Abstract
Background:
Lippia javanica (Burm.F.) Spreng is one of the spice plants commonly found in almost every part of South Africa. Apart from its culinary uses, it is also traditionally used as an insect repellant and infusion for fever, flu, kidney stone treatment, cough, common cold, and chest pain.
Materials and Methods:
The antioxidant activities of the aqueous and acetone extracts were determined by measuring their effects against 1,1-Diphenyl-2-picryl-hydrazyl, 2,2’azino-bis (3-ethylbenzthiazoline-6-sulfonic acid), nitric oxide, phosphomolybdate, lipid peroxidation, hydrogen peroxide, and reducing power. The antimicrobial activities were evaluated against four bacterial (two Gram-positive, two Gram-negative) strains and 9 fungal pathogens using the agar well diffusion and microdilution methods. Anti-inflammatory activity was assessed by determining the inhibition against protein denaturation and membrane stabilizing effects.
Objective:
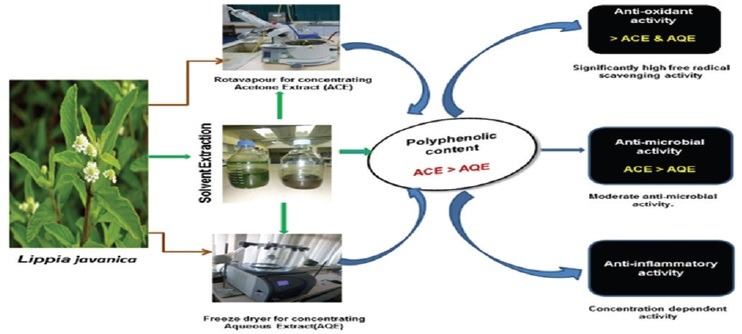
The polyphenolic content, free radical scavenging, anti-inflammatory, and antimicrobial activities of the aqueous and acetone extracts of the plant were evaluated.
Results:
A significantly high total phenolic content and free radical scavenging activities were observed in the acetone extracts of the plants. The study also revealed a concentration-dependent inhibition of protein denaturation and membrane stabilization effects by both the aqueous and acetone extracts at the concentrations studied. The ability of L. javanica extracts to inhibit protein denaturation and maintain membrane stability could be responsible for its folkloric use. The overall antimicrobial activity indicates that both extracts were active against the bacterial strains but the acetone extract exhibited the most potent antifungal activity higher than even the reference drugs.
Conclusion:
Overall, the acetone extract of L. javanica exhibited a more pronounced antioxidant, anti-inflammatory, and antimicrobial effects than the aqueous extract.
SUMMARY
The polyphenolic content and biological activities of the spice plant, Lippia javanica from South Africa was evaluated
Significantly high polyphenolic content and free radical scavenging activities were observed for both extracts
Moderate antimicrobial action, concentration-dependent inhibition of protein denaturation and membrane haemolysis were also observed.
Abbreviations used: AA: ascorbic acid, ABTS: 2,2’azino-bis (3-ethylbenthiazoline-6-sulfonic acid), BHT: Butylated hydroxytoluene, DPPH: 1,1-Diphenyl-2-picryl-hydrazyl, NBT: 2,2’-di-pnitrophenyl-5,5’-diphenyl-(3,3’-dimethoxy-4,4’-diphenylene)- ditetrazolium chloride, PMS: Potassium metabisulfite, ROS: Reactive oxygen species, TBA: Thiobarbituric acid, TCA: Trichloroacetic acid.
Keywords: Anti-inflammatory properties, antimicrobial, antioxidant activities, Lippia javanica, membrane stability, protein denaturation
INTRODUCTION
Biologically active compounds from natural sources have always been of great interest to scientists. It has been documented severally that some naturally occurring substances in plants possess antioxidant activity.[1,2,3,4] These naturally occurring substances include a wide variety of free radical scavenging molecules such as flavonoids, anthocyanins, carotenoids, dietary glutathiones, vitamins, and endogenous metabolites.[5] A large number of phytochemicals belonging to several chemical classes have been reported to show biological activities such as antioxidants, antimicrobial, anti-inflammatory, antiviral, antitumor, antimalarial, antiurolithiatic, and analgesic.[6] It has been reported that plants owe their antimicrobial properties mostly to the presence of alkaloids, phenols, glycosides, steroids, essential oils, coumarins, and tannins.[4,6] Several anti-inflammatory, digestive, antinecrotic, neuroprotective, and hepatoprotective drugs have recently been shown to have radical scavenging activity as part of their mechanism of action.[7]
Many pathological disorders have been associated with oxidative stress and inflammation. Reactive oxygen species (ROS) are involved in a diversity of important pathological processes such as inflammatory and neurodegenerative diseases, atherosclerosis, cancer, and reperfusion injury. Inflammation is the foundation of most chronic diseases; the presence of inflammation makes most disease perceptible to an individual. It can often occur for years before it exists at levels sufficient to be apparent or clinically significant. How long it has been smoldering really determines the degree of severity of a disease and often the prognosis assuming the inflammation can be controlled. Hence, one could also argue that without inflammation most disease would not even exist.[8]
The mechanism of inflammatory injury is attributed in part to the release of ROS from activated neutrophils and macrophages. This overproduction leads to tissue injury by damaging macromolecules and lipid peroxidation of membranes.[7] Free radicals are important mediators that provoke or sustain inflammatory processes, and consequently, their neutralization by antioxidants and radical scavengers can reduce inflammation.[9] Currently, researchers are searching for powerful and nontoxic natural antioxidants from edible plants not only to prevent autoxidation and lipid peroxidation but also to replace synthetic antioxidants.
Lippia javanica (Burm.F.) Spreng belongs to the family Verbenaceae, and the genus includes about 200 species.[10,11,12,13] It is a woody shrub with aromatic leaves that gives a lemon-like smell, which probably accounts for its common name lemon bush plant. The plant is widely distributed in the Eastern Cape Province of South Africa, and different bioactive compounds have been isolated from it. The plant is traditionally used as a culinary spice and medicinally to treat coughs, colds, fever, chest ailments, kidney stones, measles, rashes, and stomach problems.[12] The main objective of this study was to evaluate the phenolic content, antioxidant, anti-inflammatory, and antimicrobial activities of the acetone and aqueous extracts of the aerial parts of this plant to validate some of its traditional medicinal uses.
MATERIALS AND METHODS
Collection and preparation of the plant
The fresh leaves of L. javanica were collected in June 2014 from the University of Fort Hare research farm in Alice, Eastern Cape. It was authenticated by Tony Dold in Albany Herbarium, Rhodes University, and voucher specimen (ASO/01) was deposited at Giffen herbarium of the University of Fort Hare. The aerial parts of L. javanica were oven-dried at 40°C, milled into fine powder, packed into airtight plastic bottles, and stored at 4°C until needed. The acetone extract was obtained by extracting 100 g of the powdered plant in 70% acetone (560 mL) and shaking for 24 h in an orbital shaker. The extract was filtered using a Buchner funnel and a Whatman No. 1 filter paper. The filtrate was then concentrated to dryness under reduced pressure at 40°C using a rotary evaporator (Laborota 4000-efficient, Heidolph, Germany). For the aqueous extract, 50 g of the powdered plant was extracted in 1000 mL of distilled water heated at 100°C for 10 min. It was allowed to cool, filtered, and then freeze-dried (Vir Tis bench top K, Vir Tis Co., Gardiner, NY, USA). The freeze-dried sample was reconstituted with distilled water to give the desired concentrations used in the study.
Chemicals and reagents
1,1-Diphenyl-2-picryl-hydrazyl (DPPH), absolute ethanol, ascorbic acid (AA), Folin–Ciocalteu phenol reagent, sodium carbonate, aluminum chloride, vanillin, sodium acetate, phosphate buffer, potassium ferricyanide (K3 Fe[CN]6), trichloroacetic acid (TCA), 2-thiobarbituric acid (TBA), potassium metabisulfite, 2,2’-di-p-Nitrophenyl-5,5’-diphenyl-3,3’-(3,3’-dimethoxy-4,4’-diphenyl)- ditetrazolium chloride catechin, quercetin, 2,2’azino-bis (3-ethylbenthiazoline- 6-sulfonic acid) (ABTS), potassium persulfate, sodium nitroprusside, hydrogen peroxide (H2O2), sulfanilic acid, glacial acetic acid, butylated hydroxytoluene (BHT), and tannic acid were purchased from Sigma Chemical Co., (St. Louis, MO, USA). Butanol-HCl reagents (butanol-HCl, 95:5 v/v) and ferric reagents were also purchased from Merck Chemical Supplies (Darmstadt, Germany). All chemicals used including the solvents were of analytical grade.
Determination of total phenols
The amount of phenol was determined spectrophotometrically using the modified method of Wolfe et al.,[14] with Folin–Ciocalteu reagent. About 1 mg/mL aliquot of the extract was mixed with 5 mL Folin–Ciocalteu reagent (previously diluted with water at a concentration of 1:10 v/v) and 4 mL (75 g/L) of sodium carbonate. The tubes were vortexed for 15 s and left to stand for 30 min at 40°C for color development. Absorbance was then measured at 765 nm using the AJI-C03 ultraviolet-visible spectrophotometer. Results were expressed as mg/g of tannic acid equivalent (TAE) using the calibration curve:
Y = 2.0573x + 2.635, R2 = 0.9985
where x is the concentration and Y is the TAE.
Estimation of total flavonoids
Total flavonoid content was determined using the method of Ordonez et al.[15] A volume of 0.5 mL of 2% AlCl3 ethanol solution was added to 0.5 mL of the sample solution and allowed to stand for 1 h at room temperature. The absorbance was measured at 420 nm. A yellow color indicated the presence of flavonoids. Plant extracts were evaluated at a concentration of 0.1 mg/mL. Total flavonoid content was calculated as mg/g of quercetin using the following equation based on the calibration curve:
Y = 0.3705x + 1.1779, R2 = 0.7601
where x is the concentration and Y is the quercetin equivalent.
Determination of total flavonols
Total flavonol content was determined by adopting the procedure described by Kumaran and Karunakaran.[16] The mixture consisted of 2.0 mL of the sample, 2.0 mL of AlCl3 which was prepared in ethanol, and 3.0 mL of (50 g/L) sodium acetate solution. The absorbance at 440 nm was measured after 2.5 h at 20°C. Total flavonol content was calculated as mg/g of quercetin equivalent from the calibration curve using the equation:
Y = 0.3705x + 1.1779, R2 = 0.7601
where x is the concentration and Y is the quercetin equivalent.
Determination of total proanthocyanidin
The method of Sun et al.[17] was used for the determination of total proanthocyanidin. To 0.5 mL of 1 mg/mL of the extract, 3 mL of vanillin-methanol (4% v/v) was added; 1.5 mL of hydrochloric acid was also added and vortexed. The mixture was allowed to stand for 15 min at room temperature, and the absorbance was measured at 500 nm. Total proanthocyanidin content was evaluated at a concentration of 0.1 mg/mL and expressed as catechin equivalent (mg/g) using the calibration curve equation:
Y = 0.6845x + 0.7147, R2 = 0.7932
where x is the concentration and Y is the catechin equivalent.
Antioxidant assays
The antioxidant activities of the aqueous and acetone extracts were determined by measuring their effect against DPPH, ABTS, reducing power, lipid peroxidation, H2O2, nitric oxide (NO), and phosphomolybdate.
Determination of ferric reducing power of the extracts
The reducing power of the plant extracts was evaluated according to the method described by Shiddhuraju et al.[18] The mixture containing 2.5 mL of 0.2 M phosphate buffer (pH 6.6) and 2.5 mL of K3 Fe (CN)6 (1% w/v) was added to 1.0 mL of the extracts and standards (0.025–0.5 mg/mL) prepared in distilled water. The resulting mixture was incubated for 20 min at 50°C, followed by the addition of 2.5 mL of TCA (10% w/v), which was then centrifuged at 3000 rpm for 10 min. A volume of 2.5 mL of the supernatant was mixed with 2.5 mL of distilled water and 0.5 mL of FeCl3 (0.1% w/v). The absorbance was then measured at 700 nm against the blank sample. Increased absorbance of the reaction mixture indicated higher reducing power of the plant extract.
1,1-Diphenyl-2-picryl-hydrazyl radical scavenging assay
The method of Liyana-Pathirana and Shahidi[19] was used for the determination of scavenging activity of DPPH free radical. DPPH (1 mL, 0.135 mM) prepared in methanol was mixed with 1.0 mL of aqueous extract ranging from 0.025 to 0.5 mg/mL. The mixture was vortexed thoroughly and left in dark at room temperature for 30 min. The absorbance was measured at 517 nm. The scavenging ability of the plant extract was calculated using the equation:
DPPH scavenging activity (%) = ([Abs control − Abs sample]/[Abs control]) × 100
where Abs control is the absorbance of DPPH + methanol and Abs sample is the absorbance of DPPH radical + sample (sample or standard).
2,2’azino-bis (3-ethylbenthiazoline-6-sulfonic acid) radical scavenging activity
The method described by Pandey et al.[20] was adopted for the determination of ABTS activity of the plant extract. The working solution was prepared by mixing two stock solutions of 7 mM ABTS and 2.4 mM potassium persulfate in equal amounts and allowed to react for 12 h at room temperature in the dark. The resulting solution was further diluted by mixing 1 mL ABTS + solution with 60 mL methanol to obtain an absorbance of 0.706 ± 0.001 units at 734 nm after 7 min using a spectrophotometer. The percentage inhibition (% inhibition) of ABTS + by the extract was calculated as follows:
% inhibition = ([Abs control − Abs sample]/[Abs control]) × 100.
Nitric oxide scavenging activity
The modified method described by Green et al.[21] was used to determine the NO radical scavenging activity of aqueous and acetone extracts. A volume of 2 mL of 10 mM of sodium nitroprusside prepared in 0.5 mM phosphate buffered saline (pH 7.4) was mixed with 0.5 mL of plant extracts, gallic acid, and BHT individually at 0.025–0.5 mg/mL. The mixture was incubated at 25°C for 150 min. Then, 0.5 mL of the incubated solution was mixed with 0.5 mL of Griess reagent (1.0 mL sulfanilic acid reagent [0.33% prepared in 20% glacial acetic acid] at room temperature for 5 min with 1 mL of naphthylene diamine dichloride [0.1% w/v]). The mixture was incubated at room temperature for 30 min followed by the measurement of the absorbance at 540 nm. The amount of NO radicals inhibited by the extract was calculated using the following equation:
NO radical scavenging activity (%) = ([Abs control − Abs sample]/[Abs control]) × 100
where Abs control is the absorbance of NO radicals + methanol and Abs sample is the absorbance of NO radical + extract or standard.
Hydrogen peroxide scavenging activity
H2O2 scavenging activity of the plant extract was estimated as described by Ruch et al.[22] Plant extract (4 mg/mL) prepared in distilled water at various concentrations was mixed with 0.6 mL of 4 mM H2O2 solution prepared in phosphate buffer (0.1 M, pH 7.4) and incubated for 10 min. The absorbance of the solution was measured at 230 nm using the Biomate thermospectronoic, against a blank solution containing the plant extract without H2O2. The amount of H2O2 radical inhibited by the extract was calculated using the following equation:
H2O2 radical scavenging activity = ([Abs control − Abs sample])/(Abs control) ×100
where Abs control is the absorbance of H2O2 radicals + methanol and Abs sample is the absorbance of H2O2 radical + sample or extract or standard.
Estimation of lipid peroxidation
A modified TBA-reactive species assay described by Dasgupta and De[23] was used to measure the lipid peroxide formed, using liver homogenates as lipid-rich media. Liver homogenate (0.5 mL, 10% in distilled water, v/v) and 0.1 mL of the plant extracts were mixed in a test tube, and the volume was made up to 1 mL with distilled water. Finally, 0.05 mL FeSO4 (0.07 M) was added to the mixture and incubated for 30 min to induce lipid peroxidation. Thereafter, 1.5 mL of 20% acetic acid (pH adjusted to 3.5 with NaOH) and 1.5 mL of 0.8% TBA (w/v) (prepared in 1.1% sodium dodecyl sulfate) and 0.05 mL 20% TCA were added, vortexed, and heated in a boiling water bath for 60 min. After cooling, 5.0 mL of n-butanol was added to each tube and centrifuged at 3000 rpm for 10 min. The absorbance of the organic upper layer was measured at 532 nm. The control was made up of FeSO4+ acetic acid + TBA + SDS + TCA + butanol − the extract.
Phosphomolybdate assay
The total antioxidant activity of the plant sample was determined using phosphomolybdenum method according to the protocol of Rabia et al.[24] The plant extract 0.1 mL was taken into test tubes and dissolved in 1 mL of reagent solution containing 0.6 M sulfuric acid, 4 mM ammonium molybdate, and 28 mM sodium phosphate. Then, the test tubes were covered with silver foil and incubated in a water bath at 95°C for 95 min. The sample was allowed to cool at room temperature after which the absorbance of the mixture was measured at 765 nm against a blank. AA was used as standard. Higher absorbance indicates higher total antioxidant potential.
Anti-inflammatory assay
In vitro protein denaturation
The reaction mixture (0.5 mL, pH 6.3) consisted of 0.45 mL of bovine serum albumin (5% aqueous solution) and 0.05 mL of distilled water; pH was adjusted at 6.3 using 1 N HCl (83 mL of concentrated HCl was diluted in a volumetric flask to 1000 mL). Around 1000 µg of L. javanica aqueous and acetone extracts (mg/mL of respective organic solvents) was added to the reaction mixture and was incubated at 37°C for 30 min and then heated at 57°C for 5 min. After cooling the samples, 2.5 mL of phosphate buffer was added. Turbidity was measured spectrophotometrically at 600 nm. For negative control, 0.05 mL distilled water and 0.45 mL of bovine serum albumin were used. The % inhibition of protein denaturation was calculated as follows.[25]
% inhibition of protein denaturation = ([Abs control − Abs sample]/[Abs control]) × 100
Ex vivo membrane stabilizing activity
Preparation of erythrocyte suspension
The red blood cell (RBC) was obtained with heparinized syringes from a rat through cardiac puncture. The blood was washed 3 times with isotonic buffered solution (154 mM NaCl) in 10 mM sodium phosphate buffer (pH 7.4). The blood was centrifuged each time for 10 min at 3000 g.
Induced rat erythrocyte hemolysis
Membrane stabilizing activity of the extract was assessed using hypotonic solution-induced rat erythrocyte hemolysis.[26] The test sample consisted of stock erythrocyte (RBC) suspension (0.50 mL) mixed with 5 mL of hypotonic solution (50 mM NaCl) in 10 mM sodium phosphate buffered saline (pH 7.4) containing the extract (0.25–2.0 mg/mL) or diclofenac (0.1 mg/mL). The control sample consisted of 0.5 mL of RBC mixed with hypotonic buffered saline solution alone. The mixtures were incubated for 10 min at room temperature (25°C) and centrifuged for 10 min at 3000 g (13,200 rpm). The absorbance of the supernatant was measured at 540 nm. Membrane stabilization of the extract was determined to be the % inhibition of hemolysis and calculated as follows:
% inhibition of hemolysis = 100 × (OD1 − OD2/OD1)
where OD1 = optical density of hypotonic buffered saline solution alone and OD2 = optical density of test sample in hypotonic solution.
Antimicrobial assay
Bacterial strains
The reference strains used in this study were chosen based on their pathological effects on human and deterioration of food products: Two Gram-positive (Staphylococcus aureus and Listeria monocytogenes) and two Gram-negative (Salmonella typhimurium and Escherichia coli) bacteria were obtained from the Department of Microbiology, University of Fort Hare.
The agar well diffusion-based method of Deans and Ritchie[27] modified by Oyedeji et al.[28] was used to determine the susceptibility of bacteria. A 100 µL of 18 h bacterial cultures was used to spread a bacterial lawn on nutrient agar. The cultures were adjusted to approximately 105 CFU/mL using McFarland standard. Twenty-five microliters (25 µL) of various concentrations of plant extracts was added to each well (diameter of 4 mm) bored on nutrient agar plates under aseptic condition. The plates were left for 30 min at room temperature for the diffusion of the extracts and incubated at 37°C for 18 h. The zones of inhibition were measured after 18 h using ruler. Each concentration of the extract was repeated 3 times. The minimum bactericidal concentration of L. javanica extracts against the test bacteria was determined by agar dilution method as described by the National Committee for Clinical Laboratory Standards.[29] The minimum inhibitory concentrations (MICs) were the lowest concentrations of the extract resulting in completing inhibition of visible growth of the test organisms.
Antifungal assay
Pathogens and media
The fungi used in this study were chosen primarily on the basis of their importance as opportunistic pathogens of humans. Strains from the American Type Culture Collection (ATCC) were used: Aspergillus fumigatus ATCC 204305, Aspergillus niger ATCC 16888, Microsporum canis ATCC 36299, Microsporum gypseum ATCC 24102, Trichophyton tonsurans ATCC 28942, Trichophyton rubrum ATCC 28188, Trichophyton mucoides ATCC 201382, Penicillium aurantiogriseum ATCC 16025, and Penicillium chrysogenum ATCC 1010. Both Sabouraud dextrose agar (SDA) and Sabouraud dextrose broth (SDB) were prepared according to the manufacturer's instructions. The fungi were maintained at 4°C on SDA plates and the inoculum for the assays was prepared by diluting scraped cell mass in 0.85% NaCl solution, adjusted to 0.5 McFarland standards, and confirmed by spectrophotometric reading at 580 nm.[30,31] Cell suspensions were finally diluted to 104 CFU mL − 1 for the use in the assays.
Antifungal susceptibility assays
The agar diffusion and microdilution methods were used to determine the antifungal activities of the plant extracts against the opportunistic fungi.[32,33]
Agar well diffusion assay
The agar diffusion assay was carried out with slight modifications.[34] Using the micropipette, 100 μL of 0.5 McFarland solution of each fungus culture in 0.85% sterile distilled water (SDW) was placed over the surface of an agar plate and spread using a sterile inoculation loop. The same procedure was followed for the other fungi. Using a sterile cork borer, four holes (5 mm in diameter) were punched in each of the culture plates. In the first hole, 50 μL of a positive control drug was added (nystatin); 50 μL of the corresponding extract solvent was added as a negative control in the second hole; 50 μL of the plant extract was added in the third and last holes at concentrations of 25 and 50 mg/mL, respectively. Each test was duplicated. The culture plates were then incubated at 37°C and the results were observed after 24 h, up to 6 days depending on each fungal growth. The clear zone around each well was measured in mm, indicating the activity of the plant extracts against the fungal organisms.
Microdilution assay
The microdilution method was employed to determine the MIC of the plant extracts using 96-well microtiter plate.[34] Initially, 120 μL of SDW was added into each well of the first (A) and last (H) rows and also into all the wells of the last column.[35] Then, 120 μL of SDB was added into each well of the second row (B) and 150 μL of SDB was added into the remaining wells of the first column and 100 μl into the rest of the wells from the second column rightward. Fifty microliters of the plant extract was then added into the third well of the first column while 50 μL of the positive and negative control was separately added into the remaining wells of the first column. A 2-fold serial dilution was done by mixing the contents in each well of the first column (starting from the third row) and transferring 100 μL into the second well of the same row and the procedure was repeated up to the 11th well of the same row and the last 100 μL from the 11th well was discarded. Hence, various concentrations of the plant extracts ranging from 0.005 mg/mL to 5 mg/mL were prepared in the wells, following the 2-fold dilution method. Thereafter, 20 μL of 0.5 McFarland fungal suspensions was inoculated into the wells. The growth of the fungi was measured by determining the absorbance at 620 nm with a microtiter plate reader before and after incubation. The plates were incubated at 37°C at various durations (24 h to 48 h). The lowest concentration which inhibited the growth of the fungi was considered as the MIC of the extract.
Determination of the minimum fungicidal concentration
The minimum fungicidal concentration (MFC) was determined by inoculating the contents from the MIC plates onto SDA plates, and the results were observed after incubation at 37°C at various durations depending on the fungi. The presence of the fungal colonies on the agar plates was an indication that the plant extract only inhibited the growth of the fungi without killing them, and the absence indicated that the plant extract was able to kill the fungal organisms.[36] The smallest concentration of the plant extract that was able to kill the microorganisms was considered MFC.
Statistical analysis
Results were expressed as mean ± standard deviation of three determinations. One-way analysis of variance was used to determine the differences of means among the samples. A significant difference was considered at the level of P < 0.05.
RESULTS
Polyphenolic constituents
The flavonoid and flavonol contents of L. javanica acetone and aqueous extracts were expressed as quercetin equivalents while total phenol and proanthocyanidin were expressed as tannic acid and catechin equivalents, respectively. The acetone extract of L. javanica showed a higher content of phenol, flavonoid, and proanthocyanidin compared to the aqueous extract [Table 1]. This could be attributed to the better extracting power of acetone over water. According to Eloff,[37] acetone extracts are more bioactive components from plants. Further, the lower total polyphenolic content could be as a result of extraction by boiling which has been reported to reduce/destroy total phenolic contents in some foods.[38] The total phenolic content of acetone and aqueous extracts were 4.49 ± 0.411 mg/g and 3.73 ± 0.498 mg/g TAE, respectively.
Table 1.
Polyphenolic constituents of Lippia javanica

Free radical scavenging activity
2,2’azino-bis (3-ethylbenthiazoline-6-sulfonic acid)
The % inhibition of ABTS by L. javanica extracts is shown in Figure 1. All the tested samples inhibited the ABTS radicals, but the acetone extract exhibited a higher inhibitory activity compared to the aqueous extract. The inhibition concentration (IC50) values [Table 2] confirmed that the acetone extract exhibited a greater inhibition than the aqueous extract, as well as comparable activity with the standards. The ABTS radical scavenging ability of the samples can be ranked as rutin > acetone extract > BHT > aqueous extract.
Figure 1.
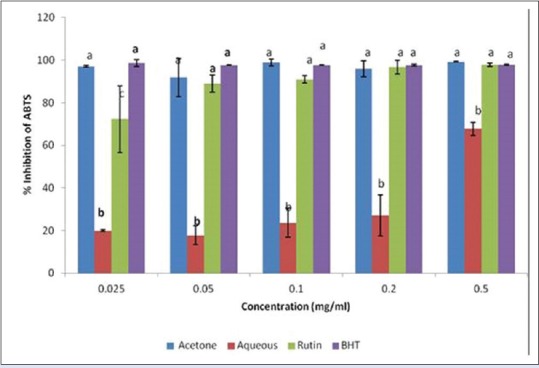
Inhibition of 2,2’azino-bis (3-ethylbenthiazoline-6-sulfonic acid) radicals by acetone and aqueous extracts of Lippia javanica. Columns with different letters are significantly different (P ≤ 0.05)
Table 2.
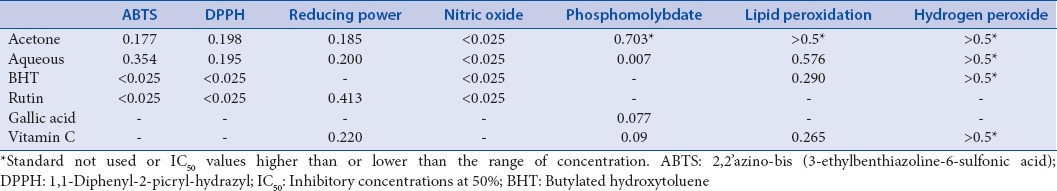
Inhibitory concentrations at 50% of the antioxidant activities of Lippia javanica (mg/mL)
1,1-Diphenyl-2-picryl-hydrazyl
[Figure 2] shows the DPPH radical scavenging capacity of the extracts and standards. The extracts exhibited good DPPH scavenging capacity in the order rutin > aqueous extract > BHT > acetone extract. Although the antioxidant potential of fractions was found to be lower (P < 0.05) than those of rutin, the IC50 [Table 2] revealed that both the aqueous and acetone extracts have prominent antioxidant activities.
Figure 2.
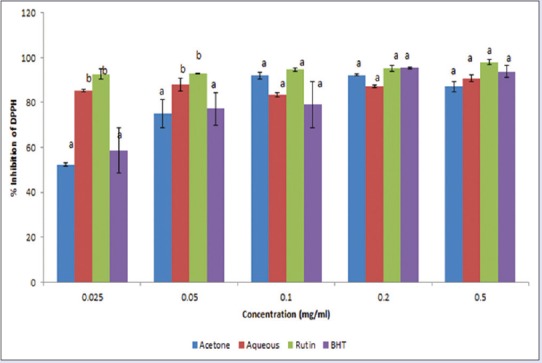
Inhibition of 1,1-Diphenyl-2-picryl-hydrazyl radicals by acetone and aqueous extracts of Lippia javanica. Columns with different letters are significantly different (P ≤ 0.05)
Nitric oxide
NO radical scavenging was in a concentration-dependent manner [Figure 3] and was in the order: Rutin > aqueous extract > BHT > acetone extract. The IC50 values obtained for the aqueous extract (0.205 mg/mL) and acetone extract (0.215 mg/mL) showed a comparable activity with the standards rutin (0.184 mg/mL) and BHT (0.222 mg/mL). The data demonstrated that both the aqueous and acetone extracts are potent scavengers of NO.
Figure 3.
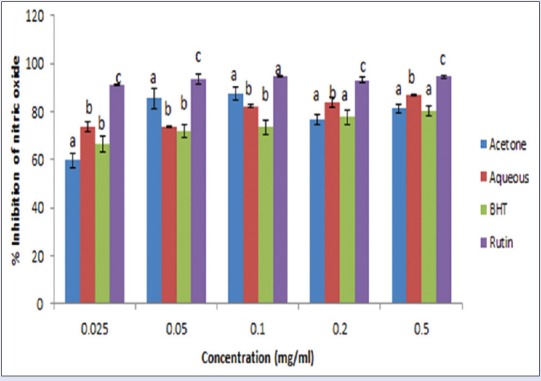
Inhibition of nitric oxide radicals by acetone and aqueous extracts of Lippia javanica. Columns with different letters are significantly different (P ≤ 0.05)
Lipid peroxidation
The liver homogenate from a rat was used for this assay. The extracts exhibited significant (P < 0.05) lipid peroxidation quenching activities in a concentration-dependent manner, reaching the peak at concentrations of 0.5 mg/mL [Figure 4]. The results from IC50 values [Table 2] showed that the aqueous extract showed a significantly higher scavenging capacity than the acetone extract though the standards BHT and Vitamin C exhibited the greatest inhibition. The inhibitory capacity is ranked in the order: BHT > Vitamin C > aqueous extract > acetone extract.
Figure 4.
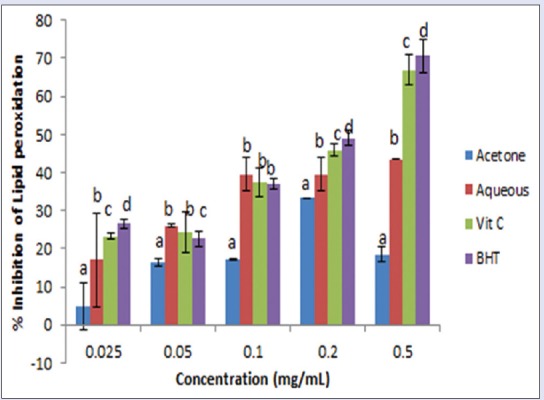
Percentage inhibition of lipid peroxidation by acetone and aqueous extracts of Lippia javanica. Columns with different letters are significantly different (P ≤ 0.05)
Phosphomolybdate
The extracts exhibited a concentration-dependent activity against phosphomolybdate radical in the order: Aqueous extract > gallic acid > Vitamin C > acetone extract [Figure 5]. The IC50 value [Table 2] for the aqueous extract was 0.007, while the acetone extract, Vitamin C, and gallic acid had values of 0.703, 0.113, and 0.184 mg/mL, respectively. This is an indication of the potent overall antioxidant capacity of the aqueous extract of L. javanica.
Figure 5.
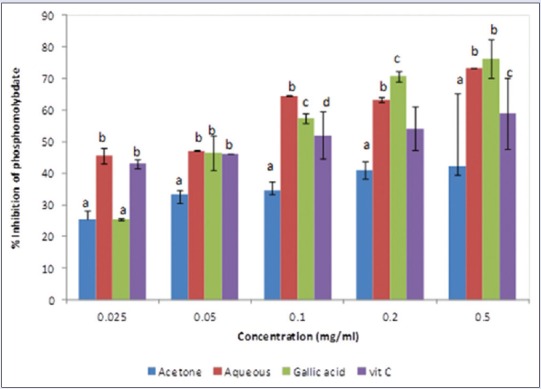
Percentage inhibition of phosphomolybdate radicals by acetone and aqueous extracts of Lippia javanica. Columns with different letters are significantly different (P ≤ 0.05)
Reducing power
The potentials of the plant extracts to reduce Fe3+ to Fe2+ by electron transfer are an indication of their antioxidant ability. The reducing power of the extracts in comparison with the standards (BHT, rutin, and Vitamin C) is presented in Figure 6. BHT and Vitamin C exhibited better-reducing power compared to the extracts while the aqueous extract exhibited a higher reducing power compared with the acetone extract.
Figure 6.
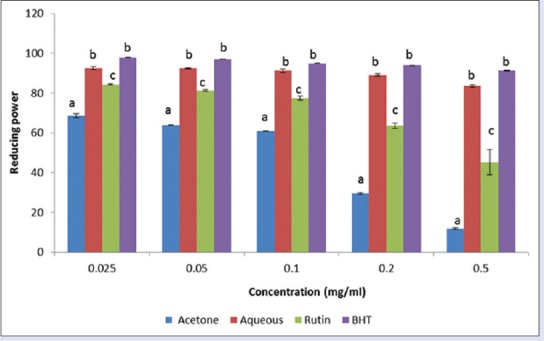
Ferric reducing power effect of acetone and aqueous extracts of Lippia javanica. Columns with different letters are significantly different (P ≤ 0.05)
Hydrogen peroxide
The acetone extract exhibited a strong activity against H2O2 compared to the aqueous extract and standards [Figure 7]. The order of activity was aqueous extract > Vitamin C > BHT > acetone extract.
Figure 7.
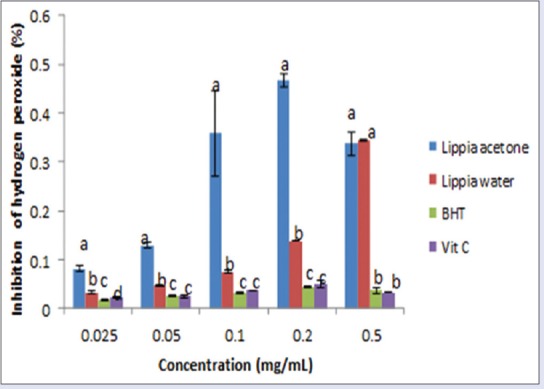
Effect of acetone and aqueous extracts of Lippia javanica on hydrogen peroxide radicals. Columns with different letters are significantly different (P ≤ 0.05)
Anti-inflammatory activity
Results on anti-inflammatory activity of the plant extracts showed that both extracts were able to inhibit protein denaturation in a concentration-dependent manner [Figure 8]. Aqueous extract was very active at IC50 = 0.250 mg/mL followed by the acetone extract 0.300 mg/mL and the anti-inflammatory drug diclofenac with IC50 of 0.6905 mg/mL [Figure 9]. The aqueous extract exhibited a higher membrane stability potential, compared with the acetone extract and the anti-inflammatory drug diclofenac [Figure 10]. The IC50 values [Figure 9] were 0.228, 0.314, and 0.314 mg/mL for the aqueous, acetone, and diclofenac, respectively.
Figure 8.
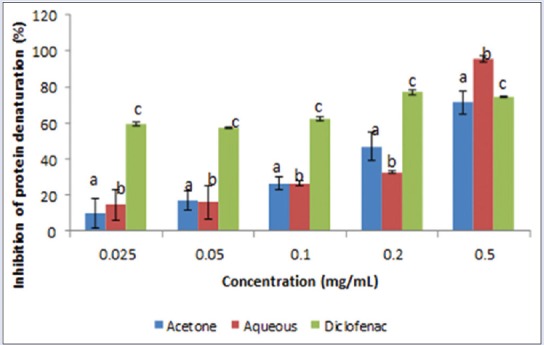
Inhibition of protein denaturation (%) by aqueous and acetone extracts of Lippia javanica. Columns with different letters are significantly different (P ≤ 0.05)
Figure 9.
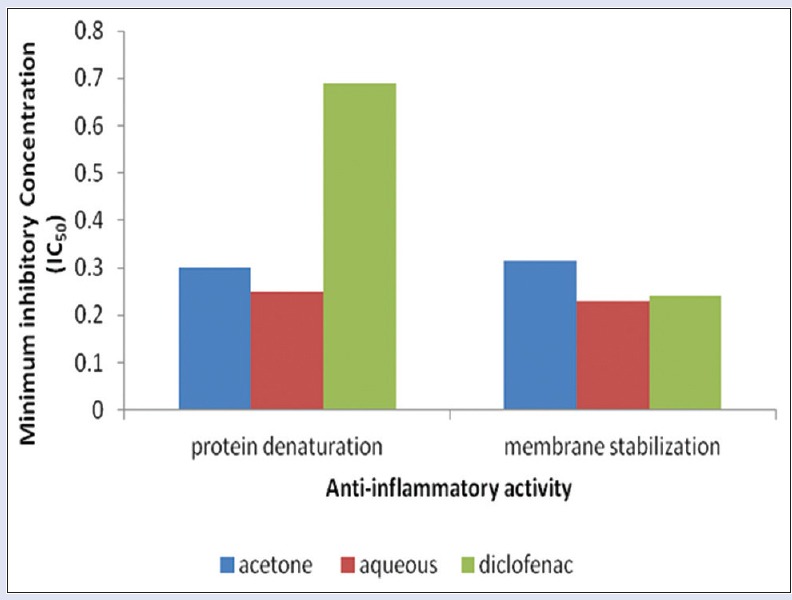
Minimum inhibitory concentration of anti-inflammatory activities of aqueous and acetone extracts of Lippia javanica
Figure 10.
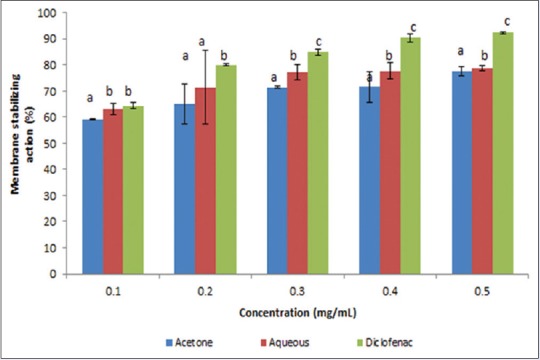
Membrane stabilizing action (%) of acetone and aqueous extracts of Lippia javanica. Columns with different letters are significantly different (P ≤ 0.05)
Antimicrobial activities
The aqueous and acetone extracts of L. javanica exhibited antibacterial effect against S. typhimurium, E. coli, L. Monocytogenes, and S. aureus. The two extracts showed similar activities on the tested bacteria [Table 3]. In addition, both extracts were active against all the fungi with zones of inhibition varying from 5 to 35 mm; the reference drug, nystatin, was also active on all the tested pathogens, but the most active was the acetone extract [Table 4]. The most susceptible fungi based on the overall mean diameter of growth inhibition were T. mucoides, T. rubrum, M. Gypseum, and T. tonsurans. A. niger, M. canis, P. aurantiogriseum, and A. fumigatus were quite resistant to the extracts even at a highest concentration of 5 mg/mL [Table 5].
Table 3.
Zone of inhibition (mm) and antibacterial minimum inhibitory concentration of Lippia javanica extracts
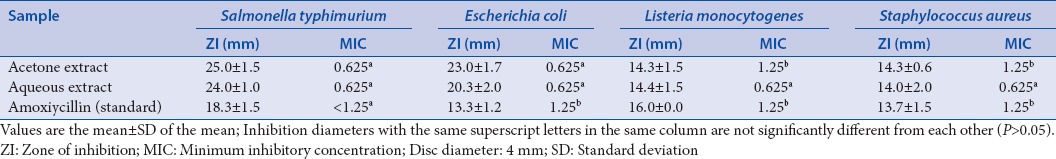
Table 4.
Antifungal activity of Lippia javanica extracts
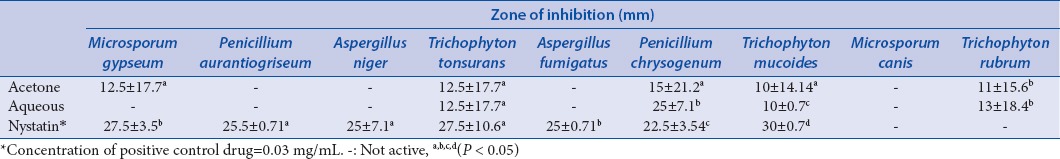
Table 5.
Minimum fungicidal concentration of Lippia javanica extracts

DISCUSSION
The data presented in this study demonstrate that both acetone and aqueous extracts of L. javanica possess excellent antioxidant and free radical scavenging activities. The observed in vitro activities suggest that the plant extracts could exert protective effects also in vivo against oxidative and free radical injuries occurring in different pathological conditions. According to Saeed et al.,[39] substances that have low antioxidant activity in vitro will probably show little activity in vivo. It is therefore assumed that substances with high antioxidant activity in vitro will probably show the same type of activity in vivo.
The total phenol, flavonoids, flavonol, and proanthocyanidin contents of both extracts were determined in this study. Phenolic compounds aid in the preservation of food, fresh flavor, taste, color and help in prevention of oxidative deterioration. In particular, many phenolic compounds are attracting the attention of food and medical scientists because of their antioxidative, anti-inflammatory, antimutagenic, and anticancer properties as well as their capacity to modulate some key cellular enzyme functions.[40] Phenolic compounds are very important plant constituents because they exhibit antioxidant activity by inactivating lipid free radicals or preventing decomposition of H2O2 into free radicals.[41] In addition, flavonoids and phenolic compounds are effective in preventing the formation of ROS and protecting low-density lipoprotein from iron- and copper-mediated free radical production.[42] Flavonoids are hydroxylated phenolics and are potent water soluble antioxidants which help in radical scavenging and the prevention of oxidative cell damage.[43] Proanthocyanidins are polyphenolic bioflavonoids which have a protective effect in eliminating hydroxyl radicals. The concentration of flavonoids and proanthocyanidin was higher in the acetone extracts compared with the aqueous extracts in this study which agree with several studies that acetone extracts had higher concentration of phenolic compound.[43,44] The presence of these polyphenols may be responsible for the therapeutic effects of L. javanica.
The scavenging activity of ABTS by the aqueous and acetone extracts was found to vary with concentration used and IC50 values; the acetone extracts exhibited better scavenging effect with ABTS compared to the aqueous extracts. The solubility of extracts in different testing systems and radical reactivity confirming the removal of odd electron are believed to be responsible for the higher scavenging activity of ABTS. The scavenging activity of ABTS radical by the plant extracts justifies the presence of compounds with free radical scavenging activity as well as the possibility of the extracts being used for treating radical-related pathological ailments.[43]
The electron donating ability of natural products can be measured by the decolorization of the DPPH radical. The degree of color change is proportional to the concentration and potency of the antioxidant. Both extracts and standards tested exhibited strong antioxidant effect against DPPH radicals. This implies that the high total polyphenol content could be responsible for the strong DPPH scavenging power since they can readily donate hydrogen atom, thereby quenching the radical.[45]
NO is an important chemical mediator generated by endothelial cells, macrophages, and neurons and is involved in the regulation of various physiological processes. Excess concentration of NO is associated with several diseases. Oxygen reacts with the excess NO to generate nitrite and peroxynitrite anions which act as free radicals. Although the standard (gallic acid) exhibited greater NO inhibition, both the aqueous and acetone extracts were also potent scavengers of NO and could be useful in the management of inflammatory reactions that are detrimental to human health.
Lipid peroxidation of cellular membranes by free radicals generates malondialdehyde which reacts with DNA to cause mutations.[46] Active components of L. javanica significantly inhibit the generation of lipid peroxides. This implies that the combination of phenols, flavonoids, and other bioactive compounds in the plant are actively involved in the antioxidant role of L. javanica extracts.
When converted to hydroxyl radical, H2O2 becomes toxic and may initiate lipid peroxidation and irreversible damage to DNA.[47] Both the aqueous and acetone extracts of L. javanica were able to quench these radicals. This is an indication of the presence of potent phytoconstituents which have the ability to scavenge hydroxyl radicals though the activities of such components may be shielded by the presence of other components in the extracts.
Phosphomolybdate assay is used to assess the overall antioxidant capacity of plant extracts. Extracts of L. javanica exhibit a high total antioxidant capacity against molybdenum radicals. This could be attributed to the presence of flavonoids in the extracts since many flavonoids and related phenols have been reported to contribute significantly to the phosphomolybdate scavenging activity of medicinal plants.[48]
Overall L. javanica exhibits high polyphenolic contents, outstanding reducing power, and good radical scavenging activity against DPPH, ABTS, NO, and inhibition of lipid peroxidation, H2O2, and phosphomolybdate. The free radical scavenging ability of L. javanica is dependent on the polyphenol content. Several studies have evaluated the relationships between antioxidant activity of plant products and their phenolic content. Some authors found a correlation between the phenolic content and antioxidant activity while others found no strong relationship.[49,50] In this study, a significant total phenolic content and free radical scavenging activities were found in both the acetone and aqueous extracts supporting the claim that a correlation exists between the total phenolic content and antioxidant activity.[7,50,51]
In addition, numerous monoterpenoids have been identified in the volatile extract of L. javanica including myrcene, caryophyllene, linalool, p-cymene, and ipsdienol.[41] L. javanica contains various organic acids and alcohols iridoid glycosides and toxic triterpenoids which have been detected in some Lippia species.[52,53,54] The presence of all these compounds in L. javanica could probably account for the medicinal uses of the plant as these metabolites are well known for their biological activities.[55] This finding seems to justify the folkloric use of infusing a combination of the aerial plant parts of L. javanica for therapeutic purposes. Previous studies have reported that the essential oils of L. javanica possess moderate antioxidant activity.[56,57]
Denaturation of proteins is a well-documented cause of inflammation. Some anti-inflammatory drugs have been reported to show dose-dependent ability to prevent thermally-induced protein denaturation.[58,59] In the present investigation, the in vitro anti-inflammatory effects of aqueous and acetone extracts were evaluated against denaturation of bovine serum albumin and stability of rat erythrocyte membrane. Both extracts exhibited a concentration-dependent inhibition of protein denaturation and membrane stability within the concentration ranges studied and was compared with diclofenac, a standard anti-inflammatory drug. Protein denaturation and membrane lysis have been reported to be the cause of inflammation in conditions such as rheumatism and arthritis;[60,61] therefore, any substance that can prevent or inhibit protein denaturation and enhance membrane stability will be a good anti-inflammatory agent. The ability of L. javanica extracts to enhance these two as observed in this study could account for its reported folkloric use as an anti-inflammatory agent.[60,61] The precise mechanism of this anti-inflammatory action is yet to be elucidated and will be further investigated.
The results from the antimicrobial studies are quite encouraging as almost all the extracts exhibited moderate antimicrobial activity against most of the tested pathogens, which included Gram-negative and Gram-positive bacteria as well as fungi. Although some pathogens such as M. canis, P. aurantiogriseum, A. niger, and A. fumigatus showed resistance to both the aqueous and acetone extracts of L. javanica. This study agrees with the reports of Mangena and Muyima[62] that Tagetes minuta, L. javanica, and Foeniculum vulgare oils displayed remarkable antimicrobial activity against all the tested organisms.[62]
CONCLUSION
The polyphenolic content, free radical scavenging, anti-inflammatory, and antimicrobial activities of the aqueous and acetone extracts of L. javanica (Burm.F.) Spreng were evaluated. The data presented in this study demonstrate that both extracts possess excellent antioxidant, free radical scavenging, anti-inflammatory, and antimicrobial activities. There was correlation between the antioxidant activities and the total phenolic and flavonoids content. A concentration-dependent inhibition of protein denaturation and membrane stabilization of the aqueous and acetone extracts were also observed. Therefore, the ability of L. javanica extracts to inhibit protein denaturation and maintain stability as reported in this study could account for its folkloric use in the treatment of inflammatory diseases. The overall antimicrobial activity evaluated revealed that both extracts were active, but the acetone extract exhibited a higher antifungal activity compared to the reference drugs. Further studies on the characterization of various antioxidant compounds present and the mechanism of anti-inflammatory action are in progress.
Financial support and sponsorship
This study was supported by the Govan Mbeki Research and Development Centre (GMRDC) University of Fort Hare, South Africa; and Medical Research Council (MRC), South Africa.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
This study was supported by the Govan Mbeki Research and Development Centre and Medicinal Plants and Economic Development Research Centre, both of the University of Fort Hare, South Africa.
ABOUT AUTHOR

Gloria A Otunola
Gloria A Otunola, PhD, is a Researcher at the Medicinal Plants and Economic Development (MPED) Research Centre, Department of Botany, University of Fort Hare, South Africa. Her research interest is on the biochemical, pharmacological and metabolomic elucidation of medicinal plants and food fortification in the management of chronic and metabolic diseases.
REFERENCES
- 1.Halliwell B, Gutteridge JM, Cross CE. Free radicals, antioxidants, and human disease: Where are we now? J Lab Clin Med. 1992;119:598–620. [PubMed] [Google Scholar]
- 2.Feher M, Schmidt JM. Property distributions: Differences between drugs, natural products, and molecules from combinatorial chemistry. J Chem Inf Comput Sci. 2003;43:218–27. doi: 10.1021/ci0200467. [DOI] [PubMed] [Google Scholar]
- 3.Galm U, Shen B. Natural product drug discovery: The times have never been better. Chem Biol. 2007;14:1098–104. doi: 10.1016/j.chembiol.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Ejele AE, Duru IA, Ogukwe CE, Iwu IC. Phytochemistry and antimicrobial potential of basic metabolites of Piper umbellatum, Piper guineense, Ocimum gratissmium and Newbouldia laevis extracts. J Emerg Trends Eng Appl Sci. 2012;3:309–14. [Google Scholar]
- 5.Cao G, Sofic E, Prior RL. Antioxidant capacity of tea and common vegetables. J Agric Food Chem. 1996;44:3426–31. [Google Scholar]
- 6.Himani R, Garima S, Nupur J, Neelam S, Navneeta B. Phytochemical importance of medicinal plants as potential sources of anticancer agents. Turk J Bot. 2014;38:1027–35. [Google Scholar]
- 7.Conforti F, Sosa S, Marrelli M, Menichini F, Statti GA, Uzunov D, et al. In vivo anti-inflammatory and in vitro antioxidant activities of Mediterranean dietary plants. J Ethnopharmacol. 2008;116:144–51. doi: 10.1016/j.jep.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 8.Libby P. Inflammatory mechanisms: The molecular basis of inflammation and disease. Nutr Rev. 2007;65(12 Pt 2):S140–6. doi: 10.1111/j.1753-4887.2007.tb00352.x. [DOI] [PubMed] [Google Scholar]
- 9.Geronikaki AA, Gavalas AM. Antioxidants and inflammatory disease: Synthetic and natural antioxidants with anti-inflammatory activity. Comb Chem High Throughput Screen. 2006;9:425–42. doi: 10.2174/138620706777698481. [DOI] [PubMed] [Google Scholar]
- 10.Hutchings A, Scott AH, Lewis G, Cunningham A. Pietermaritzburg: Universty of Natal Press; 1996. Zulu Medicinal Plants: An Inventory; p. 450. [Google Scholar]
- 11.Pascual ME, Slowing K, Carretero E, Sánchez Mata D, Villar A. Lippia: Traditional uses, chemistry and pharmacology: A review. J Ethnopharmacol. 2001;76:201–14. doi: 10.1016/s0378-8741(01)00234-3. [DOI] [PubMed] [Google Scholar]
- 12.Van Wyk BE. A review of ethnobotanical research in South Africa. S Afr J Bot. 2002;68:1–13. [Google Scholar]
- 13.Leistner OA. Pretoria: National Botanical Institute; 2000. Strelitzia. Seed Plants of Southern Africa: Families and Genera; p. 775. [Google Scholar]
- 14.Wolfe K, Wu X, Liu RH. Antioxidant activity of apple peels. J Agric Food Chem. 2003;51:609–14. doi: 10.1021/jf020782a. [DOI] [PubMed] [Google Scholar]
- 15.Ordonez AA, Gomez JD, Vattuone MA, Isla MI. Antioxidant activities of Sechium edule (Jacq) Food Chem. 2006;97:452–8. [Google Scholar]
- 16.Kumaran A, Karunakaran RJ. In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India. Food Sci Technol. 2007;40:344–52. [Google Scholar]
- 17.Sun JS, Tsuang YH, Chen IJ, Huang WC, Hang YS, Lu FJ. An ultra-weak chemiluminescence study on oxidative stress in rabbits following acute thermal injury. Burns. 1998;24:225–31. doi: 10.1016/s0305-4179(97)00115-0. [DOI] [PubMed] [Google Scholar]
- 18.Shiddhuraju P, Mohan PS, Becker K. Studies on antioxidant activity of Indian Laburnum (Cassia fistula L.): A preliminary assessment of crude extracts from stem back leaves, flower and fruit pulp. Food Chem. 2002;79:61–7. [Google Scholar]
- 19.Liyana-Pathirana CM, Shahidi F. Antioxidant activity of commercial soft and hard wheat (Triticum aestivum L.) as affected by gastric pH conditions. J Agric Food Chem. 2005;53:2433–40. doi: 10.1021/jf049320i. [DOI] [PubMed] [Google Scholar]
- 20.Pandey N, Chaurasia JK, Tiwari OP, Tripathi YB. Antioxidant properties of different fractions of tubers from Pueraria tuberosa Linn. Food Chem. 2007;105:219–222. [Google Scholar]
- 21.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem. 1982;126:131–8. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 22.Ruch RT, Cheng SJ, Klaunig JE. Spin trapping of superoxide and hydroxyl radicals. Methods Enzymol. 1984;105:198–209. doi: 10.1016/s0076-6879(84)05026-6. [DOI] [PubMed] [Google Scholar]
- 23.Dasgupta N, De B. Antioxidant activity of Piper betel L. Leaf extract in vitro. Food Chem. 2004;88:21924. [Google Scholar]
- 24.Rabia K, Muhammad A, Yamin B, Saira A, Sunbal KC. Evaluation of ethnopharmacological and antioxidant potential of Zanthoxylum armatum DC. Hindawi Publishing Corporation. J Chem 2015. 2015:8. [Google Scholar]
- 25.Sakat S, Juvekar AR, Gambhire MN. In vitro antioxidant and anti-inflammatory activity of methanol extract of Oxalis corniculata Linn. Int J Pharm Pharmacol Sci. 2010;2:146–55. [Google Scholar]
- 26.Alam MA, Ghani A, Subhan N, Rahman M, Haque M, Majumder M. Antioxidant and membrane stabilizing properties of the flowering tops of Anthocephalus cadamba. Nat Prod Commun. 2008;3:65–70. [Google Scholar]
- 27.Deans SG, Ritchie G. Antibacterial properties of plant essential oils. Int J Food Microbiol. 1987;5:165–80. [Google Scholar]
- 28.Oyedeji OO, Lawal OA, Shode FO, Oyedeji AO. Chemical composition and antibacterial activity of the essential oils of Callistemon citrinus and Callistemon viminalis from South Africa. Molecules. 2009;14:1990–8. doi: 10.3390/molecules14061990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Villanova, PA: National Committee for Clinical Laboratory Standards; 2004. National Committee for Clinical Laboratory Standards. Performance for Antimicrobial Susceptibility Testing. Standard M100-S5. [Google Scholar]
- 30.Duarte MC, Figueira GM, Sartoratto A, Rehder VL, Delarmelina C. Anti-Candida activity of Brazilian medicinal plants. J Ethnopharmacol. 2005;97:305–11. doi: 10.1016/j.jep.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 31.Afolayan AJ. Extracts from the shoots of Arctotis arctotoides inhibit the growth of bacteria and fungi. Pharm Biol. 2003;41:22–5. [Google Scholar]
- 32.Shai LJ, McGaw LJ, Masoko P, Eloff JN. Antifungal and antibacterial activity of seven traditionally used South African plant species active against Candida albicans. S Afr J Bot. 2008;74:677–84. [Google Scholar]
- 33.Otang WM, Grierson DS, Ndip RN. Ethnobotanical survey of medicinal plants used in the management of opportunistic fungal infections in HIV/AIDS patients in the Amathole District of the Eastern Cape Province, South Africa. J Med Plant Res. 2012;6:2071–80. [Google Scholar]
- 34.Samie A, Tambani T, Harshfield E, Green E, Ramalivhana JE, Bessong PO. Antifungal activities of selected Venda medicinal plants against Candida albicans, Candida krusei and Cryptococcus neoformans isolated from South African AIDS patients. Afr J Biotechnol. 2010;9:2965–76. [Google Scholar]
- 35.Dagne E, Van Wyk BE, Mueller M, Steglich W. Three dihydroanthracenones from Gasteria bicolor. Phytochemistry. 1996;41:795–9. doi: 10.1016/0031-9422(95)00704-0. [DOI] [PubMed] [Google Scholar]
- 36.Jones NP, Arnason JT, Abou-Zaid M, Akpagana K, Sanchez-Vindas P, Smith ML. Antifungal activity of extracts from medicinal plants used by first nations peoples of eastern Canada. J Ethnopharmacol. 2000;73:191–8. doi: 10.1016/s0378-8741(00)00306-8. [DOI] [PubMed] [Google Scholar]
- 37.Eloff JN. Which extractant should be used for the screening and isolation of antimicrobial components from plants? J Ethnopharmacol. 1998;60:1–8. doi: 10.1016/s0378-8741(97)00123-2. [DOI] [PubMed] [Google Scholar]
- 38.Xu BJ, Chang SK. Total phenolic content and antioxidant properties of eclipse black beans (Phaseolus vulgaris L.) as affected by processing methods. J Food Sci. 2008;73:H19–27. doi: 10.1111/j.1750-3841.2007.00625.x. [DOI] [PubMed] [Google Scholar]
- 39.Saeed N, Khan MR, Shabbir M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement Altern Med. 2012;12:221. doi: 10.1186/1472-6882-12-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jimoh FO, Adedapo AA, Aliero AA, Afolayan AJ. Polyphenolic contents and biological activities of Rumex ecklonianus. Pharm Biol. 2008;46:333–40. [Google Scholar]
- 41.Pokorny J. Introduction. In: Pokorny J, Yanishlieva N, Gordon NH, editors. Antioxidant in Foods: Practical Applications. Cambridge: Woodhead Publishing Limited; 2001. pp. 1–3. [Google Scholar]
- 42.Owen PI, John T. Antioxidants in medicines and spices as cardioprotective agents in Tibetan highlanders. Pharm Biol. 2002;40:346–57. [Google Scholar]
- 43.Wintola OA, Afolayan AJ. Phytochemical constituents and antioxidant activities of the whole leaf extract of Aloe ferox Mill. Pharmacogn Mag. 2011;7:325–33. doi: 10.4103/0973-1296.90414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loots DT, van der Westhuizen FH, Botes L. Aloe ferox leaf gel phytochemical content, antioxidant capacity, and possible health benefits. J Agric Food Chem. 2007;55:6891–6. doi: 10.1021/jf071110t. [DOI] [PubMed] [Google Scholar]
- 45.Awah FM, Verla A. Antioxidant activity, nitric oxide scavenging activity and phenolic content of Ocimum gratissimum leaf extract. J Med Plant Res. 2010;4:2481–2. [Google Scholar]
- 46.Marnett LJ. Oxyradicals and DNA damage. Carcinogenesis. 2000;21:361–70. doi: 10.1093/carcin/21.3.361. [DOI] [PubMed] [Google Scholar]
- 47.Halliwell B, Gutteridge JM. 3rd ed. New York: Oxford University Press; 1999. Free Radical in Biology and Medicine. [Google Scholar]
- 48.Khan TA, Mazid M, Mohammad F. Potential of ascorbic acid against oxidative burst in plants under biotic stress. A review. J Ind Res Technol. 2012;2:72–80. [Google Scholar]
- 49.Velioglu Y, Mazza G, Gao L, Oomah B. Antioxidant activity and total phenolics in selected fruits, vegetables and grain products. J Agric Food Chem. 1998;46:4113–7. [Google Scholar]
- 50.Mwangi JW, Addae-Mensah I, Munavu RM, Lwande W. Essential oils of Kenyan Lippia species. Part III. Flavour Fragr J. 1991;6:221–4. [Google Scholar]
- 51.Buckingham J. London: Chapman and Hall; 2006. Dictionary of Natural Products on CD-ROM. [Google Scholar]
- 52.Nzira L, Per M, Peter F, Claus B. Lippia javanica (Burm F) Spreng: Its general constituents and bioactivity on mosquitoes. Trop Biomed. 2009;26:85–91. [PubMed] [Google Scholar]
- 53.Mujovo SF, Hussein AA, Meyer JJ, Fourie B, Muthivhi T, Lall N. Bioactive compounds from Lippia javanica and Hoslundia opposita. Nat Prod Res. 2008;22:1047–54. doi: 10.1080/14786410802250037. [DOI] [PubMed] [Google Scholar]
- 54.Olivier DK, Shikanga EA, Combrinck S, Krause RW, Regnier T, Dlamini TP. Phenylethanoid glycosides from Lippia javanica. S Afr J Bot. 2010;76:58–63. [Google Scholar]
- 55.Muyima N, Nziweni S, Mabinya L. Antimicrobial and antioxidative activities of Tagetes minuta, Lippia javanica and Foeniculum vulgare essentials oil from the Eastern Cape Province of South Africa. J Essent Oil Bearing Plants. 2004;7:68–78. [Google Scholar]
- 56.Dzoyem JP, Eloff JN. Anti-inflammatory, anticholinesterase and antioxidant activity of leaf extracts of twelve plants used traditionally to alleviate pain and inflammation in South Africa. J Ethnopharmacol. 2015;160:194–201. doi: 10.1016/j.jep.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 57.Kähkönen MP, Hopia AI, Vuorela HJ, Rauha JP, Pihlaja K, Kujala TS, et al. Antioxidant activity of plant extracts containing phenolic compounds. J Agric Food Chem. 1999;47:3954–62. doi: 10.1021/jf990146l. [DOI] [PubMed] [Google Scholar]
- 58.Nickavar B, Kamalinejad M, Izadpanah H. In vitro free radical scavenging activity of five Salvia species. Pak J Pharm Sci. 2007;20:291–4. [PubMed] [Google Scholar]
- 59.Mizushima Y, Kobayashi M. Interaction of anti-inflammatory drugs with serum proteins, especially with some biologically active proteins. J Pharm Pharmacol. 1968;20:169–73. doi: 10.1111/j.2042-7158.1968.tb09718.x. [DOI] [PubMed] [Google Scholar]
- 60.Umapathy EU, Ndebia EJ, Meeme A, Adam B, Menziwa P, Nkeh-Chungag BN, et al. An experimental evaluation of Albuca setosa aqueous extract on membrane stabilization, protein denaturation and white blood cell migration during acute inflammation. J Med Plants Res. 2010;4:789–95. [Google Scholar]
- 61.Chandra S, Chatterjee P, Dey S, Bhattacharya S. Evaluation of anti-inflammatory effect of ashwagandha: A preliminary study in vitro. Pharmacogn J. 2012;4:47–9. [Google Scholar]
- 62.Mangena T, Muyima NY. Comparative evaluation of the antimicrobial activities of essential oils of Artemisia afra, Pteronia incana and Rosmarinus officinalis on selected Bacteria and yeast strains. Lett Appl Microbiol. 1999;28:291–6. doi: 10.1046/j.1365-2672.1999.00525.x. [DOI] [PubMed] [Google Scholar]


