Abstract
Background:
Protein–ligand interaction plays a major role in identification of the possible mechanism by which a ligand can bind with the target and exerts the pharmacological action.
Objective:
The aim is to identify the best candidate of Cocculus hirsutus which binds with the hepatocellular carcinoma (HCC) targets by docking studies.
Materials and Methods:
The reported phytoconstituents such as coclaurine, hirsutine, cohirsine, cohirsinine, lirioresinol, cohirsitinine, haiderine, jamtinine, isotrilobine, shaheenine, jamtine, and cocsoline present in the plant, C. hirsutus were docked with the HCC targets such as Aurora kinase, c-Kit, fibroblast growth factor, nuclear factor kappa B (NF-kB), B-cell lymphoma-extra large, and vascular endothelial growth factor (VEGF) using in silico technique with the software Grid-Based Ligand Docking with Energies.
Results:
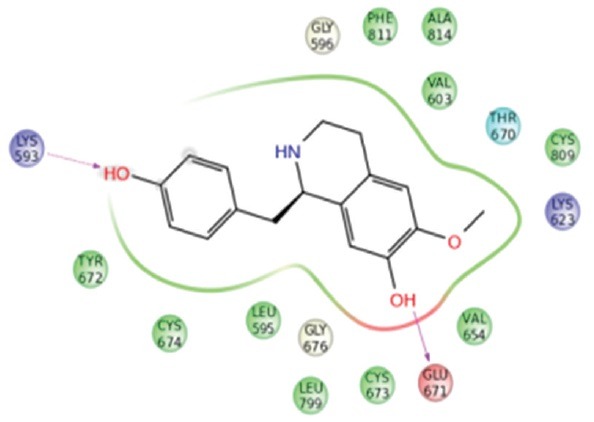
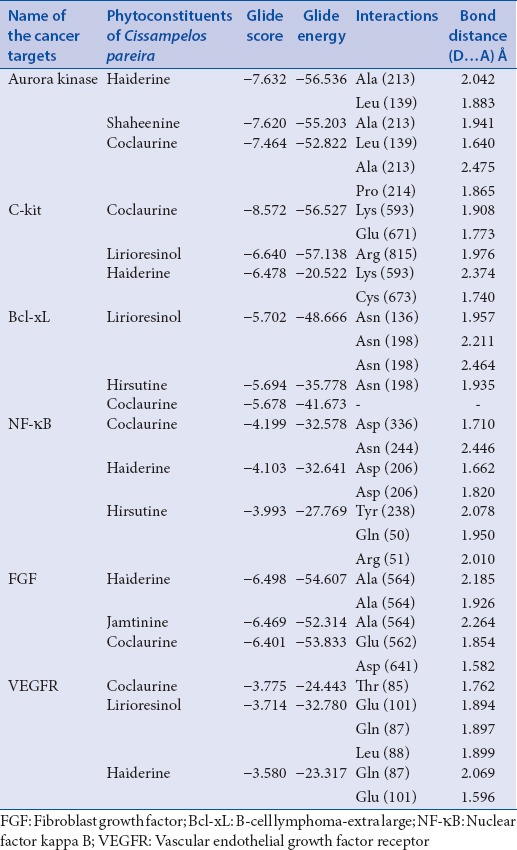
Haiderine, shaheenine, and coclaurine had good interaction with Aurora kinase with the glide score and glide energy of − 7.632, −7.620, −7.464; and − 56.536, −55.203, −52,822, respectively. Coclaurine, lirioresinol, and haiderine possess good binding with c-Kit with the glide score and glide energy of − 8.572, −6.640, −6.478; and − 56.527, −57.138, −20,522, respectively. Lirioresinol, hirsutine, and coclaurine exhibit good binding with c-Kit with the glide score and glide energy of − 5.702, −5.694, −5.678; and − 48.666, −35.778, −41,673, respectively. Similarly, coclaurine, haiderine, and hisutine had good interaction with NF-kB. Haiderine, jamtinine, and coclaurine had good binding with VEGF receptors (VEGFR) and coclaurine, lirioresinol, and haiderine exhibit good bonding with VEGFR.
Conclusion:
Coclaurine, haiderine, and lirioresinol exibited good hydrogen bonding interactions and binding energy with the select targets. Hence, these compounds have to be taken up for experimental work against hepatocellular carcinoma.
SUMMARY
Compounds of interest showed good interaction and binding with the selected targets. Hence these compounds has to be explored further to study their anticancer potentials.
Abbreviations used: HCC: Hepatocellular Carcinoma, Bcl-xL: B-cell lymphoma-extra large, FGF: Fibroblast Growth Factor, VEGF: Vascular Endothelial Growth Factor, DLA: Dalton's Lymphoma Ascites.
Keywords: Cocculus hirsutus, hepatocellular carcinoma, molecular docking, phytoconstituents
INTRODUCTION
Hepatocellular carcinoma (HCC), the third common cancer, accounts for more than 626,000 new cases per year worldwide.[1] This study aimed to perform docking of ligands, which were reported in the plant Cocculus hirsutus with the macromolecular targets which involves in various apoptosis cell signaling pathways.[2] Our laboratory findings indicate that this plant is found to have in vitro cytotoxic activity in MCF-7, Hep-2, and Hela cancer cell line[3] and in vivo anticancer activity against DLA cells in mice.[4] To find out the best candidate of the plant constituents, the reported phytoconstituents of C. hirsutus were docked with the various targets responsible for different signaling pathways such as Aurora kinase, proto-oncogene c-Kit (c-Kit), B-cell lymphoma-extra large (Bcl-xL), nuclear factor kappa B (NF-kB), fibroblast growth factor (FGF), and vascular endothelial growth factor (VEGF).
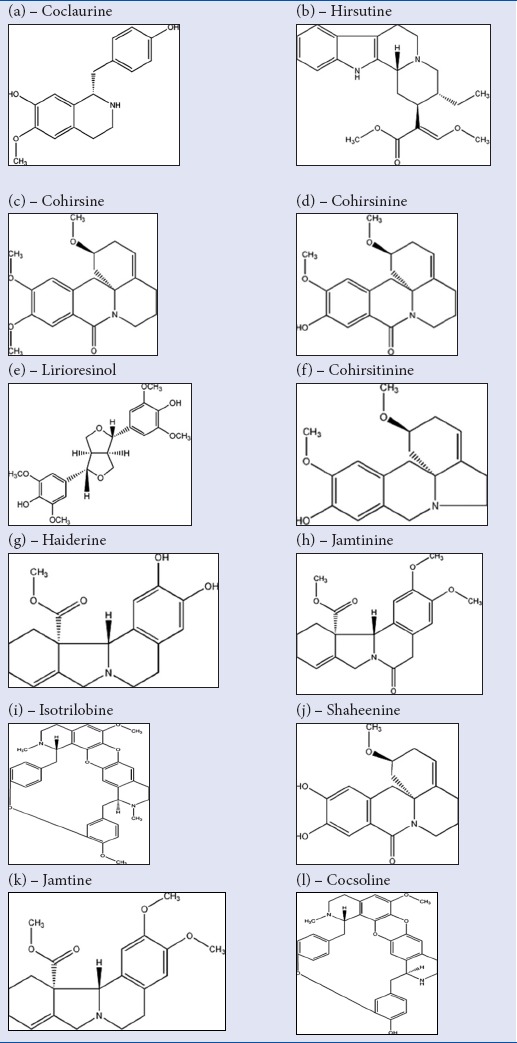
Plants are rich in secondary metabolites such as alkaloids, glycosides, tannins, terpenoids, flavanoids, etc. C. hirsutus (L.) Diels is a climbing shrub belongs to the family Menispermaceae is rich in alkaloid, flavanoids, and phenolic compounds. C. hirsutus is widely used in the indigenous system of medicine for curing various ailments due to its different medicinal properties. The plants are reported to have antioxidant, cytotoxic,[5] hepatoprotective,[6] anticancer, and hypotensive.[7] The reported phytoconstituents of Cocculus hirustus are coclaurine, cocsoline, cohirsine, cohirsinine, cohirsitinine, haiderine, hirsutine, isotrilobine, Jamtine, jamtinine, lirioresinol, and shaheenine.[8,9] Attempt has been made to dock these plant contituents with the HCC targets such as Aurora kinase, c-Kit, FGF, NF-kB, Bcl-xL, and VEGF using in silico technique with the software Grid-Based Ligand Docking with Energies (GLIDE).
MATERIALS AND METHODS
Previously reported phytoconstituents of C. hirsutus [Table 1] were downloaded from the database and subjected to docking studies. Maestro build panel was used for building the ligands. LigPrep is a utility of Schrodinger software (USA) that generates three-dimensional structures from two-dimensional representation. The active protein site with which the ligand will be docked was prepared using the wizard, protein preparation of Schrodinger software. Ligand and protein were docked using the software GLIDE. The prepared ligands were docked against such as Aurora kinase, c-Kit, FGF, NF-kB, Bcl-xL, and VEGF cancer targets.
Table 1.
Compounds of Cocculus hirsutus taken for docking studies
GScore = b × Coul + a × vdW + Metal + Lipo + RotB + Hbond + BuryP + Site
Where Coul is Coulomb's energy, vdW is Van der Waal's energy, Metal is metal-binding term, Lipo is lipophilic contact term, RotB is penalty for freezing rotatable bonds, Hbond is hydrogen-bonding term, BuryP is penalty for buried polar groups, site is polar interactions at the active site, and the coefficients of vdW and Coul are a = 0.065 and b = 0.130, respectively. All docking computations were carried out using Linux is an operating system manufactured by Linus Torvalds.
RESULTS
The results of the best candidate with good binding efficiency of the phytoconstituent with good G-score, glide energy, and amino acid interactions along with the bond distance are summarized in Table 2.
Table 2.
Phytoconstituents having good interactions with cancer targets
DISCUSSION
From the results, it is observed that out of 12 compounds of C. hirsutus, coclaurine, haiderine, and lirioresinol are found to have good glide score and glide energy toward Aurora kinase, c-Kit, FGF, NF-kB, Bcl-xL, and VEGF.
Aurora kinase which is crucial for cell cycle control is overexpressed in tumor cells.[10] Inhibiting Aurora kinase in such increased levels will control the rapid cell growth. c-Kit receptor is present in hematopoietic cells and other tissue cells. c-Kit signaling plays a vital role in regulation of the red blood cell production, lymphocyte proliferation, etc. Downstream signal transduction regulates cell growth, proliferation, and differentiation.[11] Overexpression of Bcl-xL relates with resistance to chemotherapy and radiation therapy in multiple cancer.[12] NF-kB, i.e. NF-kB is a transcription factor, which will play a vital role in carcinogenesis. Therefore, inhibition of NF-kB activation plays a tumor suppressor role in Liver.[13] FGF target is the FGF, which will increase in solid, and metastatic tumors and gives drug resistance.[14] Thus, inhibitions of FGF will results in the entry of chemical compounds into the target cell and thereby cell toxicity and retardation of cell growth.[15] VEGF-receptors (VEGFRs) are useful of the angiogenesis (development of blood vessels from pre-existing vasculature) and vasculogenesis (development of the circulatory system). Overexpression of VEGF will lead to the abnormal growth of tumor cells.[16] Therefore, an inhibitor of VEGFR will control the growth of new cells binding with the above targets and inhibiting their activity will show a significant tumor control in liver tissues. Thus, this study suggests that these phytoconstituents, especially the best candidates such as coclaurine, haiderine, and lirioresinol, will exhibit its activity by effective binding with the targets as explained by GLIDE software [Figure 1].
Figure 1.
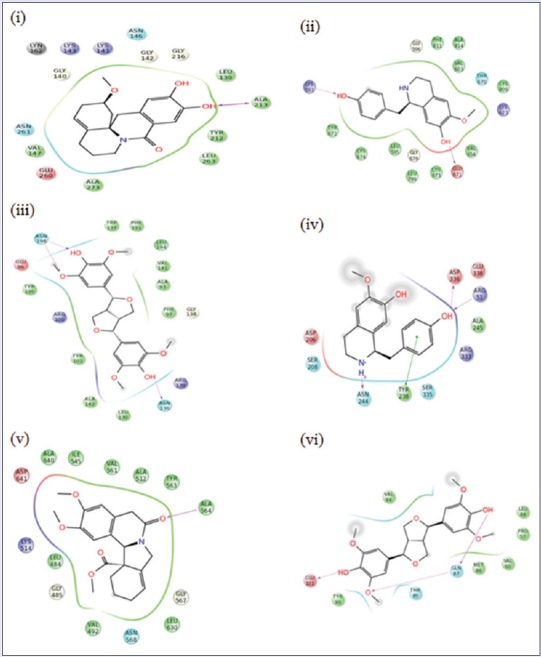
(i). Docking of Shaheenine with Arorakinase, (ii) Docking of Coclaurine with C- kit, (iii) Docking of Lirioresinol with Bcl-xL, (iv) Docking of Coclaurine with NF-κB, (v) Docking of Jamtinine with FGF, (vi) Docking of Lirioresinol with VEGF
CONCLUSION
From this study, it is clear that out of 12 compounds docked with various targets of HCC, coclaurine, haiderine, and lirioresinol have found to have good interaction and binding efficiency with all targets. Therefore, these best candidates for HCC have to be taken up for further research.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHOR

Dr. B. Samuel Thavamani
Dr. B. Samuel Thavamani is working as associate professor in the department of Pharmacognosy, PSG College of Pharmacy, Coimbatore. He has been working in the area of phytochemical studies of plants especially of anticancer property. To his credit he has national and international publication in this area.
REFERENCES
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Mabrouk MS. Discovering best candidates for hepatocellular carcinoma (HCC) by in-silico techniques and tools. Int J Bioinform Res Appl. 2012;8:141–52. doi: 10.1504/IJBRA.2012.045956. [DOI] [PubMed] [Google Scholar]
- 3.Thavamani BS, Mathew M, Dhanabal SP. In vitro cytotoxic activity of menispermaceae plants against HeLa cell line. Anc Sci Life. 2013;33:81–4. doi: 10.4103/0257-7941.139040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thavamani BS, Mathew M, Palaniswamy DS. Anticancer activity of Cocculus hirsutus against Dalton's lymphoma ascites (DLA) cells in mice. Pharm Biol. 2014;52:867–72. doi: 10.3109/13880209.2013.871642. [DOI] [PubMed] [Google Scholar]
- 5.Mon MM, Maw SS, Oo ZK. Screening of antioxidant, anti-tumor and antimicrobial herbal drugs/diets from some Myanmar traditional herbs. Int J Biosci Biochem Bioinforma. 2011;1:142–7. [Google Scholar]
- 6.Sagar PT, Hitesh NJ, Savita DP, Umesh MU. Hepatoprotective effect of Cocculus hirsutus on bile duct ligation induced liver fibrosis in albino wistar rats. Banglad J Pharmacol. 2009;4:126–30. [Google Scholar]
- 7.Khare CP. New Delhi: Springer Science, Business Media LCC, Springer India; 2007. Indian Medicinal Plants; pp. 162–3. [Google Scholar]
- 8.Viqar UA, Faryal VM, Rasheed T. Hirsudiol, a triterpenoid from Cocculus hirsutus. Phytochemistry. 1987;26:793–4. [Google Scholar]
- 9.Viqar UA, Iqbal S. Jamtinine, an alkaloid from Cocculus hirsutus. Phytochemistry. 1993;33:735–6. [Google Scholar]
- 10.Tatsuka M, Katayama H, Ota T, Tanaka T, Odashima S, Suzuki F, et al. Multinuclearity and increased ploidy caused by overexpression of the aurora- and Ipl1-like midbody-associated protein mitotic kinase in human cancer cells. Cancer Res. 1998;58:4811–6. [PubMed] [Google Scholar]
- 11.Liang J, Wu YL, Chen BJ, Zhang W, Tanaka Y, Sugiyama H. The C-kit receptor-mediated signal transduction and tumor-related diseases. Int J Biol Sci. 2013;9:435–43. doi: 10.7150/ijbs.6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwartz PS, Manion MK, Emerson CB, Fry JS, Schulz CM, Sweet IR, et al. 2-methoxy antimycin reveals a unique mechanism for Bcl-x(L) inhibition. Mol Cancer Ther. 2007;6:2073–80. doi: 10.1158/1535-7163.MCT-06-0767. [DOI] [PubMed] [Google Scholar]
- 13.Lee CH, Jeon YT, Kim SH, Song YS. NF-kappaB as a potential molecular target for cancer therapy. Biofactors. 2007;29:19–35. doi: 10.1002/biof.5520290103. [DOI] [PubMed] [Google Scholar]
- 14.Korc M, Friesel RE. The role of fibroblast growth factors in tumor growth. Curr Cancer Drug Targets. 2009;9:639–51. doi: 10.2174/156800909789057006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao H, Liu H, Chen Y, Xin X, Li J, Hou Y, et al. Oligomannurarate sulfate, a novel heparanase inhibitor simultaneously targeting basic fibroblast growth factor, combats tumor angiogenesis and metastasis. Cancer Res. 2006;66:8779–87. doi: 10.1158/0008-5472.CAN-06-1382. [DOI] [PubMed] [Google Scholar]
- 16.Saha S, Islam MK, Shilpi JA, Hasan S. Inhibition of VEGF: A novel mechanism to control angiogenesis by Withania somnifera's key metabolite withaferin A. In Silico Pharmacol. 2013;1:11. doi: 10.1186/2193-9616-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]


