Abstract
Pi transport in epithelia has both Na+-dependent and Na+-independent components, but so far only Na+-dependent transporters have been characterized in detail and molecularly identified. Consequently, in the present study, we initiated the characterization and analysis of intestinal Na+-independent Pi transport using an in vitro model, Caco2BBE cells. Only Na+-independent Pi uptake was observed in these cells, and Pi uptake was dramatically increased when cells were incubated in high-Pi DMEM (4 mM) from 1 day to several days. No response to low-Pi medium was observed. The increased Pi transport was mainly caused by Vmax changes, and it was prevented by actinomycin D and cycloheximide. Pi transport in cells grown in 1 mM Pi (basal DMEM) decreased at pH > 7.5, and it was inhibited with proton ionophores. Pi transport in cells incubated with 4 mM Pi increased with alkaline pH, suggesting a preference for divalent phosphate. Pi uptake in cells in 1 mM Pi was completely inhibited only by Pi and partially inhibited by phosphonoformate, oxalate, DIDS, SITS, SO42−, HCO3−, and arsenate. This inhibition pattern suggests that more than one Pi transporter is active in cells maintained with 1 mM Pi. Phosphate transport from cells maintained at 4 mM Pi was only partially inhibited by phosphonoformate, oxalate, and arsenate. Attempts to identify the responsible transporters showed that multifunctional anion exchangers of the Slc26 family as well as members of Slc17, Slc20, and Slc37 and the Pi exporter xenotropic and polytropic retrovirus receptor 1 are not involved.
Keywords: phosphate transport, Caco2BBE cells, Na+-independent Pi uptake, phosphate absorption, small intestine, inorganic phosphate
control of Pi homeostasis is mediated by the coordinated activity of a complex set of physiological mechanisms acting on the rate of intestinal absorption, rate of renal excretion, and eventual mobilization of the bone reservoir. These mechanisms consist of hormones and nonhormonal events that act either acutely or slowly (chronically) to modulate the activity of plasma membrane Pi transporters (2, 4, 23). The precision of Pi homeostasis control is critical not only because of the extreme relevance of the physiological roles of Pi in the organism (pH buffer, energy bonds, signal transduction, phospholipid composition, bone formation, etc.) but also because serious conditions can emerge when control of Pi homeostasis is lost, resulting in either hypophosphatemia (7) or hyperphosphatemia (20, 23).
While the kidney has historically been recognized as the major checkpoint and regulator of Pi homeostasis, more recently it has been revealed that the intestine is a relevant modulator of Pi signaling and is now a target for pharmacological interventions in phosphate disorders (23, 30, 31). The majority of Pi absorption takes place in the small intestine, with important regional differences depending on the animal species (22). With respect to the mechanisms of Pi absorption, the classical combination of both saturated (transcellular transport) and unsaturated (paracellular diffusion) components have been described (16, 21). A detailed kinetic characterization of the transport component was achieved using brush-border membrane vesicles (BBMVs) of the small intestine, thereby showing the existence of both Na+-dependent and Na+-independent components, with similar characteristics and pH dependence (3, 5).
More recently, the molecular characterization of Na+-dependent Pi transport in the intestine revealed the complementary participation of different Pi transporters: NaPi2b and retroviral receptors Pit-1 and Pit-2. These transporters are mainly located in the apical membrane of enterocytes, and they exhibit different kinetic behavior, substrate specificity, and pH dependence (11, 12) as well as regulation by several agents, including vitamin D3 and changes in dietary Pi (14, 18, 22).
In addition to classical kinetic studies, mouse models have revealed the relevance of the different transport components through NaPi2b gene deletion (26, 29). Studies with these models revealed that whereas NaPi2b seemed to be responsible for most of the Na+-dependent Pi absorption, the Na+-independent component (including downhill transport and the paracellular route) was still responsible for ∼50% of total Pi absorption. However, the exact percentage of Na+-independent Pi absorption depends on the experimental setup (3, 5, 29), among other factors. The molecular identity of Na+-independent transport is still unknown, as is the basolateral exit of Pi from the enterocyte into the blood. However, it has recently been proposed that the basolateral step could be mediated by xenotropic and polytropic retrovirus receptor 1 (XPR1), another cell surface multipass retroviral receptor (13).
In the present study, we characterized Na+-independent Pi transport in a human intestinal cell line (Caco2BBE cells) as a preliminary step toward molecular identification of the responsible transporter. In addition to physiological and regulatory experiments, we also assayed several candidate transporters according to the pattern of inhibition that we observed.
MATERIALS AND METHODS
Cell culture.
All cell culture products were from Life Technologies-GIBCO (Paisley, UK). Caco2BBE cells were obtained and grown, as previously described (15), in high-glucose (4.5 mg/ml) DMEM supplemented with 20% FCS, penicillin, streptomycin, and l-glutamine at 37°C and 5% CO2. The incubation of cells at different concentrations of Pi was initiated at 100% confluence.
Opossum kidney (OK) cells were grown, as previously described (32), in DMEM-Ham's F-12 supplemented with 10% FCS, penicillin, streptomycin, and l-glutamine at 37°C and 5% CO2. For experiments with Caco2BBE or OK cells in which the Pi concentration was below 1 mM, Pi-free DMEM was used (GIBCO).
Animals.
Male Wistar rats were purchased from Janvier SAS (Berthevin, France). Animals were cared for in accordance with European legislation, and procedures were approved by the Ethical Committee of the University of Zaragoza. Rodent fodder containing 1.2% or 0.1% Pi was purchased from Provimi Kliba SA (Penthalaz, Switzerland).
Rats were fed with 0.1% Pi diet for 4 h every day (from 8:00 AM to 12:00 PM) over 5 days. On the day of the experiment, some of the rats were fed with 1.2% Pi diet for 4 h to achieve acute adaptation to the high-Pi diet. The remaining rats once again received 0.1% Pi diet for 4 h.
Transport assays.
These assays have been extensively explained in our previous publications, either for cells grown on plastic support (32) or using intestinal BBMVs according to the rapid filtration method (14). In both cases, [32P]H3PO4 (Perkin-Elmer, Waltham, MA) was used as a radiotracer. d-[3H]glucose and d-[14C]fructose were obtained from Amersham Biosciences (Buckinghamshire, UK). Fits to either Michaelis-Menten for the kinetic parameters of transport or to the sigmoidal curve for mean inhibitory concentration (IC50) determinations were performed by nonlinear regression using GraphPad Prism 5.0 software (San Diego, CA). For Pi saturation kinetics, an equation containing both saturable and nonsaturable (diffusion plus unspecific binding) components was used: v = [Vmax × Pi concentration/(Km + Pi concentration)] + (Kd × Pi concentration). For the estimation of IC50, the following equation was used: v = V0 + (V100 − V0)/[1 + 10e(logEC50 − Pi concentration)], where V100 is the velocity of transport at zero concentration of inhibitor and V0 is the minimal transport rate obtained with the maximal concentration of inhibitor.
Pharmacological treatments.
Caco2BBE cells were incubated with either 1 or 4 mM Pi for 24 h in the presence or absence of 1 μg/ml actinomycin D (inhibitor of RNA transcription) or 100 μM cycloheximide (inhibitor of protein synthesis). To study the involvement of proton gradients in Na+-independent Pi transport, the following compounds were used: 10 μM FCCP, 10 μM CCCP, 0.1 μM bafilomycin A1, and 2 mM furosemide. All drugs were dissolved in DMSO except for furosemide, bafilomycin A1, and FCCP, which were dissolved in ethanol. The same amount of solvent was added to the corresponding control cells. All chemicals were from Sigma-Aldrich (St. Louis, MO).
RNA interference.
Validated short interfering (si)RNA molecules (Silencer Select siRNAs) were obtained from Life Technologies-Ambion (Austin, TX). siRNAs were transfected using Lipofectamine 2000 (Life Technologies) at a final concentration of 5 nM following the manufacturer's instructions. The maximal reduction of target RNAs was observed after 48 h in Caco2BBE cells after real-time quantification. The corresponding negative controls with a similar base composition were also used.
Real-time PCR.
Total RNA was purified from Caco2BBE cells using the SV Total RNA Isolation Kit (Promega, Fitchburg, WI). After being treated with DNase I, total RNA was retrotranscribed with a Transcriptor First Strand cDNA Synthesis Kit and amplified in a LightCycler 1.5 using a FastStart Master SYBR Green I kit (all from Roche, Mannheim, Germany). The primers used are shown in Table 1. Gene expression data were normalized to an endogenous reference (peptidylprolyl isomerase B, also known as cyclophilin B) and to a calibrator (a combination of identical amounts of RNA from cells incubated for 48 h in culture medium containing 1 or 4 mM Pi) as previously described (24) and according to the manufacturer's instructions.
Table 1.
Primers used in real-time PCR
| Sequences |
|||||
|---|---|---|---|---|---|
| Gene Name | Protein Name | Accession No. | Forward | Reverse | Amplification, bp |
| PPIB | Peptidylprolyl isomerase B/cyclophilin B | NM_000942 | 5′-CCTTAGCTACAGGAGAGAAAG-3′ | 5′-CCCTGGATCATGAAGTCCTTGA-3′ | 402–481 |
| SLC26A1 | Sat1 | NM_022042 | 5′-GATGACCGGGCTTTACCAG-3′ | 5′-AGGTGTTTGAGCTGCGAG-3′ | 680–810 |
| SLC26A2 | DTDST | NM_000112 | 5′-CTATGCAATTATGGTTGGCAGC-3′ | 5′-AGGCATCTGAGAGGTAGACAG-3′ | 916–1022 |
| SLC26A3 | DRA | NM_000111 | 5′-TGGTATCAGCACAGGGATTG-3′ | 5′-ATATGTGTCTGGAAGTGCCG-3′ | 448–587 |
| SLC26A4 | Pendrin | NM_000441 | 5′-ACTGCGGGTGATTGTCAAAG-3′ | 5′-TCGTCAAAGAACCCGCATTG-3′ | 2249–2356 |
| SLC26A5 | Prestin | NM_198999 | 5′-ATCATGGGAGCAAGGAGAAAG-3′ | 5′-GCATCCTCTCCATCTACTTCTG-3′ | 1935–2047 |
| SLC26A6 | PAT1 | NM_022911 | 5′-CCTCTATAGCTCCTTCTACCCTG-3′ | 5′-ATCATGGAGTCGTTCAAGGC-3′ | 466–612 |
| SLC26A7 | SUT2 | NM_052832 | 5′-GCTTCCCAAGAGCAATGACT-3′ | 5′-GCAGGGTTTCACTGTCCATT-3′ | 1651–1737 |
| SLC26A8 | TAT1 | NM_052961 | 5′-GCCCAGGATTCTTTACACAGAG-3′ | 5′-TGAGACACGGACGATACTGT-3′ | 2144–1992 |
| SLC26A9 | SLC26A9 | NM_052934 | 5′-CACCCACTGCTTGTAAATGC-3′ | 5′-TGTCCGGTCCTTCTTCTCAA-3′ | 88–204 |
| SLC26A10 | SLC26A10 | NM_133489 | 5′-AATCCTGCGAGAAGCCTTAGA-3′ | 5′-TGATCTTCCTCAGACCCTTTCC-3′ | 167–2044 |
| SLC26A11 | SUT1 | NM_173626 | 5′-GTCTCCTTCTACACCTTCCATG-3′ | 5′-GGTGAAGCCTTTAATGACGGG-3′ | 603–743 |
| SLC17A1 | NPT1/NaPi1 | NM_005074 | 5′-TTTGTCCCGAATCAGTGGGT-3′ | 5′-ACCATTGTGAGGTTCAGGCA-3′ | 52–250 |
| SLC34A2 | NaPi2b | NM_001177999 | 5′-AGCCACTGTCCATGACTTCTTC-3′ | 5′-GAGCTTTGTGAAGGGCTTAGTG-3′ | 749–923 |
| SLC20A1 | Pit-1 | NM_005415 | 5′-TGGGAGCCAATGATGTAGCA-3′ | 5′-ACATCTCCACGTCAATCAAGCC-3′ | 655–822 |
| SLC20A2 | Pit-2 | NM_001257181 | 5′-ATTTCCTTTGGTGTCGCCCT-3′ | 5′-TTGGCACCTGGTAGCTCTTT-3′ | 1346–1527 |
| XPR1 | Xenotropic and polytropic retrovirus receptor 1 | NM_004736 | 5′-TTTGCAGCCCTTTACAGCAC-3′ | 5′-GAGGAAAGTGTTCTCTCCAGCA-3′ | 1666–1833 |
Statistics.
With the exception of the animal experiments, which were confirmatory, all other experiments were repeated three times using triplicates per condition. GraphPad Prism 5.0 was also used for statistical analysis, and significances of differences were determined by one-way ANOVA and a Tukey posttest for multiple comparisons. P values are indicated in the figures when necessary. To compare the fits to the same model of Michaelian behavior, an extra sum-of-squares F-test was performed. To compare the specific parameters and to test whether the kinetic constant was shared among all data sets, a global fit was also performed.
RESULTS
Response to Pi concentration in rats and Caco2BBE cells.
In previous experiments, rats chronically adapted to a low-Pi diet (0.1%) showed an increase of duodenal BBMV Pi uptake when rats were acutely fed a high-Pi diet (1.2%) for 4 h (14). Before we studied Caco2BBE cells as a model of the intestinal absorption of Pi, we repeated and extended the previous study on rats by analyzing the duodenum, jejunum, and ileum in the presence and absence of Na+ (Fig. 1A). In this experiment, acute, 4-h feeding of a high-Pi diet (1.2%) significantly increased Pi uptake in the duodenum and jejunum. Interestingly, this increase was also significant in the absence of Na+ in the duodenum and jejunum.
Fig. 1.
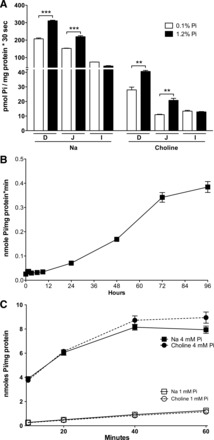
Pi transport upregulation in response to a high Pi concentration. A: acute (4 h) intestinal response to a 1.2% Pi diet in rats fed with a diet of 0.1% Pi for 5 days. D, duodenum; J, jejunum; I, ileum. ***P < 0.001; **P < 0.01. B: effect of hours of incubation at 4 mM Pi in cell culture on 32Pi uptake in the presence of Na+ in Caco2BBE cells. C: time course of Pi uptake in Caco2 cells preincubated or not with 4 mM Pi for 24 h.
We then studied the response of Caco2BBE cells incubated from 1 h to 4 days in high-Pi (4mM) culture medium (Fig. 1B). 32Pi uptake was determined during 20 min at room temperature and in the presence of Na+. The results revealed a significant increase that started at 24 h and increased thereafter up to 4 days.
Subsequently, we incubated Caco2BBE cells for 48 h with 4 mM Pi in DMEM to compare their uptake of Pi with the uptake of cells maintained in 1 mM Pi. Figure 1C shows that 32Pi uptake of Caco2BBE cells incubated with 4 mM Pi increased compared with cells maintained in DMEM with 1 mM Pi. Once again, Na+-dependent Pi transport was not observed, i.e., the effect and total uptake were similar in the presence and absence of Na+. The maximal increase (∼15 times) was observed at 10 min of uptake time. Thereafter, the increase dropped progressively as a consequence of the saturation of Pi uptake in cells preincubated with 4 mM Pi, and at 60 min the increase was only six times.
Kinetic characteristics of Pi transport in Caco2BBE cells.
The absence of Na+-dependent Pi uptake was checked under different conditions. First, it was assayed after the time of confluence, because the differentiation state could affect the expression of Na+-dependent (NaPi2b) transporters. As shown in Fig. 2A, no Na+-coupled Pi uptake was observed above the level indicated by uptake in the absence of Na+, even after 17 days postconfluence in Caco2BBE cells maintained in normal DMEM (1 mM Pi). However, uptake was drastically diminished between 0 and 5 days of confluence (see discussion).
Fig. 2.
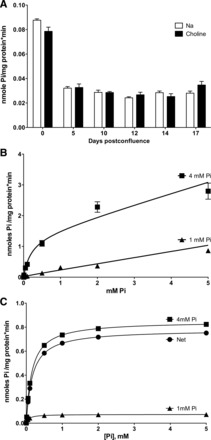
Kinetic characteristics of Pi transport in Caco2BBE cells. A: total Pi uptake in the presence and absence of Na+ as a function of postconfluence days in regular DMEM (1 mM Pi). B: nonlinear regression fits of total Pi uptake to an equation containing a Michaelis-Menten component and a nonsaturable component (see materials and methods). Both fits were significantly different, as a consequence of the Vmax increase, with P < 0.0001. C: net, nonlinear regression of theoretical transport in the same cells, according to the kinetic parameters of transport obtained in B. See text for further details.
To further characterize Pi uptake in Caco2BBE cells, Michaelis-Menten saturation kinetics were performed in cells preincubated with either 1 or 4 mM Pi for 48 h (Fig. 2B). These assays were performed in the presence of Na+ to guarantee more physiological conditions. Fits revealed that the increased uptake was mediated by an increase of 12 times Vmax: from 0.073 to 0.849 nmol Pi·mg cell protein−1·min−1. The apparent Km value increased from 0.071 to 0.16 mM, but a global (shared) fit revealed that the difference was not statistically significant. This was confirmed by a t-test using the different affinity constants (three Km per condition). To illustrate that the uptake change was mainly caused by an increase in capacity (Vmax) rather than affinity, the theoretical (i.e., saturable) transport components of the total uptake in Fig. 2B are shown in Fig. 2C.
pH effect on Pi transport in Caco2BBE cells.
The transport response to environmental pH changes is a hallmark of every Pi transporter. Therefore, we also studied this effect in Caco2BBE cells. In cells maintained in DMEM with 1 mM Pi, Pi transport was identical in the presence and absence of Na+ (Fig. 3A,1) at all pHs. From pH 6.0 to 7.5, Pi transport remained constant, and thereafter it decreased constantly at pH 8.0 and 8.5, similar to Pi transport in the small intestine (see discussion). Conversely, cells preincubated with 4 mM Pi (Fig. 3A,2) exhibited transport in which, while still Na+ independent, the behavior was similar to that of the proximal tubule of the kidney: Pi uptake increased steeply from pH 6.0 to 8.5. As a control to confirm the accuracy of our determinations, we also measured the well-known effect of pH on 50 μM Pi uptake in OK cells, a well-established cell line model of the renal proximal tubule obtained from a female American opossum, which exhibits strong type IIa Na+-dependent Pi transport. Figure 3A,3 shows that OK cells maintained in standard medium only express Na+-dependent Pi transport, which increases when the pH of the uptake medium also increases, similar to Na+-independent Pi uptake in Caco2BBE cells preincubated with 4 mM Pi.
Fig. 3.
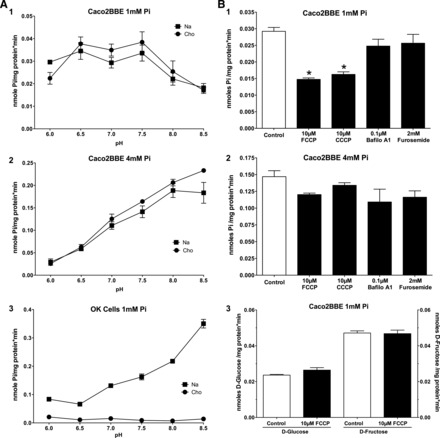
Effect of pH and proton ionophores on 50 μM Pi uptake in two cell lines. A: effect on Caco2BBE cells incubated for 48 h in 1 mM Pi (1) or 4 mM Pi (2) as well as the effect of pH on Pi uptake in opossum kidney (OK) cells incubated in standard conditions (DMEM-Ham's F-12 medium; 3). B: effect of ionophores and ATPAse inhibitors on Pi uptake in 1 mM Pi-maintained cells (1) or 4 mM Pi-maintained cells (2) as well as the absence of an effect on Na+-independent uptake of d-glucose and d-fructose (3). Cho, choline; Bafilo A1, bafilomycin A1.
The effect of pH could be interpreted as a preference of the two Pi transports (mainly being expressed in cells maintained in 1 vs. 4 mM Pi culture medium) for the two Pi species, H2PO4− versus HPO42−. Regarding the transport in 1 mM Pi-maintained cells, which exhibit higher Pi uptake at a low pH, this could also be interpreted as the use of a proton gradient as the driving force. Therefore, we treated cells with two ionophores (FCCP and CCCP) and a vacuolar-type H+-ATPase inhibitor (bafilomycin A1), and Pi transport was measured in standard conditions (pH 7.5). FCCP and CCCP only reduced Pi uptake by 50% in Caco2BBE cells maintained in 1 mM Pi (Fig. 3B,1), but they failed to modify Pi uptake in cells preincubated in 4 mM Pi (Fig. 3B,2). Similarly, bafilomycin A1 did not alter Pi transport at either 1 or 4 mM Pi. This can be explained as the proton gradient being maintained by another type of pump or, most likely, as the experimental proton gradient created by the low pH is sufficient to sustain Pi transport during the short, linear velocity time of uptake. As expected, furosemide, a Na+-ATPase inhibitor, did not modify Pi uptake in any condition. To confirm the specificity of the effect of FCCP and CCCP in 1 mM Pi, the absence of an effect by these drugs on Na+-independent uptake of d-glucose and d-fructose was also tested (Fig. 3B,3).
The response of Caco2BBE cells to a high Pi concentration is progressive.
Caco2BBE cells were incubated for 48 h in DMEM containing 0.2, 1, 2, or 4 mM Pi, and 32Pi uptake was then assayed in the presence or absence of Na+ and at different pHs (6.0, 7.5, and 8.5; Fig. 4). The changes in Pi transport as a consequence of incubation with different concentrations of Pi were not observed when uptake was assayed at pH 6.0. At pH 7.5, however, a significant increase was already observed in cells preincubated with 4 mM Pi, and at pH 8.5, even the transport in cells preincubated with 2 mM Pi was significantly different from that of cells maintained in 1 mM Pi. No changes were observed with a low concentration of Pi (0.2 mM) at any pH. In Fig. 4, each graph represents a different experiment, meaning different cell cultures, and therefore results in A–C cannot be compared in absolute terms with respect to the transport rate (according to the critical changes in transport rate depending on confluence, as shown in Fig. 2A) but rather in relation to the experimental conditions used in each assay or graph.
Fig. 4.
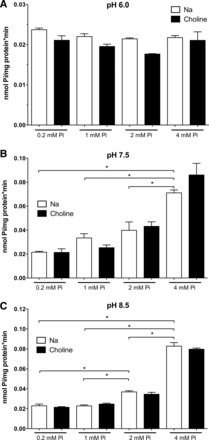
Progressive upregulation of Pi transport in Caco2BBE cells as a response to increasing Pi concentrations. The increased uptake effect is blunted when measured at pH 6.0 (A), and it can only be observed at pH 7.5 and 8.5 and at 2 and 4 mM Pi (B and C). Uptake is shown in the presence or absence of Na+. Comparisons are only shown for conditions in the presence of Na+. The same significances were obtained in the absence of Na+. *P < 0.05.
The response to a high Pi concentration is dependent on de novo RNA and protein syntheses.
To ascertain whether the increase in Pi uptake was dependent on the synthesis of new transporters or activators, or conversely if it was caused by the posttranslational reorganization or modification of preexisting molecules, Caco2BBE cells were incubated for 24 h in the presence or absence of inhibitors of transcription (1 μg/ml actinomycin D) or translation (100 μM cycloheximide). Cells were incubated for only 24 h to avoid toxic overexposure to the drugs. Figure 5 shows that, in this particular experiment, cells incubated with 4 mM Pi exhibited five times more Pi transport than cells incubated with 1 mM Pi, yet when cells were simultaneously treated with actinomycin D or cycloheximide, the increase was only twice as much. This means that both of the pharmacological agents prevented the increased Pi transport by 70%, and, therefore, the transcription and translation of new proteins is a requisite step for the response to 4 mM Pi.
Fig. 5.
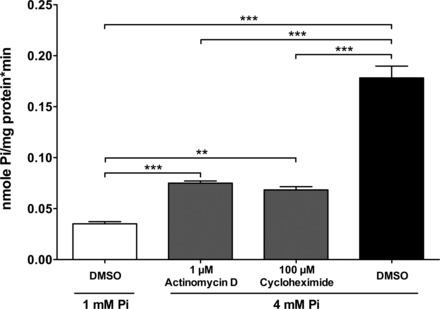
The effect by the incubation of Caco2BBE cells in a culture medium of 4 mM Pi for 24 h on Pi transport is mostly prevented by inhibitors of RNA and protein synthesis, actinomycin D and cycloheximide. P < 0.0001 by ANOVA. **P < 0.01; ***P < 0.001.
Pattern of Pi transport inhibition on Caco2BBE cells.
Inhibitions of Pi uptake using potential substrates were performed in Caco2BBE cells cultured in the presence of 1 or 4 mM Pi for 48 h to gain knowledge of the likely transport systems involved in this Na+-independent Pi transport. 32Pi uptake was assayed at 50 μM for 20 min in the absence or presence of the inhibitors shown in Fig. 6A. We mainly tested several inorganic and organic anions of biological relevance, whose transporters could also be involved in Na+-independent phosphate uptake. For example, sulfate is Na+ independently transported by Sat-1 and other members of the Slc26 family, including oxalate, formate, bicarbonate, etc. DIDS and SITS are stilbene disulphonate derivatives that inhibit most anion antiporters, whereas phosphonoformic acid (PFA) and arsenate are mainly inhibitors of type II Pi transporters. The results showed that in both cases (i.e., cells maintained at either 1 or 4 mM Pi in culture medium) no other inhibitor was as effective as 5 mM phosphate at inhibiting 32Pi uptake. In cells maintained at 1 mM Pi, incubation with 10 mM alanine, glutamate, or formate did not inhibit Pi transport, and only partial inhibition was reached with 10 mM sodium sulfate, sodium oxalate, or sodium bicarbonate, with 5 mM PFA, with 0.1 mM DIDS, with 0.1 mM SITS, and with 5 mM sodium arsenate. Regarding cells incubated for 48 h with 4 mM Pi, the pattern of inhibition was more restricted, because Pi transport was only partially inhibited with oxalate, PFA, and arsenate.
Fig. 6.
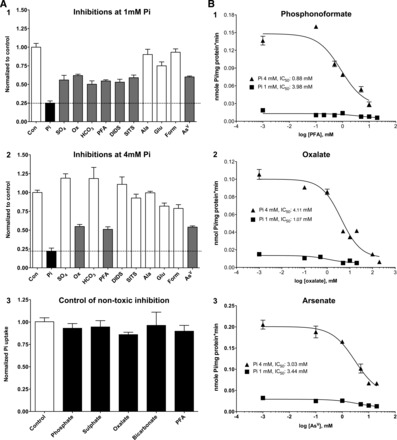
Pattern of Pi transport inhibition. A: normalized graphs of Pi uptake inhibition with several substrates. 32Pi uptake was assayed at 50 μM for 20 min. Solid and shaded bars indicate significant (complete or partial, respectively) inhibition compared with the control (Con; open bars). Pi, phosphonoformic acid (PFA), and arsenate (AsV) were used at 5 mM; alanine (Ala), glutamate (Glu), formate (Form), sulfate (SO4), oxalate (Ox), and carbonate (HCO3) were used at 10 mM; DIDS and SITS were used at 0.1 mM. The dashed line indicates the level of inhibition with 5 mM Pi as an inhibitor. A: effect on 1 mM Pi-maintained Caco2BBE cells (1), effect on 4 mM Pi-preincubated Caco2BBE cells (2), and absence of metabolic/toxic effects of the inhibitors after 20 min of preincubation in the absence of 32Pi (3). B: dose-response relationships of the indicated inhibitors using Caco2BBE cells incubated with 1 or 4 mM Pi. The corresponding mean inhibitory concentrations are indicated.
The different patterns of inhibition in cells maintained in 1 or 4 mM Pi suggest that the inhibition is not caused by toxic effects such as metabolic alterations induced by an excess of inhibitors. Nevertheless, to completely discard possible side effects induced by the inhibitors, we also treated Caco2BBE cells with the inhibitors but without 32Pi. After 20 min of incubation, cells were washed twice, and 32Pi uptake was then measured for an additional 20 min. The assay revealed no changes induced by the inhibitors during the 20 min of exposure (Fig. 6A,3).
The inhibitions of Pi transport with PFA, oxalate, and arsenate were further characterized using dose-response relationships. The results are shown in Fig. 6B: complete inhibitions were barely achieved, and apparent IC50 values were, in general, very high. Arsenate was a poor inhibitor of Pi transport in cells incubated with either 1 or 4 mM Pi, with IC50 values of ∼3 mM. Oxalate inhibited Pi transport with more intensity in cells maintained at 1 mM Pi than at 4 mM Pi, whereas the opposite was observed for PFA, because a stronger inhibition of Pi transport was observed in cells maintained at 4 mM Pi.
Toward the molecular identification of Na+-independent Pi transport.
The pattern of inhibition of the two Na+-independent Pi transport systems (i.e., in cells incubated with either 1 or 4 mM Pi) suggested the involvement of some members of the Slc26 family of multifunctional anion transporters and channels, among others. Previously known Pi transporters, such as Slc20 family members Pit-1 and Pit-2 (Slc20A1 and Slc20A2), Slc17A1 (NaPi-I), and Slc43a2 (NaPi-IIb), should not be involved based on the respective Na+ dependence. We first analyzed the expressions of the corresponding RNAs by real-time PCR using a combination of cDNAs from Caco2BBE cells grown in 1 or 4 mM Pi culture medium for 48 h (i.e., our calibrator for quantitative PCR; see materials and methods). The primers used are shown in Table 1, and significant expressions are shown in Fig. 7A. The relative abundances are plotted in Fig. 7 with respect to the abundance of Slc26a8, which is the Slc26 family member with the lowest significant amplification. Very abundant transcripts corresponded to Slc26 family members 1, 2, 3, and 6. We also found low but significant expression of Slc26A1 and Slc26A11 as well as Slc20a1 and Slc20a2.
Fig. 7.
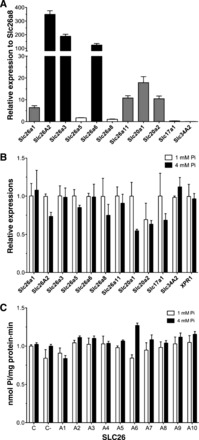
Analysis of the involvement of different carriers on Na+-independent Pi transport. A: real-time analysis of RNA expression of the indicated Slc26, Slc20, Slc17, and Slc34 family members. B: comparison of the relative abundance of the indicated RNAs after treatment with 1 or 4 mM Pi for 48 h. C: Pi transport after 48 h of small interfering (si)RNA transfections against the indicated Slc26 family members in Caco2BBE cells incubated with 1 or 4 mM Pi. C, nontransfected cells; C−, scrambled siRNA-transfected cells.
Next, we compared the expressions of those transcripts in cDNA prepared from cells grown at 1 versus 4 mM Pi for 48 h (Fig. 7B). The Na+-independent Pi exporter XPR1 was also included in this assay. No significant differences were observed between 1 and 4 mM Pi for any transcript, even for transcripts apparently inhibited at 4 mM Pi.
Even though the absence of differences in RNA expression suggest that the corresponding transporters are not involved in the increased Na+-independent Pi transport observed in cells incubated with 4 mM Pi, we also knocked down the expression of Slc26 family members with siRNA. Using this approach, the involvement of these members in the basal Pi transport observed in cells at 1 mM Pi could also be tested. As shown in Fig. 7C, the effect by the reduced expression of the indicated members of the Slc26 family did not alter the Pi transport rate in Caco2BBE cells. Real-time PCR confirmed the success of the siRNA treatment, showing a reduction of at least 75% compared with scrambled siRNA with a similar CG content (not shown).
DISCUSSION
In this work, we have functionally characterized a Na+-independent Pi transport system in the Caco2BBE cell line. This is a well-known colon carcinoma cell line from a Caucasian American male used as a model to study the enterocyte physiology of the small intestine, because it shares many characteristics of solute transport with the intestinal epithelium (27). However, contrary to a previous work (25), we did not detect a Na+-dependent Pi transport system in Caco2BBE cells. This absence could be explained by the fact that the Caco2BBE cell line corresponds to a C2 subclone of the original Caco-2 cells (28), whereas Mohrmann et al. (25) used the original cells.
Even though it is still Na+ independent, Pi transport in Caco2BBE cells also decreases after confluence, similar to the previous report (25). When epithelial cells, such as Caco2BBE cells, become confluent, they initiate a series of gene expression changes that induces differentiation (10). The change in Pi transport rate upon confluence could be related to this differentiation, e.g., it could be caused by the switch from a transport system of proliferating cells to a different system, more characteristic of intestinal, epithelial cells. In addition, the Caco2BBE cell line is an in vitro model cell line, and even if many authors have used it as a model of small intestine, this should be proved for every study and experimental condition. Consequently, we do not know yet whether the Pi transport that we measured in Caco2BBE cells is representative of either the small intestine or the colon. In this respect, not much is known about the role of the colon in Pi absorption, but findings in the literature point to the existence of, at least, a paracellular route that could explain the observed enema-induced hyperphosphatemia (17).
We also observed that Pi uptake is upregulated in Caco2BBE cells after cells were incubated with a high Pi concentration (4 mM; Fig. 1B). This increase is also strictly Na+ independent, it becomes significant at 24 h, and it increases even further with incubation time in a culture medium of 4 mM Pi (Fig. 1B). This finding is notable, because it shows that Caco2BBE cells behave similarly to the small intestine with respect to the Na+-independent component of Pi transport observed in BBMVs from the duodenum and jejunum of rats fed with an acutely high-Pi diet (Fig. 1A) (12). However, incubation of Caco2BBE cells in a low Pi culture medium (0.2 mM Pi) did not significantly change the transport rate of Pi, which is in contrast with the chronic intestinal response to a low-Pi diet (14).
In the present study, the characterization of Pi transport in Caco2BBE cells was always done under two conditions, namely, cells were maintained in a culture medium containing either 1 or 4 mM Pi. The rationale was to mimic the high concentrations of Pi that can be reached in the intestinal lumen (19). The subsequent results showed that the increased Pi uptake observed after incubation with 4 mM Pi is not simply a consequence of increased diffusion, unspecific uptake, or any other side effect resulting from Pi toxicity or the precipitation of calcium phosphates in the culture medium (for example). To the contrary, this increased rate of uptake exhibits several characteristics that are exclusive of transport mechanisms: saturation (Fig. 2, B and C), pH dependence (Fig. 3), inhibition by putative substrates (Fig. 6), and prevention by inhibitors of transcription and translation (Fig. 5). A summary of the different characteristics of Pi transport is shown in Table 2.
Table 2.
Characteristics of Pi transport in Caco2BBE cells
| Parameter | 1 mM Pi | 4 mM Pi |
|---|---|---|
| Vmax, nmol Pi·mg cell protein−1·min−1 | 0.073 ± 0.017 | 0.849 ± 0.11 |
| Km (mM) | 0.071 ± 0.020 | 0.155 ± 0.025 |
| pH correlation | Negative | Positive |
| Na+ gradient | Independent | Independent |
| Proton gradient | Dependent | Independent |
| Partial inhibitors | PFA, arsenate, oxalate, sulfate, bicarbonate, DIDS, SITS | PFA, arsenate, oxalate |
1 and 4 mM Pi refers to Pi transport in Caco2BBE cells maintained for 48 h in culture medium containing 1 or 4 mM Pi. PFA, phosphonoformate.
The increased Pi uptake observed in cells incubated with 4 mM Pi is caused by a significant change in Vmax (Fig. 2C). This could be interpreted as a consequence of the incorporation/activation of more transporters of the same kind as those observed in cells incubated with 1 mM Pi. However, other characteristics clearly differentiate between both transport systems described in cells incubated with 1 and 4 mM Pi: different pH dependence and pattern of inhibition. With respect to pH dependence, as shown in Fig. 3A, Pi transport in Caco2BBE cells incubated with 1 mM Pi remains unchanged from pH 6.0 to 7.5, and thereafter Pi transport drops. Conversely, Pi transport in cells maintained in a medium of 4 mM Pi for 48 h increases linearly to a maximum at pH 8.5, where 6.3 times the uptake of Pi at pH 6.0 is observed. Because Pi is a polyprotic acid, one possibility is that the transport observed in cells maintained with 4 mM Pi corresponds to a transporter that mainly carries divalent phosphate (HPO42−), whereas at 1 mM Pi the major transport system would prefer H2PO4− over HPO42−. pH dependence is a known characteristic of Pi transporters, because most of them transport preferentially monovalent or divalent Pi. Type II Na+-Pi cotransporters (NaPi2a, PaPi2b, and NaPi2c), for example, preferentially transport divalent Pi. Consequently, because Pi transport in the kidney (and OK cells) is mostly mediated by NaPi2a, the transport of Pi in the proximal tubule also increases with the pH, as shown when kidney cortex BBMVs are used (1, 33). The behavior of the small intestine, however, is opposite that of the kidney, i.e., Pi transport decreases as pH increases (3). This is a paradox because this behavior corresponds to type III Pi transporters, Pit-1 and Pit-2, despite the prominent expression of NaPi2b (6). In the case of Caco2BBE cells, only Na+-independent Pi transport is observed, and, therefore, type II and III Pi transporters should not be involved. In cells maintained at 1 mM Pi, Pi transport decreases as pH increases, whereas cells preincubated with 4 mM Pi show Na+-independent Pi uptake that increases with pH. Therefore, the molecular identity of the transport systems acting in cells maintained at either 1 or 4 mM Pi should be different.
The response of Pi transport to pH changes in Caco2BBE cells maintained in 1 mM Pi medium is not identical to changes observed in the intestine: the drop in transport does not initiate at pH 6.0 according to the decreasing concentration of H2PO4−; rather, it remains constant up to pH 7.5 (Fig. 3A,1). Therefore, additional causes could be involved in pH dependence at 1 mM Pi, such as the use of a proton gradient in microorganisms, as it has been recently suggested (9). The successful use of the ionophores FCCP and CCCP to reduce Pi uptake in 1 mM Pi-maintained cells strongly supports this view (Fig. 3B,1). The preference for HPO42− as a substrate of H+-mediated uptake at 1 mM Pi (or no preference by Pi species, either for H2PO4− or HPO42−) could explain the lack of inhibition of Pi transport between pH 6.0 and 7.5.
Finally, the pattern of inhibition with potential competitive substrates is also different in Caco2BBE cells maintained at 1 or 4 mM Pi. In both cases, maximal inhibition was obtained with 5 mM Pi, whereas partial inhibition was observed with 5 mM PFA and arsenate and with 10 mM oxalate (Fig. 6A). In the case of cells maintained in a medium of 1 mM Pi, Pi transport was also partially inhibited with sulfate, bicarbonate, DIDS, and SITS. These differences could once again be a consequence of more than one transport system participating in the Na+-independent uptake of Pi.
There are a large number of possible transporters that could be involved in the two Na+-independent Pi transport systems of Caco2BBE cells (observed in cells incubated at 1 or 4 mM Pi), according to the inhibitions shown in Fig. 6A. These transporters could belong to the SLC26 gene family of multifunctional anion exchangers (which include anions such as bicarbonate, sulfate, oxalate, or formate and inhibitions with DIDS or SITS), among others. With the exception of formate, all substrates behaved as partial inhibitors of Pi transport expressed in cells maintained in 1 mM Pi. In the transport observed in cells maintained with 4 mM Pi, inhibition was restricted to oxalate, PFA, and arsenate (Fig. 6A). When considering the use of a proton gradient as the source of energy in 1 mM Pi-maintained cells, the possibilities are further reduced. For example, SLC25A3 is a H+-coupled mitochondrial Pi carrier of the inner membrane, and several plasma membrane H+-coupled transporters of the SLC36 family only carry several amino acids. The same can be said for SLC45A1, which seems to transport H+/sugars, and SLC46A1, which seems to transport H+/folates.
Furthermore, we tried to identify potential transporters in several ways. For example, we analyzed RNA expression changes between cells maintained at 1 or 4 mM Pi or using siRNA-induced downregulation of specific transporters to prevent the increase of transport or to reduce basal Pi uptake (Fig. 7). None of these approaches were successful, but they help to eliminate potential candidates.
The functional and molecular nature of Na+-independent Pi transport remains elusive so far, and only a few classical works have made initial characterizations, such as Pi-HCO3− exchange in capillaries of the blood-brain barrier, which is also inhibited by DIDS, SITS, sulfate, and other substrates (8). Our work represents an initial characterization of the intestinal Na+-independent Pi transport system, and the findings could help to identify the specific carriers by using alternative approaches. For example, current work in our laboratory includes PCR subtraction of cDNAs prepared from cells maintained in 1 or 4 mM Pi as well as a proteomic strategy to identify differentially expressed proteins in cell lysates under the same conditions. This should help to understand the complex physiological mechanism of intestinal absorption of phosphate, whose knowledge is presently restricted to the Na+-dependent component of absorption.
GRANTS
This work was supported by Spanish Ministry of Economy and Competitiveness Research Grants SAF2012-33898 (to V. Sorribas) and Predoctoral Fellowship BES-2010-029965 (to E. Candeal).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: E.C., Y.A.C., and N.G. performed experiments; E.C., Y.A.C., N.G., and V.S. analyzed data; E.C., Y.A.C., N.G., M.L., and V.S. interpreted results of experiments; E.C., Y.A.C., N.G., M.L., and V.S. edited and revised manuscript; E.C., Y.A.C., N.G., M.L., and V.S. approved final version of manuscript; V.S. conception and design of research; V.S. prepared figures; V.S. drafted manuscript.
REFERENCES
- 1.Amstutz M, Mohrmann M, Gmaj P, Murer H. Effect of pH on phosphate transport in rat renal brush border membrane vesicles. Am J Physiol Renal Fluid Electrolyte Physiol 248: F705–F710, 1985. [DOI] [PubMed] [Google Scholar]
- 2.Berndt T, Kumar R. Novel mechanisms in the regulation of phosphorus homeostasis. Physiology (Bethesda) 24: 17–25, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berner W, Kinne R, Murer H. Phosphate transport into brush-border membrane vesicles isolated from rat small intestine. Biochem J 160: 467–474, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biber J, Hernando N, Forster I. Phosphate transporters and their function. Annu Rev Physiol 75: 535–550, 2013. [DOI] [PubMed] [Google Scholar]
- 5.Borowitz SM, Ghishan FK. Phosphate transport in human jejunal brush-border membrane vesicles. Gastroenterology 96: 4–10, 1989. [DOI] [PubMed] [Google Scholar]
- 6.Caldas Y, Giral H, Sorribas V, Levi M. Nuclear receptor LXR: a new partner for sodium-dependent phosphate cotransporters. Contrib Nephrol 180: 64–73, 2013. [DOI] [PubMed] [Google Scholar]
- 7.Carpenter TO. The expanding family of hypophosphatemic syndromes. J Bone Miner Metab 30: 1–9, 2012. [DOI] [PubMed] [Google Scholar]
- 8.Dallaire L, Béliveau R. Phosphate transport by capillaries of the blood-brain barrier. J Biol Chem 267: 22323–22327, 1992. [PubMed] [Google Scholar]
- 9.Dick CF, Dos-Santos AL, Majerowicz D, Gondim KC, Caruso-Neves C, Silva IV, Vieyra A, Meyer-Fernandes JR. Na+-dependent and Na+-independent mechanisms for inorganic phosphate uptake in Trypanosoma rangeli. Biochim Biophys Acta 1820: 1001–1008, 2012. [DOI] [PubMed] [Google Scholar]
- 10.Fleet JC, Wang L, Vitek O, Craig BA, Edenberg HJ. Gene expression profiling of Caco-2 BBe cells suggests a role for specific signaling pathways during intestinal differentiation. Physiol Genomics 13: 57–68, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Forster I, Hernando N, Sorribas V, Werner A. Phosphate transporters in renal, gastrointestinal, and other tissues. Adv Chronic Kidney Dis 18: 63–76, 2011. [DOI] [PubMed] [Google Scholar]
- 12.Forster IC, Hernando N, Biber J, Murer H. Phosphate transporters of the SLC20 and SLC34 families. Mol Aspects Med 34: 386–395, 2013. [DOI] [PubMed] [Google Scholar]
- 13.Giovannini D, Touhami J, Charnet P, Sitbon M, Battini JL. Inorganic phosphate export by the retrovirus receptor XPR1 in metazoans. Cell Rep 27: 1866–1873, 2013. [DOI] [PubMed] [Google Scholar]
- 14.Giral H, Caldas Y, Sutherland E, Wilson P, Breusegem S, Barry N, Blaine J, Jiang T, Wang XX, Levi M. Regulation of rat intestinal Na-dependent phosphate transporters by dietary phosphate. Am J Physiol Renal Physiol 297: F1466–F1475, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giral H, Cranston D, Lanzano L, Caldas Y, Sutherland E, Rachelson J, Dobrinskikh E, Weinman EJ, Doctor RB, Gratton E, Levi M. NHE3 regulatory factor 1 (NHERF1) modulates intestinal sodium-dependent phosphate transporter (NaPi-2b) expression in apical microvilli. J Biol Chem 287: 35047–35056, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrison HE, Harrison HC. Sodium, potassium, and intestinal transport of glucose, 1-tyrosine, phosphate, and calcium. Am J Physiol 205: 107–111, 1963. [DOI] [PubMed] [Google Scholar]
- 17.Hu MS, Kayne LH, Jamgotchian N, Ward HJ, Lee DB. Paracellular phosphate absorption in rat colon: a mechanism for enema-induced hyperphosphatemia. Miner Electrolyte Metab 23: 7–12, 1997. [PubMed] [Google Scholar]
- 18.Katai K, Miyamoto K, Kishida S, Segawa H, Nii T, Tanaka H, Tani Y, Arai H, Tatsumi S, Morita K, Taketani Y, Takeda E. Regulation of intestinal Na+-dependent phosphate co-transporters by a low-phosphate diet and 1,25-dihydroxyvitamin D3. Biochem J 343: 705–712, 1999. [PMC free article] [PubMed] [Google Scholar]
- 19.Kirchner S, Muduli A, Casirola D, Prum K, Douard V, Ferraris RP. Luminal fructose inhibits rat intestinal sodium-phosphate cotransporter gene expression and phosphate uptake. Am J Clin Nutr 87: 1028–1038, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lanzer P, Boehm M, Sorribas V, Thiriet M, Janzen J, Zeller T, St Hilaire C, Shanahan C. Medial vascular calcification revisited: review and perspectives. Eur Heart J 35: 1515–1525, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee DB, Walling MW, Corry DB. Phosphate transport across rat jejunum: influence of sodium, pH, and 1,25-dihydroxyvitamin D3. Am J Physiol Gastrointest Liver Physiol 251: G90–G95, 1986. [DOI] [PubMed] [Google Scholar]
- 22.Marks J, Srai SK, Biber J, Murer H, Unwin RJ, Debnam ES. Intestinal phosphate absorption and the effect of vitamin D: a comparison of rats with mice. Exp Physiol 91: 531–537, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Marks J, Debnam ES, Unwin RJ. The role of the gastrointestinal tract in phosphate homeostasis in health and chronic kidney disease. Curr Opin Nephrol Hypertens 22: 481–487, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martín-Pardillos A, Sosa C, Millán Á, Sorribas V. Effect of water fluoridation on the development of medial vascular calcification in uremic rats. Toxicology 318: 40–50, 2014. [DOI] [PubMed] [Google Scholar]
- 25.Mohrmann I, Mohrmann M, Biber J, Murer H. Sodium-dependent transport of Pi by an established intestinal epithelial cell line (CaCo-2). Am J Physiol Gastrointest Liver Physiol 250: G323–G330, 1986. [DOI] [PubMed] [Google Scholar]
- 26.Ohi A, Hanabusa E, Ueda O, Segawa H, Horiba N, Kaneko I, Kuwahara S, Mukai T, Sasaki S, Tominaga R, Furutani J, Aranami F, Ohtomo S, Oikawa Y, Kawase Y, Wada NA, Tachibe T, Kakefuda M, Tateishi H, Matsumoto K, Tatsumi S, Kido S, Fukushima N, Jishage K, Miyamoto K. Inorganic phosphate homeostasis in sodium-dependent phosphate cotransporter Npt2b+/− mice. Am J Physiol Renal Physiol 301: F1105–F1113, 2011. [DOI] [PubMed] [Google Scholar]
- 27.Peterson MD, Mooseker MS. Characterization of the enterocyte-like brush border cytoskeleton of the C2BBe clones of the human intestinal cell line, Caco-2. J Cell Sci 102: 581–600, 1992. [DOI] [PubMed] [Google Scholar]
- 28.Pinto M, Robin-Leon S, Apopay MD, Kedinger M, Triadou N, Dussaulx E, Lacroix B, Simon-Assmann P, Haffen K, Fogh J, Zweibaum A. Enterocyte-like differentiation and polarization of the human colon. Enterocyte-like differentiation and polarization of the human colon carcinoma cell line Caco-2 in culture. Biol Cell 47: 323–330, 1983. [Google Scholar]
- 29.Sabbagh Y, O'Brien SP, Song W, Boulanger JH, Stockmann A, Arbeeny C, Schiavi SC. Intestinal npt2b plays a major role in phosphate absorption and homeostasis. J Am Soc Nephrol 20: 2348–2358, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sabbagh Y, Giral H, Caldas Y, Levi M, Schiavi SC. Intestinal phosphate transport. Adv Chronic Kidney Dis 18: 85–90, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sabbagh Y, Schiavi SC. Role of NPT2b in health and chronic kidney disease. Curr Opin Nephrol Hypertens 23: 377–384, 2014. [DOI] [PubMed] [Google Scholar]
- 32.Sorribas V, Markovich D, Werner A, Biber J, Murer H. Expression of Na/Pi cotransport from opossum kidney cells in Xenopus laevis oocytes. Biochim Biophys Acta 1178: 141–145, 1993. [DOI] [PubMed] [Google Scholar]
- 33.Villa-Bellosta R, Sorribas V. Compensatory regulation of the sodium/phosphate cotransporters NaPi-IIc (SCL34A3) and Pit-2 (SLC20A2) during Pi deprivation and acidosis. Pflügers Arch 459: 499–508, 2010. [DOI] [PubMed] [Google Scholar]


