Abstract
Acid-sensing ion channel 1 (ASIC1) contributes to Ca2+ influx and contraction in pulmonary arterial smooth muscle cells (PASMC). ASIC1 binds the PDZ (PSD-95/Dlg/ZO-1) domain of the protein interacting with C kinase 1 (PICK1), and this interaction is important for the subcellular localization and/or activity of ASIC1. Therefore, we first hypothesized that PICK1 facilitates ASIC1-dependent Ca2+ influx in PASMC by promoting plasma membrane localization. Using Duolink to determine protein-protein interactions and a biotinylation assay to assess membrane localization, we demonstrated that the PICK1 PDZ domain inhibitor FSC231 diminished the colocalization of PICK1 and ASIC1 but did not limit ASIC1 plasma membrane localization. Although stimulation of store-operated Ca2+ entry (SOCE) greatly enhanced colocalization between ASIC1 and PICK1, both FSC231 and shRNA knockdown of PICK1 largely augmented SOCE. These data suggest PICK1 imparts a basal inhibitory effect on ASIC1 Ca2+ entry in PASMC and led to an alternative hypothesis that PICK1 facilitates the interaction between ASIC1 and negative intracellular modulators, namely PKC and/or the calcium-calmodulin-activated phosphatase calcineurin. FSC231 limited PKC-mediated inhibition of SOCE, supporting a potential role for PICK1 in this response. Additionally, we found PICK1 inhibits ASIC1-mediated SOCE through an effect of calcineurin to dephosphorylate the channel. Furthermore, it appears PICK1/calcineurin-mediated regulation of SOCE opposes PKA phosphorylation and activation of ASIC1. Together our data suggest PKA and PICK1/calcineurin differentially regulate ASIC1-mediated SOCE and these modulatory complexes are important in determining downstream Ca2+ signaling.
Keywords: store-operated calcium entry, FSC231, DEG/ENaC, Duolink, PKA, PKC
acid-sensing ion channels (ASIC) belong to the degenerin/epithelial sodium channel (DEG/ENaC) superfamily, which includes several amiloride-sensitive cation channels. Significant progress has been made in understanding the structure and function of ASIC in the nervous system; however, several questions remain regarding their physiological importance in other tissues. There is an emerging role for both ENaC and ASIC in vascular smooth muscle and endothelial cells from a variety of vascular beds (8, 10–12, 19, 20, 25, 33, 46). Consistent with a physiological role of ASIC in the vasculature, our laboratory has recently shown that ASIC1 is an important facilitator of G protein-coupled receptor signaling via store-operated Ca2+ entry (SOCE) in pulmonary arterial smooth muscle cells (PASMC) (21, 22). Furthermore, ASIC1-mediated Ca2+ entry in PASMC appears to be an important constituent of both the active vasoconstrictor and vascular remodeling components of chronic hypoxic-induced pulmonary hypertension (21, 31).
Although the conventional mode of ASIC activation is via extracellular acidosis, various nonproton ligands, protein kinases, and other signaling molecules have been shown to regulate ASIC function (52, 53). Despite advances in identifying these signaling molecules, the molecular mechanism(s) that govern the trafficking and activity of ASIC remain largely unknown. All ASIC contain a COOH-terminal PDZ (PSD-95/Dlg/ZO-1) binding motif (1, 9, 14, 15). The COOH termini of ASIC1 and ASIC2 share homology with type-II PDZ binding motifs and bind protein interacting with C-kinase-1 (PICK1) (9, 15). PICK1 is a scaffolding protein that mediates the direct interaction of many proteins that contain PDZ binding motifs. In addition to the PDZ domain, PICK1 contains a larger BAR (Bin/amphiphysin/Rvs) domain that directly binds to lipids and is important in membrane localization (23). The interaction between the PDZ domain of PICK1 and the COOH terminus of ASIC1 increases surface expression and clustering of ASIC1 (9, 15, 24). Although PICK1 is expressed in many tissues (42, 49), whether it is specifically expressed in PASMC and the relative physiological importance of PICK1 in ASIC1 membrane trafficking and regulation of channel activity is unknown. Therefore, we initially hypothesized that PICK1 facilitates ASIC-dependent Ca2+ influx in PASMC by promoting surface localization.
In addition to changes in the cellular localization of the channel, PICK1 may alter the activity of ASIC1 by facilitating the interaction with intracellular modulators of the channel (26). PICK1 was originally isolated by its ability to bind the COOH terminus of protein kinase C (PKC) through its PDZ domain and facilitate PKC phosphorylation of ASIC1 and ASIC2 (2, 16, 42). Although PKC phosphorylation leads to potentiation of ASIC2 current (2), the effect of PKC on ASIC1 activity is controversial (3, 4, 50). Additionally, PICK1 can bind the calcium/calmodulin- activated phosphatase, calcineurin B, and ASIC activity has been shown to be inhibited by calcineurin (5, 17). Therefore, we additionally assessed the role of PICK1 to indirectly regulate ASIC1 phosphorylation status and activity by providing a necessary scaffolding complex for PKC and calcineurin.
METHODS
Generation of Primary PASMC Culture
All protocols employed in this study were reviewed and approved by the Institutional Animal Care and Use Committee of the University of New Mexico School of Medicine. Male Wistar rats (∼12 wk old; Harlan Industries) were anesthetized with pentobarbital sodium (200 mg/kg ip), and the heart and lungs were removed by midline thoracotomy. Intrapulmonary arteries (∼2nd-5th order) were dissected from surrounding lung parenchyma and enzymatically digested in reduced-Ca2+ Hank's balanced salt solution (HBSS) containing papain (26 U/ml), type-I collagenase (1,750 U/ml), dithiothreitol (1 mg/ml), and BSA (2 mg/ml) at 37°C for 30 min. Single smooth muscle cells were dispersed by gentle trituration with a fire-polished pipette in Ca2+-free HBSS. The cell suspension was plated in Ham's F-12 media supplemented with 5% fetal bovine serum and 1% penicillin/streptomycin for 3–4 days in a humidified atmosphere of 5% CO2-95% air at 37°C. Cellular purity was >90%, as assessed by morphological appearance and immunofluorescence of anti-smooth muscle 22α (SM22α).
Determination of mRNA and Protein Expression of PICK1 in PASMC
Total RNA was prepared from PASMC and brain tissue using TRIzol extraction, and 1 μg of total RNA was reverse transcribed to cDNA using the Transcriptor First-Strand cDNA Synthesis kit (Roche). PCR was performed on cDNA with the iCycler PCR system (Bio-Rad) using Taq polymerase and specific primers to detect transcripts for PICK1 (forward: 5′-GGGACGTGTTCTCTGTGATT-3′; reverse: 5′-CCAGGTACTTCTTGATGGTGAG-3; product size: 201) and β-actin (forward: 5′-AGTGTGACGTTGACATCCGT-3′; reverse: 5′-GACTCATCGTACTCCTGCTT-3′; product size: 244). PCR products were electrophoresed through a 3% agarose gel and stained with ethidium bromide for visualization under UV light.
PICK1 protein expression was determined by Western blot analysis and immunofluorescence. PASMC were homogenized in 10 mM Tris·HCl homogenization buffer (containing 255 mM sucrose, 2 mM EDTA, 12 μM leupeptin, 1 μM pepstatin A, and 0.3 μM aprotinin) and centrifuged at 10,000 g for 10 min at 4°C to remove insoluble debris (21). The PASMC lysate (20 μg) was separated by SDS-PAGE (7.5% Tris/glycine) and transferred to a polyvinylidene difluoride membrane. The blot was blocked for 1 h with 5% milk and then incubated overnight at 4°C with rabbit anti-PICK1 (1:500; Abcam). For immunochemical labeling, blots were incubated with goat anti-rabbit IgG-horseradish peroxidase (1:3,000; 1 h; Bio-Rad). Following chemiluminescence labeling (ECL; Pierce), PICK1 was detected by exposing the blot to chemiluminescence-sensitive film (GeneMate).
For immunofluorescence, PASMC were fixed in 2% paraformaldehyde, permeabilized with 0.05% Triton-X and incubated with rabbit anti-PICK1 (1:100) and goat anti- SM22α (1:200) overnight at 4°C. PICK1 and SM22α were detected with donkey anti-rabbit Alexa Fluor 647 and anti-goat DyLight 549 (1:100; 1 h; Jackson ImmunoResearch), and nuclei were stained with SYTOX (1:10,000). Sections were mounted with FluoroGel (Electron Microscopy Sciences), and images were acquired using a confocal microscope (TCS SP5; Leica).
Determination of ASIC1-PICK1 Colocalization
Protein-protein interactions in PASMC were determined using the Duolink in situ proximity ligation assay (PLA) according to manufacturer's instructions (Olink Biosciences; Sigma-Aldrich). Briefly, PASMC were plated on 18-well slides (Ibidi) and grown until ∼75% confluent. In some experiments, PASMC were pretreated with the PICK1 inhibitor FSC231 (50 μM; 30 min) before the cells were fixed with 2% paraformaldehyde. PASMC were incubated with Duolink blocking buffer for 30 min at 37°C then incubated overnight with rabbit anti-PICK1 (1:100; Abcam) and goat anti-ASIC1 (1:50; Santa Cruz Biotechnology). We have previously determined the specificity of goat anti-ASIC1 using wild-type and knockout mice (31). PASMC were then incubated with anti-rabbit PLUS and anti-goat MINUS PLA probes (1:5) for 1 h at 37°C. Negative controls were completed by 1) omission of primary antibody, and 2) incubation of each primary antibody individually. Samples were amplified with Duolink In Situ Detection Reagent Orange (excitation/emission: 554/579 nm; Sigma-Aldrich) for 100 min at 37°C. SYTOX Green (1:5,000; Invitrogen) was used as a nuclear stain and actin was stained with Alexa Fluor 647 Phalloidin (1:100; Invitrogen). Samples were mounted with Duolink mounting media and Z-stack images of the PLA interaction were acquired using a confocal microscope (TCS SP5; Leica). Each puncta was considered a positive protein-protein interaction. The number and size (pixel2) of puncta per cell were determined using ImageJ (National Institutes of Health). In addition, colocalization of ASIC1-PICK1 was determined by traditional immunofluorescence in paraformaldehyde-fixed PASMC by incubating with rabbit anti-PICK1 (1:100) and goat anti-ASIC1 (1:50; Santa Cruz Biotechnology) overnight at 4°C. Nuclei were stained with SYTOX (1:10,000). Colocalization of ASIC1 and PICK1 was determined using Leica Microsystems software.
For ASIC1-PICK1 coimmunoprecipitation (co-IP) experiments, ASIC1 or PICK1 antibodies (20 μg) were incubated with M-270 Epoxy Dynabeads (1.5 mg; Life Technologies) overnight at 37°C to covalently couple the antibody to the beads. PASMC were homogenized in lysis buffer and incubated with antibody-labeled beads (1 h at 4°C). Captured proteins and protein complexes were separated from lysate using magnetic separation followed by elution buffer. IP samples were analyzed by Western blot with either rabbit anti-PICK1 (1:500; Abcam) or rabbit anti-ASIC1 (1:500; Millipore) as described above.
Role of PICK1 in Regulation of ASIC1 Surface Expression
PASMC were grown until ∼90% confluent in 75-cm2 flasks and treated with or without the PICK1 PDZ domain inhibitor FSC231 (50 uM; Calbiochem) for 30 min or 24 h. Nontreated and treated cells were then incubated with Sulfo-NHS-SS-Biotin (Pierce) for 30 min at 4°C. The reaction was quenched and PASMC were harvested and lysed with 10 mM Tris·HCl homogenization buffer as mentioned previously and spun at 10,000 g for 2 min. Clarified supernatant was added to NeutrAvidin Agarose resin columns for 1 h a room temperature. Surface protein was collected by eluting with five times sample buffer and analyzed by Western blot. Surface protein (25 μl) and total protein lysates (20 μg) were separated by SDS-PAGE (7.5% Tris/glycine) and incubated 48 h at 4°C with rabbit anti-ASIC1 (1:500; Millipore). For immunochemical labeling, blots were incubated with goat anti-rabbit IgG-horseradish peroxidase (1:3,000; 1 h; Bio-Rad). Following chemiluminescence labeling (ECL; Pierce), ASIC1 was detected by exposing the blot to chemiluminescence-sensitive film (GeneMate). A separate blot was were probed with goat anti-SM22α (1:500) and donkey anti-goat IgG (1:3,000) to demonstrate the specificity of biotin to label cell surface vs. cytosolic proteins.
Role of PICK1 to Regulate ASIC1-Dependent SOCE
PASMC were incubated with fura-2 AM (2 μM and 0.05% pluronic acid in PSS; Molecular Probes) for 30 min at 32°C. Fura-2-loaded PASMC were alternately excited at 340 and 380 nm at a frequency of 1 Hz with an IonOptix Hyperswitch dual excitation light source (IonOptix), and the respective 510-nm emissions were detected with a photomultiplier tube. PASMC were superfused (5 ml/min at 37°C) with Ca2+-free, HEPES-based physiological saline solution [PSS (in mM): 130 NaCl, 4 KC1, 1.2 MgSO4, 4 NaHCO3, 10 HEPES, 1.18 KH2PO4, 6 glucose, and 3 EGTA; pH adjusted to 7.4 with NaOH] containing 50 μM diltiazem (Sigma-Aldrich) to prevent Ca2+ entry through L-type voltage-gated Ca2+ channel and 10 μM cyclopiazonic acid (CPA; Calbiochem) to deplete intracellular Ca2+ stores and prevent Ca2+ reuptake through the sarcoplasmic reticulum Ca2+-ATPase. The changes in intracellular Ca2+ concentration ([Ca2+]i) were determined upon repletion of HEPES-based PSS containing 1.8 mM CaCl2 in the continued presence of diltiazem and CPA. Area under the curve was calculated as previously described (36). Experiments were conducted in the absence or presence of the PICK1 inhibitor FSC231 (50 μM; Calbiochem), the ASIC1 inhibitor PcTX1 (20 nM; Phoenix Peptides), or in PASMC transfected with the negative or PICK1 shRNA.
shRNA transfection.
PASMC (1 × 106) were suspended in Nucleofector Solution (Lonza) with 5 μg PICK1 shRNA plasmid (Qiagen). The cell/DNA suspension was transferred to an Ingenio cuvette (Mirus Bio) and was electroporated using a Nucleofector Device (program P-024; Lonza). Samples were then plated onto 100-mm2 dishes and incubated for 24 h, and media were changed to reduced serum (1% FBS) and incubated an addition 24 h. PASMC were then used for quantitative PCR (RT2 SYBR green; Qiagen), protein expression, or [Ca2+]i measurements. β-Actin was used as the reference gene and relative quantification of gene expression was determined by the 2−ΔΔCT method. PICK1 protein expression was determined as described above using GAPDH as the housekeeping protein.
Role of PKC, Calcineurin, and PKA in PICK1 Regulation of ASIC1-Mediated SOCE
SOCE responses were determined as described above in the presence, absence, or combination of the PKC activator VII CGK062 (30 μM; Calbiochem), myristoylated-PKC inhibitor (myr-PKC, 10 μM; Calbiochem), calcineurin inhibitor cyclosporin A (1 μM; Cayman Chemicals), PICK1 inhibitor FSC231 (50 μM; Calbiochem), ASIC1 inhibitor PcTX1 (20 nM; Phoenix Peptides), PKA activator forskolin (10 μM; Calbiochem), or PKA inhibitor KT 5720 (300 nM; Calbiochem).
To determine the effect of PKA on ASIC1 phosphorylation, co-IP experiments were performed in PASMC treated with or without forskolin (10 μM), KT 5720 (1 μM), FSC231 (50 μM), or cyclosporin A (1 μM) for 30 min at 37°C. M-270 Epoxy Dynabeads were incubated with ASIC1 antibody (20 μg) followed by PASMC lysate as described above. IP samples were analyzed by Western blot for anti-rabbit phosphoserine (3 μg/ml; Abcam). Total ASIC1 levels were determined as an internal control.
Calculations and Statistics
All data are expressed as means ± SE. Values of n refer to number of animals in each group unless otherwise stated. A one sample t-test, two sample t-test, one-way ANOVA, or two-way ANOVA was used to make comparisons. The statistical test performed for each experiment is indicated in the figure legends. If differences were detected by ANOVA, individual groups were compared with the Student-Newman-Keuls test. A probability of P < 0.05 was accepted as significant for all comparisons.
RESULTS
PICK1 Expression in PASMC
PICK1 is widely distributed in many different tissues, with highest levels in the brain and testis (42, 49). Using brain tissue as a positive control, we show mRNA expression of PICK1 in PASMC by RT-PCR (Fig. 1A). Western blot analysis of PICK1 expression in PASMC reveals a prominent single band present at the predicted molecular mass (52 kDa; Fig. 1B). Based on immunofluorescence imaging, PICK1 expression is fairly diffuse throughout the cell with prominent perinuclear staining (Fig. 1C), as reported previously in heterologous expression systems (9, 15, 23, 42). Taken together, these data demonstrate the presence of PICK1 in PASMC.
Fig. 1.
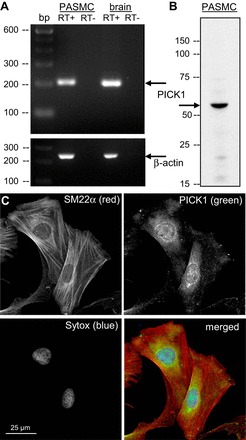
Protein interacting with C kinase 1 (PICK1) is expressed in rat pulmonary arterial smooth muscle cells (PASMC). A: PCR analysis of PICK1 (top: 201 bp) and β-actin (bottom: 244 bp) mRNA expression in PASMC and rat brain tissue as a positive control. B: immunoblot showing protein expression of PICK1 (∼52 kDa) in PASMC. C: immunofluorescence in PASMC showing smooth muscle 22 α (SM22α; red), PICK1 (green), and the nuclear dye SYTOX (blue).
PICK1 Colocalizes with ASIC1
PICK1 has been shown to interact with ASIC1 in yeast two-hybrid assays, heterologous expression systems and neuronal cells (9, 15, 16, 23, 42). Figure 2, A and D, demonstrates this interaction in PASMC using a COOH-terminal anti-ASIC1 and anti-PICK1 antibody via Duolink PLA. According to the manufacturer, the theoretical maximum distance between the two target proteins must be <40 nm to create a PLA signal. As a negative control, either one or both primary antibodies were omitted at a time. We observed very few puncta under these conditions (Fig. 2, B and C). The distribution of ASIC1-PICK1 PLA signal is diffuse throughout the cells (Fig. 2A), with apparent membrane localization as well (Fig. 2, D and E). To confirm this association, we performed co-IP assays in PASMC. Immunoprecipitation of ASIC1 from PASMC lysates resulted in the co-IP of PICK1 (Fig. 2F). The reciprocal immunoprecipitation assays of PICK1 showed that ASIC1 co-IP in PASMC (Fig. 2G). Inhibition of PICK1 with FSC231 tended to reduce the number of puncta per cell (P = 0.063) and significantly decreased the size of the puncta detected indicating diminished clustering of the proteins (Fig. 3).
Fig. 2.
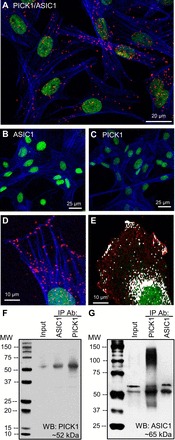
Colocalization of PICK1 and acid-sensing ion channel 1 (ASIC1) in PASMC. A: representative confocal images of the Duolink PLA interaction between goat anti-ASIC1 and rabbit anti-PICK1 (red puncta). For negative controls, PASMC were incubated with each primary alone (B and C) and both PLA probes. Actin is labeled with Alexa Fluor 647 phalloidin (blue) and the nuclei are labeled with SYTOX (green). Zoomed Duolink (D) and traditional colocalization (E) images showing ASIC1-PICK1 localize at the cell edge. Endogenous PICK1 and ASIC1 were immunoprecipitated (IP) from PASMC lysates with anti-ASIC1 and anti-PICK1-labeled beads. Coimmunoprecipitated ASIC1 and PICK1 were detected by Western blotting (WB) using either anti-PICK1 (F) or anti-ASIC1 (G) antibodies.
Fig. 3.
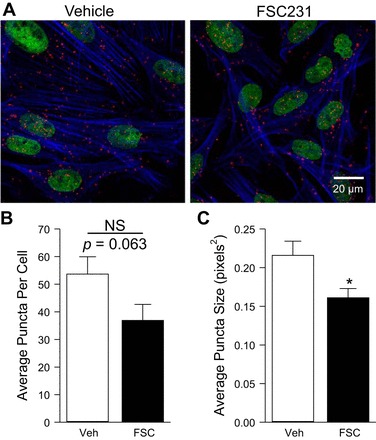
Inhibition of PICK1 diminishes colocalization and clustering with ASIC1. Representative confocal images (A) of the Duolink interaction between goat anti-ASIC1 and rabbit anti-PICK1 (A; red puncta) in the absence or presence of the PICK1 inhibitor FSC231 (50 μM for 30 min). Actin is labeled with Alexa Fluor 647 Phalloidin (blue) and the nuclei are labeled with SYTOX (green). Summary data for average number of puncta per cell (B) and puncta size (C). Values are means ± SE; n = 9–11 images from 5 separate experiments/group; *P < 0.05 vs. vehicle; analyzed by unpaired t-test.
PICK1 Does Not Alter ASIC1 Plasma Membrane Localization
To determine whether PICK1 regulates trafficking of ASIC1 to and from the plasma membrane, we used cell surface biotinylation and subsequent Western blot analysis. We found that inhibition of PICK1 with FSC231 for 30 min did not alter membrane localization of ASIC1 (data not shown). Since the time required for ASIC1 trafficking is unknown, we also examined ASIC1 membrane localization following a 24-h inhibition of PICK1. Again, inhibition of PICK1 did not significantly alter ASIC1 surface expression (Fig. 4A) or total ASIC1 expression (Fig. 4B) in PASMC. The absence of the cytosolic protein SM22α in the biotinylated protein samples demonstrates the specificity of the assay for cell surface verses intracellular proteins (Fig. 4C).
Fig. 4.
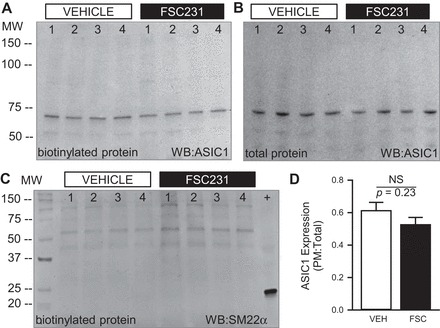
PICK1 does not alter ASIC1 plasma membrane (PM) localization in PASMC. Representative Western blots (A and B) and summary data (D) showing the ratio of cell surface biotinylated (A) to total (B) ASIC1 expression following 24-h treatment with the PICK1 inhibitor FSC231 (50 μM). To verify specificity of biotin labeling for cell surface proteins, a separate blot of cell surface biotinylated proteins was probed for SM22α (23kDa; C). The blot also contains a total protein sample (+) as a positive control. Values are means ± SE; n = 6/group; analyzed by unpaired t-test.
Store Depletion Increases Colocalization Between ASIC1 and PICK1
Our initial hypothesis was that PICK1 facilitates ASIC-dependent Ca2+ influx in PASMC by promoting surface localization. However, we did not detect changes in plasma membrane localization after PICK1 was inhibited (Fig. 4). We next examined the association of ASIC1 and PICK1 upon stimulation of store depletion (SD; Ca2+-free plus CPA and diltiazem) and SOCE (Ca2+-repletion plus CPA and diltiazem). Compared with baseline (BL) conditions (data taken from Fig. 3, vehicle), Ca2+ store depletion largely increased the number of puncta per cell as well as puncta size (Fig. 5), suggesting greater interactions between ASIC1 and PICK1. Replenishing extracellular Ca2+, in the presence of CPA and diltiazem, resulted in a fall in the number of puncta per cell and puncta size (Fig. 5). However, the average number of puncta per cell was still elevated compared with baseline.
Fig. 5.
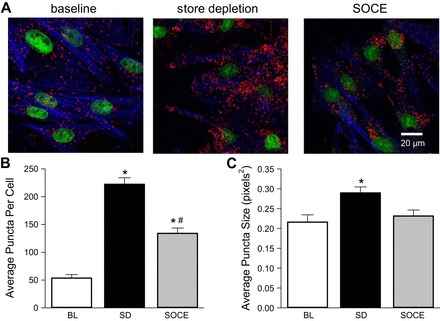
Store depletion increases the interaction and clustering between PICK1 and ASIC1. Representative confocal images (A) of the Duolink interaction between goat anti-ASIC1 and rabbit anti-PICK1 (A; red puncta) upon stimulation of store depletion [SD; Ca2+-free plus cyclopiazonic acid (CPA) and diltiazem] and store-operated Ca2+ entry (SOCE: Ca2+-repletion plus CPA and diltiazem). Actin is labeled with Alexa Fluor 647 Phalloidin (blue) and the nuclei are labeled with SYTOX (green). Summary data for average number of puncta per cell (B) and puncta size (C). Baseline (BL) values are the same data from Fig. 3 vehicle. Values are means ± SE; n = 3–5 images from 7 separate experiments/group; *P < 0.05 vs. baseline; #P < 0.05 vs. store depletion (SD), analyzed with one-way ANOVA and individual groups compared with the Student-Newman-Keuls test.
PICK1 Inhibits ASIC1-Dependent SOCE
The functional significance of the interaction between PICK1 and ASIC1 is unknown; therefore, we examined the effect of PICK1 inhibition on ASIC1-dependent SOCE. In contrast to our hypothesis, inhibition of PICK1 with FSC231 (24-h incubation) augmented SOCE (Fig. 6A). A 30-min incubation with FSC231 produced a similar effect (Fig. 6B). Inhibition of ASIC1 with PcTX1 inhibited SOCE and normalized the response between the vehicle and FSC231-treated groups suggesting the augmented SOCE response is ASIC1 dependent (Fig. 6B). Similar results were obtained following knockdown of PICK1 with shRNA (Fig. 7). Figure 7A shows ∼65% knockdown of PICK1 mRNA (Fig. 7A) and ∼30% knockdown of PICK1 protein (Fig. 7B) in PASMC by shRNA. Consistent with pharmacological inhibition of PICK1, shRNA knockdown of PICK1 significantly augmented SOCE (Fig. 7C). Together, these findings suggest PICK1 limits ASIC1-dependent SOCE and this is independent of ASIC1 membrane trafficking by PICK1.
Fig. 6.
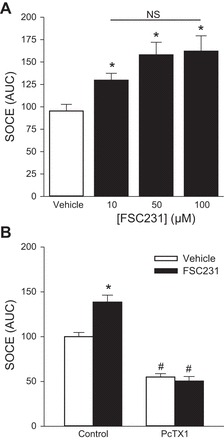
PICK1 inhibits ASIC1-dependent SOCE. A: SOCE responses as determined by area under the curve (AUC) for PASMC pretreated with increasing concentrations of the PICK1 inhibitor, FSC231 (10–100 μM) for 24 h. Values are means ± SE; n = 4–6/group; *P < 0.05 vs. vehicle. B: SOCE responses (AUC) for PASMC pretreated for 30 min with FSC231 (50 μM) with or without pretreatment with the ASIC1 inhibitor psalmotoxin 1 (PcTX1; 20 nM). Values are means ± SE; n = 4–6/group; *P < 0.05 vs. vehicle; #P < 0.05 vs. control; analyzed with one-way (A) and two-way (B) ANOVA and individual groups compared with the Student-Newman-Keuls test.
Fig. 7.
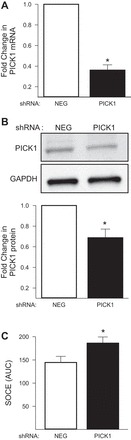
PICK1 inhibits SOCE. Fold change in PICK1 mRNA (A) and protein (B) expression in PASMC following treatment with PICK1 shRNA compared with the negative (NEG) control shRNA. Values are means ± SE; n = 4/group; *P < 0.05 vs. NEG shRNA, analyzed by one sample t-test. C: SOCE responses as determined by AUC in PASMC treated with NEG or PICK1 shRNA. Values are means ± SE; n = 8–10/group; *P < 0.05 vs. NEG shRNA, analyzed by unpaired t-test.
PKC Inhibits SOCE
In addition to changes in the cellular localization of the channel, PICK1 may alter the activity of ASIC1 by facilitating the interaction with intracellular modulators of the channel (26). PICK1 binds the COOH terminus of PKC through its PDZ domain (42) and can regulate ASIC1. However, the effect of PKC on ASIC1 activity is controversial (3, 4, 50); therefore, we first determined the effect of PKC on SOCE. Activation of PKCα significantly attenuated SOCE, whereas inhibition of PKC augmented SOCE (Fig. 8A). It is possible that PICK1 facilitates PKC phosphorylation of ASIC1, thereby inhibiting ASIC1-mediated SOCE. Therefore, we repeated these experiments in the presence of the PICK1 inhibitor, FSC231 (Fig. 8B). Although inhibiting PICK1 improved SOCE during PKC activation, PKC still had an inhibitory effect on SOCE in the presence of PICK1 inhibition. PKC activation resulted in ∼42% inhibition under vehicle conditions and ∼25% inhibition in the presence of FSC231 (Fig. 8C). Although PICK1 may facilitate PKC-induced inhibition of SOCE, it does not appear to be a necessary component of this response.
Fig. 8.
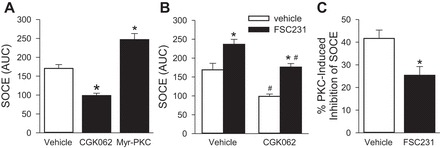
PKC inhibits SOCE. SOCE responses as determined by area under the curve (AUC) for PASMC pretreated with the PKC activator VII CGK062 (30 μM) or myristoylated-PKC inhibitor (myr-PKC, 10 μM; A). Responses in the presence of CGK062 were additionally assessed in the presence of FSC231 (B) and the percent PKC-induced inhibition of SOCE under vehicle and FSC231 treatments was determined (C). Values are means ± SE; n = 5–6/group; *P < 0.05 vs. vehicle; #P < 0.05 vs. non-CGK062, analyzed with t-test (C), one-way (A) or two-way (B) ANOVA, and individual groups compared with the Student-Newman-Keuls test.
Calcineurin Inhibits SOCE and ASIC1 Phosphorylation
The activity of ASIC can further be regulated by the calcium/calmodulin-activated phosphatase calcineurin, and PICK1 has been shown to bind calcineurin B (5, 17). Therefore, we examined the role of calcineurin to regulate SOCE and the dependency of PICK1 in this response. We found that the calcineurin inhibitor cyclosporine A augmented SOCE similarly to FSC231 (Fig. 9A). There was no further effect of combining the inhibitors suggesting these proteins function together. Moreover, SOCE responses in the presence of FSC231/cyclosporin A were largely reduced by the ASIC1 inhibitor PcTX1 (Fig. 9A). Together, these data suggest PICK1 may facilitate inactivation of ASIC1 via calcineurin-dependent dephosphorylation of the channel.
Fig. 9.

PICK1/Calcineurin inhibit PKA-stimulated SOCE. SOCE responses were determined by AUC in PASMC pretreated with the PICK1 inhibitor FSC231 (50 μM), calcineurin inhibitor cyclosporin A (CsA; 1 μM), ASIC1 inhibitor psalmotoxin 1 (PcTX1; 20 nM), PKA activator forskolin (10 μM), or PKA inhibitor KT 5720 (300 nM) as indicated on graphs. Values are means ± SE; n = 4–6/group. A: *P < 0.05 vs. vehicle; #P < 0.05 vs. FSC231/CsA. B: *P < 0.05 vs. non-FSC231/CsA treatment and #P < 0.05 vs. non-forskolin or -KT 5720 treatment; analyzed with one-way (A) or two-way (B) ANOVA and individual groups compared with the Student-Newman-Keuls test.
Consistent with reports in neurons (5), we found that activation of PKA with forskolin augmented SOCE, while inhibition of PKA with KT 5720 decreased SOCE responses in PASMC (Fig. 9B). To determine whether PICK1-calcineurin limits SOCE by reversing PKA-dependent ASIC1 phosphorylation, we examined the effect of PKA stimulation on SOCE following combined inhibition of PICK1 and calcineurin. The ability of forskolin to increase SOCE was further potentiated in the presence of PICK1-calcineurin inhibition, while the decrease in SOCE by KT 5720 was not enhanced in the presence of PICK1-calcineurin inhibition (Fig. 9B), suggesting inhibition of PKA prevents any further regulation of ASIC1 by PICK1-calcineurin.
Leonard et al. (26) reported that PKA-dependent phosphorylation of Ser-479 in the ASIC1 COOH terminus interferes with PICK1 binding. Therefore, to examine serine phosphorylation of ASIC1, we treated PASMC with agents to activate or inhibit PKA, PICK1, and calcineurin. ASIC1 was immunoprecipiated from PASMC lysates and probed for phosphoserine by Western blot. Consistent with other studies showing ASIC1 is a PKA substrate (26), we observed basal levels of ASIC1 phosphorylation in PASMC that are significantly enhanced by forskolin and diminished KT 5720 (Fig. 10). Both FSC231 and cyclosporin A largely augmented ASIC1 serine phosphorylation (Fig. 10), suggesting both PICK1 and calcineurin play a role in dephosphorylating ASIC1.
Fig. 10.
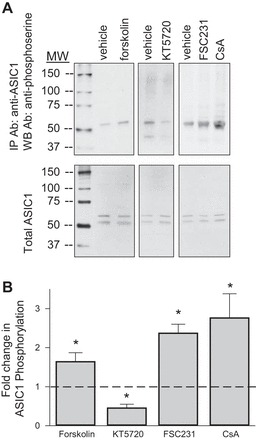
PICK1 and calcineurin inhibit ASIC1 phosphorylation. PASMC were pretreated with vehicle; PKA activator forskolin (10 μM), PKA inhibitor KT 5720 (300 nM), PICK1 inhibitor FSC231 (50 μM), or calcineurin inhibitor CsA (1 μM). Endogenous ASIC1 was immunoprecipitated from PASMC lysates with anti-ASIC1-labeled beads. Phosphorylation of ASIC1 was detected by Western blotting using anti-phosphoserine antibody (A). PASMC lysates were additionally probed for total ASIC1 (B). Summary data showing the fold change in ratio of co-IP ASIC1-phosphoserine to total ASIC1 relative to vehicle-treated samples (dotted line; C). Values are means ± SE; n = 3/group; *P < 0.05 vs. vehicle, analyzed with one-way ANOVA and individual groups compared with the Student-Newman-Keuls test.
DISCUSSION
Our overall goal of this study was to determine the contribution of the scaffolding protein PICK1 to the regulation of both ASIC1 plasma membrane localization and ASIC1-mediated Ca2+ influx in PASMC. Our results demonstrate that PASMC express PICK1, which colocalizes with ASIC1. Inhibition of PICK1 diminished the interaction with ASIC1 but did not limit ASIC1 plasma membrane localization. Intracellular Ca2+ store depletion increased the interaction between ASIC1 and PICK1; however, studies using both pharmacological inhibition of PICK1 and shRNA knockdown of PICK1 suggest that PICK1 imparts a basal inhibitory effect on ASIC1 Ca2+ entry in PASMC. These data argue against our original hypothesis that PICK1 facilitates ASIC1-dependent Ca2+ influx in PASMC by promoting plasma membrane localization but rather suggest that PICK1 negatively regulates ASIC1-mediated SOCE. Further investigation uncovered a role for PICK1 to facilitate calcineurin-dependent dephosphorylation of ASIC1. Moreover, it appears PICK1/calcineurin-mediated regulation of SOCE opposes PKA phosphorylation and activation of ASIC1 (Fig. 11). Together these data demonstrate the ability of PICK1 to regulate PASMC signal transduction by providing a scaffold for multiple signaling proteins important to overall Ca2+ homeostasis in PASMC.
Fig. 11.
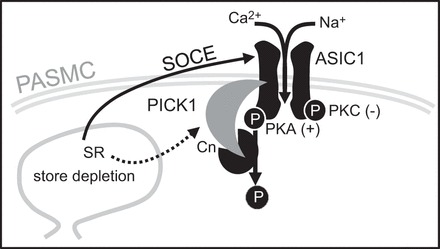
Summary of major findings. Ca2+ depletion of the sarcoplasmic reticulum (SR) stimulates ASIC1-dependent SOCE (solid line) in PASMC. ASIC1-mediated SOCE is augmented by PKA-mediated phosphorylation and inhibited by PKC-mediated phosphorylation. Store depletion also increases the association between ASIC1 and PICK1 which limits SOCE by facilitating calcineurin (Cn)-dependent dephosphorylation of ASIC1.
PDZ domains are well-characterized protein-protein interaction modules that play an essential role in a number of cellular processes by facilitating protein scaffolding and assembly of protein complexes. These supramolecular signaling complexes mediate signaling and regulatory cascades that can either promote or inhibit the activation of certain proteins and thus can regulate channel activity, trafficking, and localization. PDZ domains generally consist of 80 to 100 amino acids and can be divided into three basic types based on the short COOH-terminal sequence it recognizes on the target proteins (51). ASIC1 and ASIC2 COOH termini share homology with type-II PDZ binding motifs and bind PICK1 (9, 15). This interaction with PICK1 is known to change the subcellular clustering of ASIC1. Indeed, we observed less clustering (smaller puncta size) in PASMC in the presence of the PICK1 PDZ-domain inhibitor. Although the interaction between PICK1 and ASIC1 is well characterized, the functional significance of this interaction is not well understood.
PICK1 regulates the trafficking of its binding partner by altering either its subcellular targeting and/or surface expression. Both the PDZ and lipid binding BAR domains are essential for vesicular formation and protein trafficking (23, 24, 35). Moreover, PICK1 is associated with both promoting and prohibiting membrane localization of various proteins (6, 27, 34, 44, 48, 49). In terms of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR), which has been the most widely studied, PICK1 promotes the intracellular retention and prolonged reinsertion of membrane proteins upon internalization (6, 27). With regards to ASIC1, PICK1 has been shown to increase ASIC1 surface levels in heterologous expressing HEK293T cells (24). However, compared with ASIC1 expression alone, coexpression of PICK1 resulted in a marginal (∼2–3%) increase in ASIC1 surface levels suggesting the majority of ASIC1 trafficking to the membrane may be PICK1 independent (24). Although we did not detect an effect of PICK1 to regulate ASIC1 membrane localization in naive PASMC, we cannot rule out a role for PICK1 in regulating subcellular trafficking of ASIC1. Further investigation will determine the mechanisms involved in ASIC1 membrane trafficking but likely involve other trafficking proteins. The current finding regarding PICK1 suppression of SOCE suggests that PICK1 binding to ASIC1 is associated with phosphorylation rather than changes in the cellular localization of the channel.
Although PICK1 potentiates the activity of ASIC2a (2, 7), the influence of PICK1 on the activity of ASIC1 is less clear. Whether PICK1 inhibition of SOCE observed in the present study is a result of direct ASIC1 interaction or a broader regulation of the SOCE complex is unknown. The scaffolding properties of PICK1 enable macromolecular complexes to form thereby assimilating molecules that might have important functions to regulate store-operated channels. PICK1 binds the COOH terminus of PKC through its PDZ domain (42) and can regulate ASIC. The effect of PKC on ASIC1 has been shown to be either stimulatory or inhibitory, depending on the PKC isoform and phosphorylation site (3, 4, 16, 50). Consistent with previous data from our laboratory in isolated pulmonary arteries and pulmonary arterial endothelial sheets (32, 39), activation of PKC suppressed SOCE in PASMC. These data are in line with reports that PKC activation reduces current amplitude of human ASIC1 in glioma cells and Xenopus oocytes (3, 4). Although PICK1 may contribute to PKC-mediated inhibition of SOCE, it does not appear to be required, since inhibition of PICK1 did not prevent PKC-mediated attenuation of SOCE. Therefore, other PICK1-dependent mechanisms are likely involved in the regulation of ASIC1-mediated SOCE.
Pulldown assays in combination with mass spectrometric analyses identified A kinase-anchoring protein 150 (AKAP150) and the Ca2+/calmodulin-dependent protein phosphatase 2B, also called calcineurin, as proteins interacting with ASIC1 (5). PICK1 binds calcineurin B (17), and calcineurin-dependent dephosphorylation has been shown to be involved in the inactivation of ASIC in neuronal cells (5). Consistent with this observation, our current study showed that inhibition of calcineurin with cyclosporin A augments SOCE and increases ASIC1 serine phosphorylation similarly to FSC231 in PASMC. A recent study demonstrates calcineurin plays a role in regulation of SOCE in pulmonary artery endothelial cells; however, in contrast to our study the calcineurin inhibitory peptide increases the phosphorylation of the endoplasmic reticulum Ca2+ sensor STIM1 and decreases SOCE (45). The reason for this discrepancy is currently unclear.
The ability of PICK1 and calcineurin to suppress SOCE in PASMC suggests they may be involved in a feedback mechanism that limits excessive SOCE. Furthermore, this association between ASIC1 and PICK1 appears to be largely influenced by levels of [Ca2+]i since Ca2+ store depletion resulted in a dramatic increase in colocalization of PICK1 and ASIC1. PICK1 contains two acidic stretches adjacent to both the NH2 and COOH termini that bind Ca2+ and can function as potential calcium sensors (13). Therefore, it is possible Ca2+ plays a significant role in regulating the functional interaction between ASIC1 and PICK1 and calcineurin. Although we currently do not know the significance of this interaction, it is additionally possible that Ca2+ entry through ASIC1 directly activates calcineurin. Several studies provide evidence that Ca2+ store depletion triggers the dynamic recruitment of AKAP79/calcineurin to activated store-operated channel complexes (29, 37, 40, 41). This is an area that we are currently investigating in more detail.
Consistent with other reports, we also show ASIC1 is a substrate for PKA (5, 26). Concerning the role of PKA in ASIC1 function, there are conflicting data. Chai et al. (5) show that anchoring of PKA in AKAP150 is required for the full activation of ASIC in neurons and CHO cells, whereas Leonard et al. (26) report that neither activation of PKA with forskolin nor inhibition with KT 5720 altered acid-evoked currents in hippocampal neurons even though the drugs increased and decreased ASIC1 phosphorylation, respectively. Interestingly, Leonard et al. (26) demonstrated that PKA-mediated phosphorylation of ASIC1 reduces ASIC1 colocalization with PICK1 and clustering of ASIC1. Consistent with this, we observed that inhibition of PICK1 increased ASIC1 serine phosphorylation. Based on our current findings and those of others, it would seem possible that inhibition of PICK1 increases PKA phosphorylation and activity of ASIC1 by preventing calcineurin-dependent dephosphorylation in PASMC.
In the pulmonary circulation, ASIC1 contributes to agonist-induced vasoconstriction and increases in pulmonary vascular smooth muscle [Ca2+]i (21, 31). It therefore seems somewhat counterintuitive that PKA, a known vascular mediator of vasodilation, stimulates SOCE in PASMC. However, SOCE is the central mechanism involved in replenishing the level of Ca2+ in the sarcoplasmic reticulum (SR) and PKA is a prominent regulator of the SR Ca2+-cycling proteins. When phospholamban is phosphorylated by PKA, its ability to inhibit the SR calcium ATPase (SERCA) is lost, leading to rapid increases in SR Ca2+ load (38). Although this will decrease [Ca2+]i and lead to smooth muscle relaxation, increasing the Ca2+ load of the SR will also lead to faster turn around and increased release of Ca2+ from the stores upon subsequent and/or continuous activation. Indeed, PKA increases Ca2+-spark frequency mediated by SR ryanodine receptor channels (RyR) (18, 30, 47). It is possible that PKA not only regulates SR Ca2+ through SERCA and RyR channels but also by activating the store-operated channels responsible for replenishing the SR. PKA has recently been shown to activate the heteromeric Orai1/Orai3 channel through phosphorylation of STIM1 in HEK-293 cells (43). These heteromeric Orai channels are arachidonic acid-regulated calcium-selective (ARC), and in contrast to the related homomeric Orai1 channel (CRAC channel), their activation can be store independent (28). Together these studies suggest the potential for PKA to be a fundamental modulator of agonist-induced Ca2+ entry and the subsequent downstream cellular responses in a variety of cell types. Further investigation is needed to determine the functional relevance of PKA vs. PICK1/calcineurin in regulating ASIC1-mediated SOCE and how these modulatory complexes facilitate either SR Ca2+ load or downstream Ca2+ signaling.
In summary, this study demonstrates a regulatory interaction between ASIC1 and PICK1 in PASMC. More specifically, we found that PICK1 binding to ASIC1 is associated with changes in the phosphorylation state of the channel, rather than plasma membrane localization. PKA phosphorylates ASIC1 and stimulates channel activity (Fig. 11). This activation of ASIC1 can be counteracted by PICK1/calcineurin-mediated dephosphorylation of ASIC1. PICK1 suppression of SOCE suggests that PICK1 may be involved in a Ca2+-sensitive feedback mechanism that limits excessive SOCE. However, we cannot presently rule out the ability of PICK1 to facilitate downstream signaling by providing the scaffold for the store-operated complex. The regulatory role of PICK1 on SOCE suggests PICK1 is an important determinant of overall Ca2+ homeostasis in PASMC and that loss of PICK1 could potentially result in the dysregulation of Ca2+ handling observed in many disease states.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-111084 (to N. L. Jernigan).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: L.M.H., C.H.N., T.R.Y., C.B., and N.L.J. performed experiments; L.M.H., C.H.N., and N.L.J. analyzed data; L.M.H. and N.L.J. prepared figures; L.M.H. and N.L.J. drafted manuscript; L.M.H., C.H.N., L.V.G.B., T.C.R., and N.L.J. edited and revised manuscript; L.M.H., C.H.N., T.R.Y., C.B., L.V.G.B., T.C.R., and N.L.J. approved final version of manuscript; L.V.G.B., T.C.R., and N.L.J. conception and design of research; T.C.R. and N.L.J. interpreted results of experiments.
REFERENCES
- 1.Anzai N, Deval E, Schaefer L, Friend V, Lazdunski M, Lingueglia E. The multivalent PDZ domain-containing protein CIPP is a partner of acid-sensing ion channel 3 in sensory neurons. J Biol Chem 277: 16655–16661, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Baron A, Deval E, Salinas M, Lingueglia E, Voilley N, Lazdunski M. Protein kinase C stimulates the acid-sensing ion channel ASIC2a via the PDZ domain-containing protein PICK1. J Biol Chem 277: 50463–50468, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Bashari E, Qadri Y, Zhou Z, Kapoor N, Anderson S, Meltzer R, Fuller C, Benos D. Two PKC consensus sites on human acid-sensing ion channel 1b differentially regulate its function. Am J Physiol Cell Physiol 296: C372–C384, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berdiev BK, Xia J, Jovov B, Markert JM, Mapstone TB, Gillespie GY, Fuller CM, Bubien JK, Benos DJ. Protein kinase C isoform antagonism controls BNaC2 (ASIC1) function. J Biol Chem 277: 45734–45740, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Chai S, Li M, Lan J, Xiong Z, Saugstad J, Simon R. A kinase-anchoring protein 150 and calcineurin are involved in regulation of acid-sensing ion channels ASIC1a and ASIC2a. J Biol Chem 282: 22668–22677, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Citri A, Bhattacharyya S, Ma C, Morishita W, Fang S, Rizo J, Malenka RC. Calcium binding to PICK1 is essential for the intracellular retention of AMPA receptors underlying long-term depression. J Neurosci 30: 16437–16452, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deval E, Salinas M, Baron A, Lingueglia E, Lazdunski M. ASIC2b-dependent regulation of ASIC3, an essential acid-sensing ion channel subunit in sensory neurons via the partner protein PICK-1. J Biol Chem 279: 19531–19539, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Drummond HA, Gebremedhin D, Harder DR. Degenerin/epithelial Na+ channel proteins: components of a vascular mechanosensor. Hypertension 44: 643–648, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Duggan A, Garcia-Anoveros J, Corey DP. The PDZ domain protein PICK1 and the sodium channel bnac1 interact and localize at mechanosensory terminals of dorsal root ganglion neurons and dendrites of central neurons. J Biol Chem 277: 5203–5208, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Gannon KP, Vanlandingham LG, Jernigan NL, Grifoni SC, Hamilton G, Drummond HA. Impaired pressure-induced constriction in mouse middle cerebral arteries of ASIC2 knockout mice. Am J Physiol Heart Circ Physiol 294: H1793–H1803, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Grifoni SC, Gannon KP, Stec DE, Drummond HA. ENaC proteins contribute to VSMC migration. Am J Physiol Heart Circ Physiol 291: H3076–H3086, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Grifoni SC, Jernigan NL, Hamilton G, Drummond HA. ASIC proteins regulate smooth muscle cell migration. Microvasc Res 75: 202–210, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanley JG, Henley JM. PICK1 is a calcium-sensor for NMDA-induced AMPA receptor trafficking. EMBO J 24: 3266–3278, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hruska-Hageman A, Benson C, Leonard A, Price M, Welsh M. PSD-95 and Lin-7b interact with acid-sensing ion channel-3 and have opposite effects on H+-gated current. J Biol Chem 279: 46962–46968, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Hruska-Hageman A, Wemmie J, Price M, Welsh M. Interaction of the synaptic protein PICK1 (protein interacting with C kinase 1) with the non-voltage gated sodium channels BNC1 (brain Na+ channel 1) and ASIC (acid-sensing ion channel). Biochem J 361: 443–450, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu ZL, Huang C, Fu H, Jin Y, Wu WN, Xiong QJ, Xie N, Long LH, Chen JG, Wang F. Disruption of PICK1 attenuates the function of ASICs and PKC regulation of ASICs. Am J Physiol Cell Physiol 299: C1355–C1362, 2010. [DOI] [PubMed] [Google Scholar]
- 17.Iida T, Egusa H, Saeki M, Yatani H, Kamisaki Y. PICK1 binds to calcineurin B and modulates the NFAT activity in PC12 cells. Biochem Biophys Res Commun 375: 655–659, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Jeffries O, McGahon MK, Bankhead P, Lozano MM, Scholfield CN, Curtis TM, McGeown JG. cAMP/PKA-dependent increases in Ca sparks, oscillations and SR Ca stores in retinal arteriolar myocytes after exposure to vasopressin. Invest Ophthalmol Vis Sci 51: 1591–1598, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeggle P, Callies C, Tarjus A, Fassot C, Fels J, Oberleithner H, Jaisser F, Kusche-Vihrog K. Epithelial sodium channel stiffens the vascular endothelium in vitro and in liddle mice. Hypertension 61: 1053–1059, 2013. [DOI] [PubMed] [Google Scholar]
- 20.Jernigan NL, Drummond HA. Myogenic vasoconstriction in mouse renal interlobar arteries: role of endogenous beta and gammaENaC. Am J Physiol Renal Physiol 291: F1184–F1191, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Jernigan NL, Herbert LM, Walker BR, Resta TC. Chronic hypoxia upregulates pulmonary arterial ASIC1: a novel mechanism of enhanced store-operated Ca2+ entry and receptor-dependent vasoconstriction. Am J Physiol Cell Physiol 302: C931–C940, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jernigan NL, Paffett ML, Walker BR, Resta TC. ASIC1 contributes to pulmonary vascular smooth muscle store-operated Ca2+ entry. Am J Physiol Lung Cell Mol Physiol 297: L271–L285, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin W, Ge W, Xu J, Cao M, Peng L, Yung W, Liao D, Duan S, Zhang M, Xia J. Lipid binding regulates synaptic targeting of PICK1, AMPA receptor trafficking, and synaptic plasticity. J Neurosci 26: 2380–2390, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin W, Shen C, Jing L, Zha Xm, Xia J. PICK1 regulates the trafficking of ASIC1a and acidotoxicity in a BAR domain lipid binding-dependent manner. Mol Brain 3: 39, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim EC, Ahn DS, Yeon SI, Lim M, Lee YH. Epithelial Na+ channel proteins are mechanotransducers of myogenic constriction in rat posterior cerebral arteries. Exp Physiol 97: 544–555, 2012. [DOI] [PubMed] [Google Scholar]
- 26.Leonard A, Yermolaieva O, Hruska-Hageman A, Askwith C, Price M, Wemmie J, Welsh M. cAMP-dependent protein kinase phosphorylation of the acid-sensing ion channel-1 regulates its binding to the protein interacting with C-kinase-1. Proc Natl Acad Sci USA 100: 2029–2034, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madsen KL, Thorsen TS, Rahbek-Clemmensen T, Eriksen J, Gether U. Protein interacting with C kinase 1 (PICK1) reduces reinsertion rates of interaction partners sorted to Rab11-dependent slow recycling pathway. J Biol Chem 287: 12293–12308, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mignen O, Shuttleworth TJ. I ARC, a novel arachidonate-regulated, noncapacitative Ca2+ entry channel. J Biol Chem 275: 9114–9119, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Mullins FM, Chan YP, Dolmetsch RE, Lewis RS. STIM1 and calmodulin interact with Orai1 to induce Ca(2+)-dependent inactivation of CRAC channels. Proc Natl Acad Sci USA 106: 15495–15500, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Navedo MF, Takeda Y, Nieves-Cintrón M, Molkentin JD, Santana LF. Elevated Ca2+ sparklet activity during acute hyperglycemia and diabetes in cerebral arterial smooth muscle cells. Am J Physiol Cell Physiol 298: C211–C220, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nitta CH, Osmond DA, Herbert LM, Beasley BF, Resta TC, Walker BR, Jernigan NL. Role of ASIC1 in the development of chronic hypoxia-induced pulmonary hypertension. Am J Physiol Heart Circ Physiol 306: H41–H52, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paffett ML, Riddle MA, Kanagy NL, Resta TC, Walker BR. Altered protein kinase c regulation of pulmonary endothelial store- and receptor-operated Ca2+ entry after chronic hypoxia. J Pharmacol Exp Ther 334: 753–760, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pérez FR, Venegas F, González M, Andrés S, Vallejos C, Riquelme G, Sierralta J, Michea L. Endothelial epithelial sodium channel inhibition activates endothelial nitric oxide synthase via phosphoinositide 3-kinase/Akt in small-diameter mesenteric arteries. Hypertension 53: 1000–1007, 2009. [DOI] [PubMed] [Google Scholar]
- 34.Perez J, Khatri L, Chang C, Srivastava S, Osten P, Ziff E. PICK1 targets activated protein kinase Calpha to AMPA receptor clusters in spines of hippocampal neurons and reduces surface levels of the AMPA-type glutamate receptor subunit 2. J Neurosci 21: 5417–5428, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pinheiro PS, Jansen AM, de Wit H, Tawfik B, Madsen KL, Verhage M, Gether U, Sørensen JB. The BAR domain protein PICK1 controls vesicle number and size in adrenal chromaffin cells. J Neurosci 34: 10688–10700, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plomaritas DR, Herbert LM, Yellowhair TR, Resta TC, Gonzalez Bosc LV, Walker BR, Jernigan NL. Chronic hypoxia limits H2O2-induced inhibition of ASIC1-dependent store-operated calcium entry in pulmonary arterial smooth muscle. Am J Physiol Lung Cell Mol Physiol 307: L419–L430, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Samanta K, Kar P, Mirams Gary R, Parekh Anant B. Ca2+ channel re-localization to plasma-membrane microdomains strengthens activation of Ca2+-dependent nuclear gene expression. Cell Rep 12: 203–216, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simmerman HK, Jones LR. Phospholamban: protein structure, mechanism of action, and role in cardiac function. Physiol Rev 78: 921–947, 1998. [DOI] [PubMed] [Google Scholar]
- 39.Snow JB, Kanagy NL, Walker BR, Resta TC. Rat strain differences in pulmonary artery smooth muscle Ca(2+) entry following chronic hypoxia. Microcirculation 22: 1–12, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Somasundaram A, Shum AK, McBride HJ, Kessler JA, Feske S, Miller RJ, Prakriya M. Store-operated CRAC channels regulate gene expression and proliferation in neural progenitor cells. J Neurosci 34: 9107–9123, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Srikanth S, Gwack Y. Orai1-NFAT signalling pathway triggered by T cell receptor stimulation. Mol Cell 35: 182–194, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Staudinger J, Zhou J, Burgess R, Elledge SJ, Olson EN. PICK1: a perinuclear binding protein and substrate for protein kinase C isolated by the yeast two-hybrid system. J Cell Biol 128: 263–271, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thompson JL, Shuttleworth TJ. Anchoring protein AKAP79-mediated PKA phosphorylation of STIM1 determines selective activation of the ARC channel, a store-independent Orai channel. J Physiol 593: 559–572, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Torres G, Yao W, Mohn A, Quan H, Kim K, Levey A, Staudinger J, Caron M. Functional interaction between monoamine plasma membrane transporters and the synaptic PDZ domain-containing protein PICK1. Neuron 30: 121–134, 2001. [DOI] [PubMed] [Google Scholar]
- 45.Vasauskas AA, Chen H, Wu S, Cioffi DL. The serine-threonine phosphatase calcineurin is a regulator of endothelial store-operated calcium entry. Pulm Circ 4: 116–127, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang SU, Meng FE, Mohan S, Champaneri B, Gu Y. Functional ENaC channels expressed in endothelial cells: a new candidate for mediating shear force. Microcirculation 16: 276–287, 2009. [DOI] [PubMed] [Google Scholar]
- 47.Wellman GC, Santana LF, Bonev AD, Nelson MT. Role of phospholamban in the modulation of arterial Ca2+ sparks and Ca2+-activated K+ channels by cAMP. Am J Physiol Cell Physiol 281: C1029–C1037, 2001. [DOI] [PubMed] [Google Scholar]
- 48.Williams ME, Wu SCY, McKenna WL, Hinck L. Surface expression of the netrin receptor UNC5H1 is regulated through a protein kinase C-interacting protein/protein kinase-dependent mechanism. J Neurosci 23: 11279–11288, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xia J, Zhang X, Staudinger J, Huganir R. Clustering of AMPA receptors by the synaptic PDZ domain-containing protein PICK1. Neuron 22: 179–187, 1999. [DOI] [PubMed] [Google Scholar]
- 50.Xiong Z, Liu Y, Hu L, Ma B, Ai Y, Xiong C. A rapid facilitation of acid-sensing ion channels current by corticosterone in cultured hippocampal neurons. Neurochem Res 38: 1446–1453, 2013. [DOI] [PubMed] [Google Scholar]
- 51.Xu J, Xia J. Structure and function of PICK1. Neurosignals 15: 190–201, 2006. [DOI] [PubMed] [Google Scholar]
- 52.Yu Y, Chen Z, Li WG, Cao H, Feng EG, Yu F, Liu H, Jiang H, Xu TL. A nonproton ligand sensor in the acid-sensing ion channel. Neuron 68: 61–72, 2010. [DOI] [PubMed] [Google Scholar]
- 53.Zha XM. Acid-sensing ion channels: trafficking and synaptic function. Mol Brain 6: 1, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]


