Abstract
The extent of lateral neck dissection (LND) in surgical resection of papillary thyroid carcinoma (PTC) with clinically lateral LNM (LLNM) remains controversial. We aimed to explore the frequency of and risk factors for level V LNM in patients with solitary PTC and clinically LLNM. To analyze the frequency and risk factors for level V LNM, we retrospectively reviewed 220 solitary PTC patients who underwent total thyroidectomy, bilateral central neck dissection, and therapeutic LND. LLNM were present in 82.3% patients, and levels II–V LNM were present in 45.9%, 62.7%, 55.5%, and 12.3% patients, respectively. Ipsilateral level V LNM was significantly associated with tumor size >10 mm, extrathyroidal extension, ipsilateral central LNM ratio ≥50%, and contralateral central LNM (CLNM), bilateral CLNM, and simultaneous levels II–IV LNM. Contralateral CLNM was an independent risk factor for level V LNM. In patients with solitary PTC and clinically LLNM, level V LNM was relatively uncommon. Therefore, routine level V lymphadenectomy may be unnecessary in these patients unless level V LNM is suspected on preoperative examination or associated risk factors, especially contralateral CLNM, are present.
Keywords: Lymph nodes, neck dissection, papillary, risk factors, thyroid cancer
Introduction
Cervical lymph node metastases (LNM) in papillary thyroid carcinoma (PTC), the most common histological type of thyroid cancer with an increasing worldwide incidence 1, are frequent and occur in approximately 30–80% patients 2, 3. Cervical LNM in PTC have been identified as an independent risk factor for regional recurrence 4, 5, 6, 7, and emerging evidences from large population‐based studies have indicated decreased disease‐free survival rate and increased mortality associated with regional LNM 7, 8, 9, 10. There is universal agreement that therapeutic lateral neck dissection (LND) should be undertaken in patients with PTC and clinically lateral LNM (LLNM) on the basis of palpation or imaging examination 11, 12. However, determining the appropriate extent of LND remains controversial. Radical operations, such as those with increased extent of LND, may lead to clinically important postoperative morbidities (shoulder dysfunction, neck numbness, and neuropathic pain) because of injury to the spinal accessory nerve or the cervical plexus despite gross preservation of these nerves 13, 14, 15. Therefore, an oncologically effective therapeutic LND is critical to postoperative outcome.
In general, the extent of therapeutic LND includes levels II–V. However, it is debatable whether routine level V lymphadenectomy is necessary in patients with PTC with clinically LLNM 16, 17, 18, 19, 20, 21, 22. In addition, there have been few studies to explore the risk factors for level V LNM in solitary PTC with clinically LLNM. To determine a correlation that could define the rational extent of therapeutic LND in PTC, we aimed to explore the frequency of and the risk factors for level V LNM in solitary PTC patients with clinically LLNM.
Patients and Methods
Study population
We retrospectively reviewed the medical records of consecutive patients with histologically proven solitary (without pathological evidence of multifocality) PTC who underwent simultaneous total thyroidectomy (TT), bilateral central neck dissection (CND), and LND (at least from levels II to V) at the Department of Thyroid and Breast Surgery, West China Hospital of Sichuan University, between January 2011 and December 2014. All patients underwent preoperative physical examination, high‐quality thyroid ultrasonography (US), and US‐guided fine‐needle aspiration biopsy (USgFNAB) of the primary tumor. At our institution, preoperative US is routinely performed to access cervical lymphadenopathy, with therapeutic LND performed mostly on the basis of the US findings; therefore, our study included solitary PTC patients with clinically LLNM suspected on US according to at least one of the following metastatic criteria: round shape (long/short ratio <2), microcalcification, cystic change, hyperechogenicity, and heterogeneous inner structure 23. The final diagnosis of primary tumors and cervical LNM was based on pathological examination of surgical specimens. Patients were excluded from the study if they had thyroid carcinoma with a diffuse sclerosis variant, mixed histology, cancer localized to the isthmus, reoperation, or undivided lymph node specimens. Consequently, 220 patients were enrolled in the study. Of these, 25 and 195 patients had undergone bilateral and unilateral LND, respectively. This study was approved by the institutional review board of the West China Hospital of Sichuan University.
Tumor and lymph node classification
In our study, solitary PTC was defined as PTC without pathological evidence of multifocality within the thyroid. The largest diameter and location of the primary tumor within the thyroid were determined from pathology reports. The location of the tumor was classified according to which third (superior, middle, or inferior) of the affected thyroid lobe was involved. If a tumor extended into an adjacent lobe, it was categorized according to all sections involved 24.
The entire thyroid gland was excised first, followed by bilateral CND and LND. The maximum extent of CND was the hyoid bone superiorly, the innominate vein inferiorly, and the carotid sheaths laterally. Central lymph node (CLN) specimens were classified as prelaryngeal, pretracheal, ipsilateral paratracheal, or contralateral paratracheal (Figure 1). Next, the prelaryngeal, pretracheal, and ipsilateral paratracheal lymph nodes were defined as ipsilateral CLN and the contralateral paratracheal lymph node as contralateral CLN, according to the definition of laterality proposed by Keum et al. 22. The surgeon separately removed the nodes in each of these categories. The LND was carried out in the usual fashion from at least level II to V, sparing the internal jugular vein, spinal accessory nerve, and sternocleidomastoid muscle. The surgeon also separated the LND specimens according to neck levels. All thyroid and LND specimens were sent to the department of pathology for paraffin fixation and histological analysis. All neck dissection specimens were recorded according to neck regions, but only the ipsilateral lateral specimens that had a bilateral LND were analyzed in this study.
Figure 1.
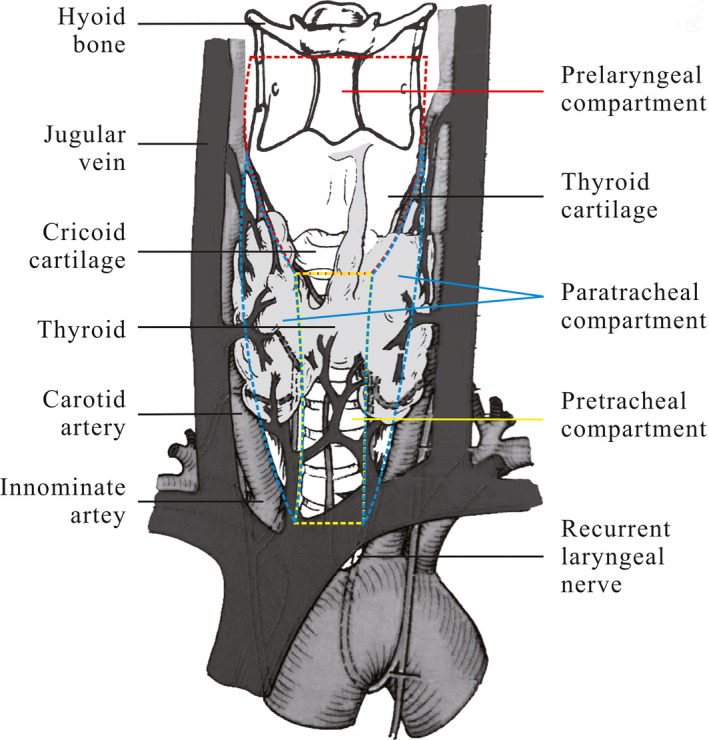
Subgroups of the central compartment (Prelaryngeal lymph node demarcated superiorly by the inferior border of the hyoid bone and inferiorly by the inferior border of the cricoid cartilage; Ipsilateral/contralateral paratracheal lymph node demarcated laterally by the carotid artery and medially by the trachea; Pretracheal lymph node demarcated superiorly by the inferior border of the cricoid cartilage and inferiorly by the innominate artery) 210x297mm (300 x 300 DPI).
Information on the following risk factors was obtained: sex, age, size, and location of the primary tumor, capsular invasion, extrathyroidal extension, presence of coexistent lymphocytic thyroiditis, and the extent of LNM.
Statistical analysis
In univariate analysis, categorical variables were analyzed using Pearson's chi‐square test and continuous variables were analyzed using the Student's t‐test or the Wilcoxon rank‐sum test. Binary logistic regression analysis was used for the multivariate analysis of categorical variables, with P < 0.100 on univariate analysis; P < 0.05 was considered statistically significant. Statistical analysis was performed using STATA version 12.0 (Stata Corporation, College Station, TX, USA).
Results
Of the eligible patients with solitary PTC (n = 220), 51 (23.2%) were male and 169 (76.8%) were female. The median age was 41.0 ± 13.5 years (range: 13–79 years) and the mean size of the primary thyroid tumor was 21.4 ± 13.3 mm (range: 3–89 mm). A summary of patient and tumor characteristics is shown in Table 1. LNM was histologically confirmed to involve the central compartment in 177 patients (80.5%) and the lateral compartment in 181 patients (82.3%). Twenty‐two patients (10%) had skip metastases (metastases to the lateral cervical compartment without metastasis to the central cervical compartment). Of the 177 patients with central LNM (CLNM), 172 patients (78.2%) had LNM in the central compartment ipsilateral to the primary tumor, 87 (39.5%) had LNM in the contralateral central compartment, and 82 (39.1%) had LNM in the bilateral central compartments. Of the 181 patients with LLNM, 44 patients (20.0%) had single‐level metastases, and 137 patients (62.3%) had multiple‐level metastases. Level III metastases were most common (138/220; 62.7%), followed by level IV (122/220; 55.5%), level II (101/220; 45.9%), and level V (27/220; 12.3%) metastases (Table 1). The mean ± SD (Standard Deviation) number of excised and metastatic lymph nodes in the central compartment was 10.19 ± 5.71 (range: 0–32) and 4.18 ± 3.90 (range: 0–20), respectively. The median total metastatic lymph node ratio was 0.41 ± 0.31 (range: 0.00–1.00) in the central compartment. In the lateral compartment, the mean ± SD number of excised and metastatic lymph nodes, respectively, was 24.95 ± 11.31 (range: 5–63) and 4.07 ± 3.79 (range: 0–23). The median total metastatic lymph node ratio was 0.18 ± 0.16 (range: 0.00–0.86) in the lateral compartment. The mean ± SD number of excised lymph nodes, metastatic lymph nodes, and median metastatic lymph node ratio in each compartment are shown in Table 2. The mean number of excised and metastatic lymph nodes and the median metastatic lymph node ratio were lower in contralateral compartment than those in ipsilateral compartment in the central compartment (P < 0.001, Table 2). The mean number of excised and metastatic lymph nodes and the median metastatic lymph node ratio were lower in level V than those in level II, III, and IV in the lateral compartment (P < 0.001, Table 2).
Table 1.
Demographics and clinical characteristics of 220 solitary papillary thyroid carcinoma patients
| Characteristics | Values (%) |
|---|---|
| No. of patients | 220 |
| Gender | |
| Male | 51 (23.2) |
| Female | 169 (76.8) |
| Age (years) | |
| Mean ± SD | 41.0 ± 13.5 |
| ≥45 | 82 (37.3) |
| <45 | 138 (62.7) |
| Size (mm) | |
| Mean ± SD | 21.4 ± 13.3 |
| >10 | 170 (77.3) |
| ≤10 | 50 (22.7) |
| Location of the primary tumor | |
| Superior lobe | 111 (50.5) |
| Middle lobe | 127 (57.7) |
| Inferior lobe | 76 (34.5) |
| Capsular invasion | 176 (80.0) |
| Extrathyroidal extension | 99 (45.0) |
| Lymphocytic thyroiditis | 48 (21.8) |
| Central lymph node metastases | 177 (80.5) |
| Ipsilateral | 172 (78.2) |
| Contralateral | 87 (39.5) |
| Bilateral | 82 (39.1) |
| Lateral lymph node metastases | 181 (82.3) |
| Level II | 101 (45.9) |
| Level III | 138 (62.7) |
| Level IV | 122 (55.5) |
| Level V | 27 (12.3) |
| Single‐level | 44 (20.0) |
| Multiple‐level | 137 (62.3) |
Table 2.
The harvested lymph nodes in 220 solitary papillary thyroid carcinoma patients
| Compartment | Excised number (Mean ± SD) | P‐value | Metastatic number (Mean ± SD) | P‐value | Metastatic ratio (Mean ± SD) | P‐value |
|---|---|---|---|---|---|---|
| Central lymph node | ||||||
| Ipsilateral | 6.34 ± 4.57 | <0.001b | 3.26 ± 3.33 | <0.001b | 0.50 ± 0.36 | <0.001a |
| Contralateral | 3.85 ± 3.01 | 0.92 ± 1.47 | 0.26 ± 0.37 | |||
| Lateral lymph node | ||||||
| Level II | 7.12 ± 4.31 | <0.001a, c | 0.90 ± 1.29 | <0.001b, c | 0.27 ± 0.29 | <0.001b, c |
| Level III | 6.51 ± 3.99 | <0.001b, d | 1.60 ± 1.79 | <0.001b, d | 0.22 ± 0.29 | <0.001b, d |
| Level IV | 7.36 ± 5.00 | <0.001b, e | 1.38 ± 1.90 | <0.001b, e | 0.22 ± 0.29 | <0.001b, e |
| Level V | 3.95 ± 4.03 | — | 0.22 ± 0.94 | — | 0.04 ± 0.13 | — |
The Student's t‐test was adopted.
The Wilcoxon rank‐sum test was adopted.
Level V versus Level II.
Level V versus Level III.
Level V versus Level IV.
Table 3 shows the association between level V LNM and several risk factors in the 220 solitary PTC patients who underwent LND for clinically LLNM. The mean age of the patients with level V LNM was similar to that of patients without level V LNM (Table 3). The mean size of the primary tumor in patients with level V LNM was larger than that in patients without level V LNM (Table 3). Univariate analysis showed that the presence of level V LNM was significantly associated with tumor size >10 mm, extrathyroidal extension, ipsilateral central LNM ratio ≥50%, and the presence of contralateral CLNM, bilateral CLNM, and simultaneous levels II + III + IV LNM (Table 3). Variables with P < 0.100 were included in the multivariate logistic regression analysis. The multivariate analysis showed that only contralateral CLNM was an independent risk factor associated with level V LNM [odds ratio (OR): 14.001; 95% confidence interval (CI): 1.528–128.257; P = 0.020; Table 4]. Gender, age, location of the primary tumor, capsular invasion, coexisting lymphocytic thyroiditis, ipsilateral CLNM, and level II, level III, level IV, simultaneous levels II + III and simultaneous levels III + IV LLNM were not found to be associated with level V LNM.
Table 3.
Univariate analysis of risk factors related to level V lymph node metastases
| Variables | Level V metastases | P‐value | |
|---|---|---|---|
| Present, n (%) | Absent, n (%) | ||
| Total | 27 (12.3) | 193 (87.7) | |
| Gender | |||
| Male | 6 (22.2) | 45 (23.3) | 0.900 |
| Female | 21 (77.8) | 148 (76.7) | |
| Age (years) | |||
| Mean ± SD | 41.9 ± 15.5 | 40.9 ± 13.2 | 0.708a |
| ≥45 | 9 (33.3) | 73 (37.8) | 0.651 |
| <45 | 18 (66.7) | 120 (62.2) | |
| Size (mm) | |||
| Mean ± SD | 29.2 ± 20.7 | 20.3 ± 11.6 | 0.021b |
| >10 | 26 (96.3) | 144 (74.6) | 0.012 |
| ≤10 | 1 (3.7) | 49 (25.4) | |
| Location of primary tumor | |||
| Superior lobe | 15 (55.6) | 96 (49.7) | 0.571 |
| Middle lobe | 19 (70.4) | 108 (56.0) | 0.156 |
| Inferior lobe | 11 (40.7) | 65 (33.7) | 0.470 |
| Capsular invasion | 24 (88.9) | 152 (78.8) | 0.218 |
| Extrathyroidal extension | 17 (63.0) | 82 (42.5) | 0.045 |
| Lymphocytic thyroiditis | 4 (14.8) | 44 (22.8) | 0.347 |
| Central lymph node metastases | |||
| Ipsilateral | 24 (88.9) | 149 (77.2) | 0.165 |
| Metastatic ratio ≥50% | 21 (77.8) | 107 (55.4) | 0.028 |
| Contralateral | 17 (63.0) | 70 (36.3) | 0.008 |
| Bilateral | 15 (55.6) | 67 (34.7) | 0.036 |
| Lateral lymph node metastases | |||
| Level II | 16 (59.3) | 85 (44.0) | 0.137 |
| Level III | 20 (74.1) | 118 (61.1) | 0.193 |
| Level IV | 18 (66.7) | 104 (53.9) | 0.211 |
| Level II + III | 13 (48.1) | 68 (35.2) | 0.193 |
| Level III + IV | 15 (55.6) | 72 (37.3) | 0.069 |
| Level II + III + IV | 11 (40.7) | 41 (21.2) | 0.026 |
The Student's t‐test was adopted.
The Wilcoxon rank‐sum test was adopted.
Table 4.
Multivariate analysis of risk factors related to level V lymph node metastases
| Variables | OR | 95% CI | P‐value |
|---|---|---|---|
| Tumor size >10 mm | 6.349 | 0.802–50.245 | 0.080 |
| Extrathyroidal extension | 1.418 | 0.575–3.495 | 0.448 |
| Ipsilateral central lymph node metastatic ratio ≥50% | 2.531 | 0.738–8.683 | 0.140 |
| Contralateral central lymph node metastases | 14.001 | 1.528–128.257 | 0.020 |
| Bilateral central lymph node metastases | 0.107 | 0.010–1.097 | 0.060 |
| Level III + IV lymph node metastases | 0.927 | 0.251–3.422 | 0.910 |
| Level II + III + IV lymph node metastases | 1.620 | 0.424–6.192 | 0.481 |
OR, odds ratio; 95% CI, 95 % confidence interval.
Additional subgroup analysis for 181 PTC patients with pathological LLNM by univariate analysis showed that level V LNM was significantly associated only with tumor size >10 mm (P = 0.030), and multivariate analysis showed that there was no independent risk factor associated with level V LNM.
Discussion
Patients with PTC have excellent prognosis; however, the presence of LNM significantly increases the risk of locoregional recurrence, and some groups have demonstrated decreased disease‐free survival rate and increased mortality associated with regional LNM 4, 5, 6, 7, 8, 9, 10. Extensive LND has the potential to decrease regional recurrence and increase disease‐free survival, but may lead to clinically important postoperative morbidities 13, 14, 15. Therefore, determining a rational extent of therapeutic LND is vital. Whether level V should be included in therapeutic LND continues to be debated 16, 17, 18, 19, 20, 21, 22. A majority of previous studies had explored some predictors for level V LNM in PTC patients with clinically LLNM, but only a few studies have explored the risk factors for level V LNM in solitary PTC with clinically LLNM. Therefore, the frequency of and the risk factors for level V LNM in solitary PTC with clinically LLNM were analyzed to determine the rational extent of therapeutic LND.
Several imaging modalities, including US, computed tomography (CT), magnetic resonance imaging (MRI), and [18F]‐fluoro‐2‐deoxy‐D‐glucose‐positron emission tomography/CT (18FDG‐PET/CT) have been used to evaluate cervical LNM. However, US is more easily accessible and cheaper than other imaging modalities, and was determined to be the most sensitive method for assessing cervical LNM 25, 26. Therefore, US performed by an experienced ultrasonographer is considered, by most clinicians and by the American Thyroid Association, as the screening and surveillance imaging modality of choice for detecting LLNM 27. Although USgFNAC was showed to be the most specific and accurate imaging modality to detect cervical LNM 25, most studies report that USgFNAC has a relatively lower sensitivity than US, and the false‐negative rate of USgFNAC could be as high as 45–52% 28, 29. In addition, given the closer relationship of the node to the surrounding vascular structures and the body habitus of the patient (such as some small nodes, which are not easily punctured by fine needle), routine preoperative USgFNAC is not done to guide LND at our institution. In our study, the sensitivity of US was 82.3% (181/220) for predicting LLNM, and this is in the range of the sensitivities reported for US, with 63–97% specified in the meta‐analysis by de Bondt 25. The clinical evaluation of US that established the presence of LLNM in 17.7% (39/220) patients was inaccurate, which might have an inaccuracy that is lower than USgFNAC and other imaging modalities. Of course, USgFNAC and other imaging modalities would, to some extent, have helped prevent unnecessary LND in the 17.7% patients with a negative LLNM, but they might have contributed more to the omission of LND in patients with positive LLNM according to the reasons discussed earlier.
Our present study demonstrated that the mean number of excised and metastatic lymph nodes and the median metastatic lymph node ratio were lower in contralateral compartment than that in ipsilateral compartment in the central compartment, which were caused by that the ipsilateral CLM included prelaryngeal, pretracheal and ipsilateral paratracheal lymph nodes, and LNM generally follow a nearby principle. Secondly, the mean number of excised and metastatic lymph nodes and the median metastatic lymph node ratio were also lower in level V than that in other levels in the lateral compartment, which was consistent with the reported result by Lim et al. 16. Of course, those results might have been affected by the lower sampling lymph nodes on those areas. However, each compartment or level nodes were thoroughly swept by surgeons at our institution. Therefore, there was a smaller possibility to the lower sampling lymph nodes on those areas in our study.
Almost all previous studies have indicated that, in patients with PTC and clinical LNM, most LLNM were levels II, III, and IV. This is consistent with the findings of this study, which found that LLNM mainly occurred at levels II, III, and IV with frequencies of 45.9%, 62.7%, and 55.5%, respectively. These frequencies are in the ranges of the percentages reported for levels II, III, and IV LNM, respectively, 27–65%, 57–82%, and 41–82% in a meta‐analysis by Eskander et al. 30. In addition, most patients (137/220, 62.3%) in this study had multiple‐level metastases, which is consistent with the values reported in previous studies 16, 22. On the basis of the above reasons and the prognostic significance, it is universally agreed that therapeutic LND should include at least a comprehensive but selective LND of levels II–IV. However, whether therapeutic LND should routinely include level V lymphadenectomy remains controversial 13, 14, 15, 16, 17, 18, 19, 20, 21, 22.
In our study, level V LNM in solitary PTC with clinically LLNM was the least frequent (12.7%) type of metastasis. The rate was slightly lower than that reported in the 15 articles included in the meta‐analysis by Eskander et al. 30. This difference may be attributable to the fact that our study only included patients with solitary PTC. Moreover, our study showed that the mean number of excise and metastatic lymph nodes and the median metastatic lymph node ratio were far lower in level V than that in other levels in the lateral compartment. The risk of postoperative morbidity (especially injury to the spinal accessory nerve and cervical plexus) that can lead to increased morbidity and affect the quality of life increases with the extent of LND. Therefore, the necessity of routine level V dissection has been questioned 13, 14, 15. Furthermore, because level V LNM are comparatively rare, some researchers have suggested that routine level V dissection is not necessary for patients with PTC and lateral cervical lymphadenopathy 16, 17, 18. However, others hold the opposite view according to the high rates of metastasis seen with this cancer 19, 20, 21, 22. On the basis of the comparatively low frequency of level V LLNM in this study and the risk of postoperative complications, we propose that therapeutic LND should not routinely include level V lymphadenectomy except when level V LLNM is suspected on the basis of preoperative examination, such as US and CT, or there are associated risk factors.
Level V LNM may not be identified reliably on preoperative US and/or CT 17, 18. Therefore, we further explored the possible risk factors for level V LNM to guide level V lymphadenectomy. Our study demonstrated that the mean size of the primary tumor in patients with level V LNM was greater than that in patients without level V LNM; moreover, tumor size >10 mm was associated with level V LNM. In contrast, this association was not found by previous studies 17, 18, 19, 21, which might have been affected by multifocality. Of 27 patients with level V LNM, only one patient had a tumor size <10 mm (microcarcinoma). Thus, in solitary papillary thyroid microcarcinoma with clinically LLNM, the LNM seldom spread to level V. Zhang et al. 21 found that extrathyroidal extension was an independent risk factor for level V LNM. However, our study showed that extrathyroidal extension was a risk factor, although not an independent risk factor, for level V LNM. Our study also showed that level V LNM was associated with simultaneous level II–IV LNM, which is similar to that reported in the study by Lim et al. 16, Shim et al. 17, and Zhang et al. 21. Furthermore, Shim et al. 17 and Zhang et al. 21 found that simultaneous level II–IV LNM was also an independent risk factor for level V LNM. Our study demonstrated that level V LNM was not associated with ipsilateral CLNM, but an ipsilateral CLNM ratio ≥50%, contralateral LNM, and bilateral CLNM were risk factors for level V LNM. One important finding in our study was that contralateral CLNM was an independent risk factor for level V LNM. Therefore, we suggest that, when patients with solitary PTC and clinically LLNM present with the above mentioned associated risk factors (especially contralateral CLNM), level V lymphadenectomy may be considered in therapeutic LND. To the best of our knowledge, this is the first study to investigate the risk value of CLNM with separate compartments for level V LNM in solitary PTC with clinically LLNM.
There are some potential limitations of this study. Because this was a retrospective study based on the review of medical records, wherein clinical LLNM were not partitioned by preoperative US according to the lateral neck level, the detected value of US for each level LNM could not be evaluated. Moreover, the specimens of level V LNM were not routinely divided into levels Va and Vb by the surgeon; therefore, levels Va and Vb could not be assessed separately. Furthermore, other risk factors such as histological subtype, lymphovascular invasion, distant metastasis, and lymph nodal volume were not included because they were not routinely reported in the pathological report in our institution. Finally, patients who underwent elective (<4 levels) or prophylactic LND were not enrolled, which may have weakened the impact of some of the study outcomes.
Conclusions
This study showed a high prevalence of level II–IV LNM and a relatively low prevalence of level V LNM in patients with solitary PTC and clinically LLNM. A comprehensive selective level II–IV LND should be included in the therapeutic LND for solitary PTC patients with clinically LLNM. Routine level V lymphadenectomy may not be necessary for the treatment of solitary PTC in patients with clinically LLNM unless level V LNM is suspected according to preoperative findings on examination or associated risk factors, especially contralateral CLNM, are present. However, further prospective studies will be warranted to elucidate risk factors for level V LNM and to evaluate the impact of level V lymphadenectomy on prognostic outcomes in these patients.
Conflict of Interest
None declared.
Cancer Medicine 2016; 5(8):2161–2168
References
- 1. Davies, L. , and Welch H. G.. 2006. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 295:2164–2167. [DOI] [PubMed] [Google Scholar]
- 2. Mazzaferri, E. L. , and Jhiang S. M.. 1994. Long‐term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am. J. Med. 97:418–428. [DOI] [PubMed] [Google Scholar]
- 3. Shaha, A. R. 2004. Prognostic factors in papillary thyroid carcinoma and implications of large nodal metastasis. Surgery 135:237–239. [DOI] [PubMed] [Google Scholar]
- 4. Scheumann, G. F. , Gimm O., Wegener G., et al. 1994. Prognostic significance and surgical management of locoregional lymph node metastases in papillary thyroid cancer. World J. Surg. 18:559–567. [DOI] [PubMed] [Google Scholar]
- 5. Mercante, G. , Frasoldati A., Pedroni C., et al. 2009. Prognostic factors affecting neck lymph node recurrence and distant metastasis in papillary microcarcinoma of the thyroid: Results of a study in 445 patients. Thyroid 19:707–716. [DOI] [PubMed] [Google Scholar]
- 6. Hasney, C. P. , and Amedee R. G.. 2010. What is the appropriate extent of lateral neck dissection in the treatment of metastatic well‐differentiated thyroid carcinoma. Laryngoscope 120:1716–1717. [DOI] [PubMed] [Google Scholar]
- 7. Beasley, N. J. , Lee J., Eski S., et al. 2002. Impact of nodal metastases on prognosis in patients with well‐differentiated thyroid cancer. Arch. Otolaryngol. Head Neck Surg. 128:825–828. [DOI] [PubMed] [Google Scholar]
- 8. Grogan, R. H. , Kaplan S. P., Cao H., et al. 2013. A study of recurrence and death from papillary thyroid cancer with 27 years of median follow‐up. Surgery 154:1436–1446. [DOI] [PubMed] [Google Scholar]
- 9. Lundgren, C. I. , Hall P., Dickman P. W., et al. 2006. Clinically significant prognostic factors for differentiated thyroid carcinoma: a population‐based, nested case‐control study. Cancer 106:524–531. [DOI] [PubMed] [Google Scholar]
- 10. Tisell, L. E. , Nilsson B., Mölne J., et al. 1996. Improved survival of patients with papillary thyroid cancer after surgical microdissection. World J. Surg. 20:854–859. [DOI] [PubMed] [Google Scholar]
- 11. Ito, Y. , Tomoda C., Uruno T., et al. 2004. Preoperative ultrasonographic examination for lymph node metastasis: usefulness when designing lymph node dissection for papillary microcarcinoma of the thyroid. World J. Surg. 28:498–501. [DOI] [PubMed] [Google Scholar]
- 12. Stack, B. C. Jr , Ferris R. L., Goldenberg D., et al. 2012. American Thyroid Association consensus review and statement regarding the anatomy, terminology, and rationale for lateral neck dissection in differentiated thyroid cancer. Thyroid 22:501–508. [DOI] [PubMed] [Google Scholar]
- 13. Laverick, S. , Lowe D., Brown J. S., et al. 2004. The impact of neck dissection on health‐related quality of life. Arch. Otolaryngol. Head Neck Surg. 130:149–154. [DOI] [PubMed] [Google Scholar]
- 14. Inoue, H. , Nibu K., Saito M., et al. 2006. Quality of life after neck dissection. Arch. Otolaryngol. Head Neck Surg. 132:662–666. [DOI] [PubMed] [Google Scholar]
- 15. Terrell, J. E. , Welsh D. E., Bradford C. R., et al. 2000. Pain, quality of life, and spinal accessory nerve status after neck dissection. Laryngoscope 110:620–626. [DOI] [PubMed] [Google Scholar]
- 16. Lim, Y. C. , Choi E. C., Yoon Y. H., et al. 2010. Occult lymph node metastases in neck level V in papillary thyroid carcinoma. Surgery 147:241–245. [DOI] [PubMed] [Google Scholar]
- 17. Shim, M. J. , Roh J. L., Gong G., et al. 2013. Preoperative detection and predictors of level V lymph node metastasis in patients with papillary thyroid carcinoma. Br. J. Surg. 100:497–503. [DOI] [PubMed] [Google Scholar]
- 18. Kang, B. C. , Roh J. L., Lee J. H., et al. 2014. Candidates for limited lateral neck dissection among patients with metastatic papillary thyroid carcinoma. World J. Surg. 38:863–871. [DOI] [PubMed] [Google Scholar]
- 19. Kupferman, M. E. , Weinstock Y. E., Santillan A. A., et al. 2008. Predictors of level V metastasis in well‐differentiated thyroid cancer. Head Neck 30:1469–1474. [DOI] [PubMed] [Google Scholar]
- 20. Farrag, T. , Lin F., Brownlee N., et al. 2009. Is routine dissection of level II‐B and V‐A necessary in patients with papillary thyroid cancer undergoing lateral neck dissection for FNA‐confirmed metastases in other levels. World J. Surg. 33:1680–1683. [DOI] [PubMed] [Google Scholar]
- 21. Zhang, X. J. , Liu D., Xu D. B., et al. 2013. Should level V be included in lateral neck dissection in treating papillary thyroid carcinoma? World J. Surg. Oncol. 11:304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Keum, H. S. , Ji Y. B., Kim J. M., et al. 2012. Optimal surgical extent of lateral and central neck dissection for papillary thyroid carcinoma located in one lobe with clinical lateral lymph node metastasis. World J. Surg. Oncol. 10:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Leboulleux, S. , Girard E., Rose M., et al. 2007. Ultrasound criteria of malignancy for cervical lymph nodes in patients followed up for differentiated thyroid cancer. J. Clin. Endocrinol. Metab. 92:3590–3594. [DOI] [PubMed] [Google Scholar]
- 24. Hunt, J. P. , Buchmann L. O., Wang L., et al. 2011. An analysis of factors predicting lateral cervical nodal metastases in papillary carcinoma of the thyroid. Arch. Otolaryngol. Head Neck Surg. 137:1141–1145. [DOI] [PubMed] [Google Scholar]
- 25. de Bondt, R. B. , Nelemans P. J., Hofman P. A., et al. 2007. Detection of lymph node metastases in head and neck cancer: a meta‐analysis comparing US, USgFNAC, CT and MR imaging. Eur. J. Radiol. 64:266–272. [DOI] [PubMed] [Google Scholar]
- 26. Morita, S. , Mizoguchi K., Suzuki M., et al. 2010. The accuracy of (18)[F]‐fluoro‐2‐deoxy‐D‐glucose‐ positron emission tomography/computed tomography, ultrasonography, and enhanced computed tomography alone in the preoperative diagnosis of cervical lymph node metastasis in patients with papillary thyroid carcinoma. World J. Surg. 34:2564–2569. [DOI] [PubMed] [Google Scholar]
- 27. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer : Cooper, D. S. , Doherty G. M., et al. 2009. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 19:1167–1214. [DOI] [PubMed] [Google Scholar]
- 28. Takes, R. P. , Righi P., Meeuwis C. A., et al. 1998. The value of ultrasound with ultrasound‐guided fine‐needle aspiration biopsy compared to computed tomography in the detection of regional metastases in the clinically negative neck. Int. J. Radiat. Oncol. Biol. Phys. 40:1027–1032. [DOI] [PubMed] [Google Scholar]
- 29. Jun, H. H. , Kim S. M., Kim B. W., et al. 2015. Overcoming the limitations of fine needle aspiration biopsy: detection neck node metastasis in papillary thyroid carcinoma. Yonsei Med. J. 56:182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Eskander, A. , Merdad M., Freeman J. L., et al. 2013. Pattern of spread to the lateral neck in metastatic well‐differentiated thyroid cancer: a systematic review and meta‐analysis. Thyroid 23:583–592. [DOI] [PubMed] [Google Scholar]


