Abstract
Background:
Rauwolfia serpentina and Solanum khasianum are well-known medicinally important plants contained important alkaloids in their different parts. Elicitation of these alkaloids is important because of associated pharmaceutical properties. Targeted metabolites were ajmaline and ajmalicine in R. serpentina; solasodine and α-solanine in S. khasianum.
Objective:
Enhancement of secondary metabolites through biotic and abiotic elicitors in hairy root cultures of R. serpentina and S. khasianum.
Materials and Methods:
In this report, hairy root cultures of these two plants were established through Agrobacterium rhizogenes mediated transformation by optimizing various parameters as age of explants, duration of preculture, and co-cultivation period. NaCl was used as abiotic elicitors in these two plants. Cellulase from Aspergillus niger was used as biotic elicitor in S. khasianum and mannan from Saccharomyces cerevisiae was used in R. serpentina.
Results:
First time we have reported the effect of biotic and abiotic elicitors on the production of important metabolites in hairy root cultures of these two plants. Ajmalicine production was stimulated up to 14.8-fold at 100 mM concentration of NaCl after 1 week of treatment. Ajmaline concentration was also increased 2.9-fold at 100 mg/l dose of mannan after 1 week. Solasodine content was enhanced up to 4.0-fold and 3.6-fold at 100 mM and 200 mM NaCl, respectively, after 6 days of treatments.
Conclusion:
This study explored the potential of the elicitation strategy in A. rhizogenes transformed cell cultures and this potential further used for commercial production of these pharmaceutically important secondary metabolites.
SUMMARY
Hairy roots of Rauwolfia serpentina were subjected to salt (abiotic stress) and mannan (biotic stress) treatment for 1 week. Ajmaline and ajmalicine secondary metabolites were quantified before and after stress treatment
Ajmalicine yield was enhanced up to 14.8-fold at 100 mM concentration of NaCl. Ajmaline content was also stimulated 2.9-fold at 100 mg/l dose of mannan after 1 week
Hairy roots of Solanum khasianum were treated with cellulase (biotic elicitor) and salt (abiotic stress)
Solasodine content was improved up to 4.0-fold and 3.6-fold at 100 mM and 200 mM NaCl, respectively, after 6.days of treatments
The α-solanine content increased to 1.6-fold after 24 h of treatment at 100 μg/mL cellulase concentration.
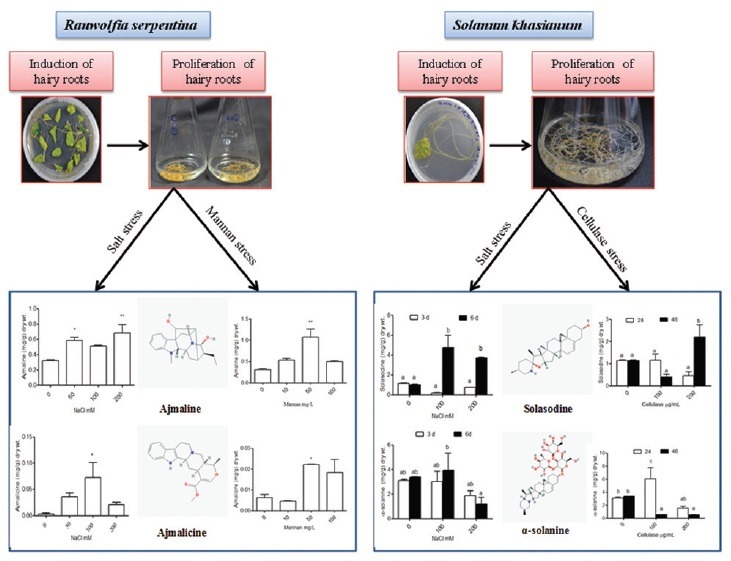
Abbreviations used: MS medium: Murashige and Skoog medium, B5 medium: Gamborg B5 medium, OD: Optical Density, NaCl: Sodium Chloride.
Keywords: Elicitation, hairy root cultures, Rauwolfia serpentina, secondary metabolites, Solanum khasianum
INTRODUCTION
Significant increase in world population enhanced the demand of medicines worldwide. Synthetic drugs cannot replace the natural drugs due to high cost value and associated numerous side effects. In the present scenario, 80% of world population rely on herbal products for their primary health care.[1]
Rauwolfia serpentina has been categorized under endangered plants category by International Union for the Conservation of Nature and Natural Resources.[2] This plant contains numerous medicinal properties and therefore strongly suffered from the loss of habitat distortion.[3] Hairy root cultures of Rauwolfia plants proved to be very beneficial among all conservational strategies. Lots of reports are available regarding the biosynthetic stability and uniformity in the long-term production of bioactive compounds in hairy root cultures. Besides, fast growth in growth hormone free medium made this technique cost effective also.[4,5] Medicinal plants are hubs of pharmaceutically important compounds therefore, Agrobacterium rhizogenes-mediated transformations were extensively studied in these plants. Phytochemical analysis revealed that transformed roots usually showed a similar level of secondary metabolites as in nontransformed roots.[6] Ajmaline and ajmalicine are root-specific indole alkaloids in R. serpentina plants. Ajmalicine is effective in circulatory disorders while ajmaline is mainly known for its antihypertensive and antiarrhythmic activities.[7]
In Solanaceae family, steroidal glycoalkaloids comprise an essential group of pharmaceutically important bioactive compounds.[8] Solasodine and α-solanine are among those present in large amount in Solanum plants. These two alkaloids are blessed with many important pharmacological properties. These alkaloids could be used as precursor in the synthesis of steroids at a commercial level. Solasodine exclusively used as a contraceptive in different areas of world.[9] Glycoalkaloids used as defensive agent when the plant is subjected to attack by insect and fungi. At higher concentration, glycoalkaloids showed some harmful effects to humans although they enhanced anti-inflammatory and anticancer activity in humans.[10]
Medicinal plants respond differently when subjected to different types of stress. Stress was proved to be harmful in case of agricultural crops as it reduces the total yield of crops but in case of medicinal plants the whole entity of medicinal plants is dependent on the presence of therapeutically compounds, often stress enhances the level of these valuable compounds.[11] Among all major crops, medicinal plants hold exclusive position due to the presence of their valuable secondary metabolites. Use of medicinal plants for medication is very old, starting from ancient times. Even in the United States, 19% mature population use herbal medicines. Significance and amount of secondary metabolites are strongly influenced through variation in the environment.[12,13,14]
In in vitro cultures biotic and abiotic elicitors were used for enhancing the yield of secondary metabolites.[15] For plant prospective, elicitors are a substance used to elicitate metabolic changes in plants which execute in the form of elevated level of bioactive compounds.[16] Biotic and abiotic elicitors are extensively used because they had shown a great impact on indole alkaloid biosynthesis.[17] Carbohydrate and protein are being used as biotic elicitors. Cellulase from Aspergillus niger (protein) and mannan from Saccharomyces cerevisiae (carbohydrate) were used in this study. There are few reports available on induction of hairy root cultures in R. serpentina and S. khasianum[4,18,19,20,21,22] but as per our knowledge, there is no report, regarding elicitation based enhancement of ajmaline, ajmalicine, solanine, and solasodine in hairy root cultures of these two plants. However, Jacob and Malpathak (2004 and 2005) was optimized culture condition as light, temperature, CO2 concentration and proportion of major and minor salt in the medium for enhancing the solasodine content in hairy root cultures of S. khasianum. Presence of ajmaline, ajmalicine, and α-solanine are being reported for the first time, in hairy root cultures of R. serpentina and S. khasianum, respectively.
MATERIALS AND METHODS
Chemicals
Cellulase, from A. niger and mannan from S. cerevisiae, were purchased from Sigma Chemicals Co., ajmaline, ajmalicine and α-solanine as high-performance liquid chromatography (HPLC) reference standards were also purchased from Sigma Co., HPLC grade acetonitrile bought from Merck.
Hairy root induction and proliferation
Leaves were excised from the in vitro proliferating cultures of R. serpentina and S. khasianum being maintained in our laboratory and used as explant for transformation. Bacterial plates were maintained on yeast mannitol broth (YMB) medium. Single colony were picked from this plate and immersed in liquid YMB medium. This bacterial culture was incubated at 28°C temperature with 220 rpm for 16–18 h. Bacterial cells were harvested in log phase OD600 = 0.6. Centrifugation conditions were 4000 rpm, temperature 4°C, and time 10 min. Supernatant was discarded, and pellet was dissolved in liquid MS medium (containing MES buffer and glucose). This bacterial solution along with 100 mM acetosyringone considered as infection medium, incubated at 28°C with 100–150 rpm for at least 1 h.
Precultured and pierced leaves were immersed in this suspension for 20–30 min with gentle shaking. Infected leaves were then dried on sterile blotting sheets for removal of excess moisture. Leaves were placed on solid ½ MS and B5 medium for 3–4 days in dark at 28°C. After co-cultivation leaves were washed with sterile MQ water containing cefotaxime at 250 mg/L concentration. Blotted dry leaves were placed on ½ MS medium, full MS medium and B5 medium containing cefotaxime (250 mg/L) for root induction. Explants, pricked with a needle devoid of the bacterial suspension, were used as a control. These explants remained nonresponded in any of the medium.
Elicitor preparation
All elicitors were prepared in 1 mg/mL concentration. NaCl and mannan were dissolved in milli-Q water; cellulase in citrate buffer. NaCl solution was autoclaved prior to use although filter sterilized solutions of mannan and cellulase were used in elicitation experiments.
Elicitor treatment in hairy root cultures
Hairy roots were subcultured in 250 mL flask, containing 100 mL modified MS medium (pH 5.8) under constant agitation 70–90 rpm on a rotatory shaker. Thirty days old hairy roots in exponential growth phase were used for elicitation experiment. NaCl as abiotic stress was used in this study. In case of R. serpentina 50, 100 and 200 mM concentrations were used and data were recorded after 1 week of treatment. In S. khasianum 100 and 200 mM doses were subjected and samples were collected at 3 days and 6 days interval. Cellulase (A. niger) was used as biotic elicitor in S. khasianum at two different concentrations, i.e., 100 and 200 µg/mL. Harvesting of samples were performed after 24 and 48 h. Mannan (S. cerevisiae) was subjected to R. serpentina hairy roots for 1 week at three different concentration 10, 50 and 100 mg/L. Ajmaline, ajmalicine, α-solanine, and solasodine were quantified from these treated and control samples through HPLC. All treatments were performed in triplicate.
Preparation of standard solutions
All standard stock solutions were prepared in 1 mg/mL concentration. Ajmaline and ajmalicine have shown complete solubility in methanol. Solasodine was also dissolved in methanol; however, α-solanine was dissolved in pyridine then diluted with ethanol.
Preparation of extract
Hairy roots of R. serpentina were dried in oven at 25°C. Fine powder of roots was extracted overnight in ethanol at 25°C. Ethanol was evaporated at room temperature, remaining residue, after dissolving in 1 mL of ethanol was used for HPLC quantification. Harvested samples of the S. khasianum plant were also dried in an oven at 37°C. Fine powered root samples were three times extracted in 1 mL methanol for 6 h at room temperature. Pooled extract was evaporated to 1 mL and used for HPLC analysis.
High-performance liquid chromatography
Analysis of all treated and nontreated samples were performed through HPLC (Waters Pvt. Ltd., Milford, USA) consisting of 717 autosampler, µBondapak C18 Column (4.0 mm × 25.0 mm), M 6000 pump, 996 PDA detector and Millennium software. Quantification of R. serpentina alkaloids were performed according to modified protocol of Deshmukh et al., isocratic mode of elution was used with mobile phase of acetonitrile and phosphate buffer (35:65) combination. 20 µL of sample was injected; flow rate was 1 mL/min. Detection wavelength was 268 nm at PDA detector.[23] In the case of S. khasianum, it was very difficult to separate α-solanine (glycoalkaloids) and solasodine (aglycone) in single run due to the similarity in their structures. Therefore, two separate mobile phases were used to separate these two compounds. Mobile phase 1 - acetonitrile and ammonium dihydogen phosphate buffer (30:70) and mobile phase 2 - acetonitrile and ammonium dihydogen phosphate buffer (60:40), respectively, used for α-solanine and solasodine separately. Detection wavelength 205 nm was used in both cases while injection volume was 5 µL.
Statistical analysis
All experiments were performed in triplicate. Bar represent the standard error. A comparison was conducted using one-way analysis of variance. Dunnett's Multiple Comparison test was performed, and all treatments were compared with their respective control in case of the yield of ajmaline and ajmalicine. Production of solasodine and α-solanine were statistically analyzed through SPSS software (IBM corporation) using Duncan's multiple range test. In all cases, the confidence coefficient was set at P < 0.05.
RESULTS
Hairy root cultures of these two plants were established through A. rhizogenes (A4 strain) mediated transformation. Three-to-four weeks old leaves responded best as explant material. Age of explant proved to be very important factor for the induction of hairy roots. Leaves were precultured in ½ MS medium before transformation event. Two-to-three days preculture period was optimum whereas a further increase in the period caused necrosis at leaf margins, probably due to the lack of any additional growth supplement. Co-cultivation time was 3–4 days, extension in this time showed no significant effect on R. serpentina but leaves of S. khasianum were deformed due to its sensitive nature. Putative hairy roots were induced after 3–4 weeks in R. serpentina in ½ MS medium with no growth hormone, proliferation of hairy roots was performed in modified MS liquid medium [Figure 1a and b] whereas, in S. khasianum first emergence of hairy root was observed after 10 days in B5 medium further proliferation of hairy root was occurred in modified MS liquid medium [Figure 1c and d]. Although ½ MS medium could also induce hairy roots but high transformation efficiency was obtained in B5 medium.
Figure 1.

Establishment of hairy roots in Rauwolfia serpentina and Solanum khasianum. (a and c) Induction; (b and d) proliferation and (e and f) PCR confirmation of positive lines respectively
Genomic DNA was isolated from putative hairy root lines and also from normal roots using 5 prime DNA isolation kit as according to manufacturer's instructions. Polymerase chain reaction amplification was performed with TL region specific primers forword- 5’AAGTTGAATGAGTATGACTGCC 3’ reverse-5’GGACGACATGGCACTCTGGGAG 3’ [Figure 1e and f]. Many positive lines were obtained but the best performing line was selected among them on the basis of high growth rate. Selected hairy root lines were subjected to biotic and abiotic stress.
Increase in ajmaline content was observed, i.e., 1.7-fold (0.563 mg/g dry weight) at 50 mM, 1.5-fold (0.510 mg/g dry weight) at 100 mM and 1.9-fold (0.629 mg/g dry weight) at 200 mM NaCl in contrast to control cultures (0.328 mg/g dry weight) after 1 week of NaCl treatment [Figure 2a]. Mannan was also subjected to hairy root cultures for 1 week. Ajmaline production was stimulated 1.5-fold, 2.9-fold and 1.48-fold at 50 mg/L, 100 mg/L and 200 mg/L doses of mannan, respectively. Content of ajmaline was 0.521, 0.975 and 0.488 mg/g dry weight at respective doses and in control cultures it was 0.328 mg/g dry weight [Figure 2b].
Figure 2.
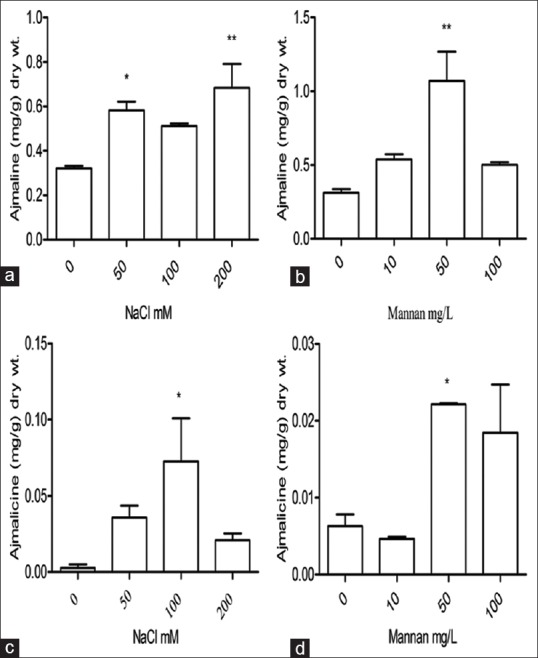
Ajmaline and ajmalicine production in Rauwolfia serpentina in control and treated hairy roots. (a and c) After one week of NaCl treatment; (b and d) after one week of mannan treatment respectively
In case of ajmalicine, the enhancement was 8.1-fold (0.032 mg/g dry weight), 14.8-fold (0.058 mg/g dry weight) and 4.7-fold (0.018 mg/g dry weight) at 50 mM, 100 mM and 200 mM NaCl concentration respectively, ajmalicine concentration in nontreated hairy root cultures was 0.003 mg/g dry weight [Figure 2c]. Decrease in ajmalicine content was observed at 10 mg/l mannan concentration but at 50 mg/l, 3.1-fold (0.022 mg/g dry weight) and at 100 mg/l, 2.0-fold (0.014 mg/g dry weight) enhancements were occurred in comparison to control (0.007 mg/g dry weight) [Figure 2d].
Solasodine content was enhanced up to 4.0-fold (7.161 mg/g dry weight) and 3.6-fold (3.751 mg/g dry weight) in comparison to control nontreated cultures (1.023 mg/g dry weight) at 100 mM and 200 mM NaCl, respectively, after 6 days of treatments [Figure 3b]. At 200 µg/mL cellulase concentration, 1.6-fold (1.930 mg/g dry weight) enhancements were observed in solasodine production in comparison to control (1.172 mg/g dry weight) after 48 h of treatment [Figure 3a]. At 100 µg/mL cellulase concentration, 1.6-fold (5.213 mg/g dry weight) increased in α-solanine content was reported as compared to nontreated hairy roots where α-solanine content was 3.157 mg/g dry weight, after 24 h of treatment [Figure 3c].
Figure 3.
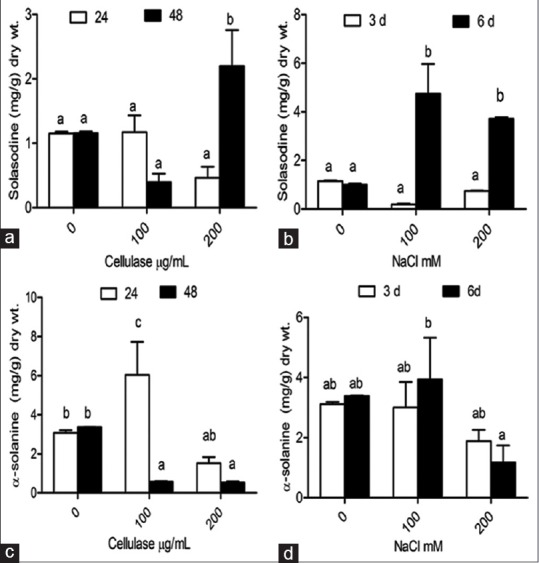
Solasodine and α-solanine content in hairy roots of Solanum khasianum. (a and c) 0 μg/mL, 100 μg/mL and 200 μg/mL concentration of cellulase treatment; (b and d) 0 mM, 100 mM and 200 mM NaCl concentration respectively
DISCUSSION
Salt stress causes osmotic imbalance in plants as the plants do not take water from the surroundings as in the case of drought stress. Ionic disproportion, especially Na+ and Cl− caused the formation of reactive oxygen species (ROS). This may lead to disturbance in cell homeostasis and activation of the defense-related gene.[24,25] In our study NaCl enhanced the ajmaline and ajmalicine production at all tested concentration [Figure 2a and 2c], however, synthesis of solasodine was decreased at 100 mM and 200 mM concentration of NaCl but 200 mM accumulated higher solasodine in comparison to 100 mM concentration after 3 days of treatment [Figure 3b].
Significant increase in production of solasodine was occurred at 100 mM concentration after 6 days of treatment in comparison to respective control although also at 200 mM concentration yield of solasodine was higher in comparison to nontreated cultures but it was lower than the yield at 100 mM concentration of NaCl [Figure 3b]. Yield of α-solanine was less affected in comparison to solasodine [Figure 3d] but increased in synthesis of α-solanine was observed at 100 mM NaCl concentration in comparison to control cultures after 6 days of treatment [Figure 3d]. A similar study was also conducted by Manikonda et al., for observing the effect of salt stress on production of daidzein in hairy root cultures of Psoralea corylifolia. They reported 300 mM NaCl, just doubled the content of daidzein after 2 weeks of treatment.[26] Significant increase in solasodine content was observed at 150 mM concentration after 6th week of NaCl treatment in in vitro raised callus of Solanum nigrum.[27] Estimation of ajmalicine content before and after salt stress was examined in Catharanthus roseus, an important medicinal plant, increase in ajmalicine content was observed with increase in salt stress.[28] In C. roseus, the effect of arbuscular mycorrhizal fungi and different doses of phosphorus was also evaluated for stimulating ajmalicine content. The highest increase in ajmalicine content was observed in the combination of these two stresses.[29]
Study of stress on the complete plant is a highly complex phenomenon because various factors are associated with it. Hence, targeting root for optimizing doses and duration provide an additional advantage, roots are in direct contact with medium or soil (in the case of in vivo studies).[30] It was more useful in our study because maximum alkaloids are root specific in nature. Cholesterol is a key intermediate in solasodine biosynthesis, during salinity stress level of cholesterol was increased leading to enhancement in the production of solasodine.[27] Accumulation of proline was observed in salt stress facing cells, perhaps proline plays a protective role and prevent cells against the ionic imbalance created by salt.[31,32,33] In this study, mannan stimulated the ajmaline and ajmalicine production most effectively at 50 mg/L concentration, production was also higher at 100 mg/L in comparison to control but it was lesser than 50 mg/L [Figure 2b and d]. Solasodine content was showed initial decline [Figure 3a] but it was enhanced at 200 µg/mL cellulase after 48 h of treatment [Figure 3a]. The reverse situation was observed in case of α-solanine as it showed enhancement at 100 µg/mL cellulase after 24 h of treatment [Figure 3c] after that production of α-solanine was dramatically reduced.
In plant cell cultures, extract of fungal cell wall was proved to be efficient elicitating agent. The cell wall extract of two fungi Phytophthora megasperma and Alternaria carthami were investigated for stimulating the content of umbelliferone, a plant phenolic compound in Ammi majus L. plant. Significant increase was observed in comparison to nonelicitated cultures. P. megasperma was found to be more efficient in contrast to A. carthami.[34] The glutathione S-cinnamoyltransferases (GSTs), are detoxifying enzymes catalyzing the inactivation of ROS, basically GSTs are anti-carcinogenic in nature. Colletotrichum lidemuthianum, cell wall extract was used as biotic elicitor for enhancing the synthesis of GSTs in cell culture of Pharsalus vulgaris. Remarkable increase (125%) was found after the addition of elicitor.[35]
Polysaccharides and oligosaccharides from fungal cell wall are considered as effective alternative of fungal elicitation.[16] Mannan is used as biotic elicitor in stimulation of ajmaline and ajmalicine production in this study. Mannan is an essential part of yeast cell wall.[36] Effects of mannan on the biosynthesis of hypericins and pseudohypericin production was estimated in in vitro seedlings of Hypericum adenotrichum, where mannan enhanced hypericin synthesis up to 1.7-fold and pseudohypericin synthesis up to 2.8-fold.[37] Kirakosyan et al., also reported that mannan enhanced the production of pseudohypericin and hypericin up to 4- and 2-fold, respectively, in shoot cultures of Hypericum perforatum. They also proved the effectiveness of commercially synthesized mannan.[38] Suspension cultures of Capsicum annuum subjected to cellulase treatment, increased the capsidiol content, after 24 h of treatment at 3 µg/mL concentration.[39] There are two possibilities of the entrance of cellulase either it may enter through pore formation or by disrupting the cell wall of substrate.[40] Exact mechanism of action of elicitation of cellulase and mannan is not clearly known; probably it activate the gene involved in biosynthetic pathways of these secondary metabolites.
Elicitation is the most important strategy for enhancing the level of bioactive compounds in plant tissue culture, after identification of the upregulated genes and further its cloning through transgenic approaches we could generate the plants with desired level of secondary metabolites. Additional information regarding signal perception and its transduction proved to be more beneficial for manipulating the whole pathway through suppression and activation of related gene. Although elicitation was commonly used approach for stimulating the production of bioactive compounds in medicinal plants but it was first time introduced in the hairy root cultures of R. serpentina and S. khasianum.
CONCLUSION
This study showed the presences of pharmaceutical important secondary metabolites in hairy root cultures. Transformed cultures were known for its higher growth rate so hairy roots alone capable of commercial level production of valuable metabolites. The combination of elicitation with hairy roots undoubtedly enhances the yield of targeted metabolites.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHOR

Dr. Pratibha Misra
Dr. Pratibha Misra, is Principle Scientist at Tissue Culture and Genetic Transformation Lab of CSIR- National Botanical Research Institute Lucknow. Her research interests are mainly in the area of Plant tissue culture, genetic transformation and functional characterization of genes. She has good SCI indexes (high impact factor) articles in these fields. She is also a member of Plant Tissue Culture Association of India and International Association of Plant Biotechnology.
REFERENCES
- 1.Kumari R, Rathi B, Rani A, Bhatnagar S. Rauvolfia serpentina L. Benth. ex Kurz: Phytochemical, pharmacological and therapeutic aspects. Int J Pharm Sci Rev Res. 2013;23:348–55. [Google Scholar]
- 2.Shetty MR, Harisha GA, Jayanth Y, Kumar AH. Production of secondary metabolites from in vitro cultures of Rauwolfia serpentina (L.) Benth. Int J Sci Technol Res. 2014;2:844–52. [Google Scholar]
- 3.Mehrotra S, Goel MK, Srivastava V, Rahman LU. Hairy root biotechnology of Rauwolfia serpentina: A potent approach for the production of pharmaceutically important terpenoid indole alkaloids. Biotechnol Lett. 2015;37:253–63. doi: 10.1007/s10529-014-1695-y. [DOI] [PubMed] [Google Scholar]
- 4.Madhusudanan KP, Banerjee S, Khanuja SP, Chattopadhyay SK. Analysis of hairy root culture of Rauvolfia serpentina using direct analysis in real time mass spectrometric technique. Biomed Chromatogr. 2008;22:596–600. doi: 10.1002/bmc.974. [DOI] [PubMed] [Google Scholar]
- 5.Guillon S, Trémouillaux-Guiller J, Pati PK, Rideau M, Gantet P. Harnessing the potential of hairy roots: Dawn of a new era. Trends Biotechnol. 2006;24:403–9. doi: 10.1016/j.tibtech.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Sheludko Y, Gerasimenko I, Kolshorn H, Stöckigt J. New alkaloids of the sarpagine group from Rauvolfia serpentina hairy root culture. J Nat Prod. 2002;65:1006–10. doi: 10.1021/np0200919. [DOI] [PubMed] [Google Scholar]
- 7.Srivastava A, Tripathi AK, Pandey R, Verma RK, Gupta MM. Quantitative determination of reserpine, ajmaline, and ajmalicine in Rauvolfia serpentina by reversed-phase high-performance liquid chromatography. J Chromatogr Sci. 2006;44:557–60. doi: 10.1093/chromsci/44.9.557. [DOI] [PubMed] [Google Scholar]
- 8.Attoumbré J, Lesur D, Giordanengo P, Baltora-Rosset S. Preparative separation of glycoalkaloids a-solanine and a-chaconine by centrifugal partition chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;908:150–4. doi: 10.1016/j.jchromb.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 9.Kuo CI, Chao CH, Lu MK. Effects of auxins on the production of steroidal alkaloids in rapidly proliferating tissue and cell cultures of Solanum lyratum. Phytochem Anal. 2012;23:400–4. doi: 10.1002/pca.1371. [DOI] [PubMed] [Google Scholar]
- 10.Patel K, Singh RB, Patel DK. Medicinal significance, pharmacological activities, and analytical aspects of solasodine: A concise report of current scientific literature. J Acute Dis. 2013;2:92–8. [Google Scholar]
- 11.Gorelick J, Bernstein N. Elicitation: An underutilized tool for the development of medicinal plants as a source for therapeutic secondary metabolites. Adv Agron. 2014;124:201–30. [Google Scholar]
- 12.Aghaei K, Komatsu S. Crop and medicinal plants proteomics in response to salt stress. Front Plant Sci. 2013;4:8. doi: 10.3389/fpls.2013.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rehm S, Espig G. The Cultivated Plants of the Tropics and Subtropics. Cultivation, Economic value, Utilization, Verlag, Josef. 1991:522–528. [Google Scholar]
- 14.Williamson EM. Drug interactions between herbal and prescription medicines. Drug Saf. 2003;26:1075–92. doi: 10.2165/00002018-200326150-00002. [DOI] [PubMed] [Google Scholar]
- 15.Rezaeieh KA, Gurbuz B, Uyanık M. Biotic and abiotic stresses mediated changes in secondary metabolites induction of medicinal plants. Aromatic Herbs Medicine Symposium. 2012;13-15:218–22. [Google Scholar]
- 16.Zhao J, Davis LC, Verpoorte R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol Adv. 2005;23:283–333. doi: 10.1016/j.biotechadv.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Siddiqui ZH, Mujib A, Aslam J, Hakeem KR, Parween T. US: Springer; 2013. In vitro Production of Secondary Metabolites Using Elicitor in Catharanthus roseus: A Case Study.”Crop Improvement; pp. 401–419. [Google Scholar]
- 18.Jacob A, Malpathak N. Green hairy root cultures of Solanum khasianum Clarke – A new route to in vitro solasodine production. Curr Sci. 2004;87:1442–7. [Google Scholar]
- 19.Goel MK, Goel S, Banerjee S, Shanker K, Kukreja AK. Agrobacterium rhizogenes-mediated transformed roots of Rauwolfia serpentina for reserpine biosynthesis. Med Aromat Plant Sci Biotechnol. 2010;4:8–14. [Google Scholar]
- 20.Mehrotra S, Goel MK, Rahman LU, Kukreja A. Molecular and chemical characterization of plants regenerated from Ri-mediated hairy root cultures of Rauwolfia serpentina. Plant Cell Tissue Organ. 2013;114:31–8. [Google Scholar]
- 21.Jacob A, Malpathak N. Plantlet regeneration enhances solasodine productivity in hairy root cultures of Solanum khasianum clarke. In Vitro Cell Dev Biol Plant. 2005;41:291–5. [Google Scholar]
- 22.Putalun W. Technology of compact MAb and its application for medicinal plant breeding named as missile type molecular breeding. Curr Drug Discov Technol. 2011;8:24–31. doi: 10.2174/157016311794519965. [DOI] [PubMed] [Google Scholar]
- 23.Deshmukh SR, Ashrit DS, Patil BA. Extraction and evaluation of indole alkaloids from Rauwolfia serpentina for their antimicrobial and antiproliferative activities. Int J Pharm Pharm Sci. 2012;4:329–34. [Google Scholar]
- 24.Timperio AM, Egidi MG, Zolla L. Proteomics applied on plant abiotic stresses: Role of heat shock proteins (HSP) J Proteomics. 2008;71:391–411. doi: 10.1016/j.jprot.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Du CX, Fan HF, Guo SR, Tezuka T, Li J. Proteomic analysis of cucumber seedling roots subjected to salt stress. Phytochemistry. 2010;71:1450–9. doi: 10.1016/j.phytochem.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 26.Manikonda PK, Abhyanikarn G, Rao KV, Reddy VD, Subramanyam C. Salt stress enhances daidzein production in hairy root cultures of Psoralea corylifolia L. (Fabaceae) Proc AP Akad Sci. 2009;13:35–49. [Google Scholar]
- 27.Šutković J, Ler D, Gawwad MR. In vitro production of solasodine alkaloid in Solanum nigrum under salinity stress. J Phytol. 2011;3:43–9. [Google Scholar]
- 28.Behzadifar M, Chehrazi M, Aboutalebi A. Effect of salt stress by using unconventional water on some morphological characters and ajmalicine alkaloid amount in the roots of Catharanthus roseus Cvs. rosea and alba. Ann Biol Res. 2013;4:229–31. [Google Scholar]
- 29.Karthikeyan B, Jaleel CA, Changxing Z, Joe MM, Srimannarayan J, Deiveekasundaram M. The effect of AM fungi and phosphorous level on the biomass yield and ajmalicine production in Catharanthus roseus. Eurasia J Biosci. 2008;2:26–33. [Google Scholar]
- 30.Andreu P, Arbeloa A, Lorente P, Marín JA. Early detection of salt stress tolerance of Prunus rootstocks by excised root culture. HortScience. 2011;46:80–5. [Google Scholar]
- 31.Ashraf M, Harris P. Potential biochemical indicators of salinity tolerance in plants. Plant Sci. 2004;166:3–16. [Google Scholar]
- 32.Munns R, Tester M. Mechanisms of salinity tolerance. Annu Rev Plant Biol. 2008;59:651–81. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- 33.Lu S, Chen C, Wang Z, Guo Z, Li H. Physiological responses of somaclonal variants of triploid bermudagrass (Cynodon transvaalensis × Cynodon dactylon) to drought stress. Plant Cell Rep. 2009;28:517–26. doi: 10.1007/s00299-008-0649-z. [DOI] [PubMed] [Google Scholar]
- 34.Hamerski D, Beier RC, Kneusel RE, Matern U, Himmelspacht K. Accumulation of coumarins in elicitor-treated cell suspension cultures of Ammi majus. Phytochemistry. 1990;29:1137–42. doi: 10.1016/0031-9422(90)85417-e. [DOI] [PubMed] [Google Scholar]
- 35.Radman R, Saez T, Bucke C, Keshavarz T. Elicitation of plants and microbial cell systems. Biotechnol Appl Biochem. 2003;37(Pt 1):91–102. doi: 10.1042/ba20020118. [DOI] [PubMed] [Google Scholar]
- 36.Jones GH, Ballou CE. Studies on the structure of yeast mannan. II. Mode of action of the Arthrobacter alpha-mannosidase on yeast mannan. J Biol Chem. 1969;244:1052–9. [PubMed] [Google Scholar]
- 37.Yamaner Ö, Erdağ B, Gökbulut C. Stimulation of the production of hypericins in in vitro seedlings of Hypericum adenotrichum by some biotic elicitors. Turk J Bot. 2013;37:153–9. [Google Scholar]
- 38.Kirakosyan A, Hayashi H, Inoue K, Charchoglyan A, Vardapetyan H. Stimulation of the production of hypericins by mannan in Hypericum perforatum shoot cultures. Phytochemistry. 2000;53:345–8. doi: 10.1016/s0031-9422(99)00496-3. [DOI] [PubMed] [Google Scholar]
- 39.Ma CJ. Cellulase elicitor induced accumulation of capsidiol in Capsicum annumm L. suspension cultures. Biotechnol Lett. 2008;30:961–5. doi: 10.1007/s10529-007-9624-y. [DOI] [PubMed] [Google Scholar]
- 40.Klüsener B, Weiler EW. Pore-forming properties of elicitors of plant defense reactions and cellulolytic enzymes. FEBS Lett. 1999;459:263–6. doi: 10.1016/s0014-5793(99)01261-2. [DOI] [PubMed] [Google Scholar]


