Abstract
Background:
Essential oil of Ocimum sanctum Linn. exhibited various pharmacological activities including antifungal and antimicrobial activities. In this study, we analyzed the anticancer and apoptosis mechanisms of Ocimum sanctum essential oil (OSEO).
Objective:
To trigger the apoptosis mechanism in human breast cancer cells using OSEO.
Materials and Methods:
OSEO was extracted using hydrodistillation of the leaves. Cell proliferation was determined using different concentrations of OSEO. Apoptosis studies were carried out in human breast cancer cells using propidium iodide (PI) and Hoechst staining.
Results:
We found that OSEO inhibited proliferation (IC50 = 170 μg/ml) of Michigan cancer foundation-7 (MCF-7) cells in a dose-dependent manner. The OSEO also induced apoptosis as evidenced by the increasing number of PI-stained apoptotic nucleic of MCF-7 cells. Flow cytometry analysis revealed that treatment with OSEO (50–500 μg/ml) increased the apoptotic cells population (16–84%) dose dependently compared to the control. OSEO has the ability to up-regulate the apoptotic genes p53 and Bid and as well as elevates the ratio of Bax/Bcl-2.
Conclusion:
Our findings indicate that OSEO has the ability as proapoptotic inducer and it could be developed as an anticancer agent.
SUMMARY
OSEO inhibited proliferation of MCF-7 cells with an IC50 of 170 μg/mL
OSEO at 500 μg/mL increased the population of apoptotic cells by 84%
OSEO up-regulated the expression of apoptotic genes and as well increased the Bax/Bcl2 ratio.
Abbreviations used: BAX: BAX BCL2-associated X protein; BCL2: B-cell CLL/lymphoma 2; BID: BH3 Interacting domain death agonist; OSEO: Ocimum sanctum essential oil; DMSO: Dimethyl sulfoxide; DMEM: Dulbecco's modified Eagle medium; MCF-7: Michigan cancer foundation-7; RT-PCR: Real Time Polymerase Chain Reaction.
Keywords: Apoptosis, breast cancer, essential oil, gene expression, Ocimum sanctum
INTRODUCTION
Breast cancer is the most frequent type of cancer affecting women in Malaysia.[1] The chance of developing breast cancer in Malaysian women is estimated to be about one in nine.[2] The current approach of combating breast cancer includes operation, radiotherapy, hormone remedy, and chemotherapy. Although some of the present treatments have been successful in treating cancer, these always come with vulnerable side effects. Therefore, recent attention has focused on finding natural chemotherapeutic agents to combat breast cancer.
It is widely recognized that the prevention of cancer could be associated with the intake of fresh fruits and vegetables. Ocimum sanctum Linn. commonly known as tulsi or holy basil is widely known across South Asia as an aromatic medicinal herb and is distributed and cultivated worldwide.[3,4] O. sanctum leaves are categorized as functional foods and have a variety of pharmacological effects such as antimicrobial,[5] hypolipidemic,[6] antioxidant,[7] antibacterial,[8] immunomodulatory, antistress, anti-inflammatory, antiulcer, antidiabetic, hepatoprotective, chemoprotective, cardioprotective, antitussive, radioprotective, memory-enhancing, antiarthritic, antifertility, antihypertensive, anticoagulant, anticataract, anthelmintic, and antinociceptive.[9] On the other hand, the essential oil from the leaves of O. sanctum (OSEO) has been evaluated pharmacologically for antimicrobial,[10] anticandidal,[11] and antifungal[12,13] activities. Our recent study on OSEO showed its antimetastatic and anti-inflammatory potentials.[14] However, to the best of our knowledge, there is limited information about the anticancer and apoptosis mechanisms of OSEO. The aim of the present study was to investigate the anticancer and apoptosis activities of OSEO against human breast cancer cells.
MATERIALS AND METHODS
Essential oil extraction
Freshly collected leaves of O. sanctum (1 kg) were hydrodistilled for 4 h using Clevenger apparatus for essential oil extraction. Extracted oil was treated with sodium sulfate anhydrous to remove excess water. The purified oil was then filled in small vials, tightly sealed, and stored in a refrigerator (4°C) for further studies.
Cell culture
Michigan cancer foundation-7 (MCF-7) cell line was obtained from ATCC and routinely maintained in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum, L-glutamine (1%), streptomycin, and penicillin (1%) at 37°C in a humidified incubator containing 5% CO2.
Cell proliferation (MTT assay)
The MCF-7 cell line was seeded at a density of 5 × 103 cells/well in 96-well plates. The cells were allowed to adhere for 24 h and then treated with OSEO at various concentrations (50–500 μg/mL) for 24 h. The culture medium was removed and 20 μL of MTT (5 mg/mL in DMEM) was added to each well, followed by incubation for 2 h. The formation of formazan crystals was visualized under a light microscope. The formazan crystals were dissolved by adding 100 μL Dimethyl sulfoxide (DMSO) to each well. The absorbance was measured using a microplate reader (Bio-Rad, Hercules, CA, USA) at a wavelength of 570 nm.[15] The effect of OSEO on MCF-7 cell proliferation was assessed as percentage cell viability over that of control, where vehicle-treated control cells (0.1% DMSO) were taken as 100% viable.
Propidium iodide and Hoechst 33342 staining
Apoptosis in MCF-7 cells was assessed using uptake of fluorescent dye propidium iodide (PI) and Hoechst 33342 as described.[16] MCF-7 cells (1 × 106 cells/well) were seeded in a 24-well plate and grown until 90% confluent. The cells were treated with OSEO at various concentrations (50–500 μg/mL) for 24 h. The cells were washed with ice cold PBS and fixed with 70% ethanol for 30 min. After fixation, the plates were rinsed with ice cold PBS and stained with 3 mM of Hoechst 33342 for 30 min. Then, the plates were rinsed again with ice cold PBS to remove the excess stain and followed by counterstaining with 500 μM PI for 30 min. The nuclear staining of the apoptotic cells (red color) and live cells (blue color) was observed under inverted fluorescence microscope ECLIPSE Ti-E (NIKON Instruments Inc, UK).
Flow cytometry analysis (Annexin-V-fluorescein isothiocyanate)
Double staining of Annexin-V-fluorescein isothiocyanate (Annexin V-FITC) and PI was performed according to the manufacturer's protocol (eBioscience, San Diego, USA). MCF-7 cells grown in 25 cm2 flasks (1 × 106 cells/flask) were treated with various concentrations of OSEO (50–500 μg/mL) for 24 h. Cells were trypsinized, washed with ice cold PBS, and resuspended in 1 mL of binding buffer. They were then stained with 5 μL of Annexin V-FITC for 15 min, followed by 5 μL of PI stain according to the manufacturer's protocol (eBioscience, San Diego, USA). The early and late apoptosis were visualized by constructing a dot plot using a FACSCanto II flow cytometer (Becton Dickinson, California, USA). Green fluorescence from the Annexin-V-FITC was determined using an FL1 detector having a band pass filter with specifications of 530 ± 15 nm. Red fluorescence from PI was determined using an FL2 detector having a band pass filter with specifications of 585 ± 21 nm. A total of 10,000 events were recorded for each sample.
Quantification of apoptotic gene expression using real-time polymerase chain reaction
MCF-7 cells grown in 25 cm2 flasks (1 × 107 cells/flask) were treated with 100 μg/mL and 200 μg/mL of OSEO for 24 h. Total mRNA was obtained from the MCF-7 cells after treatment using QiagenRNeasy Mini Kit (Qiagen, Valencia, CA, USA) according to the protocol provided by the manufacturer. The concentration, purity, and integrity of the mRNA extracted from the sample were verified and quantified using NanoDrop 2000 UV-Vis Spectrophotometer (Thermo Scientific, Wilmington, USA) and the Agilent 2100 Bioanalyzer (Agilent Technologies, CA, USA).First-strand cDNA was synthesized after the total mRNA was extracted by using the high capacity RNA to cDNA kits (Applied Biosystems, California, USA). The expression of p53, Bid, Bax, and Bcl2 was assessed by quantitative real-time reverse transcriptase-polymerase chain reaction (PCR) using the StepOnePlus real-time PCR system (Applied Biosystems, California, USA). All the primers and probes for Taqman® real-time PCR were made by Applied Biosystems as shown in Table 1. The cycle conditions were 95.0°C for 20 min, followed by 40 cycles of 95.0°C for 1 min, and 60.0°C for 20 min. The comparison of Bax, Bcl2, and p53 expression levels between control and treated cells was performed using the comparative Ct (ΔΔCt) method, and was normalized using 18S RNA as an endogenous control.
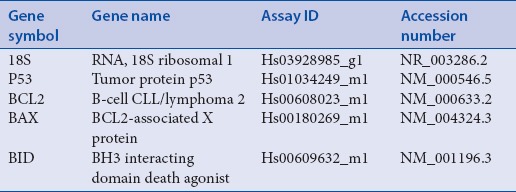
Table 1.
List of genes used in real time RT-PCR
Statistical analysis
Analysis at every time point from each experiment was carried out in triplicate. Means, standard errors, standard deviations, Student's paired t-test, and one-way ANOVA were calculated from replicates within the experiments, and analyses were done using SPSS version 16 (IBM SPSS Statistics, Armonk, NY, USA). Statistical significance was accepted at a level of P < 0.05.
RESULTS
Effect of Ocimum sanctum essential oil on Michigan cancer foundation-7 cell proliferation
In this study, we evaluated the antiproliferative effect of OSEO against human breast cancer cell line (MCF-7). As shown in Figure 1a, OSEO inhibited the proliferation of MCF-7 cells dose dependently with an IC50 value of 170 μg/mL. The oil showed better cytotoxic effect over resveratrol, a naturally occurring polyphenolic compound that induces apoptosis and inhibits the growth of several cancer cell lines.
Figure 1.
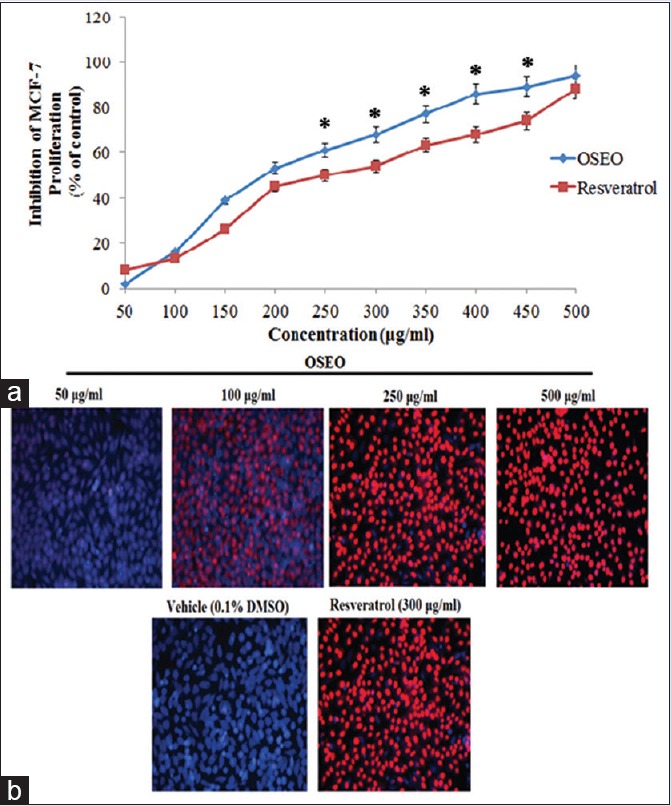
(a) The effect of OSEO on MCF-7 cells. Cell proliferation after 24 h of treatment with OSEO at indicated concentrations (50–500 μg/ml) was determined by MTT assay. (b) Propidium iodide and Hoechst 33342 staining of MCF-7 cells after 24 h treatment with OSEO (50–500 μg/ml). The control cells treated with vehicle (0.1% DMSO) and the cells treated with resveratrol (300 μg/ml) served as positive control. Fluorescence-stained apoptotic nucleic visualized at ×100 magnificence. The symbol “*” denotes that the data are statistically significant (P < 0.5) when compared to resveratrol. OSEO: Ocimum sanctum essential oil; MCF-7: Michigan cancer foundation-7
Effect of Ocimum sanctum essential oil on apoptosis
The antiproliferative activity of OSEO has raised the question whether inhibition of proliferation could be through the induction of apoptosis. Double staining with PI and Hoechst 33342 was performed to visualize the apoptosis in the MCF-7 cells. PI stain was used to visualize the apoptotic nuclei whereas the Hoechst 33342 stains the nuclei of live cells. Our results revealed that treatment with OSEO (50, 100, 250, and 500 μg/mL) showed a dose-dependent increase in the apoptotic nuclei (red color) and a dose-dependent decrease in the nuclei of live cells (blue color) [Figure 1b]. Cells treated with 250 μg/mL and 500 μg/mL of OSEO exhibited more apoptotic nucleic [Figure 1b] than the control cells treated with the vehicle (0.1% DMSO) and resveratrol (300 μg/mL). These results were further evidenced by the Annexin V-FITC flow cytometry analysis. The apoptotic cell population increased at 16%, 38%, 61%, and 84% with the treatment of 50, 100, 250, and 500 μg/mL of OSEO, respectively [Figure 2]. OSEO at a concentration of 250 μg/mL and above induced more apoptosis compared to the resveratrol (300 μg/mL) [Figure 2]. Our data strongly suggest that OSEO has anticancer potential by inducing apoptosis.
Figure 2.
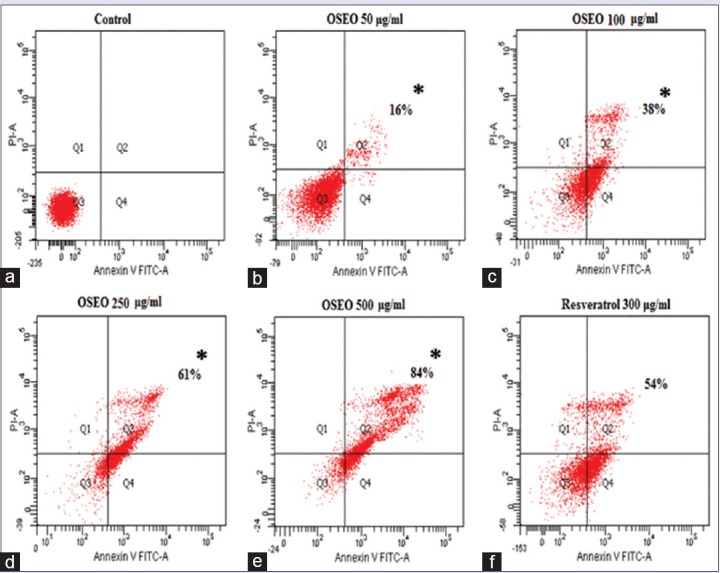
(a) The control cells treated with 0.1% DMSO showed no induction of apoptosis. The cells treated with OSEO showed gradual increased in percentage of apoptotic cells at concentration of (b) 50 μg/ml (16%), (c) 100 μg/ml (38%), (d) 250 μg/ml (61%), (e) 500 μg/ml (84%). (f) The resveratrol 300 μg/ml treated cells showed about 54% of apoptotic cells population. OSEO: Ocimum sanctum essential oil; MCF-7: Michigan cancer foundation-7; Annexin V-FITC: Annexin-V-fluorescein isothiocyanate; PI: Propidium iodide
Regulation of apoptotic genes by Ocimum sanctum essential oil
The effect of OSEO on the expression of genes involved in apoptotic pathway was studied. In the present study, the MCF-7 cells treated with essential oils increased the expression of p53 in a dose-dependent manner [Figure 3a]. Hence, apoptosis induced by OSEO on MCF-7 cells can be attributed to the induction of p53 expression. The OSEO also induced a dose-dependent increase in the expression of Bid [Figure 3a]. In this study, the ratio of Bax/Bcl-2 expression was significantly (**P < 0.001) elevated following treatment with OSEO [Figure 3b]. We postulate that increased Bax/Bcl-2 ratio by OSEO triggers the apoptosis mechanism.
Figure 3.
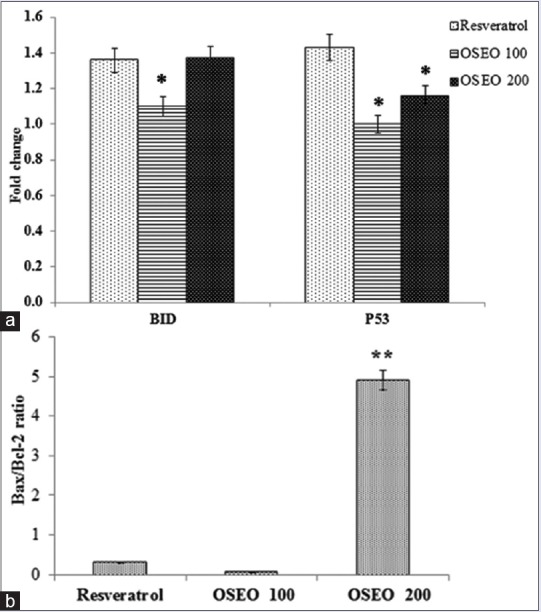
(a) Effect of OSEO on the expression of p53 and Bid. An up-regulation in the expression of gene p53 and Bid was observed following treatment with OSEO (100 and 200 μg/ml) and resveratrol (300 μg/ml). (b) An elevation of Bax/Bcl-2 ratio was observed in MCF-7 cells treated with OSEO compared to the resveratrol. Data are expressed as mean ± SD, n = 3. Statistical significance was calculated based on the mean ΔΔCT values by one-way ANOVA, **P < 0.001 versus resveratrol. OSEO: Ocimum sanctum essential oil; SD: Standard deviation
DISCUSSION
Cancer cells divide and grow uncontrollably, forming malignant tumors and invade nearby tissues of the body.[17] The goal of the most current cancer therapy is to prevent proliferation and accumulation of cancer cells. In the present study, OSEO showed a significant antiproliferative activity. A previous study has reported the anticancer potential of the crude extract of O. sanctum.[18]
The p53, Bid, and Bax/Bcl-2 expression levels were elevated in OSEO-treated cells which proved that O. sanctum oil is a potent anticancer agent. The BH3 interacting domain death agonist (Bid) is cleaved by caspase-8 and gets activated. It is translocating to mitochondria and induces the release of proapoptotic proteins such as cytochrome c, apoptosis-inducing factor, or Endo G. The release of cytochrome c by Bid is mediated by Bax. Bid facilitates the insertion of Bax into the mitochondrial membrane to form functional oligomers.[19] Indeed, activation of intracellular Bax component causes the elevation of mitochondrial outer membrane permeabilization, eventually increasing apoptotic cascade events. A recent study has proven that increased intracellular ratio of proapoptotic protein, Bax than the antiapoptotic protein, Bcl-2, stimulated the cellular apoptosis mechanism.[20] The p53 is an important tumor suppressor that functions by inducing cell cycle arrest, apoptosis, and DNA repair.[21] Recent findings proved that p53 arrests the proliferation of mutant and cancer cells by apoptosis.[16,22] To the best of our knowledge, this is the first study to report the antiproliferative activity of OSEO. This suggests that OSEO has the potential to be developed as a chemopreventive drug for the treatment of human breast cancer.
CONCLUSION
In our study, OSEO has the ability to inhibit the proliferation of MCF-7 cells by inducing apoptosis. OSEO was clearly far better than resveratrol in inducing apoptosis at a concentration of 200 µg/mL and above. About 84% of apoptosis was shown at the optimum concentration (500 µg/mL) of OSEO. Interestingly, OSEO up-regulated the expression of p53, Bid, and elevated the ratio of Bax/Bcl-2 that leads to apoptosis in MCF-7 cells. This is the first time OSEO has been reported to have anticancer and proapoptotic effects on MCF-7 cells. Thus, appropriate addition of OSEO in the diet may prevent human breast cancer.
Financial support and sponsorship
This research was supported by the High Impact Research Grant (HIR-MOHE, UM.C/625/1/HIR/E000043-20001) from the University of Malaya, Malaysia, and KASC Research Grant, India (5/2014).
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHOR

Dr. Ramaraj Thirugnanasampandan
Dr. Ramaraj Thirugnanasampandan, Assistant Professor, Department of Biotechnology, Kongunadu Arts and Science College, GN Mills, Coimbatore-641029, Tamil Nadu, India.
Acknowledgments
The authors would like to thank the Medical Lab Technologist, Mrs. NurFaezah for technical contributions in this study.
REFERENCES
- 1.Hisham AN, Yip CH. Spectrum of breast cancer in Malaysian women: Overview. World J Surg. 2003;27:921–3. doi: 10.1007/s00268-003-6976-x. [DOI] [PubMed] [Google Scholar]
- 2.Yip CH, Taib NA, Mohamed I. Epidemiology of breast cancer in Malaysia. Asian Pac J Cancer Prev. 2006;7:369–74. [PubMed] [Google Scholar]
- 3.Sethi J, Sood S, Seth S, Talwar A. Protective effect of tulsi (Ocimum sanctum) on lipid peroxidation in stress induced by anemic hypoxia in rabbits. Indian J Physiol Pharmacol. 2003;47:115–9. [PubMed] [Google Scholar]
- 4.Nweze EI, Eze EE. Justification for the use of Ocimum gratissimum L in herbal medicine and its interaction with disc antibiotics. BMC Complement Altern Med. 2009;9:37. doi: 10.1186/1472-6882-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Runyoro D, Ngassapa O, Vagionas K, Aligiannis N, Graikou K, Chinou I. Chemical composition and antimicrobial activity of the essential oils of four Ocimum species growing in Tanzania. Food Chem. 2010;119:311–6. [Google Scholar]
- 6.Harnafi H, Caid HS, Bouanani NH, Aziz M, Amrani S. Hypolipemic activity of polyphenol-rich extracts from Ocimum basilicum in Triton WR-1339-induced hyperlipidemic mice. Food Chem. 2008;108:205–12. [Google Scholar]
- 7.Kwee EM, Niemeyer ED. Variations in phenolic composition and antioxidant properties among 15 basil (Ocimum basilicum L.) cultivars. Food Chem. 2011;128:1044–50. [Google Scholar]
- 8.Carovic-Stanko K, Orlic S, Politeo O, Strikic F, Kolak I, Milos M, et al. Composition and antibacterial activities of essential oils of seven Ocimum taxa. Food Chem. 2010;119:196–201. [Google Scholar]
- 9.Prakash P, Gupta N. Therapeutic uses of Ocimum sanctum Linn (tulsi) with a note on eugenol and its pharmacological actions: A short review. Indian J Physiol Pharmacol. 2005;49:125–31. [PubMed] [Google Scholar]
- 10.Kumar A, Shukla R, Singh P, Dubey NK. Chemical composition, antifungal and antiaflatoxigenic activities of Ocimum sanctum L. essential oil and its safety assessment as plant based antimicrobial. Food Chem Toxicol. 2010;48:539–43. doi: 10.1016/j.fct.2009.11.028. [DOI] [PubMed] [Google Scholar]
- 11.Amber K, Aijaz A, Immaculata X, Luqman KA, Nikhat M. Anticandidal effect of Ocimum sanctum essential oil and its synergy with fluconazole and ketoconazole. Phytomedicine. 2010;17:921–5. doi: 10.1016/j.phymed.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 12.Khan A, Ahmad A, Akhtar F, Yousuf S, Xess I, Khan LA, et al. Ocimum sanctum essential oil and its active principles exert their antifungal activity by disrupting ergosterol biosynthesis and membrane integrity. Res Microbiol. 2010;161:816–23. doi: 10.1016/j.resmic.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Kumar A, Dubey NK, Srivastava S. Antifungal evaluation of Ocimum sanctum essential oil against fungal deterioration of raw materials of Rauvolfia serpentina during storage. Ind Crop Prod. 2013;45:30–5. [Google Scholar]
- 14.Manaharan T, Thirugnanasampandan R, Jayakumar R, Ramya G, Ramnath G, Kanthimathi MS. Antimetastatic and anti-inflammatory potentials of essential oil from edible Ocimum sanctum leaves. ScientificWorldJournal 2014. 2014:239508. doi: 10.1155/2014/239508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu JQ, Lei JC, Zhang XQ, Yu HD, Tian DZ, Liao ZX, et al. Anticancer, antioxidant and antimicrobial activities of the essential oil of Lycopus lucidus Turcz. var. Hirtus Regel Food Chem. 2011;126:1593–8. doi: 10.1016/j.foodchem.2010.12.027. [DOI] [PubMed] [Google Scholar]
- 16.Boeckler FM, Joerger AC, Jaggi G, Rutherford TJ, Veprintsev DB, Fersht AR. Targeted rescue of a destabilized mutant of p53 by an in silico screened drug. Proc Natl Acad Sci U S A. 2008;105:10360–5. doi: 10.1073/pnas.0805326105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anand P, Kunnumakkara AB, Sundaram C, Harikumar KB, Tharakan ST, Lai OS, et al. Cancer is a preventable disease that requires major lifestyle changes. Pharm Res. 2008;25:2097–116. doi: 10.1007/s11095-008-9661-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aruna K, Sivaramakrishnan VM. Anticarcinogenic effects of some Indian plant products. Food Chem Toxicol. 1992;30:953–6. doi: 10.1016/0278-6915(92)90180-s. [DOI] [PubMed] [Google Scholar]
- 19.Kim TH, Zhao Y, Barber MJ, Kuharsky DK, Yin XM. Bid-induced cytochrome c release is mediated by a pathway independent of mitochondrial permeability transition pore and Bax. J Biol Chem. 2000;275:39474–81. doi: 10.1074/jbc.M003370200. [DOI] [PubMed] [Google Scholar]
- 20.Yoon O, Roh J. Downregulation of KLF4 and the Bcl-2/Bax ratio in advanced epithelial ovarian cancer. Oncol Lett. 2012;4:1033–6. doi: 10.3892/ol.2012.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levine AJ, Oren M. The first 30 years of p53: Growing ever more complex. Nat Rev Cancer. 2009;9:749–58. doi: 10.1038/nrc2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lehmann BD, Pietenpol JA. Targeting mutant p53 in human tumors. J Clin Oncol. 2012;30:3648–50. doi: 10.1200/JCO.2012.44.0412. [DOI] [PubMed] [Google Scholar]


