Abstract
Background:
Caesalpinia gilliesii Hook is an ornamental shrub with showy yellow flowers. It was used in folk medicine due to its contents of different classes of secondary metabolites. In our previous study, dichloromethane extract of C. gilliesii flowers showed a good antioxidant activity.
Aim of the Study:
Isolation and identification of bioactive hepatoprotective compounds from C. gilliesii flowers dichloromethane fraction.
Materials and Methods:
The hepatoprotective activity of dichloromethane fraction and isolated compounds were studied in CCl4-intoxicated rat liver slices by measuring liver injury markers (alanine aminotransferase, aspartate aminotransferase and glutathione [GSH]). All compounds were structurally elucidated on the basis of electron ionization-mass spectrometry, one- and two-dimensional nuclear magnetic resonance.
Results:
A new 12,13,16-trihydroxy-14(Z)-octadecenoic acid was identified in addition to the known β-sitosterol-3-O-butyl, daucosterol, isorhamnetin, isorhamnetin-3-O-rhamnoside, luteolin-7,4’-dimethyl ether, genistein-5-methyl ether, luteolin-7-O-rhamnoside, isovanillic acid, and p-methoxybenzoic acid. Dichloromethane fraction and isorhamnetin were able to significantly protect the liver against intoxication. Moreover, the dichloromethane fraction and the isolated phytosterols induced GSH above the normal level.
Conclusion:
The hepatoprotective activity of C. gilliesii may be attributed to its high content of phytosterols and phenolic compounds.
SUMMARY
Bioactive Hepatoprotective phytosterols and phenolics from chloroform extract of Caesalpinia gilliesii
Abbreviations used: ALT: Alanine Aminotransferase; AST: Aspartate aminotransferase; GSH: Glutathione; SC50: Scavenging Capacity 50 (SC 50); COSY: Correlation spectroscopy; NMR: Nuclear Magnetic Resonance; CC: Column chromatography; EI-MS: Electron-impact mass spectrometry; HSQC: Heteronuclear single-quantum correlation.
Keywords: Caesalpinia, fatty acid, hepatoprotective activity, isorhamnetin, liver injury markers (alanine aminotransferase, aspartate aminotransferase and glutathione), octadecenoic acid, plant phenolics
INTRODUCTION
Liver cell injury through various toxic chemicals, excessive alcohol consumption, and viral infections is well documented. A potential liver damage is elicited by complex mechanisms involving free radicals inducing oxidative stress which can lead to peroxidation, DNA damage, and inflammation.[1] The hepatotoxicity of CCl4 is due to the metabolic formation of the highly reactive trichloromethyl radical which attacks polyunsaturated fatty acids of the endoplasmic reticulum membrane resulting in a loss of cytochrome p450 leading to its functional failure with a decrease in protein modification and accumulation of triglycerides.[2] Antioxidants, which mop up free radicals, can reduce that process. Around 170 phytoconstituents isolated from 110 plants have been reported to possess strong antioxidant[3] and hepatoprotective activities such as Silybum marianum,[4] Tridax procumbens,[5] Strychnos potatorum,[6] and Andrographis paniculata.[7]
The Caesalpinioideae (family Fabaceae) represent approximately 11% of all legume taxa with more than 2250 mostly tropical and subtropical trees and shrubs. The genus Caesalpinia, which comprises more than forty mostly tropical trees and shrubs, are widely used in folk medicine.[8] For example, Caesalpinia bonducella seeds eliminate the symptoms of diabetes mellitus.[9] Genus Caesalpinia was also reported to possess anticancer,[10] antioxidant, and hepatoprotective properties.[11] Caesalpinia gilliesii Hook (known as Yellow Bird of Paradise) is an ornamental shrub with showy yellow flowers [Figure 1]. The plant is native to Argentina but has been cultivated worldwide. Caesalpinia was reported to contain different classes of secondary metabolites include terpenoids, flavonoids, and phenolics.[8,12,13] Since oxidative stress is one of the main causes of liver toxicity, agents with the ability to protect the liver against reactive pro-oxidant species may be therapeutically useful. This is true for several polyhydroxylated flavonoids, which have already been shown to be hepatoprotective, as in the case of catechins[14] and quercetin.[15] Dihydrobonducellin and 2-methoxy dihydro-bonducellin, isolated from Caesalpinia pulcherrima, showed radical scavenging activity that were better than the positive antioxidants controls with SC50 values of 352 μM and 325 μM, respectively, compared with vitamin C (SC50 = 852 μM).[16]
Figure 1.

Caesalpinia gilliesii flowers (x = 1/4)
However, the dichloromethane extract of C. gilliesii flowers showed antioxidant activity (SC50 = 45.5 μg/mL comparable to standard rutin SC50: 24 µg/ml).[11] In this context and in continuation of our previous work on C. gilliesii,[17] this study was designed to evaluate the in vitro hepatoprotective activity of the dichloromethane fraction of C. gilliesii flowers. In addition, a detailed phytochemical investigation of that fraction was carried out to isolate and identify its bioactive compounds. The hepatoprotective activity of the isolated compounds was also evaluated.
MATERIALS AND METHODS
General experimental procedures
1H and 13C nuclear magnetic resonance (1H NMR) spectra were measured on a Varian 300 MHz and Bruker 400 MHz AC NMR spectrometers. Based on their solubility, samples were dissolved in different deuterated solvents Deutero® (Kastellaun, Germany). Electron ionization-mass spectrometry (EI-MS) spectra were recorded on Thermo Scientific, Trace gas chromatograph Ultra coupled with ISQ Single Quadruple MS Capillary column (National Research Center, Egypt). Two-dimensional (2D) NMR experiments (double quantum filter correlated spectroscopy [COSY], heteronuclear single-quantum correlation spectroscopy [HSQC]) were carried out with all isolated compounds using the pulse sequences from the Varian and Bruker user library. On the basis of 2D-NMR analyses, assignments of 1H and 13C signals were established. Column chromatography (CC) was carried out on silica gel 60 (0.063–0.2 mm) (Sigma-Aldrich Co., USA) and Sephadex LH-20 (25–100 mm) (Sigma-Aldrich Co., USA) using mixtures of dichloromethane and methanol with different ratios for silica gel 60 and 100% methanol for Sephadex LH-20. Fractions were monitored by thin layer chromatography on precoated silica gel 60 F254 (0.25 mm) (Merck®, Darmstadt, Germany) using either p-anisaldehyde/sulfuric acid spray reagent and heating at 100°C for 7 min, NH3 or 5% AlCl3 reagent.[18]
Plant material
C. gilliesii flowers were collected from Borg El Arab (Egypt) in May 2013 [Figure 1]. The plant identity was kindly confirmed by Prof. Abd El-halem A. El-Meged (Agriculture Research Center, Cairo, Egypt). A voucher specimen (CP# 1203) is kept at the Department of Pharmacognosy, Faculty of Pharmacy, October 6 University.
Chemicals
Chemicals and solvents were of analytical grade unless mentioned and were purchased from Merck® (Darmstadt, Germany), J.T. Backer® (Deventer, The Netherlands) and Theo Seulberger® (Karlsruhe, Germany). Media and supplements for cell cultures were obtained from Gibco® (Karlsruhe, Germany) and Greiner Labortechnik® (Frickenhausen, Germany).
Extraction and isolation
Freshly collected plant flowers were washed 3 times with running tap water and then with distilled water followed by drying in shade for 8 days. A hydroalcoholic (70%) extract of flowers was prepared by powdering 500 g then defatted by n-hexane (2 L × 3 times). The solvent was filtered, and cake was exhaustively macerated with 70% ethanol. Ethanol was filtered and evaporated under reduced pressure yielding 60 g (12%) of hydroalcoholic extract. Subsequently, 55 g dried C. gilliesii flower extract was suspended in 500 ml distilled water and sonicated for 30 min. The extract was fractionated using solvent–solvent extraction with n-hexane (6 × 1 L), dichloromethane (6 × 1 L). The resulting fractions were then dried under reduced pressure in a rotary evaporator (Sineco Technology Co. Ltd, Shanghai, China).
The dichloromethane residue (1.5 g) was subjected to silica gel CC (2 cm × 50 cm, 120 g) using CH2Cl2:CH3 OH as eluents with different ratios in increasing polarity manner to give six fractions (I-VI). In our previous work,[17] Fraction I (100 mg) that eluted with CH2Cl2 (100%) was purified to give compound 1 (20 mg). However, Fraction II (200 mg) that eluted with CH2Cl2:CH3 OH (90:10) gave compound 2 (20 mg) after being further separated on silica gel CC. Fraction III (450 mg) that eluted with CH2Cl2:CH3 OH (75:25) was further chromatographed on silica gel CC with the same mobile phase to give subtractions 1–15. Subfractions 1 and 2 were pure compounds 3, 4 (25 and 15 mg, respectively). In continuation; subfractions 3–7 of that fraction were collected together afforded compound 5 (30 mg). Subfractions 10–15 were collected together afforded compound 6 (55 mg). Fraction IV (50 mg) that eluted with CH2Cl2:CH3 OH (50:50) was further purified afforded compound 7 (5 mg). Fraction V (200 mg) that eluted with CH2Cl2:CH3 OH (25:75), was further chromatographed on silica gel CC with the same mobile phase afforded compound 8 (40 mg). Fraction VI (400 mg) that eluted with 100% CH3 OH was further chromatographed on Sephadex LH 20 CC afforded compound 9 and 10 (5 mg and 30 mg, respectively). All isolated compounds were evaporated under reduced pressure and subjected to further purification on Sephadex LH-20 CC (1 cm × 40 cm, 15 g) using 100% methanol as mobile phase before being analyzed.
Compound 5
Rosette crystals sprayed with p-anisaldehyde to give a dark blue color indicative for its phytosterol nature. EI-MS (m/z) 330 [M], 312 [M-H2O], 294 [M-2 H2O], 287 [M-CO2]+, 276 [M-3H2O]+, 244 [M-C5H10O], 184 [M-C7H14O3], 147 [M-C11H22O2]+, and 86 [M-C13H24O4]. 1H NMR (400 MHz, CD3 OD): δ (ppm), J (Hz) 7.04 (s, OH), 5.69 (2H, t, J = 8 Hz, H-14, 15), 4.05 (1H, m, H-16), 3.89 (1H, t, J = 8 Hz, H-13), 3.40 (1H, m, H-12), 2.26 (2H, t, J = 8 Hz, H-2), 1.60–1.50 (6H, m, H-3, 11, 17), 1.31 (14H, remaining H-4, 5, 6, 7, 8, 9, 10), 0.89 (3H, t, J = 8 Hz, H-18). 13C NMR DEPT Q (100 MHz, CD3 OD) 177.71 (C = O), 34.95 (CH2-2), 23.73 (CH2-3), 26.83 (CH2-4), 30.21 (CH2-5), 30.38 (CH2-6), 30.64 (CH2-7), 33.00 (CH2-8), 136.52 (CH-15), 130.94 (CH-14), 38.32 (CH2-11), 73.07 (CH-12), 75.76 (CH-13), 26.24 (CH2-9), 33.51 (CH2-10), 76.53 (CH-16), 26.08 (CH2-17), 14.41 (CH3-18).
Hepatoprotective protocol
Hepatoprotective assays were carried out following the protocols developed by Wormser.[19] After cervical dislocation of the normal rats, liver lobes were removed and transferred to prewarmed Krebs Ringer Hepes (KRH) (Hepes 2.5 mM pH 7.4, NaCl 118 mM, KCl 2.85 mM, CaCl2 2.5 mM, KH2PO4 1.5 mM, MgSO4 1.18 mM, and glucose 4.0 mM). The liver was then cut into thin slices using sharp scalpel blades. The slices were weighed, and slices weighing between 4 and 6 mg were used for the experiment. Each experiment consisted of 20–22 slices weighing together 100–120 mg. These slices were washed with 10 ml KRH medium, every 10 min over a period of 1 h. These were then preincubated for 60 min in small plugged beakers containing 2 ml KRH on a shaker water bath at 37°C. After incubation period, the slices were divided into groups according to the experimental design; control group were left in the medium without any additives, CCl4 group was treated with CCl4 (15.5 mM) alone, the silymarin group plus CCl4 (15.5 mM) and silymarin (100 µg/ml), other experimental groups contained CCl4 (15.5 mM) plus examined fraction or isolated compounds (100 µg/ml). All the groups were incubated at 37°C for 24 h., and then the supernatant of each group was collected separately and kept at −20°C till further investigations.
Biochemical assays
Reduced glutathione (GSH) is the major intracellular low-molecular-weight thiol that plays a critical role in the cellular defense against oxidative stress in mammalian cells. BioVision's ApoGSH™ Glutathione Colorimetric Assay Kit provides a convenient colorimetric method for analyzing either total glutathione or the reduced form glutathione alone using a microtiter plate reader. Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) serum concentration is elevated in hepatic disease associated with necrosis, cholestasis, cirrhosis, liver carcinoma, and after administration of various drugs. The activities of serum AST and ALT were assayed according to Reitman and Frankel.[20]
Data analysis
All biological experiments were repeated at least 6 times. Data are presented as mean ± standard error of the mean. The IC50 values were calculated using a four parameter logistic curve (SigmaPlot1 11.0, Systat Software, Inc., Richmond, California, USA) and data were drawn and statistically analyzed using Student's t-test using GraphPad Prism1 5.0 (GraphPad Prism Software, Inc., San Diego, USA). The criterion for statistical significance was taken as P < 0.05.
RESULTS AND DISCUSSION
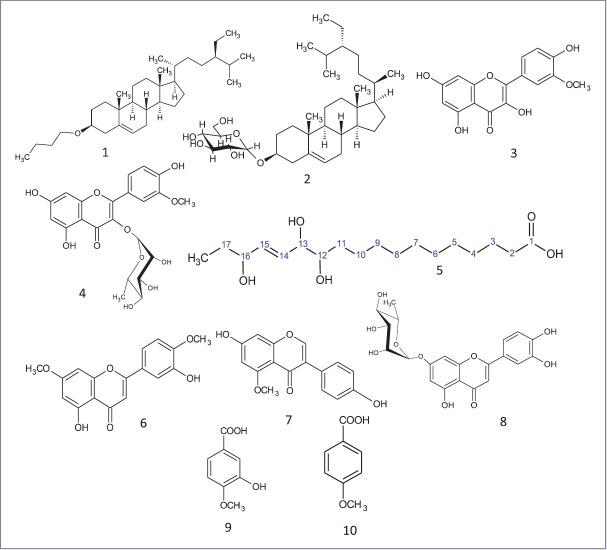
Our previous study had resulted in the isolation of four compounds β-sitosterol-3-O-butyl (1), daucosterol (2), isorhamnetin (3), and isorhamnetin-3-O-rhamnoside (4).[17] A consecutive phytochemical investigation of the dichloromethane fraction from flowers using CC resulted in the isolation of a new 12,13,16-trihydroxy-14(Z)-octadecenoic acid (5), furthermore luteolin-7,4’-dimethyl ether (6), genistein-5-methyl ether (7), luteolin-7-O-rhamnoside (8), isovanillic acid (9), and p-methoxy benzoic acid (10) were isolated [Figure 2]. Identification of all known compounds was achieved by comparing their spectral data with that published.[21,22]
Figure 2.
1) 3-O-butyl- β- sitosterol, 2) daucosterol, 3) isorhamnetin,4) isorhamnetin-3-O-rhamnoside, 5) 12,13,16-trihydroxy-14(Z)-octadecenoic acid, 6) 5,3’-dihydroxy-7,4’ dimethoxyflavone, 7) genistein-5-methyl ether, 8) Luteolin-7-O-rhamnoside, 9) Isovanillic acid and 10) p- methoxy benzoic acid
Compound (5) gives a dark blue color with p-anisaldehyde indicative for its phytosterol nature. EI-MS spectral analysis [Figure 3] showed a molecular weight of m/z 330 [C18H34O5] and illustrated by 13C NMR DEPT Q spectrum of 18 carbon signals accounting for 1 CH3, 11 CH2, 5 CH groups and 1 quaternary carbon atoms. Other molecular ions peaks at m/z 312 [M-H2O], 294 [M-2 H2O], and 277 [M-3 H2O]+ suggested for trihydroxy groups[23] that confirmed by three downfield proton signals at δ (ppm) 4.05 (1H, m), 3.89 (1H, t, J = 8 Hz), 3.40 (1H, m) and appeared at 73.07, 75.76, and 76.53 in 13C NMR DEPT Q meaning three CH-oxygenated carbons. Other m/z 287 [M-CO2]+ revealed the presence of carboxyl moiety illustrated by one signal at 177.71 ppm in 13C DEPT Q spectra. Cis olefinic CH carbons appeared in 1H NMR spectrum at δ (ppm) 5.69 (2H, t, J = 8 Hz) and at 136.52 130.94 in 13C DEPT Q spectra.[24] It showed also signal at 2.26 (2H, t, J = 8 Hz) characteristic for α CH2 of carboxyl group, signals at 1.50–1.60 (6H, m, 3CH2) were assigned to be 1 β CH2 of carboxyl group and 2 other α CH2 of oxygenated carbon.[25] Signals at 1.31 (14 H) and appeared from 38.32–23.73 in 13C DEPT Q spectra were for 11 CH2 carbons.[26] Signal at 0.89 (3H, t, J = 8 Hz) and 14.41 in 1H NMR and 13C DEPT Q spectra, respectively, were assigned for terminal methyl group. The hydroxyl and unsaturation positions were assigned based on their correlations in COSY and HSQC spectrum. Hence, compound 5 was identified to be the new natural product 12,13,16-trihydroxy-14(Z)-octadecenoic acid.
Figure 3.
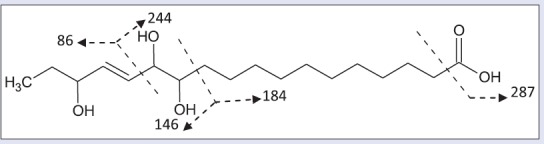
Mass fragmentation of 12, 13, and 16-trihydroxy-14(Z)- octadecenoic acid
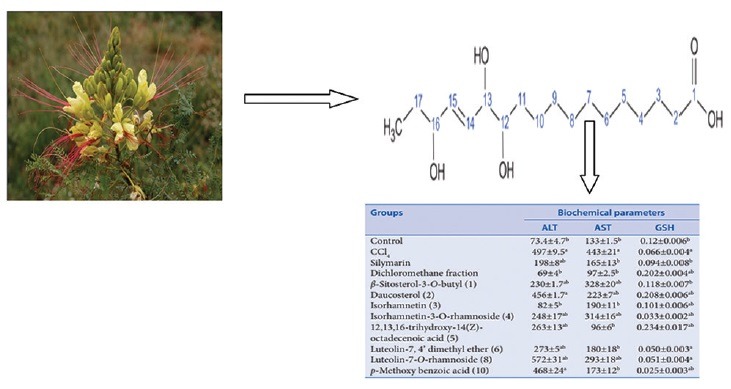
The liver is involved in almost all the biochemical pathways, and it functions as a center of metabolism of carbohydrates, proteins, lipids, and excretion of waste metabolites. Silymarin, a flavonolignan mixture from S. marianum, is used as standard hepatoprotective compound since it was reported to have a protective effect on the plasma membrane of the hepatocytes, and clinical studies have shown its efficacy in patients with Amanita poisoning and liver cirrhosis.[2] The hepatotoxicity of CCl4 is due to its active metabolite, trichloromethyl radical, which attacks macromolecules inducing degradation of biomembranes of the endoplasmic reticulum.[2] Liver injury parameters including ALT and AST were studied, which reflect the condition of hepatocytes and inflammatory destruction. A significant (P < 0.0001) elevation of the biochemical parameters ALT and AST in the CCL4 group (497 ± 9, 443 ± 21 U/L, respectively) compared to the control group (73.4 ± 4.7, 133 ± 1.5U/L, respectively) was detected. Liver slices (hepatocytes) that were treated with standard silymarin were protected considerably against the toxicity indicated by the level of the biochemical parameters (198 ± 8, 165 ± 13 U/L, respectively) comparable to CCl4 group (P < 0.001). The enzyme levels in liver slices treated with the dichloromethane fraction (69 ± 4, 97 ± 2.5 U/L, respectively) were similar to that of the control and silymarin group (P > 0.05) [Table 1].
Table 1.
Effect of the dichloromethane fraction of C. gilliesii flowers and its isolated compounds on biochemical parameters of CCl4-intoxicated rat liver slices (values are mean±SEM of 6 replicates)
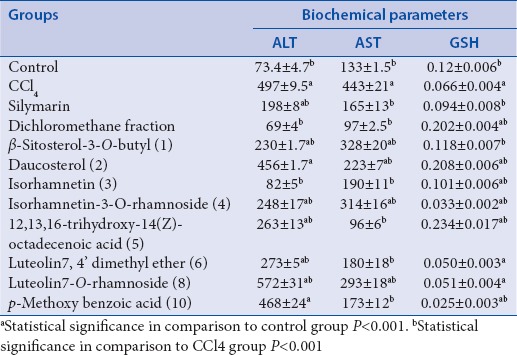
Isorhamnetin showed a significant protection as compared to CCl4 group (P < 0.001) and nonsignificant to control group. Our previous study[17] had suggested that because isorhamnetin has potent antioxidant activity, it might be promising as hepatoprotective agent. In our present study, we found that the sugar affects that activity as glycosides residue has a low influence on hepatoprotective activity. Furthermore, compounds 1 and 2 exhibited a significant reduction for ALT and AST as compared to CCl4 group (P < 0.001) but less active than isorhamnetin [Figure 4].
Figure 4.
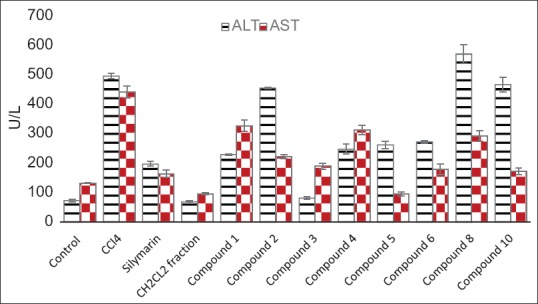
Hepatoprotective effects of the dichloromethane fraction of C. gilliesii flowers and its isolated compounds indicated by ALT and AST(U/L) activities (values are mean± SEM of 6 replicates)
The GSH level was decreased in the CCl4 group (0.066 ± 0.004 mmol/L), which reached about half its value as compared to control group (0.12 ± 0.006 mmol/L). Liver slices that were treated with standard silymarin were protected considerably against reduction in GSH level (0.094 ± 0.008 mmol/L) when compared with the CCl4 group (P < 0.001). Treatment with the dichloromethane fraction significantly increased GSH above its normal level (0.202 ± 0.004 mmol/L) (P < 0.001). The isolated new compound 5 and compound 2 also increased GSH above its normal level (0.234 ± 0.017, 0.208 ± 0.006 mmol/L, respectively) (P < 0.001) [Figure 5]. It is likely that the phytosterols (compound 5 and 2) interact with the biomembranes by forming a complex with cholesterol[27] while flavonoids, in general, are nonselective inhibitors of many enzymes and other proteins. Under normal physiological conditions, the polyphenols dissociate into negatively charged phenolate ions, which can interact with positively charged function groups of different enzymes and proteins in the cell through ionic and hydrogen bonds, which dramatically inhibit their 3D structure and, in consequence, their functions.[27] These properties might explain the hepatoprotective effects seen in this study. Based on these results, we conclude that the hepatoprotective activity of C. gilliesii may be attributed to its high content of phytosterols and phenolic compounds.[28] It may become a lead in the future for the development of hepatoprotective drug. However, animal experiments must still show its efficacy under in vivo conditions.
Figure 5.
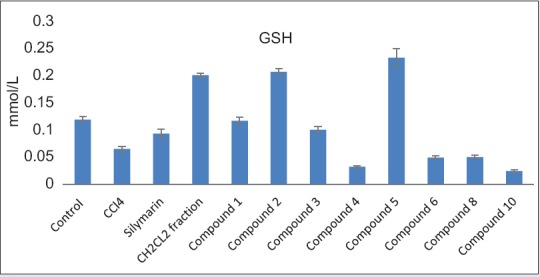
Effect of the dichloromethane fraction of C. gilliesii flowers and its isolated compounds on reduced glutathione (GSH mmol/L) of CCl4- intoxicated rats hepatocytes (values are mean ± SEM of 6 replicates)
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHOR

Dr. Michael Wink
Prof. Dr. Michael Wink, Head of the Institute of Pharmacy and Molecular Biotechnology (IPMB), Heidelberg University, Germany.
Research Scopes:
I. Active natural products biotechnology
II. Molecular evolution
Bibliometrics (March 2016):
Google Scholar: h-index = 70 (h = 44 since 2010), 24190 total citations
Web of Knowledge: 655 ISI-indexed publications; total number of citations = 12750; H-Index = 50
Research Gate: 804 publications; 1194 Impact points, R6Score 48.30, 35.500 reads
REFERENCES
- 1.Järveläinen H. Inflammatory Responses in Alcoholic Liver Diseases. Ph.D. Thesis- University of Helsinki. 2000:1–75. [Google Scholar]
- 2.Srilakshmi VS, Vijayan P, Raj PV, Dhanaraj SA, Chandrashekhar HR. Hepatoprotective properties of Caesalpinia sappan Linn. heartwood on carbon tetrachloride induced toxicity. Indian J Exp Biol. 2010;48:905–10. [PubMed] [Google Scholar]
- 3.Maheshwari DT, Yogendra Kumar MS, Verma SK, Singh VK, Singh SN. Antioxidant and hepatoprotective activities of phenolic rich fraction of Seabuckthorn (Hippophae rhamnoides L.) leaves. Food Chem Toxicol. 2011;49:2422–8. doi: 10.1016/j.fct.2011.06.061. [DOI] [PubMed] [Google Scholar]
- 4.Flora K, Hahn M, Rosen H, Benner K. Milk thistle (Silybum marianum) for the therapy of liver disease. Am J Gastroenterol. 1998;93:139–43. doi: 10.1111/j.1572-0241.1998.00139.x. [DOI] [PubMed] [Google Scholar]
- 5.Ravikumar V, Shivashangari KS, Devaki T. Hepatoprotective activity of Tridax procumbens against d-galactosamine/lipopolysaccharide-induced hepatitis in rats. J Ethnopharmacol. 2005;101:55–60. doi: 10.1016/j.jep.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 6.Pramyothin P, Ngamtin C, Poungshompoo S, Chaichantipyuth C. Hepatoprotective activity of Phyllanthus amarus Schum. et. Thonn extract in ethanol treated rats: In vitro and in vivo studies. J Ethnopharmacol. 2007;114:169–73. doi: 10.1016/j.jep.2007.07.037. [DOI] [PubMed] [Google Scholar]
- 7.Pramyothin P, Udomuksorn W, Poungshompoo S, Chaichantipyuth C. Hepatoprotective effect of Andrographis paniculata and its constituent, andrographolide on ethanol hepatotoxicity in rats. Asia Pac J Pharmacol. 1994;9:73–8. [Google Scholar]
- 8.Wink M. Evolution of secondary metabolites from an ecological and molecular phylogenetic perspective. Phytochemistry. 2003;64:3–19. doi: 10.1016/s0031-9422(03)00300-5. [DOI] [PubMed] [Google Scholar]
- 9.Rao V, Dwivedi SK, Swarup D. Hypoglycaemic effect of Caesalpinia bonducella in rabbits. Fitoterapia. 1994;65:245–7. [Google Scholar]
- 10.Nakamura ES, Kurosaki F, Arisawa M, Mukainaka T, Okuda M, Tokuda H, et al. Cancer chemopreventive effects of constituents of Caesalpinia ferrea and related compounds. Cancer Lett. 2002;177:119–24. doi: 10.1016/s0304-3835(01)00708-x. [DOI] [PubMed] [Google Scholar]
- 11.Osman SM, Alazzouni AS, Abd El-Khalek SM, Koheil MA, EL-Haddad AE. Phytoconstituents and biological activities of the aerial parts of Caesalpinia gilliesii growing in Egypt. Life Sci J. 2013;10:2418–30. [Google Scholar]
- 12.Wink M. Evolution of secondary metabolites in legumes (Fabaceae) S Afr J Bot. 2013;89:164–75. [Google Scholar]
- 13.Wu M, Wang YF, Zhang ML, Huo CH, Dong M, Shi QW, et al. Chemical constituents of plants from the genus Caesalpinia. Chem Biodivers. 2011;8:1370–99. [Google Scholar]
- 14.Kagaya N, Tagawa Y, Nagashima H, Saijo R, Kawase M, Yagi K. Suppression of cytotoxin-induced cell death in isolated hepatocytes by tea catechins. Eur J Pharmacol. 2002;450:231–6. doi: 10.1016/s0014-2999(02)02157-x. [DOI] [PubMed] [Google Scholar]
- 15.Gilani AH, Janbaz KH, Shah BH. Quercetin exhibits hepatoprotective activity in rats. Biochem Soc Trans. 1997;25:S619. doi: 10.1042/bst025s619. [DOI] [PubMed] [Google Scholar]
- 16.Zanin JL, de Carvalho BA, Martineli PS, dos Santos MH, Lago JH, Sartorelli P, et al. The genus Caesalpinia L. (Caesalpiniaceae): Phytochemical and pharmacological characteristics. Molecules. 2012;17:7887–902. doi: 10.3390/molecules17077887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osman SM, Abd El-Khalek SM, Koheil MA, EL-Haddad AE, Wink M. A new steroidal compound (β-sitosterol-3-O-butyl) isolated from Caesalpinia gilliesii flowers. Int J Appl Res Nat Prod. 2015;8:14–9. [Google Scholar]
- 18.Wagner H. Berlin: Springer; 1996. Plant Drug Analysis: A Thin Layer Chromatography Atlas. [Google Scholar]
- 19.Wormser U, Ben-Zakine S. The liver slice system: An in vitro acute toxicity test for assessment of hepatotoxins and their antidotes. Toxicol Vitr. 1990;4:449–51. doi: 10.1016/0887-2333(90)90098-e. [DOI] [PubMed] [Google Scholar]
- 20.Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 21.Mabry TJ, Markham KR, Thomas MB. Berlin: Springer Verlag; 1970. The Systematic Identification of Flavonoids. [Google Scholar]
- 22.Markham KR. London: Academic Press; 1982. Techniques of Flavonoid Identification. [Google Scholar]
- 23.Hou CT, Gardner H, Brown W. Production of polyhydroxy fatty acids from linoleic acid by Clavibacter sp. ALA2. J Am Oil Chem Soc. 1998;75:1483–7. [Google Scholar]
- 24.Rakoff H, Weisleder DE. 13C nuclear magnetic resonance spectroscopy of cyanolipids and cyanolipid-containing seed oils. Lipids. 1978;14:81–3. [Google Scholar]
- 25.Knothe G, Kenar J. Determination of the fatty acid profile by 1H NMR spectroscopy. Eur J Lipid Sci Technol. 2004;106:88–96. [Google Scholar]
- 26.Tulloch AP, Mazurek M. 13C nuclear magnetic resonance of mono-and dihydroxy saturated and unsaturated fatty methyl esters. Lipids. 1974;11:228–34. [Google Scholar]
- 27.Wink M. Evolutionary advantage and molecular modes of action of multi-component mixtures used in phytomedicine. Curr Drug Metab. 2008;9:996–1009. doi: 10.2174/138920008786927794. [DOI] [PubMed] [Google Scholar]
- 28.Miyake T, Shibamoto T. Antioxidative activities of natural compounds found in plants. J Agric Food Chem. 1997;45:1819–22. [Google Scholar]