Abstract
Background:
Sucrase enzyme inhibitor considered as an oral anti-diabetic therapy that delays the absorption of eaten carbohydrates, reducing the postprandial glucose and insulin peaks to reach normoglycemia.
Materials and Methods:
Chromatographic fractionation of the hydroalcoholic extract of leaves of Azadirachta indica growing in KSA, followed by in-vitro assay of sucrase enzyme inhibition activity.
Results:
This investigation led to the isolation of a new remarkable sucrase enzyme inhibitor; 4’-methyl Quercetin-7-O-β-D-glucuronopyranoside (1) alongside with four known compounds; 2,3-hexahydroxydiphenoyl-(α/β)-D-4C1-glucopyranose (2), Avicularin (3), Castalagin (4) and Quercetin-3-O-glucoside (5). The structure of the new compound (1) was elucidated on the basis of its spectral data, including ESI-MS, UV, 1H NMR, 13C NMR, 1H-1H COSY, HSQC, NOESY and HMBC.
Conclusion:
Under the assay conditions, hydroalcoholic extract of A. indica and compounds 1-5 exhibited significant sucrase enzyme inhibitory activity.
SUMMARY
Chromatographic fractionation of the hydroalcoholic extract of leaves of Azadirachta indica, led to the Isolation of a new flavonoid glycoside named 4’-methyl Quercetin-7-O-β-D-glucuronopyranoside, alongside to other 4 known polyphenols. The hydroalcoholic extract as well as the isolated compounds exhibited significant sucrase enzyme inhibitory activity.
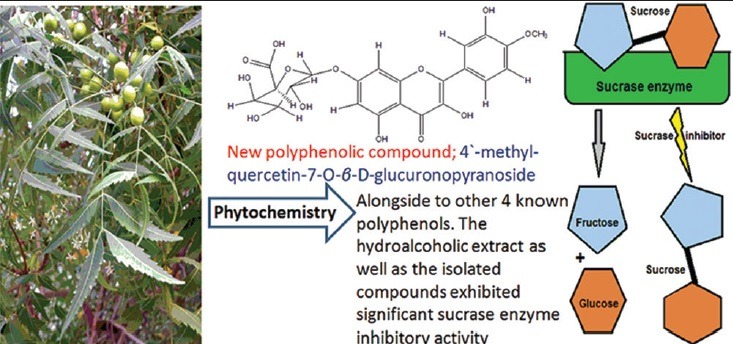
Abbreviations used: ESI-MS; electrospray ionization-mass spectrometry, UV; ultraviolet, NMR; nuclear magnetic resonance, 1H-1H COSY; 1H-1H correlation spectroscopy, NOESY; nuclear overhauser effect spectroscopy, and HSQC; heteronuclear multiple bond correlation. A. indica; Azadirachta indica.
Keywords: Azadirachta indica, polyphenols, sucrase inhibitor
INTRODUCTION
Any defect in insulin secretion or action results in hyperglycemia which leads to a metabolic disease, i.e. diabetic mellitus. Medicinal plants have been used since centuries by various cultures worldwide for the treatment of diabetes. Food and herbal control of postprandial hyperglycemia is an essential step in the early treatment of diabetes mellitus as hyperglycemia may induce nonenzymatic glycosylation of various proteins, resulting in the improvement of accompanied complications such as macrovascular and cardiovascular diseases.[1,2,3,4] Phytochemical constituents isolated from plants exhibit several hypoglycemic mechanisms, through the inhibition of carbohydrate-metabolizing enzymes, manipulation of glucose transporters, regeneration of α-cell, and enhancement of the insulin-releasing activity.[5,6] Hyperglycemia could be controlled by decreasing the absorption of glucose through the inhibition or decreasing the effect of certain enzymes responsible for the hydrolysis of carbohydrate such as sucrase enzyme in the digestive tract.[7]
Azadirachta indica A. Juss is a famous plant of the Meliaceae family. It is native to the Indian subcontinent and grows in many countries of the world such as Egypt and the Kingdom of Saudi Arabia (KSA). A massive cultivation of this tree occurred in the plains of Arafat, holy Makkah, the KSA. In 1971, approximately, 50,000 neem trees were cultivated to provide shade for the millions of pilgrims.[8] It has enormous therapeutic, biological activity, and ethno-medicinal significance, so it is considered as a source of many biological active agents, due to its contents of various active constituents with diverse medicinal properties. It was practiced by different types of vaidyas and traditional healers in almost all the countries in the world such as the KSA, China, India, Egypt, Rome, and Greek.[9,10,11,12,13,14,15] Anti-diabetic activity of medicinal plants has a strong relationship with their antioxidant property and polyphenolic contents.[16,17] Our previous study revealed that the leaves of A. indica contain a considerable amount of polyphenolic compounds with significant antioxidant and cytotoxic activity.[18] Hence, it is interesting to investigate the in vitro anti-diabetic activity of alcoholic extract of A. indica and the isolated polyphenolic compounds through the performance of sucrase inhibitory activity test.
MATERIALS AND METHODS
Equipment and materials
Pure samples were measured separately as MeOH solutions and various diagnostic shift reagents, Shimadzu ultraviolet (UV) 240 spectrophotometer,[19] and with sprayed Naturstoff reagent.[20] Nuclear magnetic resonance (NMR) analyses were run on Varian Mercury 300 MHz and Bruker 500 MHz spectrometers relative to TMS in different deuterated solvents. Electrospray ionization-mass spectrometry (ESI-MS) spectra were measured according to previously published conditions.[18]
Extraction and isolation
Leaf samples of A. indica were freshly collected from Bahra in the KSA and identified by Dr. Kadry Abdelkhaliq, Faculty of applied sciences, Umm Al-qura University, Makkah, the KSA. These samples were dried and grinded to get 1000 g which were extracted with hot 80% aqueous ethanol (2.5 L × 5 L) at 70°C. After evaporation of solvent under reduced pressure, the residue (115 g) was defatted with petroleum ether. The methanolic extract of the defatted residue was preliminarily fractionated on a polyamide 6S (Riedel De Haen AG, Seelze, Hannover, Germany) column (C) (300 g, 120 cm × 5 cm) using a step gradient of H2O–MeOH 100: 0–0:100 for elution to give 25 fractions of 1 L each, which were collected and monitored by Comp-PC using Whatman No. 1 paper (systems S1 and S2); S1: n-BuOH-AcOH-H2O (4:1:5, top layer) and S2: 15% aqueous AcOH and UV-light into three major collective fractions. Fraction I (180 mg) was subjected to repeated column chromatography (CC) on microcrystalline cellulose (E. Merk, Darmstadt, Germany) using n-BuOH-isopropanol-H2O BIW, 4:1:5, organic layer as an eluent followed by repeated and separate cellulose column for each major subfraction using methanol/BIW (50%) to give pure samples of compound 1 (17 mg), compound 3 (27 mg), and compound 5 (17 mg). Fraction II (85 mg) was chromatographed on a Sephadex LH-20 CC and eluted with MeOH, then pure compound 4 (26 mg) was obtained by precipitation from MeOH by excess EtOAc. The rest was dried (20 g) and then it was re-dissolved in water:methanol (1:10), filtered and dried under vacuum to give 15.5 g dry residue. Therefore, it was fractionated on cellulose CC (70 cm × 5 cm) using MeOH (10%), then it was chromatographed on Sephadex C (10% aqueous MeOH as eluent) followed by Sephadex LH-20 CC and eluted with 10% MeOH to give pure compound 2 (16 mg). These compounds were visualized by spraying with Naturstoff (for flavonoids) and nitrous acid or KIO3 reagents (for tannins) as illustrated previously by Abdelhady et al., 2015.[18]
Assay of sucrase inhibitory activity
A sucrase enzyme solution of rat intestine has been prepared according to Dahlqvist's method. It occurred as a complex of sucrase and isomaltase, this hydrolyzes sucrose into glucose and fructose.[21] Honda and Hara created a method to assay the effect of samples on sucrase enzyme activity.[22] Enzyme solutions (10 µL) were incubated together with buffered solubilized sample (25–200 µg/ml in maleate buffer with pH 6.0) for 10 min at 37°C, while the volume was completed to 200 µL with maleate buffer (pH 6.0) in case of control, then the reaction was initiated by the addition of 100 µL of sucrose solution (60 mM). About 30 min later, the reaction was stopped by the addition of 200 µL of 3, 5-dinitrosalysilic acid reagent. The mixture was incubated in a boiling water bath for 5 min. The absorbance of each reaction was read at 540 nm. The percentages of inhibitory activities were calculated using the following formula:

Abs control represents the absorbance of the control reaction (containing all reagents except the tested sample), whereas the Abs sample is the absorbance of the tested sample. An untreated enzyme solution was used as control. All experiments were carried out 3 times.
RESULTS
The dried residue of 80% EtOH extract, which was extracted with pet-ether for defatting, was chromatographed on a polyamide column followed by successive separation on Sephadex LH-20 CC and cellulose CC for purification. The isolated pure compounds were identified by different chromatographic and spectral techniques such as UV, 1H, 13C NMR, two-dimensional NMR, and negative ESI-MS. As discussed in the published data,[18] the isolated known compounds have been identified as 2, 3-hexahydroxydiphenoyl-(α/β)-D-4C1-glucopyranose (2), avicularin (3), castalagin, (4) and quercetin-3-O-glucoside (5). In addition to the new compound isolated for the 1st time from nature identified as 4’-methyl quercetin-7-O-β-D-glucuronopyranoside (1) is identified according to the following:
Compound 1 was obtained as pale yellow amorphous powder (17 mg) with the following chromatographic properties: Rf values; 0.32 (S1), 0.51 (S2); purple color under UV light turned to green color with FeCl3 and orange color with Naturstoff spray reagents. UV-spectral data λmax (nm) (MeOH): 281, 352; (+NaOMe): 287, 382; (+NaOAC): 280, 351; (+AlCl3): 280, 302 (sh), 352, 391; (+AlCl3/HCl): 281, 301 (sh), 348, 390. 1H NMR (500 MHz, DMSO-d6): δ ppm 12.49 (1H, s, H-bonded OH-5), 7.58 (2H, dd, J = 8.1, 2.4 Hz, 1H-6’), 7.47 (1H, d, J = 2.0 Hz, H-2’), 7.12 (1H, d, J = 8.4 Hz, H-5’), 6.74 (1H, d, J = 1.9 Hz, H-8), 6.67 (1H, d, J = 1.9 Hz, H-6), 5.68 (1H, d, J = 6.1 Hz, H-1”), 3.76 (3H, s, O-Me), 4.29-2.85 (remaining sugar protons). 13C NMR (125 MHz, DMSO-d6): δ ppm 177.24 (C-4), 172.02 (C-6”), 164.2 (C-2), 163.21 (C-5), 156.22 (C-7), 156.06 (C-9), 148.65 (C-4’), 148.60 (C-3’), 131.71 (C-3), 121.21 (C-6’), 120.07 (C-1’), 116.24 (C-5’), 113.63 (C-2’), 104.06 (C-10), 100.85 (C-1”), 97.65 (C-6), 96.48 (C-8), 78.63 (C-3”), 76.45 (C-5”), 71.06 (C-2”), 70.26 (C-4”), 54.78 (OCH3-4’)-ve ESI-MS: m/z 491.18 (M-H)−, 477.39 (M-CH3)−, 315.44 (M-deoxyglucuronide)−, 300.43 (quercetin-H)−.
Results of the anti-diabetic activity (sucrase-inhibitory activity)
The effect of the hydroalcoholic extract of A. indica and its selected isolated polyphenolic compounds on carbohydrate-hydrolyzing enzyme, namely rat intestinal sucrase, has been studied using in vitro model system.[22] These samples significantly inhibited (P > 0.05) sucrase activity [Table 1 and Figure 1]. The sucrase inhibitory activity of hydroalcoholic extract of A. indica and the isolated polyphenolic compounds supposed to be due to the presence of hydrolysable tannins and flavonoids.[17,23] It was observed that compound 1 exhibited the highest sucrase inhibitory activity with IC50 (68.45 μg/ml) followed by compound 5 with IC50 (90.26 μg/ml), compound 3 with IC50 (94.31 μg/ml), compound 4 with IC50 (138.3 μg/ml), total extract with IC50 (160.6 μg/ml), and then compound 2 with IC50 (195.62 μg/ml).
Table 1.
Percentage sucrase inhibitory activity using Honda and Hara assay
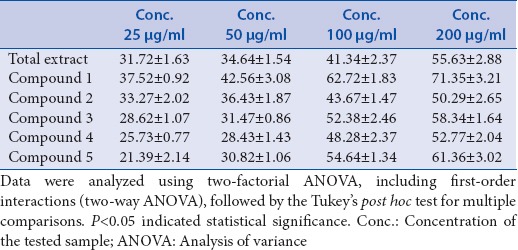
Figure 1.
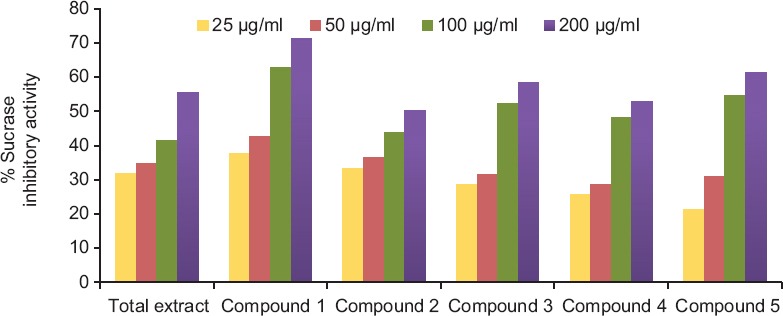
Sucrase enzyme inhibition activity
Data were analyzed using two-factorial analysis of variance (ANOVA), including first-order interactions (two-way ANOVA), followed by the Tukey's post hoc test for multiple comparisons. P < 0.05 indicated statistical significance.
DISCUSSION
According to the chromatographic properties and UV-spectral data, compound 1 was expected to be 4’, 7-quercetin derivative. The UV spectrum in MeOH exhibited the two characteristic absorption bands at λmax (nm) 281 nm (band II) and 352 nm (band I) of quercetin nucleus. Upon addition of NaOAc, no bathochromic shift of band II was observed which is diagnostic of a substituted 7-OH group. The remaining diagnostic shift reagents were in complete agreement with the 3’, 5 dihydroxy-4’-7 disubstituted flavonol structure.[19] Negative ESI-MS spectrum exhibited the molecular ion peak at m/z 491 [M-H]− which corresponds to a molecular weight of 492 and a molecular formula of C22H20O13. In addition, a fragment ion peak at m/z 477 after a loss of a methyl moiety indicates a methyl quercetin glucuronide structure. Mild acid hydrolysis and CoPC showed the presence of glucuronic acid in the aqueous phase and quercetin in the organic phase. 1H NMR spectrum showed the AM coupling system of the two meta-coupled doublets at δ ppm 6.74 and 6.67 assignable for H-8 and H-6, respectively, characteristic for ring-A of flavonol, in addition to the signals at δ ppm 7.58 (dd), 7.47 (d), 7.12 (d), assignable to H-6’, H-2’, and H-5’, respectively, of ring-B with the absence of H-3’ resonance signal suggesting the presence of quercetin moiety. The resonance singlet signal which integrated for three protons at δ ppm 3.76 was indicative for the presence of methoxy group, the location of the methoxy group on C-4’(ring-B) was deduced from the downfield shift of H-5’ at δ ppm 7.12 (+0.3), in comparison with previously published data describing related structures.[24,25] This was approved by the heteronuclear multiple bond correlation (HMBC) that showed 2,3JCH coupling between O-Me protons at δ ppm 3.76 with C-4’ at δ ppm 148.65. Further, structure confirmation was proved by the two-dimensional spectrums of 1H-1H COSY, HSQC, NOESY and HMBC, and by comparison with related compounds reported in published data.[24,25,26,27,28] This complete assignment confirmed the structure of compound 1 to be 4’-methyl Quercetin-7-O-β-D-glucuronopyranoside, which has not been reported previously in nature [Figure 2].
Figure 2.
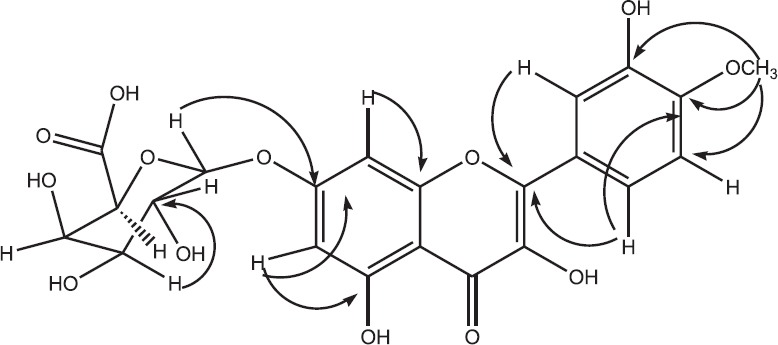
Heteronuclear multiple bond correlation correlations for compound 1
It is appeared worldwide that the drugs used for anti-diabetic effect with complementary mechanisms should be developed to control and inhibit the hydrolysis of carbohydrates in a reversible way. These consequently reduce the rise of postprandial blood glucose in diabetics.[3] Medicinal plants suggested to inhibit sucrase activity due to several possible factors such as the presence of polyphenolic constituents.[17,23] Anti-diabetic activity of medicinal plants has a strong relationship with their antioxidant property.[17] Hence, hydroalcoholic extract of A. indica and the isolated polyphenolic compounds may have anti-diabetic activity, due to their significant antioxidant and reducing power activities, as approved in the previous investigation.[18] Hence, the tested samples (i.e. A. indica and compounds 1–5) may offer a support in the treatment of diabetic disease. In the present investigation, the effect of the tested samples on carbohydrate-hydrolyzing enzyme, namely rat intestinal sucrase, has been studied using in vitro model systems. The hydroalcoholic extract of A. indica and its selected isolated polyphenolic compounds; 4’-methyl quercetin-7-O-β-D-glucuronopyranoside (1), 2,3-hexahydroxydiphenoyl-(α/β)-D-4C1-glucopyranose (2), avicularin (3), castalagin (4), and quercetin-3-O-glucoside (5) significantly decreased the sucrase enzyme activity [Figure 1]. These findings are in accordance with the previous study as the sucrase-inhibitory activity of tested plants supposed to be due to the presence of flavonoid glycosides and/or hydrolysable tannins.[16] Due to the rise in the incidence of diabetic patients around the world, it appears that more anti-diabetic drugs with complementary actions should be discovered and improved to reduce the blood glucose level by inhibiting the hydrolysis of carbohydrates in a reversible way. The World Health Organization suggested the evaluation of traditional plant treatments for diabetes as they are effective, less toxic, with fewer side effects, and are considered to be excellent candidates for oral therapy.[29] The antihyperglycemic activity of the plants is mainly due to their ability to restore the function of pancreatic tissues by causing an increase in insulin output or inhibiting the intestinal absorption of glucose or to the facilitation of metabolites in insulin-dependent processes. Glycosides, flavonoids, and carotenoids, from the plants, are frequently implicated in having anti-diabetic effect.[30] In the present study, the hydroalcoholic extract of A. indica and its isolated polyphenolic compounds 1–5 may have anti-diabetic activity due to their potent inhibitory activity against sucrase enzyme as shown in Figure 1.
CONCLUSION
The hydroalcoholic extract of the leaves of A. indica (family Meliaceae) and the tested compounds; 4’-methyl quercetin-7-O-β-D-glucuronopyranoside (1), 2,3-hexahydroxydiphenoyl-(α/β)-D-4C1-glucopyranose (2), avicularin (3), castalagin (4), and quercetin-3-O-glucoside (5) exhibited a significant in vitro anti-diabetic activity using sucrase enzyme inhibitory activity test. The hydroalcoholic extract of the leaves of A. indica contains a considerable amount of polyphenolic compounds that have significant antioxidant, cytotoxic,[18] and sucrase-inhibitory activities, thus have a great potential as a source for natural health products. Hence, the authors recommend in vitro and in vivo toxic tests to be done to evaluate the safety of A. indica to be used as a complementary medicine.
Financial support and sponsorship
The Institute of Scientific Research and Revival of Islamic Heritage at Umm Al-Qura University, Makkah, the KSA (Project ID: 4331014).
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to thank the Institute of Scientific Research and Revival of Islamic Heritage at Umm Al-Qura University, Makkah, the KSA (Project ID: 4331014), for the financial support.
ABOUT AUTHOR

Mohamed I. S. Abdelhady
Mohamed I. S. Abdelhady, is an Associate Professor at the Department of Pharmacognosy, college of Pharmacy (Helwan University, Egypt and UQU, KSA). He is a specialist in phytochemistry and plant biotechnology and has experience in the area of pharmacognosy and chemistry of Natural Products. His research interest is in the area of phytochemistry and biological activity of medicinal plants.
REFERENCES
- 1.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2009;32:62–7. [Google Scholar]
- 2.Baron AD. Postprandial hyperglycaemia and alpha-glucosidase inhibitors. Diabetes Res Clin Pract. 1998;40(Suppl 1):51–5. doi: 10.1016/s0168-8227(98)00043-6. [DOI] [PubMed] [Google Scholar]
- 3.Blickle JF, Andres E, Brogard JM. Current status of the treatment of type 2 diabetes mellitus: Alpha-glucosidase inhibitors. Food Chem. 2008;106:247–52. doi: 10.1016/s0248-8663(99)80511-6. [DOI] [PubMed] [Google Scholar]
- 4.Ceriello A. The emerging role of post-prandial hyperglycaemic spikes in the pathogenesis of diabetic complications. Diabet Med. 1998;15:188–93. doi: 10.1002/(SICI)1096-9136(199803)15:3<188::AID-DIA545>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 5.Shim YJ, Doo HK, Ahn SY, Kim YS, Seong JK, Park IS, et al. Inhibitory effect of aqueous extract from the gall of Rhus chinensis on alpha-glucosidase activity and postprandial blood glucose. J Ethnopharmacol. 2003;85:283–7. doi: 10.1016/s0378-8741(02)00370-7. [DOI] [PubMed] [Google Scholar]
- 6.Tiwari AK, Rao JM. Diabetes mellitus and multiple therapeutic approaches of phytochemicals: Present status and future prospects. Curr Sci. 2002;83:30–3. [Google Scholar]
- 7.Rhabasa-Lhoret R, Chiasson JL. 3rd ed. Vol. 1. UK: John Wiley and Sons Ltd; 2004. International Textbook of Diabetes Mellitus; pp. 901–14. [Google Scholar]
- 8.Mardiha MA, Al-Shyhaibani NA. Prospect of neem plantation at Arafat, Saudi Arabia. Curr World Environ. 2014;9:81–6. [Google Scholar]
- 9.Dholi SK, Ramakrishna R, Mankala SK, Nagappan K. in vivo anti-diabetic evaluation of neem leaf extract in alloxan induced rats. J Appl Pharm Sci. 2011;7:100–5. [Google Scholar]
- 10.Sonia B, Srinivasan BP. Investigation into the anti-diabetic activity of Azadirachta indica. Indian J Pharmacol. 1999;31:138–41. [Google Scholar]
- 11.El-Mahmood AM, Ogbonna OB, Raji M. The antibacterial activity of Azadirachta indica (Neem) associated with eye and ear infections. J Med Plant Res. 2010;4:1414–421. [Google Scholar]
- 12.Maragathavalli S, Brindha S, Kaviyarasi NS, Annadurai B, Gangwar SK. Anti-microbial activity in leaf extract of neem (Azadirachta indica linn.) Int J Secur Netw. 2012;3:110–3. [Google Scholar]
- 13.Amer H, Helmy WA, Taie HA. in vitro antitumor activities of seeds and leaves of neem (Azadirachta indica) extracts. Int J Acad Res. 2010;2:165–71. [Google Scholar]
- 14.van der Nat JM, van der Sluis WG, de Silva KT, Labadie RP. Ethnopharmacognostical survey of Azadirachta indica A. Juss (Meliaceae) J Ethnopharmacol. 1991;35:1–24. doi: 10.1016/0378-8741(91)90131-v. [DOI] [PubMed] [Google Scholar]
- 15.Koul O, Isman MB, Ketkar CM. Properties and uses of neem, Azadirachta indica. Can J Bot. 1990;68:1–11. [Google Scholar]
- 16.Andrade-Cetto A, Heinrich M. Mexican plants with hypoglycaemic effect used in the treatment of diabetes. J Ethnopharmacol. 2005;99:325–48. doi: 10.1016/j.jep.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 17.Sabu MC, Kuttan R. Anti-diabetic activity of medicinal plants and its relationship with their antioxidant property. J Ethnopharmacol. 2002;81:155–60. doi: 10.1016/s0378-8741(02)00034-x. [DOI] [PubMed] [Google Scholar]
- 18.Abdelhady MI, Bader A, Shaheen U, El-Malah Y, Abourehab MA, Barghash MF. Azadirachta indica as a source for antioxidant and cytotoxic polyphenolic compounds. Biosci Biotechnol Res Asia. 2015;12:1209–22. [Google Scholar]
- 19.Mabry TJ, Markham KR, Thomas MB. The Ultraviolet Spectra of Flavones and Flavonols, Part II. Ch. V. Berlin: Springer-Verlag; 1970. The systematic identification of flavonoids; pp. 41–164. [Google Scholar]
- 20.Brasseur T, Angenot L. Reagents for densitometric determination of flavonoids. J Chromatogr. 1986;351:351–5. [Google Scholar]
- 21.Dahlqvist A. Method for assay of intestinal disaccharidases. Anal Biochem. 1964;7:18–25. doi: 10.1016/0003-2697(64)90115-0. [DOI] [PubMed] [Google Scholar]
- 22.Honda M, Hara Y. Inhibition of rat small intestinal sucrase and α-glucosidase activities by tea polyphenols. Biosci Biotechnol Biochem. 1993;57:123–4. doi: 10.1271/bbb.57.123. [DOI] [PubMed] [Google Scholar]
- 23.Ou S, Kwok K, Li Y, Fu L. In vitro study of possible role of dietary fiber in lowering postprandial serum glucose. J Agric Food Chem. 2001;49:1026–9. doi: 10.1021/jf000574n. [DOI] [PubMed] [Google Scholar]
- 24.Agrawal PK. Studies in organic chemistry, 13C NMR of flavonoids. In: Agrawal PK, Bansal MC, editors. Flavonoid Glycosides. New York: Elsevier Science; 1989. pp. 283–364. [Google Scholar]
- 25.Mahmoud II, Moharram FA, Marzouk MS, Linscheid MW, Saleh MI. Polyphenolic constituents of Callistemon lanceolatus leaves. Pharmazie. 2002;57:494–6. [PubMed] [Google Scholar]
- 26.Azimova SS, Vinogradova VI. Vol. 113. New York: Springer Science and Business Media; 2013. Physicochemical and pharmacological properties of flavonoids. Natural Compounds-Flavonoids; pp. 187–8. [Google Scholar]
- 27.Sikorska M, Matlawska I. Quercetin and its glycosides in the flowers of Asclepias syriaca L. Acta Pol Pharm. 2000;57:321–4. [PubMed] [Google Scholar]
- 28.Wang AQ, Wang XK, Li JL, Cui XY. Isolation and structure identification of chemical constituents from the seeds of Descurainia sophia (L.) Webb ex Prantl. Acta Pharm Sin. 2004;39:46–51. [PubMed] [Google Scholar]
- 29.Maritim AC, Sanders RA, Watkins JB., 3rd Diabetes, oxidative stress, and antioxidants: A review. J Biochem Mol Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 30.Malviya N, Jain S, Malviya S. Antidiabetic potential of medicinal plants. Acta Pol Pharm. 2010;67:113–8. [PubMed] [Google Scholar]


