Abstract
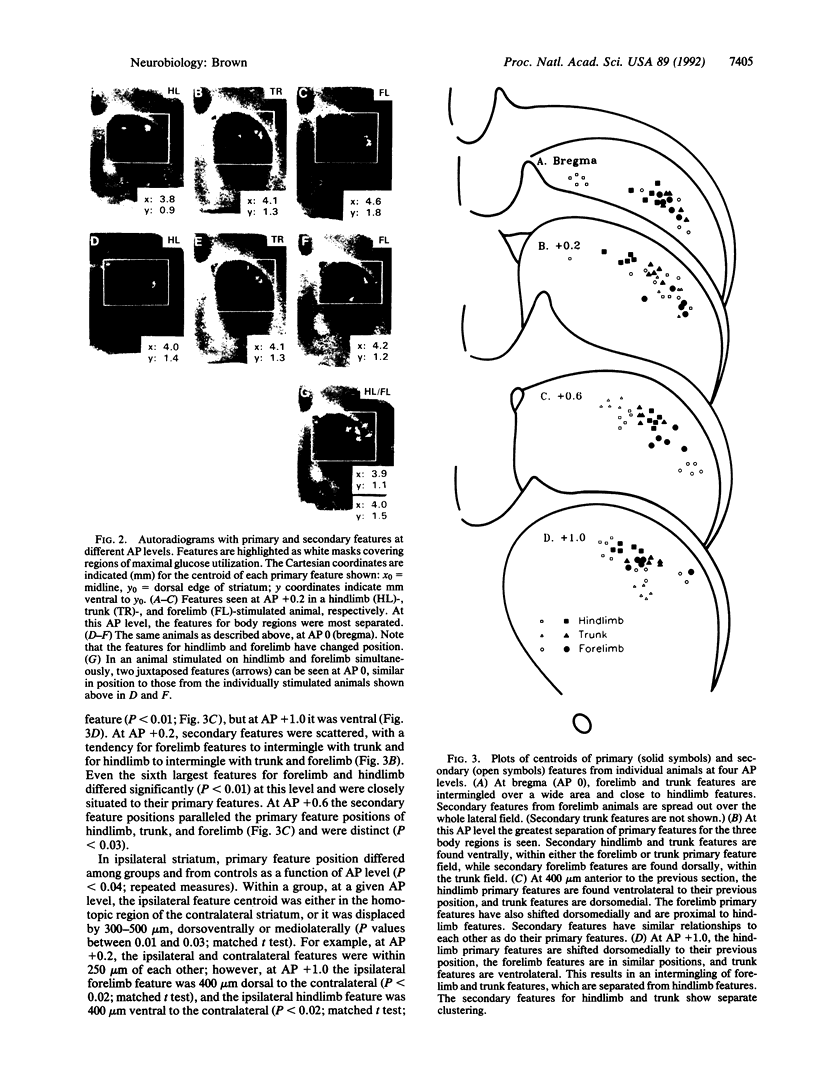
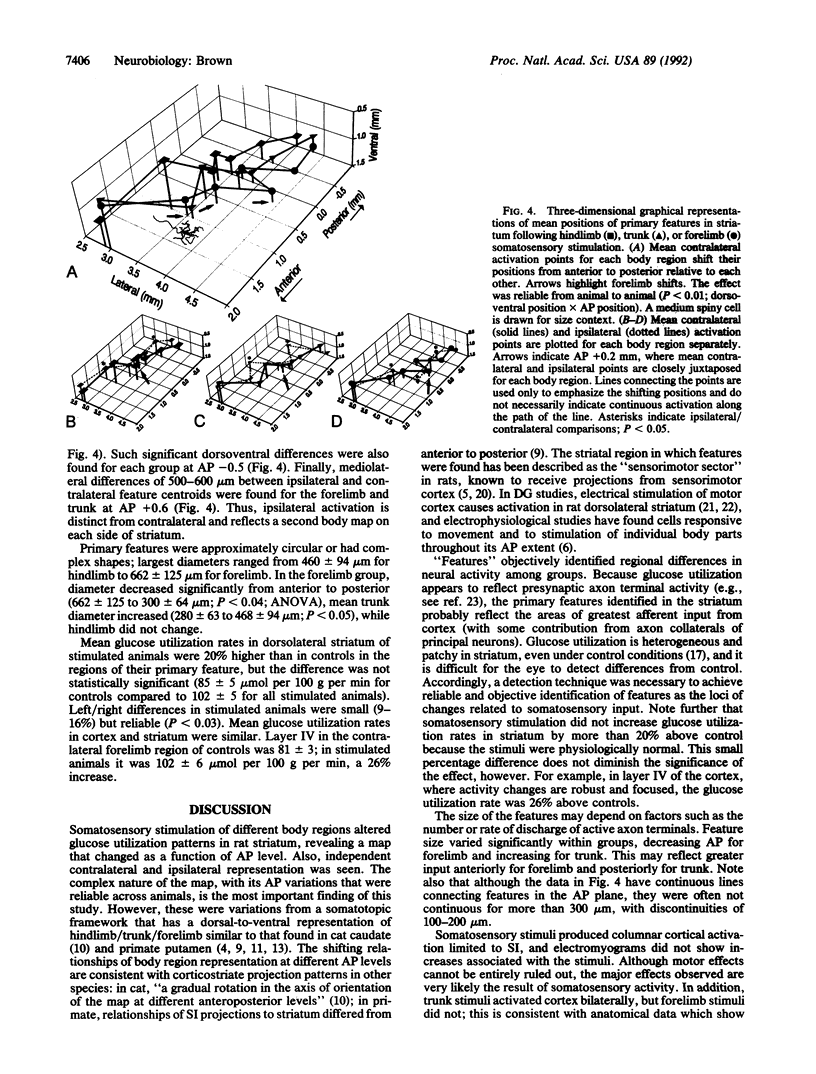
2-Deoxy-D-[14C]glucose autoradiography was used in awake rats to map neural activity in the sensorimotor sector of striatum. Stimulation of hindlimb, trunk, or forelimb activated primary sensory cortex in a localized columnar pattern, indicating activation of somatosensory receptors and a discrete cortical functional unit. In sensorimotor striatum, an image analysis detection technique revealed regions of maximal activity, or features, that formed a patchy pattern of activation reminiscent of the known anatomic patterns of cortico-striate terminals. Ipsilateral as well as contralateral activation was observed. The activated areas revealed a body map in striatum that was organized in a manner consistent with cortical topography (dorsoventrally: hindlimb, trunk, forelimb) at most anteroposterior levels, similar to that found in other species. However, at other levels, a different organization (e.g., trunk, hindlimb, forelimb) was observed. Furthermore, the arrangements of body region and side were also unique at different anteroposterior levels. Thus, functional activity showed multiple, different juxtapositions of body elements--i.e., a combinational map. The data suggest that striatum may provide an anatomic substrate for different combinations of inputs necessary to select and integrate movement.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander G. E., DeLong M. R. Microstimulation of the primate neostriatum. II. Somatotopic organization of striatal microexcitable zones and their relation to neuronal response properties. J Neurophysiol. 1985 Jun;53(6):1417–1430. doi: 10.1152/jn.1985.53.6.1417. [DOI] [PubMed] [Google Scholar]
- Brown L. L., Wolfson L. I., Feldman S. M. Functional neuroanatomic mapping of the rat striatum: regional differences in glucose utilization in normal controls and after treatment with apomorphine. Brain Res. 1987 May 12;411(1):65–71. doi: 10.1016/0006-8993(87)90681-0. [DOI] [PubMed] [Google Scholar]
- Canteras N. S., Shammah-Lagnado S. J., Silva B. A., Ricardo J. A. Somatosensory inputs to the subthalamic nucleus: a combined retrograde and anterograde horseradish peroxidase study in the rat. Brain Res. 1988 Aug 16;458(1):53–64. doi: 10.1016/0006-8993(88)90495-7. [DOI] [PubMed] [Google Scholar]
- Carelli R. M., West M. O. Representation of the body by single neurons in the dorsolateral striatum of the awake, unrestrained rat. J Comp Neurol. 1991 Jul 8;309(2):231–249. doi: 10.1002/cne.903090205. [DOI] [PubMed] [Google Scholar]
- Chapin J. K., Lin C. S. Mapping the body representation in the SI cortex of anesthetized and awake rats. J Comp Neurol. 1984 Oct 20;229(2):199–213. doi: 10.1002/cne.902290206. [DOI] [PubMed] [Google Scholar]
- Collins R. C. Kindling of neuroanatomic pathways during recurrent focal penicillin seizures. Brain Res. 1978 Jul 21;150(3):503–517. doi: 10.1016/0006-8993(78)90816-8. [DOI] [PubMed] [Google Scholar]
- Cospito J. A., Kultas-Ilinsky K. Synaptic organization of motor corticostriatal projections in the rat. Exp Neurol. 1981 May;72(2):257–266. doi: 10.1016/0014-4886(81)90221-1. [DOI] [PubMed] [Google Scholar]
- Crane A. M., Porrino L. J. Adaptation of the quantitative 2-[14C]deoxyglucose method for use in freely moving rats. Brain Res. 1989 Oct 9;499(1):87–92. doi: 10.1016/0006-8993(89)91137-2. [DOI] [PubMed] [Google Scholar]
- Crutcher M. D., DeLong M. R. Single cell studies of the primate putamen. I. Functional organization. Exp Brain Res. 1984;53(2):233–243. doi: 10.1007/BF00238153. [DOI] [PubMed] [Google Scholar]
- Flaherty A. W., Graybiel A. M. Corticostriatal transformations in the primate somatosensory system. Projections from physiologically mapped body-part representations. J Neurophysiol. 1991 Oct;66(4):1249–1263. doi: 10.1152/jn.1991.66.4.1249. [DOI] [PubMed] [Google Scholar]
- Gerfen C. R. The neostriatal mosaic: multiple levels of compartmental organization. Trends Neurosci. 1992 Apr;15(4):133–139. doi: 10.1016/0166-2236(92)90355-c. [DOI] [PubMed] [Google Scholar]
- Graybiel A. M. Neurotransmitters and neuromodulators in the basal ganglia. Trends Neurosci. 1990 Jul;13(7):244–254. doi: 10.1016/0166-2236(90)90104-i. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N., Stryker M. P. Anatomical demonstration of orientation columns in macaque monkey. J Comp Neurol. 1978 Feb 1;177(3):361–380. doi: 10.1002/cne.901770302. [DOI] [PubMed] [Google Scholar]
- Jones E. G., Coulter J. D., Wise S. P. Commissural columns in the sensory-motor cortex of monkeys. J Comp Neurol. 1979 Nov 1;188(1):113–135. doi: 10.1002/cne.901880110. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y., Wilson C. J., Emson P. C. Projection subtypes of rat neostriatal matrix cells revealed by intracellular injection of biocytin. J Neurosci. 1990 Oct;10(10):3421–3438. doi: 10.1523/JNEUROSCI.10-10-03421.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Künzle H. Bilateral projections from precentral motor cortex to the putamen and other parts of the basal ganglia. An autoradiographic study in Macaca fascicularis. Brain Res. 1975 May 2;88(2):195–209. doi: 10.1016/0006-8993(75)90384-4. [DOI] [PubMed] [Google Scholar]
- Künzle H. Projections from the primary somatosensory cortex to basal ganglia and thalamus in the monkey. Exp Brain Res. 1977 Dec 19;30(4):481–492. doi: 10.1007/BF00237639. [DOI] [PubMed] [Google Scholar]
- Lidsky T. I., Manetto C., Schneider J. S. A consideration of sensory factors involved in motor functions of the basal ganglia. Brain Res. 1985 Jun;356(2):133–146. doi: 10.1016/0165-0173(85)90010-4. [DOI] [PubMed] [Google Scholar]
- Malach R., Graybiel A. M. Mosaic architecture of the somatic sensory-recipient sector of the cat's striatum. J Neurosci. 1986 Dec;6(12):3436–3458. doi: 10.1523/JNEUROSCI.06-12-03436.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J. F. Somatosensory inattention after dopamine-depleting intracerebral 6-OHDA injections: spontaneous recovery and pharmacological control. Brain Res. 1979 Nov 16;177(2):311–324. doi: 10.1016/0006-8993(79)90782-0. [DOI] [PubMed] [Google Scholar]
- Mata M., Fink D. J., Gainer H., Smith C. B., Davidsen L., Savaki H., Schwartz W. J., Sokoloff L. Activity-dependent energy metabolism in rat posterior pituitary primarily reflects sodium pump activity. J Neurochem. 1980 Jan;34(1):213–215. doi: 10.1111/j.1471-4159.1980.tb04643.x. [DOI] [PubMed] [Google Scholar]
- McGeorge A. J., Faull R. L. The organization of the projection from the cerebral cortex to the striatum in the rat. Neuroscience. 1989;29(3):503–537. doi: 10.1016/0306-4522(89)90128-0. [DOI] [PubMed] [Google Scholar]
- Sokoloff L., Reivich M., Kennedy C., Des Rosiers M. H., Patlak C. S., Pettigrew K. D., Sakurada O., Shinohara M. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977 May;28(5):897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]