Abstract
Angiotensin II (ANG II) is secreted by the proximal tubule resulting in a luminal concentration that is 100- to 1,000-fold greater than that in the blood. Luminal ANG II has been shown to stimulate sodium transport in the proximal tubule and distal nephron. Surprisingly, luminal ANG II inhibits NaCl transport in the medullary thick ascending limb (mTAL), a nephron segment responsible for a significant amount of NaCl absorption from the glomerular ultrafiltrate. We confirmed that addition of 10−8 M ANG II to the lumen inhibited mTAL chloride transport (220 ± 19 to 165 ± 25 pmol·mm−1·min−1, P < 0.01) and examined whether an interaction with basolateral norepinephrine existed to simulate the in vivo condition of an innervated tubule. We found that in the presence of a 10−6 M norepinephrine bath, luminal ANG II stimulated mTAL chloride transport from 298 ± 18 to 364 ± 42 pmol·mm−1·min−1 (P < 0.05). Stimulation of chloride transport by luminal ANG II was also observed with 10−3 M bath dibutyryl cAMP in the bathing solution and bath isoproterenol. A bath of 10−5 H-89 blocked the stimulation of chloride transport by norepinephrine and prevented the effect of luminal ANG II to either stimulate or inhibit chloride transport. Bath phentolamine, an α-adrenergic agonist, also prevented the decrease in mTAL chloride transport by luminal ANG II. Thus luminal ANG II increases chloride transport with basolateral norepinephrine; an effect likely mediated by stimulation of cAMP. Alpha-1 adrenergic stimulation prevents the inhibition of chloride transport by luminal ANG II.
Keywords: NKCC2, chloride transport, norepinephrine, hormonal interaction, microperfusion
angiotensin ii (ang ii) levels increase with volume depletion, which results in an increase in renal sodium absorption that helps to maintain a constant extracellular fluid volume. In addition to the systemic renin-angiotensin system, the proximal tubule has all of the metabolic machinery to generate the peptide hormone ANG II and secrete it into the tubular lumen (8, 19, 22, 23, 25). The concentration of ANG II in the blood is approximately 10–100 pM (8, 25), whereas studies that have directly measured ANG II from the proximal tubule lumen using in vivo micropuncture demonstrate that the concentration of ANG II is 100- to 1,000-fold higher in the tubular lumen than in the blood (8, 25, 34). In vivo microperfusion studies in which the glomerular ultrafiltrate is blocked and an artificial ultrafiltrate is used to directly perfuse the proximal tubule have shown that the proximal tubule generates and secretes ANG II into the tubular lumen (8). The effect of luminal ANG II may thus play a significant role in the regulation of tubular transport.
ANG II has been shown to stimulate transport of sodium in the proximal tubule when added to the bathing solution of proximal tubules perfused in vitro (33). Addition of ANG II had no effect on transport when added to the luminal perfusate of proximal tubules perfused in vitro, but it stimulated proximal tubule sodium transport when the endogenous production of ANG II was inhibited by enalaprilat, which is consistent with endogenous luminal secretion affecting proximal tubule transport of sodium (6). In vitro microperfusion studies of the distal convoluted tubule have also found that ANG II had a direct effect on the apical membrane by stimulating sodium transport (39). ANG II also increases sodium transport in the cortical collecting tubule, which is dependent on pendrin, an apical Cl−/HCO3− exchanger (27).
The medullary thick ascending limb (mTAL) reabsorbs a significant amount of sodium via the apical membrane NKCC2. Although all other nephron segments studied have shown that ANG II stimulates sodium transport, in vitro microperfusion studies have demonstrated that addition of ANG II to the apical or basolateral solution results in inhibition of sodium chloride absorption (20). Although in vitro microperfusion allows precise measurement of the effect of a hormone on solute transport in a specific nephron segment, it sometimes fails to replicate the in vivo condition. Specifically, nephron segments are innervated, and the effect of ANG II on mTAL sodium chloride transport may be modulated by peritubular norepinephrine. Although we previously confirmed an inhibition in mTAL chloride transport with bath ANG II, we found that inhibition did not occur when norepinephrine was in the bathing solution (4). However, no studies have examined the important issue of whether luminal ANG II interacts with basolateral catecholamines. The purpose of this study was to examine whether luminal ANG II modulates mTAL transport of chloride and whether interaction with bath norepinephrine occurs to simulate an innervated tubule.
METHODS
Animals.
Adult male and female Sprague-Dawley rats weighing between 140 and 250 g were used in these studies. Rats were administered furosemide (2 mg/100 g body wt) by intraperitoneal injection ∼5 min before receiving intraperitoneal Inactin (10 mg/100 g body wt) similar to what has been described previously in studies that examined transport in the mTAL (4, 14, 20). After the rats were anesthetized, a vertical incision was made and the abdomen was opened. The left kidney was bathed in ice-cold PBS for 1 min. The kidney was then quickly removed and cut into several coronal slices for microdissection. These studies were approved by the Institutional Animal Care and Use Committee of the University of Texas Southwestern Medical Center.
In vitro microperfusion flux studies.
Segments of rat mTAL were dissected using sharpened forceps and needles in ice-cold Hanks' solution that contained the following (in mM): 137 NaCl, 5 KCl, 0.8 MgSO4, 0.33 Na2HPO4, 0.44 KH2PO4, 1 MgCl2 10 Tris·HCl, 0.25 CaCl2, 2 glutamine, and 2 l-lactate at 4°C. Tubules were transferred to a 1-ml bathing chamber and perfused with concentric glass pipettes. The distal end of the tubule was placed in a concentric glass pipette and cannulated so that the fluid leaving the tubule could be collected under oil with a constant-volume pipette to avoid contamination with the bathing solution. The perfusate was an ultrafiltrate-like solution that contained the following (in mM): 115 NaCl, 25 NaHCO3, 4.0 Na2HPO4, 10 sodium acetate, 1.8 mM CaCl2, 1 MgSO4, 5 KCl, 8.3 glucose, 5 alanine, 2 lactate, and 2 glutamine at 3–5 nl/min. The bathing solution was similar to the perfusate except that it contained 6 g/dl of BSA. The osmolality of all solutions was adjusted to 300 mOsmol/kg H2O. The bathing solution was heated to 38°C and exchanged at 0.5 ml/min to maintain a constant pH and osmolality.
Chloride transport was calculated as JCl = (Vl)(Cl0 − Cll)P0/Cl0L, where Vl is the collection rate; Cl0 and Cll are chloride concentrations in the perfusion solution and collected fluid, respectively, in counts per minute per nanoliter; P0 is the perfusate chloride concentration in millimoles; and L is the tubular length in millimeters. An eyepiece micrometer was used to measure the tubular length, which averaged 0.6 ± 0.1 mm. The perfusion solution contained 36Cl at a concentration of 10 μCi/ml. A 30-nl constant-volume pipette was used to collect fluid for assessment of chloride transport and to measure the collection rate. Tubules were incubated for ∼15 min before initiation of the measurements for chloride absorption and between periods. In one series of experiments a time control was performed in which ANG II was added to the luminal perfusate 45 min after the initial control period was completed to determine whether inhibition by luminal ANG II was time-dependent. After addition of luminal ANG II in the time-control experiment, there was a 15-min incubation before collections measuring chloride transport were performed. At least four measurements were carried out per period, and the mean of the collections in each period was used to represent the chloride absorption for that period. We have previously demonstrated that passive chloride transport in mTAL is not detectable (11).
To examine the effect of luminal ANG II on chloride transport, we performed a luminal fluid exchange. Luminal fluid exchange was performed at a rate of ∼0.2 ml/min by gravity. The tubing in which the luminal fluid drained was clamped after the exchange to avoid any distention of the tubule during the procedure.
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) except N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), which was purchased from Cayman Chemical (Ann Arbor, MI), and isoproterenol, which was purchased from Santa Cruz Biotechnology (Dallas, TX).
Statistical analysis.
Data are expressed as means ± SE. ANOVA for paired samples was used to determine statistical significance for studies with more than two groups. Paired Student's t-tests were used to assess the difference between experimental periods when only two periods were compared. A value of P ≤ 0.05 was considered significant.
RESULTS
In the first series of experiments we examined the effect of 10−8 M luminal ANG II on chloride transport in the mTAL. We used that concentration because 1) it was comparable to that measured in the luminal fluid of the proximal tubule (8, 25, 34), and 2) it was the concentration used previously by Lerolle et al. (20), who showed that lower doses did not affect transport in this segment. Consistent with their findings, addition of 10−8 M ANG II to the luminal fluid resulted in inhibition of chloride transport in mTAL (Fig. 1). To determine whether the incubation time affected inhibition of luminal ANG II on chloride transport, a time-control experiment was performed. Chloride transport was 228.7 ± 25.1 pmol·mm−1·min−1 in the control period and 165.1 ± 21.3 pmol·mm−1·min−1 (n = 6) after a 45-min time-control period before adding 10−8 M ANG II to the luminal perfusate (P < 0.05). Thus the ability of ANG II to inhibit chloride transport is not affected by the time of incubation of the tubule.
Fig. 1.
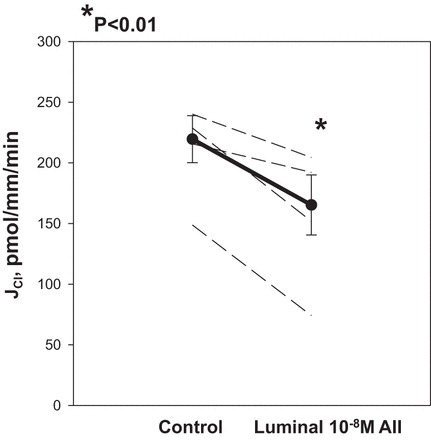
Effect of luminal ANG II on medullary thick ascending limb (mTAL) chloride transport. Rat mTAL were dissected by freehand and perfused in vitro with concentric glass pipettes. Tubules were perfused with an ultrafiltrate-like solution and bathed with a similar solution containing albumin. After the control period, 10−8 M ANG II was added to the luminal perfusate. There was a significant decrease in chloride transport with addition of luminal ANG II (P < 0.01). Dashed lines represent chloride transport in individual tubules, the dark line represents the mean and SE of all tubules. JCl indicates rate of chloride transport in pmol·mm−1·min−1.
In the next series of experiments we examined the effect of 10−6 M bath norepinephrine on chloride transport in mTAL to determine whether that would modulate the effect of luminal 10−8 M ANG II. As shown in Fig. 2, norepinephrine increased chloride transport, as was shown previously (4, 29). Addition of 10−8 M ANG II to the luminal perfusate in the presence of 10−6 M bath norepinephrine resulted in a further stimulation of chloride transport.
Fig. 2.
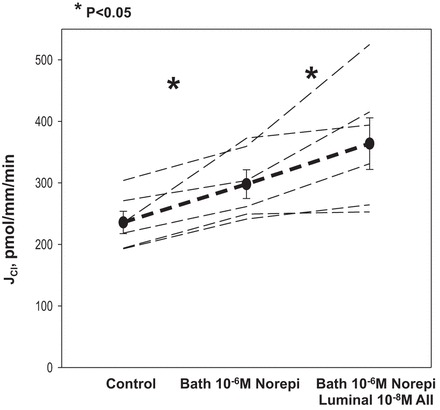
Effect of bath norepinephrine and subsequent addition of luminal ANG II on mTAL chloride transport. mTAL were perfused in vitro and the rate of chloride transport (JCl) was measured under basal conditions. After the control period, 10−6 M norepinephrine (Norepi) was added to the bathing solution, which caused an increase in chloride transport (P < 0.05). Addition of luminal 10−8 M ANG II in the presence of bath Norepi resulted in a further increase in chloride transport (P < 0.05). Dashed lines represent chloride transport in individual tubules, the dark line represents the mean and SE of all tubules.
Norepinephrine may act on the mTAL either by stimulating α- or β-adrenergic receptors; the latter increases intracellular cAMP. We had previously shown that stimulation of chloride transport by norepinephrine in the mTAL was blocked by propranolol, which is consistent with the stimulation resulting from β-adrenergic receptors, and that isoproterenol, a β-adrenergic receptor agonist, increases chloride transport (4). To determine whether AMP potentially mediates ANG II stimulation in chloride transport via norepinephrine, we added 10−3 M dibutyryl cAMP to the bathing solution followed by luminal 10−8 M ANG II. As shown in Fig. 3, 10−3 M dibutyryl cAMP increased chloride transport in the mTAL, an effect found previously by others (18, 26). Thus in the presence of bath dibutyryl cAMP, luminal 10−8 M ANG II stimulated chloride transport in the mTAL.
Fig. 3.
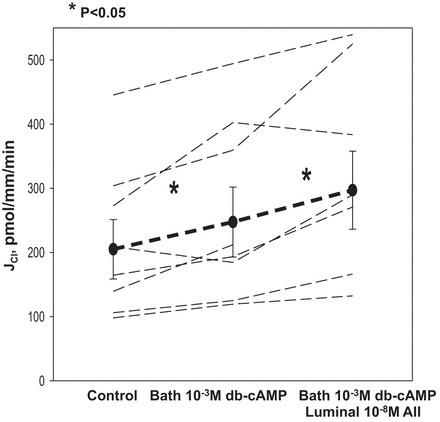
Effect of dibutyryl cAMP (db-cAMP) and subsequent addition of luminal ANG II on chloride transport in the mTAL. After measurement of chloride transport in the control period, 10−3 M db-cAMP was added to the bathing solution, which resulted in an increase in mTAL chloride transport (P < 0.05). Addition of 10−8 M ANG II in the presence of bath db-cAMP resulted in a further stimulation in chloride transport (P < 0.05, all tubules). Dashed lines represent chloride transport in individual tubules, the dark line represents the mean and SE of all tubules.
We next examined the effect of norepinephrine and luminal ANG II in the presence of H-89, a PKA inhibitor. H-89 previously has been shown to block the actions of cAMP in the mTAL (1, 21). Results are shown in Fig. 4. In the presence of 10−5 M H-89, 10−6 M norepinephrine did not stimulate chloride transport in the mTAL. In the presence of both bath H-89 and norepinephrine, addition of 10−8 M luminal ANG II did not increase or decrease chloride transport.
Fig. 4.
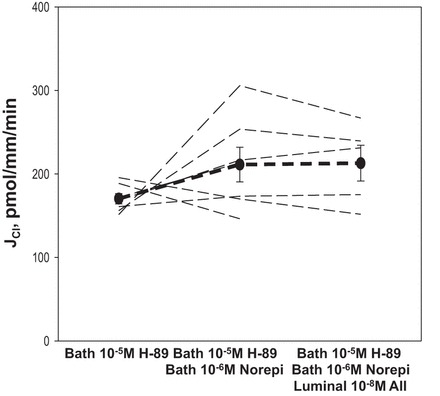
Effect of norepinephrine (Norepi) on chloride transport in the mTAL in the presence of H-89 and subsequent addition of luminal ANG II. In this experiment 10−5 M H-89, a PKA inhibitor, was present in the initial period and the effect of 10−6 M Norepi was examined. There was no stimulation in chloride transport by Norepi in the presence of H-89. Subsequent addition of 10−8 M ANG II resulted in no change in mTAL chloride transport. Dashed lines represent chloride transport in individual tubules, the dark line represents the mean and SE of all tubules.
Bath isoproterenol increases cAMP and stimulates chloride transport in the mTAL (4, 29). We examined the effect of luminal 10−8 M ANG II on chloride transport in the presence of bath 10−6 M isoproterenol. Figure 5 shows that with isoproterenol in the bathing solution, addition of luminal ANG II resulted in increased chloride transport.
Fig. 5.
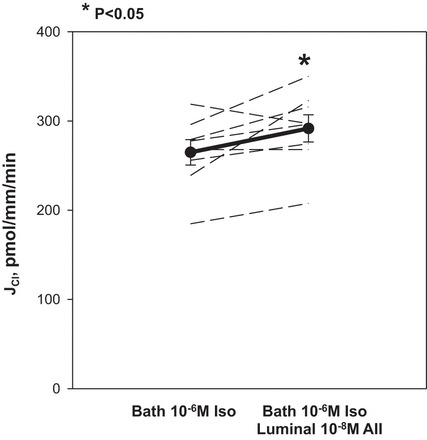
Effect of luminal ANG II on chloride transport in the presence of bath isoproterenol (Iso). mTAL were incubated in the presence of 10−6 M Iso. Addition of 10−8 M luminal ANG II in the presence of Iso resulted in stimulation of chloride transport (P = 0.05). Thin dashed lines represent chloride transport in individual tubules, the dark line represents the mean and SE of all tubules.
Because norepinephrine prevented the decline in mTAL chloride transport by ANG II in the presence of H-89, we examined whether this could be due to the effect of norepinephrine on the α-receptor. Previous studies by researchers in our laboratory and elsewhere have shown that phenylephrine, an α-1 agonist, does not affect sodium chloride transport in the mTAL (4, 29), but phenylephrine has been shown to prevent the inhibitory basolateral effect of ANG II (4). Figure 6 shows that the inhibitory effect of luminal ANG II on mTAL chloride transport was not present with 10−6 M phenylephrine in the bathing solution.
Fig. 6.
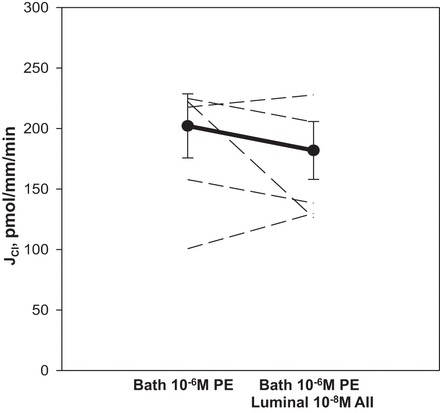
Effect of luminal ANG II on chloride transport in the presence of bath phenylephrine (PE). mTAL were incubated in the presence of 10−6 M PE. Addition of 10−8 M luminal ANG II in the presence of PE prevented inhibition of chloride transport observed with luminal ANG II alone. Dashed lines represent chloride transport in individual tubules, the dark line represents the mean and SE of all tubules.
DISCUSSION
ANG II can modulate proximal tubule transport in a dose-dependent fashion in vivo and in vitro (17, 33). Our laboratory has previously found that a synergistic effect of luminal ANG II and sympathetic nerve activity modulates proximal tubular transport of sodium (31, 32). Because ANG II is secreted into the proximal tubule, we assessed the contribution of secreted ANG II to proximal tubule sodium transport by the decrement in volume absorption by adding either luminal enalaprilat or losartan (6, 30). These studies were performed in vitro or in proximal tubules perfused in vivo beyond an oil block so that glomerular filtrate could not enter the tubular lumen. In both rabbit tubules in vitro and rat tubules perfused with an artificial ultrafiltrate-like solution in vivo, addition of enalaprilat or losartan produced a significant inhibition of proximal tubular sodium transport. Of relevance to the present studies, in proximal tubules perfused in vivo from acutely and chronically denervated kidneys, there was no inhibition in proximal tubule sodium transport with luminal enalaprilat compared with the 40–50% inhibition by luminal enalaprilat in innervated kidneys (31). Furthermore, renal nerve stimulation augmented the inhibition of volume absorption by luminal enalaprilat, which is consistent with an enhanced effect of endogenous luminal ANG II by increased sympathetic nerve activity (32). Luminal ANG II was found to stimulate sodium transport in both the early and late rat distal tubule perfused in vivo (39). Luminal ANG II also stimulates sodium transport in the rabbit cortical collecting duct perfused in vitro (28).
It was thus surprising to find that there was discordance between the stimulatory effect of luminal ANG II in the proximal tubule and distal nephron and the inhibition found in the thick ascending limb (20). In the present study we validated the inhibitory effect of luminal ANG II in isolated perfused rat mTAL. However, we found that in the presence of norepinephrine, luminal ANG II stimulates mTAL chloride transport. Others and we have previously found that stimulation of mTAL chloride transport by norepinephrine was due to β-adrenergic receptor activation (2, 4, 24, 29) and that α1-receptor activation has no effect on mTAL chloride transport (4, 29). β-Adrenergic receptor stimulation of NKCC2 in the thick ascending limb is mediated by an increase in cAMP via activation of PKA (15, 35). By activating PKA, cAMP causes trafficking of NKCC2 to the apical membrane (9, 26). We confirmed that dibutyryl cAMP stimulates chloride transport in the mTAL (26) and found that in the presence of cAMP activation, there is stimulation of chloride transport by luminal ANG II. The stimulatory effect of norepinephrine on chloride transport was blocked by H-89, a PKA inhibitor, consistent with cAMP mediating the stimulatory effect of norepinephrine on chloride transport. Further confirmation came from experiments in which luminal addition of ANG II stimulated chloride transport in the presence of isoproterenol. Although these experiments point to luminal ANG II being responsible for the increase in chloride transport in the presence of cAMP, the mechanism of inhibition of chloride transport in in vitro perfused tubules observed in the present and previous studies is unclear (20). Whether ANG II inhibits the generation of cAMP to inhibit chloride transport is unknown.
Previous studies have shown that the ability of ANG II to inhibit sodium chloride transport is mediated by AT1 receptors in that the effect of ANG II was blocked by losartan but not by PD-23319, an AT2 receptor antagonist (20). The receptors involved in stimulation of chloride transport in the presence of bath norepinephrine were not examined in this study, but it is quite likely that the same receptor for stimulation by luminal ANG II was involved in the presence of norepinephrine. It is quite likely that an increase in cAMP altered the signal transduction of luminal ANG II, resulting in a stimulation of chloride transport. However, the mechanism of how this occurs is unclear at present.
Although H-89 blocked the increase in chloride transport by ANG II, as was observed with addition of norepinephrine to the bath, inhibition of chloride transport by luminal ANG II was also inhibited. We had previously shown that despite the lack of an effect of phenylephrine on chloride transport, inhibition of chloride transport by bath ANG II was blocked (4). In this study we also found that in the presence of bath phenylephrine, inhibition of chloride transport by luminal ANG II on mTAL chloride transport was abrogated.
The mTAL is richly innervated (3). Rats with congestive heart failure induced by ligation of the left anterior descending coronary artery have sodium retention (36). Congestive heart failure mediated by this lesion results in an increase in NKCC2 expression in the outer medulla, which is inhibited by renal denervation (36–38). Renal denervation also caused a reduction in cAMP levels in mTAL in both sham-experimental rats and rats with congestive heart failure, which is consistent with renal nerves increasing NKCC2 expression and cAMP levels in this segment (38). In this rat model of congestive heart failure, the increase in NKCC2 abundance is also reduced by oral administration of losartan (36, 37), which has also been shown to reduce mTAL cAMP levels in rats with congestive heart failure (37). In summary, these data are consistent with both the local renin-angiotensin system and sympathetic nerves acting in concert to regulate transport via generation of cAMP in the mTAL.
The interaction between hormones that modulate transport is not unique to this study. Atrial natriuretic factor has no effect on proximal tubule transport in vivo or in vitro (7, 10), but it inhibits proximal tubule transport in the presence of either bath norepinephrine or ANG II (12, 13, 16). We had previously shown that luminal dopamine has no effect on transport in the proximal convoluted tubule but it inhibits transport in the presence of bath norepinephrine (5). As previously noted, the effect of luminal ANG II on proximal tubule transport is modulated by renal sympathetic nerve activity (31, 32). Finally, we have shown that the inhibitory effect of bath ANG II on mTAL chloride transport is abrogated by bath norepinephrine (4).
In conclusion, luminal ANG II inhibits chloride transport in the mTAL. However, when norepinephrine is present in the bathing solution to simulate an innervated tubule, luminal ANG II stimulates mTAL chloride transport. These results are consistent with others showing that norepinephrine modulates the effect of luminal ANG II on chloride transport, which is likely mediated by β-receptor activation and an increase in cAMP. These results are consistent with those found in the proximal tubule in which the effect of luminal ANG II is modulated by sympathetic nerve activity (31, 32).
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-41612, DK-078596, and P30-DK-079328 (an O'Brien Urology Research Centers Program grant).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
M.B. conception and design of research; M.B. performed experiments; M.B. analyzed data; M.B. interpreted results of experiments; M.B. prepared figures; M.B. drafted manuscript; M.B. edited and revised manuscript; M.B. approved final version of manuscript.
REFERENCES
- 1.Amlal H, Legoff C, Vernimmen C, Paillard M, Bichara M. Na+-K+(NH4+)-2Cl− cotransport in medullary thick ascending limb: control by PKA, PKC, and 20-HETE. Am J Physiol Cell Physiol 271: C455–C463, 1996. [DOI] [PubMed] [Google Scholar]
- 2.Bailly C, Imbert-Teboul M, Roinel N, Amiel C. Isoproterenol increases Ca, Mg, and NaCl reabsorption in mouse thick ascending limb. Am J Physiol Renal Fluid Electrolyte Physiol 258: F1224–F1231, 1990. [DOI] [PubMed] [Google Scholar]
- 3.Barajas L, Powers KV. Innervation of the thick ascending limb of Henle. Am J Physiol Renal Fluid Electrolyte Physiol 255: F340–F348, 1988. [DOI] [PubMed] [Google Scholar]
- 4.Baum M. Effect of catecholamines on rat medullary thick ascending limb chloride transport: interaction with ANG II. Am J Physiol Regul Integr Comp Physiol 298: R954–R958, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baum M, Quigley R. Inhibition of proximal convoluted tubule transport by dopamine. Kidney Int 54: 1593–1600, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baum M, Quigley R, Quan A. Effect of luminal ANG II on rabbit proximal convoluted tubule bicarbonate absorption. Am J Physiol Renal Physiol 273: F595–F600, 1997. [DOI] [PubMed] [Google Scholar]
- 7.Baum M, Toto RD. Lack of a direct effect of atrial natriuretic factor in the rabbit proximal tubule. Am J Physiol Renal Fluid Electrolyte Physiol 250: F66–F69, 1986. [DOI] [PubMed] [Google Scholar]
- 8.Braam B, Mitchell KD, Fox J, Navar LG. Proximal tubular secretion of ANG II in rats. Am J Physiol Renal Fluid Electrolyte Physiol 264: F891–F898, 1993. [DOI] [PubMed] [Google Scholar]
- 9.Caceres PS, Ares GR, Ortiz PA. cAMP stimulates apical exocytosis of the renal Na+-K+-2Cl− cotransporter NKCC2 in the thick ascending limb: role of protein kinase A. J Biol Chem 284: 24965–24971, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cogan MG. Atrial natriuretic factor can increase renal solute excretion primarily by raising glomerular filtration. Am J Physiol Renal Fluid Electrolyte Physiol 250: F710–F714, 1986. [DOI] [PubMed] [Google Scholar]
- 11.Dagan A, Habbib S, Gattineni J, Dwarakanath V, Baum M. Prenatal programming of rat thick ascending limb chloride transport by low-protein diet and dexamethasone. Am J Physiol Regul Integr Comp Physiol 297: R93–R99, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garvin JL. Inhibition of Jv by ANF in rat roximal straight tubules requires angiotensin. Am J Physiol Renal Fluid Electrolyte Physiol 257: F907–F911, 1989. [DOI] [PubMed] [Google Scholar]
- 13.Garvin JL. ANF inhibits norepinephrine-stimulated fluid absorption in rat proximal straight tubules. Am J Physiol Renal Fluid Electrolyte Physiol 263: F581–F585, 1992. [DOI] [PubMed] [Google Scholar]
- 14.Good DW, Knepper MA, Burg MB. Ammonia and bicarbonate transport by thick ascending limb of rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 247: F35–F44, 1984. [DOI] [PubMed] [Google Scholar]
- 15.Haque MZ, Caceres PS, Ortiz PA. β-Adrenergic receptor stimulation increases surface NKCC2 expression in rat thick ascending limbs in a process inhibited by phosphodiesterase 4. Am J Physiol Renal Physiol 303: F1307–F1314, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris PJ, Thomas D, Morgan TO. Atrial natriuretic peptide inhibits angiotensin-stimulated proximal tubular sodium and water reabsorption. Nature 326: 697–698, 1987. [DOI] [PubMed] [Google Scholar]
- 17.Harris PJ, Young JA. Dose-dependent stimulation and inhibition of proximal tubular sodium reabsorption by ANG II in the rat kidney. Pflugers Arch 367: 295–297, 1977. [DOI] [PubMed] [Google Scholar]
- 18.Hebert SC, Culpepper RM, Andreoli TE. NaCl transport in mouse medullary thick ascending limbs. I. Functional nephron heterogeneity and ADH-stimulated NaCl cotransport. Am J Physiol Renal Fluid Electrolyte Physiol 241: F412–F431, 1981. [DOI] [PubMed] [Google Scholar]
- 19.Ingelfinger JR, Zuo WM, Fon EA, Ellison KE, Dzau VJ. In situ hybridization evidence for angiotensinogen messenger RNA in the rat proximal tubule. An hypothesis for the intrarenal renin angiotensin system. J Clin Invest 85: 417–423, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lerolle N, Bourgeois S, Leviel F, Lebrun G, Paillard M, Houillier P. Angiotensin II inhibits NaCl absorption in the rat medullary thick ascending limb. Am J Physiol Renal Physiol 287: F404–F410, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Lessa LM, Carraro-Lacroix LR, Crajoinas RO, Bezerra CN, Dariolli R, Girardi AC, Fonteles MC, Malnic G. Mechanisms underlying the inhibitory effects of uroguanylin on NHE3 transport activity in renal proximal tubule. Am J Physiol Renal Physiol 303: F1399–F1408, 2012. [DOI] [PubMed] [Google Scholar]
- 22.Marchetti J, Roseau S, Alhenc-Gelas F. Angiotensin I converting enzyme and kinin-hydrolyzing enzymes along the rabbit nephron. Kidney Int 31: 744–751, 1987. [DOI] [PubMed] [Google Scholar]
- 23.Moe OW, Ujiie K, Star RA, Miller RT, Widell J, Alpern RJ, Henrich WL. Renin expression in renal proximal tubule. J Clin Invest 91: 774–779, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morgunov NS, You YD, Hirsch DJ. Response of mouse proximal straight tubule and medullary thick ascending limb to beta-agonist. J Am Soc Nephrol 4: 1151–1158, 1993. [DOI] [PubMed] [Google Scholar]
- 25.Navar LG, Lewis L, Hymel A, Braam B, Mitchell KD. Tubular fluid concentrations and kidney contents of angiotensins I and II in anesthetized rats. J Am Soc Nephrol 5: 1153–1158, 1994. [DOI] [PubMed] [Google Scholar]
- 26.Ortiz PA. cAMP increases surface expression of NKCC2 in rat thick ascending limbs: role of VAMP. Am J Physiol Renal Physiol 290: F608–F616, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Pech V, Kim YH, Weinstein AM, Everett LA, Pham TD, Wall SM. Angiotensin II increases chloride absorption in the cortical collecting duct in mice through a pendrin-dependent mechanism. Am J Physiol Renal Physiol 292: F914–F920, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Peti-Peterdi J, Warnock DG, Bell PD. Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT1 receptors. J Am Soc Nephrol 13: 1131–1135, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Plato CF. α-2 and β-adrenergic receptors mediate NE's biphasic effects on rat thick ascending limb chloride flux. Am J Physiol Regul Integr Comp Physiol 281: R979–R986, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Quan A, Baum M. Endogenous production of ANG II modulates rat proximal tubule transport. J Clin Invest 97: 2878–2882, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quan A, Baum M. The renal nerve is required for regulation of proximal tubule transport by intraluminally produced ANG II. Am J Physiol Renal Physiol 280: F524–F529, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Quan A, Baum M. Renal nerve stimulation augments effect of intraluminal ANG II on proximal tubule transport. Am J Physiol Renal Physiol 282: F1043–F1048, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Schuster VL, Kokko JP, Jacobson HR. Angiotensin II directly stimulates sodium transport in rabbit proximal convoluted tubules. J Clin Invest 73: 507–515, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seikaly MG, Arant BS Jr, Seney FD Jr. Endogenous angiotensin concentrations in specific intrarenal fluid compartments of the rat. J Clin Invest 86: 1352–1357, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sonalker PA, Jackson EK. Norepinephrine, via beta-adrenoceptors, regulates bumetanide-sensitive cotransporter type 1 expression in thick ascending limb cells. Hypertension 49: 1351–1357, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Staahltoft D, Nielsen S, Janjua NR, Christensen S, Skott O, Marcussen N, Jonassen TE. Losartan treatment normalizes renal sodium and water handling in rats with mild congestive heart failure. Am J Physiol Renal Physiol 282: F307–F315, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Torp M, Brond L, Hadrup N, Nielsen JB, Praetorius J, Nielsen S, Christensen S, Jonassen TE. Losartan decreases vasopressin-mediated cAMP accumulation in the thick ascending limb of the loop of Henle in rats with congestive heart failure. Acta Physiol (Oxf) 190: 339–350, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Torp M, Brond L, Nielsen JB, Nielsen S, Christensen S, Jonassen TE. Effects of renal denervation on the NKCC2 cotransporter in the thick ascending limb of the loop of Henle in rats with congestive heart failure. Acta Physiol (Oxf) 204: 451–459, 2012. [DOI] [PubMed] [Google Scholar]
- 39.Wang T, Giebisch G. Effects of ANG II on electrolyte transport in the early and late distal tubule in rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 271: F143–F149, 1996. [DOI] [PubMed] [Google Scholar]


