intestinal resection, real-time ultrasound elastography (US-RTE) was shown to be associated with the degree of muscularis thickness (p = 0.006), Masson’s trichrome stain scores (4 vs. 0, p < 0.001), and quantitation of high vs. low collagen content by western blot (2.01 vs. 0.87, p = 0.009).21 US-RTE offers advantages of immediate bedside results and standardization of applied freehand force by using a pressure-sensitive transducer. Shear wave elastography (SWE) is an alternative technique that generates an ultrasonic pressure wave (using acoustic radiation force impulse) which can be focused on a specific region of bowel wall. The resulting orthogonal shear wave speed through the tissue can be measured; increasing speed is correlated with increasing tissue density and stiffness (Figure 2). SWE has also demonstrated an ability to distinguish fibrotic and inflammatory intestinal damage in animal models with an AUROC of 0.971, exhibiting a PPV and NPV of 95.0% and 92.9%.22 In addition, shear wave speed has been shown to modestly correlate with the histologic grade of fibrosis (ρ = 0.60, p = 0.01), but not inflammation (ρ = 0.24, p = 0.36) in ex-vivo human intestinal specimens.23 A recent study of 23 patients undergoing surgical resection found elastography able to distinguish severe fibrosis from inflammation, though it was unable to separate mild-moderate histologic fibrosis from predominantly inflammatory disease.24 Much more study is needed prior to incorporating elastography into routine clinical care, including establishing reproducibility, dynamic range, the ability to quantify intermediate gradations of fibrosis, and ultimately predict natural history and clinical outcomes in CD.
Figure 2.
Ultrasound stiffness imaging using shear wave speed measurement. In shear wave imaging, an ultrasonic pulse is directed at a region of interest, generating a shear wave within the tissue that can be measured by the transducer. A ileal stricture is shown by traditional B-mode scan (left) with real-time quantitation of shear wave speed within the scan field (right). Shear wave speed is correlated to tissue stiffness, which itself is associated with the degree of fibrosis.
PET and metabolic imaging for intestinal fibrosis
Positron emission tomography (PET), commonplace in oncologic follow-up, has been investigated as a method to differentiate predominantly inflammatory from fibrostenotic bowel damage. Fluorodeoxyglucose (FDG)-PET, combined with CT or MR for anatomic localization, has been explored under the hypothesis that inflammatory tissue will have increased metabolic activity compared to fibrotic bowel. Small pilot studies to date have demonstrated mixed, generally discouraging, results for PET-imaging to distinguish inflammation from fibrosis. A recent study showed that PET-MR in 19 patients undergoing elective bowel resection was able to predict the histology of resected segments (fibrosis alone vs. a mixed fibro-inflammatory disease), albeit with underwhelming diagnostic accuracy (sensitivity 0.67, specificity 0.73).25 Lenze and colleagues prospectively evaluated PET-CT, MR-enteroclysis, and B-mode ultrasound for characterizing stricturing disease and predicting therapeutic outcomes. While non-significant trends in their data suggested some ability of all modalities to predicting medical response vs. surgical management within 6 months, no single technique was superior.26 Considering the cost, radiation exposure, and marginal performance, traditional metabolic imaging by FDG-PET has not demonstrated the potential for fibrosis detection relative to other modalities.
Experimental techniques: direct macromolecule detection
Methods discussed thus far have explored surrogates of fibrosis which remain susceptible to variations in coexistent inflammation. Exciting new directions in macromolecule detection, specifically collagen, offer a more direct measurement of fibrosis. Magnetization transfer MR imaging (MT-MRI) is an alternative contrast technique that detects the exchange of protons (magnetization) between fixed macromolecules and surrounding free water within a tissue.27 In this way, MT-MRI can detect collagen and other large macromolecules. Adler and colleagues showed that MT-MRI was able to quantify fibrosis and distinguish fibrosis from inflammation in an animal model of intestinal injury, with a PPV and NPV of 92% and 83%.28 MT-MRI correlated well with both histologic scoring (ρ = 0.74) and collagen quantitation (ρ = 0.72). Subsequent studies improved the histologic discrimination of MT-MRI by adding a marker of inflammation, T2 weighted signal intensity, with a resulting AUROC of 0.98 for detecting bowel wall fibrosis in the TNBS rat model of intestinal damage (Figure 3).29 Pilot studies have demonstrated the feasibility of MT-MRI in humans for separation of predominately inflammatory and fibrotic intestinal damage.30 In line with the limitations of fibrosis research discussed, these human pilots use surrogate endpoints themselves (radiologist definition of fibrotic vs. inflammatory). Within these limitations, MT-MRI, a technique available with existing hardware that incurs no additional cost, provides a novel method for fibrosis measurement in CD.
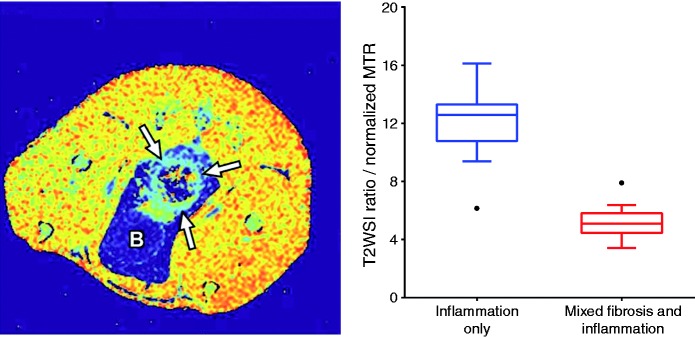
Figure 3.
Magnetization transfer (MT) MRI for quantifying fibrosis. MT-MRI detects heavy macromolecules within a tissue, including collagen. In the TNBS-rat model of intestinal inflammation and fibrosis, a parametric map of calculated MT-ratios shows the bowel (arrows) having less MT effect than surrounding muscle, but more than fat and fluid in the bladder (B). The relationship between T2-weighted signal intensity and MT-ratio (left) provides good discrimination between inflammatory and mixed fibro-inflammatory disease of the bowel wall in this animal model.29
Another experimental method gaining attention for molecular quantitation is photoacoustic imaging (PAI). PAI uses pulsed laser light to penetrate tissues at variable depths. The resulting molecular vibrations produced in the megahertz range can be detected by ultrasound for macromolecular signatures and two- or three-dimensional image production.31 Exploiting the threefold difference in the optical absorption of hemoglobin (a molecular proxy for blood flow and inflammation) and collagen (a molecular proxy for fibrosis) at 532 nm and 1370 nm, PAI may provide a means to contrast hemoglobin and collagen from surrounding macromolecules and thus quantify fibrosis. Using the TNBS-rat model of intestinal injury, PAI at 1370 nm exhibited a 2.9-fold increase in average pixel intensity within mixed fibro-inflammatory intestinal segments compared to pure inflammatory injury, p < 0.0001 (Figure 4).32 Several technical challenges remain, including adjusting for the optical absorption of water (near 1300 nm), and a more efficient means of laser light delivery with tandem ultrasound. Human studies of PAI in CD are planned, using endoscopically delivered laser light combined with transabdominal and endoscopic ultrasound.
Figure 4.
Photoacoustic imaging (PAI) for fibrosis in an animal model of Crohn’s disease. Relying on the differential optical absorption spectra of collagen and hemoglobin, PAI may separate inflammation from fibrosis. In the rat-TNBS model of intestinal damage, PA signal from the bowel at 1370 nm is higher in mixed fibro-inflammatory compared to inflammation alone.32
Endomicroscopy with molecular tagging is an exciting imaging method on the horizon with potential applications in CD. Atreya and colleagues recently showed the ability to perform in vivo membrane-bound TNF measurement using florescent antibodies and real-time endoscopic confocal laser microscopy.33 In 25 subjects, anti-TNF responders had a mean of 30 TNF+ cells per field compared to 11 TNF+ cells in non-responders (p = 0.0004). While molecular tagging and real-time confocal histology is presently restricted to the mucosa and submucosa, molecular tagging within the bowel has been accomplished in IBD. Apoptosis was assessed in animal models and 14 humans using IV administration of technetium-99m-annexin V followed by CT-scintigraphy prior to anti-TNF treatment.34 Subjects with anti-TNF response exhibited 98.7% bowel uptake of 99mTc-annexin V in affected segments compared to 15.2% in non-responders. Radiolabeling techniques could conceivably be applied to constituents of fibrosis. Finally, emerging nanotechnology is providing additional tools for intestinal assessment. Nanoparticles can be engineered for high-affinity tissue or molecular binding, can be detected by multiple non-ionizing methods, and are stable enough for both intravenous and oral administration.35 Orally administered nanoparticles have been used as a photoacoustic contrast agent to improve bowel motility assessments, with the potential for additional sophisticated intestinal imaging applications.36
Concepts of CD activity are evolving from isolated objective measures of inflammation to current efforts to objectively measure cumulative total bowel damage. Accurate and sufficiently dynamic measures of intestinal fibrosis will have an immediate impact in our understanding of the natural history of CD and an individual’s prognosis. Objective fibrosis measures will help guide decisions regarding both medical vs. surgical treatment, as well as the intensity of therapy needed to avoid disease complications. Finally, we are on the cusp of anti-fibrotic therapies. Pirfenidone and nintedanib, small molecules that inhibit or downregulate key mechanisms of fibrosis, are now approved by the EMA and FDA for the treatment of idiopathic pulmonary fibrosis. The bowel imaging techniques presented will needed to evaluate the efficacy of candidate anti-fibrotic therapies in CD. Independent measurements of inflammation and fibrosis will together provide a more comprehensive description of intestinal damage for increasingly precise and individualized treatment of CD.
Conflict of interest
The authors have no potential conflicts of interest relevant to this manuscript to declare.
Funding
This work was supported by the National Institutes of Health (NIH) (grant number 1K23DK101687)
References
- 1.Rieder F, Zimmermann EM, Remzi FH, et al. Crohn’s disease complicated by strictures: a systematic review. Gut 2013; 62: 1072–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones DW, Finlayson SRG. Trends in surgery for Crohn’s disease in the era of infliximab. Ann Surg 2010; 252: 307–312. [DOI] [PubMed] [Google Scholar]
- 3.Horsthuis K, Bipat S, Bennink RJ, et al. Inflammatory bowel disease diagnosed with US, MR, scintigraphy, and CT: meta-analysis of prospective studies. Radiology 2008; 247: 64–79. [DOI] [PubMed] [Google Scholar]
- 4.Panés J, Bouzas R, Chaparro M, et al. Systematic review: the use of ultrasonography, computed tomography and magnetic resonance imaging for the diagnosis, assessment of activity and abdominal complications of Crohn’s disease. Aliment Pharmacol Ther 2011; 34: 125–145. [DOI] [PubMed] [Google Scholar]
- 5.Ordás I, Rimola J, Rodriguez S, et al. Accuracy of magnetic resonance enterography in assessing response to therapy and mucosal healing in patients with Crohn's disease. Gastroenterology 2014; 146: 374–82.e1–374–82.e1. [DOI] [PubMed] [Google Scholar]
- 6.Adler J, Punglia DR, Dillman JR, et al. Computed tomography enterography findings correlate with tissue inflammation, not fibrosis in resected small bowel Crohn’s disease. Inflamm Bowel Dis 2012; 18: 849–856. [DOI] [PubMed] [Google Scholar]
- 7.Chiorean MV, Sandrasegaran K, Saxena R, et al. Correlation of CT enteroclysis with surgical pathology in Crohn’s disease. Am J Gastroenterol 2007; 102: 2541–2550. [DOI] [PubMed] [Google Scholar]
- 8.Pariente B, Mary J-Y, Danese S, et al. Development of the Lémann index to assess digestive tract damage in patients with Crohn’s disease. Gastroenterology 2015; 148: 52–63.e3–52–63.e3. [DOI] [PubMed] [Google Scholar]
- 9.Zappa M, Stefanescu C, Cazals-Hatem D, et al. Which magnetic resonance imaging findings accurately evaluate inflammation in small bowel Crohn’s disease? A retrospective comparison with surgical pathologic analysis. Inflamm Bowel Dis 2011; 17: 984–993. [DOI] [PubMed] [Google Scholar]
- 10.Johnson LA, Luke A, Sauder K, et al. Intestinal fibrosis is reduced by early elimination of inflammation in a mouse model of IBD: impact of a “Top-Down” approach to intestinal fibrosis in mice. Inflamm Bowel Dis 2012; 18: 460–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danese S, Sans M, la Motte de C, et al. Angiogenesis as a novel component of inflammatory bowel disease pathogenesis. Gastroenterology 2006; 130: 2060–2073. [DOI] [PubMed] [Google Scholar]
- 12.Rimola J, Planell N, Rodriguez S, et al. Characterization of inflammation and fibrosis in Crohn's disease lesions by magnetic resonance imaging. Am J Gastroenterol 2015; 110: 432–440. [DOI] [PubMed] [Google Scholar]
- 13.Rimola J, Planell N, Rodriguez S, et al. Corrigendum: Characterization of inflammation and fibrosis in Crohn's disease lesions by magnetic resonance imaging. Am J Gastroenterol 2015; 110: 480–480. [DOI] [PubMed] [Google Scholar]
- 14.Hordonneau C, Buisson A, Scanzi J, et al. Diffusion-weighted magnetic resonance imaging in ileocolonic Crohn's disease: validation of quantitative index of activity. Am J Gastroenterol 2014; 109: 89–98. [DOI] [PubMed] [Google Scholar]
- 15.Tielbeek JAW, Ziech MLW, Li Z, et al. Evaluation of conventional, dynamic contrast enhanced and diffusion weighted MRI for quantitative Crohn’s disease assessment with histopathology of surgical specimens. Eur Radiol 2014; 24: 619–629. [DOI] [PubMed] [Google Scholar]
- 16.Ripollés T, Rausell N, Paredes JM, et al. Effectiveness of contrast-enhanced ultrasound for characterisation of intestinal inflammation in Crohn’s disease: a comparison with surgical histopathology analysis. J Crohn’s Colitis 2013; 7: 120–128. [DOI] [PubMed] [Google Scholar]
- 17.Saevik F, Nylund K, Hausken T, et al. Bowel perfusion measured with dynamic contrast-enhanced ultrasound predicts treatment outcome in patients with Crohn’s disease. Inflamm Bowel Dis 2014; 20: 2029–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nylund K, Jirik R, Mezl M, et al. Quantitative contrast-enhanced ultrasound comparison between inflammatory and fibrotic lesions in patients with Crohn’s disease. Ultrasound Med Biol 2013; 39: 1197–1206. [DOI] [PubMed] [Google Scholar]
- 19.Kim K, Johnson LA, Jia C, et al. Noninvasive ultrasound elasticity imaging (UEI) of Crohn’s disease: animal model. Ultrasound Med Biol 2008; 34: 902–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stidham RW, Xu J, Johnson LA, et al. Ultrasound elasticity imaging for detecting intestinal fibrosis and inflammation in rats and humans with Crohn’s disease. Gastroenterology 2011; 141: 819–826.e1–819–826.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baumgart DC, Müller HP, Grittner U, et al. US-based real-time elastography for the detection of fibrotic gut tissue in patients with stricturing Crohn disease. Radiology 2015; 275: 889–899. [DOI] [PubMed] [Google Scholar]
- 22.Dillman JR, Stidham RW, Higgins PDR, et al. US elastography-derived shear wave velocity helps distinguish acutely inflamed from fibrotic bowel in a Crohn disease animal model. Radiology 2013; 267: 757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dillman JR, Stidham RW, Higgins PDR, et al. Ultrasound shear wave elastography helps discriminate low-grade from high-grade bowel wall fibrosis in ex vivo human intestinal specimens. J Ultrasound Med 2014; 33: 2115–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fraquelli M, Branchi F, Cribiù FM, et al. The role of ultrasound elasticity imaging in predicting ileal fibrosis in Crohn’s disease patients. Inflamm Bowel Dis 2015; 21: 2605–2612. [DOI] [PubMed] [Google Scholar]
- 25.Catalano OA, Gee MS, Nicolai E, et al. Evaluation of quantitative PET/MR enterography biomarkers for discrimination of inflammatory strictures from fibrotic strictures in Crohn disease. Radiology 2015: 150566. [DOI] [PubMed]
- 26.Lenze F, Wessling J, Bremer J, et al. Detection and differentiation of inflammatory versus fibromatous Crohn’s disease strictures: prospective comparison of 18F-FDG-PET/CT, MR-enteroclysis, and transabdominal ultrasound versus endoscopic/histologic evaluation. Inflamm Bowel Dis 2012; 18: 2252–2260. [DOI] [PubMed] [Google Scholar]
- 27.Wolff SD, Eng J, Balaban RS. Magnetization transfer contrast: method for improving contrast in gradient-recalled-echo images. Radiology 1991; 179: 133–137. [DOI] [PubMed] [Google Scholar]
- 28.Adler J, Swanson SD, Schmiedlin-Ren P, et al. Magnetization transfer helps detect intestinal fibrosis in an animal model of Crohn disease. Radiology 2011; 259: 127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dillman JR, Swanson SD, Johnson LA, et al. Comparison of noncontrast MRI magnetization transfer and T2-weighted signal intensity ratios for detection of bowel wall fibrosis in a Crohn’s disease animal model. J Magn Reson Imaging 2015; 42: 801–810. [DOI] [PubMed] [Google Scholar]
- 30.Pazahr S, Blume I, Frei P, et al. Magnetization transfer for the assessment of bowel fibrosis in patients with Crohn’s disease: initial experience. MAGMA 2013; 26: 291–301. [DOI] [PubMed] [Google Scholar]
- 31.Wang LV, Hu S. Photoacoustic tomography: in vivo imaging from organelles to organs. Science 2012; 335: 1458–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu G, Johnson LA, Hu J, et al. Detecting inflammation and fibrosis in bowel wall with photoacoustic imaging in a Crohn's disease animal model. Proc SPIE 2015; 9323: 1–5. [Google Scholar]
- 33.Atreya R, Neumann H, Neufert C, et al. In vivo imaging using fluorescent antibodies to tumor necrosis factor predicts therapeutic response in Crohn’s disease. Nat Med 2014; 20: 313–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van den Brande JMH, Koehler TC, Zelinkova Z, et al. Prediction of antitumour necrosis factor clinical efficacy by real-time visualisation of apoptosis in patients with Crohn’s disease. Gut 2007; 56: 509–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joshi BP, Wang TD. Imaging: dynamic imaging of gut function – allowing the blind to see. Nat Rev Gastroenterol Hepatol 2014; 11: 584–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y, Jeon M, Rich LJ, et al. Non-invasive multimodal functional imaging of the intestine with frozen micellar naphthalocyanines. Nat Nanotechnol 2014; 9: 631–638. [DOI] [PMC free article] [PubMed] [Google Scholar]