Abstract
Background:
As part of our search for new potential natural resources to eradicate infection, we have revealed the prominent potency of massoia bark (Massoia aromatica Becc, Lauraceae) in combating immunosuppressed-related infection.
Materials and Methods:
The extract was prepared by macerating the pulverized dried bark in ethanol 95%, followed by solvent evaporation. The oil was extracted from the dried bark by steam-hydrodistillation of which preparative thin-layer chromatography was performed on the oil to isolate the active constituent, C-10 massoia lactone (ML). Anti-biofilm assay against Candida albicans was conducted on polystyrene 96 wells microtiter plates, followed by a confocal laser scanning microscope observation to get three-dimensional profiles of the affected biofilms. Effects on the hyphae development inoculated on RPMI-1640 agar plates were observed for 7 days. Influences of samples on mice macrophage phagocytosis were examined by an in vitro technique. Samples concentration tested were in the range of 2.0–0.0625 mg/mL and done in triplicate.
Results:
Massoia bark extracts (oil and solid phase) and ML exhibited promising activities as anti-biofilm against C. albicans at IC50 0.074% v/v, 271 μg/mL and 0.026 μg/mL, respectively. The ML did not inhibit the hyphae development at the concentration tested; however, the extracts showed inhibition at 62.5 μg/mL. Macrophage phagocytosis stimulation was correlated to the ML content.
Conclusion:
Massoia bark is potential to be developed as anti-infective in immunosuppressed condition of which the C10 ML (C10H16O2) plays a major role in exerting activity.
SUMMARY
Massoia bark extracts (oily and solid phase) and C-10 Massoia lactone exhibited promising activities as antibiofilm against Candida albicans at IC50 are 0.074 %v/v, 271 μg/mL and 0.026 μg/mL respectively. The major constituent, C-10 Massoia lactone (C10H16O2) plays major role in exerting anticandida activity and potentially acts as an immunomodulator as well. However extracts showed inhibition of hyphae development of C. albicans which showed no correlation to the content of the Massoia lactone.
Abbreviations used: GC/MS: Gas Chromatography/Mass Spectrometry, ML: Massoia Lactone, TLC: Thin Layer Chromatography, ATCC: American Type Culture Collection, RPMI: Roswell Park Memorial Institute, PBS: Phosphate Buffer Sterile, LSM: Laser scanning microscope, DMSO: Dimethyl sulfoxide, UV: Ultra violet, SDB: Sabouraud dextrose agar, MeOH: Methanol, LB: Luria Bertani, EtOAc: Ethyl acetate, CLSM: Confocal Laser Scanning Microscope, PI: Propidium iodide.
Keywords: Biofilm, Candida albicans, macrophage, massoia bark, massoia lactone
INTRODUCTION
Massoia aromatica Becc. (synonym Cryptocarya massoy Oken, Lauraceae) has been used commercially to produce essential oils. Its traditional usage by Javanese and Balinese women is to prepare a warming ointment having a pleasant smell, called bobory. Detailed chemical evaluation using gas chromatography/mass spectrometry (GC/MS) revealed the major components in the bark oil as C-10 massoia lactone (ML) or 5,6-dihydro-6-pentyl-2H-pyran-2-one, (65–68%), and the C-12 ML, or 5,6-dihydro-6-heptyl-2H-pyran-2-one, (17–28%).[1] The MLs are rare essential oil components,[2] but its production by chemical synthesis route has been reported.[3,4,5] Candida albicans is a normal flora in single cell phase which can convert to an opportunistic pathogen as multicellular fungi, mostly in an immunocompromised condition.[5,6] The capacity of C. albicans to cause disease are closely associated with its ability to grow as biofilm communities by adhering both to cellular and inanimate surfaces of implanted devices such as catheters, prosthetic cardiac valves, contact lenses, artificial joints, and intrauterine devices. Microorganisms involved in biofilms are generally more resistant to antimicrobials and also against the host defense mechanism in comparison to the single cell planktonic state.[7] This fact makes them a progressive source of recurrent infections.[8] Although the drug resistance mechanisms to candida biofilms are still poorly understood, the action of antifungal may be limited by their poor penetration into the biofilm matrix.[9] Microbes in biofilm state normally have slower growth causing the antimicrobials targeting the microbial growth lose the efficacy. Moreover, biofilm heterogeneity is thought to be a contributing factor for the drug resistance phenomenon.[10] Biofilm communities develop differential gradients in nutrients and metabolic waste, so that groups of cells in the same biofilm react variably in response to environmental cues, leading to a variety of phenotypes.[11] Our previous study have suggested the prominent potency of the massoia oil in preventing and breakdown of C. albicans, Pseudomonas aeruginosa, and Staphylococcus aureus biofilms. In this research, we studied the potency of the massoia bark ethanolic extract as well as the oil major constituent, ML toward the C. albicans biofilm and hypha development as well as toward mice macrophages.[12] No effect of ML on immune response has been reported, yet. However, other lactone substances, such as homoserine,[13] macrolides, lactone, and[14] sesquiterpene lactone, do exhibit immunomodulatory properties.[14,15] Ethanolic extract of other close related plant of Lauraceae family, Cinnamomum zeylanicum, has been reported to have immunomodulatory effect.
MATERIALS AND METHODS
Cell, chemical, and biochemical
Silica gel 60 F254 aluminum precoated plate, toluene, ethyl acetate, which are analytical grade (Merck, Germany) were used in thin-layer chromatography (TLC) densitometry. GC-2010 gas chromatography (Shimadzu, Japan) equipped with a GC-MS-QP2010 plus mass spectrometer (Shimadzu, Japan). An Agilent J & W DB-1 column (30 m length, 0.25 mm diameter, 0.25 µm film thickness, Agilent) was used for the component analysis.
C-10 ML content in extract and oil were quantified by Camag TLC scanner slit dimensions 8.00 mm × 0.40 mm and a scanning speed of 80 mm/s.
C. albicans ATCC 10231, RPMI-1640 medium, L-glutamine (Sigma), sterile phosphate-buffered saline (PBS), crystal violet, fluconazole were used for biological assays. Macrophages were obtained from 3-month-old mice, taken from the peritoneal fluid with RPMI.
A Carl Zeiss LSM5 Exciter Laser Scanning Confocal Microscope (Leica Microsystems, Germany) was used to observe the architecture of the C. albicans biofilms, analog with the standardized cell suspension Candida biofilms (3 ml of a suspension containing 1.0 × 107 cells/mL in LB medium, live/dead fluorescent staining (10 µL of 3.34 µM SYTO9 and 10 µL of 20 µM propidium iodide (PI) both in dimethyl sulfoxide [DMSO]) (Molecular Probes, USA). The image was subsequently analyzed using the freely available image processing software ImageJ Version 1.46 (Rasband, National Institutes of Health [NIH], Bethesda, Maryland, USA: http://rsb.info.nih.gov/ij/) including the LSM reader plug into open LSM5 formatted image stack created by the microscope software.
Plant material
Three kilograms of M. aromatica bark were obtained from a traditional market in Yogyakarta, Indonesia, in February 2010. A voucher specimen was deposited at Department of Pharmaceutical Biology, Faculty of Pharmacy, Gadjah Mada University, Indonesia, and identified by Mr. Djoko Santosa, M. Sc., (plant taxonomist) under Nr. BF/14/Ident/Det/I/2015.
Sample preparation
The essential oil was obtained by steam-hydrodistillation process. Oil sample was stored in a sealed dark glass vial and kept at 4°C. Stock solutions of 50% (v/v) essential oils were prepared in methanol (MeOH). Ethanolic extract was prepared by macerating the pulverized bark in ethanol 95% for 24 h, repeated once. The filtrate was combined and evaporated to yield thick constituent.
Planar chromatographic separation and analysis
Chromatography was performed on 10 cm × 20 cm silica gel 60 F254 aluminum sheet TLC plates (Merck, Germany).[16,17] Before use, the plates were preconditioned by heating at 120°C for 3 h. The position of the starting line was 1.0 cm from the bottom and 1.0 cm from the left side. After sample application, the TLC plates were developed with the previously optimized mobile phase. For the separation of essential oils, toluene–ethyl acetate, 93:7 v/v, was used as a mobile phase. The development mode was ascendant in a saturated twin trough chamber (Camag, Switzerland). All TLC separations were performed at room temperature.
After chromatographic separation, the absorbent layers were dried in an oven at 90°C for 5 min to remove the solvent completely. An alcoholic vanillin–sulfuric acid reagent was used to visualize the separated spots. The developed layers were dipped into this reagent and heated for 5 min at 100°C. Detection of the separated compounds was performed according to retardation factor (Rf) value, i.e. the ratio of the distance traveled by the center of a spot to the distance traveled by the solvent front; and color of the standards.
A preparative TLC separation of M. aromatica oil was performed on a 10 cm × 20 cm silica gel 60 F254 aluminum sheet TLC plates (Merck, Germany) as described above by spotting the essential oil over the whole width of the starting line (streaking the plate). UV254 instead of stain reagent was used for the visualization. The area (band) which has similar Rf value with the inhibition zone was marked and carefully rubbed out of using a spatula. The loosened silica then was transferred onto a glass funnel of which glass wool is present, and ethyl acetate was used as a solvent. The eluted fraction that has been collected and dissolved in ethanol for further analyses using TLC, of which observation was conducted under UV254. GC-MS analysis also performed to obtain information about the chemical composition of the eluted fraction.
Effect of massoia lactone and massoia extracts on Candida albicans hyphal growth
Standard cell suspension of C. albicans was grown on RPMI-1640 agar plates supplemented with different concentrations of sample tested. The plates were incubated for 7 days at 37°C and the morphology of fungal colony was photographed using a digital camera.
Effect of massoia lactone and massoia extracts on Candida albicans planktonic growth
The antifungal activity of M. aromatica's essential oil and extracts against C. albicans planktonic cells was evaluated using microdilution method with Sabouraud Dextrose Broth (SDB) as the medium.[18] The test compounds were prepared with different concentrations using methanol. The inoculum of 5 × 107 CFU/mL in SDB was used for the assay and the final volume in each well was 200 µL including SDB, cells, and test compound. As positive control, amphotericin with concentration of 125 µg/mL in DMSO was used. Inhibition growth activity was monitored and calculated based on the optical density at 595 nm of the sample tested versus control after 48 h of incubation at 37°C.
Effect of massoia lactone and massoia extract on Candidas biofilm inhibition
Biofilms were formed on polystyrene flat bottom 96-well microtiter plates (Sarstedt, Inc., Newton, NC, USA).[19,20] Briefly, 100 µL of a standardized cell suspension (107 cells/mL) in the RPMI-1640 medium (without sodium bicarbonate) supplemented with L-glutamine (Sigma) was transferred into each well of a microtiter plate, and the plate was incubated for 90 min at 37°C of adhesion phase. The cell suspensions were then aspirated, and each well was washed twice with 150 µL of PBS to remove loosely adhered cells. A total of 100 µL RPMI media containing concentration of C-10 ML ranging from 100 µg/mL to 0.30 µg/mL, massoia solid extract ranging from 1000 µg/mL to 30 µg/mL and massoia oily extract ranging from 1% v/v to 0.03% v/v was added to the washed wells. For vehicle control, we used media containing MeOH or DMSO. Amphotericin B 125 µg/mL was used as a positive control in this study. For the negative control, biofilms were not exposed to samples while for the media control, wells should be unseeded. The plates were then incubated at 37°C for 8 h for early phase biofilm, 24 h for intermediate phase biofilm, and 48 h for mature phase biofilm. Following the incubation period, the content of each well was aspired, rinsed 3 times with distilled water, and dried at room temperature for 10 min. Then, 125 µL of 1% crystal violet stain was added to the wells and left for 15 min. The excess stain was rinsed off with tap water, and 200 µL ethanol was added to the wells. Optical density readings were obtained by a plate reader (Bio-Rad 680XR) at 595 nm. Testing was performed in triplicate. Samples found to reduce at least 50% biofilm formation were considered as biofilm preventive agents.
Effect of massoia lactone and massoia extract on fungal established biofilms
C. albicans biofilms of 24 and 48 h old were cultured at 37°C on the wells of microtiter plates using the protocol described by Pierce et al.[19] Briefly, 100 µL of a standardized cell suspension (107 cells/mL) in the RPMI-1640 medium (without sodium bicarbonate) supplemented with L-glutamine (Sigma) was transferred into each well of a microtiter plate and was incubated for 24 and 48 h at 37°C. Following biofilm formation, the medium was aspirated, and nonadherent cells were removed by rinsing the biofilms 3 times in 150 µL sterile PBS per well. After rinsing, residual PBS was removed by blotting the microtiter plates in an inverted position with paper towels. Serial double dilutions of C-10 ML in RPMI-1640 medium with concentrations ranged from 100 µg/mL to 0.30 µg/mL, massoia solid extract in RPMI-1640 medium with concentrations ranged from 1000 µg/mL to 30 µg/mL and massoia oily extract ranging from 1% v/v to 0.03% v/v, were then added to the washed wells and the plates were incubated at 37°C for another 24 and 48 h. The effects on established biofilms were estimated using crystal violet staining and read by microtiter plates (Bio-Rad 680XR) at 595 nm. Testing was performed in triplicate. Amphotericin B concentration 125 µg/mL was used as a positive control in this study.[20] Compound found to reduce at least 50% biofilm formation were considered as biofilm preventive agents.
Anti-biofilm activity observed by confocal laser scanning microscope
Preformed Candida biofilms were prepared by dispensing the standardized cell suspension (3 ml of a suspension containing 1.0 × 107 cells/mL in LB medium) onto 25 mm diameter presterilized plastic coverslips (Thermanox; Nulge Nunc International) was placed in the wells of presterilized flat bottomed six-well plates (Iwaki).[20,21,22] The plate was incubated at 37°C for 90 min (adhesion phase). Following the incubation period, the supernatant was removed, and a total of 3 mL RPMI medium containing different concentrations of tested samples oils were added to the washed wells. The plate was then incubated for 24 h (intermediate phase) and 48 h (maturation phase) at 37°C. To obtaine information about the efficacy of oil on degrading the established Candida biofilm, biofilms were performed for 24 and 48 h at 37°C on 25 mm diameter presterilized plastic coverslips (Thermanox; Nulge Nunc International) placed in the bottom of six wells microtiter plates. Following incubation period, the supernatant was removed, and a total of 3 mL RPMI medium containing different concentrations of plant oils were added to the washed wells. The plate was then incubated for another 24 and 48 h at 37°C. Afterward, the coverslips were washed twice with PBS and stained using the Bac light bacterial viability kit of Molecular Probes (10 µL of 3.34 µM SYTO9 and 10 µL of 20 µM PI both in DMSO) (Molecular Probes, USA) before examined under the Carl Zeiss LSM5 to observe the architecture of C. albicans biofilms. A 40× oil immersion objective was used with 488 nm Argon laser excitation and 500–640 nm band pass emission setting. The images were subsequently analyzed using the freely available image processing software ImageJ Version 1.46 (Rasband, NIH, Bethesda, Maryland, USA: http://rsb.info.nih.gov/ij/). The images’ scale bar used to calibrate the ImageJ area measurement algorithm. The observations were made in triplicates, and representative images are presented here.
Effect of massoia lactone, massoia extracts, and massoia oil on phagocytosis activity of macrophages
Measurement of phagocytosis activity conducted by using 3 μm latex beads suspended in PBS to obtain a concentration of 2.5 × 106 mL−1.[23] Culture of peritoneal macrophages was 24 h in wells equipped with coverslips, washed twice with RPMI-1640, and then added to the samples with various concentrations ranging from 1000 µg/mL to 30 µg/mL (200 μL/well). Incubation was performed in 5% CO2 incubator at 37°C for 4 h. The cells were washed 3 times with PBS, and then added to a suspension of latex beads (200 μL/well). Incubation was performed in 5% CO2 incubator at 37°C for 1 h. The cells were washed 3 times with PBS to remove the latex beads. Fixation with methanol took 30 s, the cover slip was allowed to dry, and 2% v/v Giemsa dye was left for 20 min. After being washed with distilled water and left to dry, the number of macrophages which engulfed the latex and total amount of enguled latex beads were counted under a light microscope to count the macrophage phagocytosis index.[24] The phagocytic index was calculated according to the following formula:
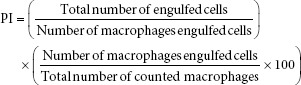
The animal handling has been approved by the Ethical Clearance Commission for preclinical studies of the LPPT-UGM (The Integrated Research and Testing Laboratory, Universitas Gadjah Mada) under Nr. 217/KEC-LPPT/II/2015.
Data analyses
One-way ANOVA was used at 95% confidence level for statistical data analyses.
RESULTS AND DISCUSSION
Yield of massoia oil was 0.4% v/w while the extract was 11.33% w/w (in total). The extract was separated into two phases, oil and solid. Phytochemical content comparison by TLC as shown in Figure 1 that describes the present of ML C-10 in the extract in much lower amount than in the oil. The oily part of the extract showed similar profile with the solid, but with different spots intensities. Content of C-10 ML in oil and extract was quantitatively determined by the TLC-densitometry at 211 nm [Figure 2]. Table 1 described the content of C-10 ML in massoia oils and extracts resulted from the TLC-densitometry measurement. Massoia oil has the highest content of C-10 ML than oil extracts and solid extracts, i.e., 46.00%; 33.68%; 10.25%, respectively. However, according to the GC-MS profiles of the massoia oil and extract (oily), and solid phase extract, C-10 ML was detected at the percentage of 48.50%, 67.62%, and 59.54%, respectively. The results described the relative percentage of C-10 ML to other volatile substances in the samples detected by GC.
Figure 1.
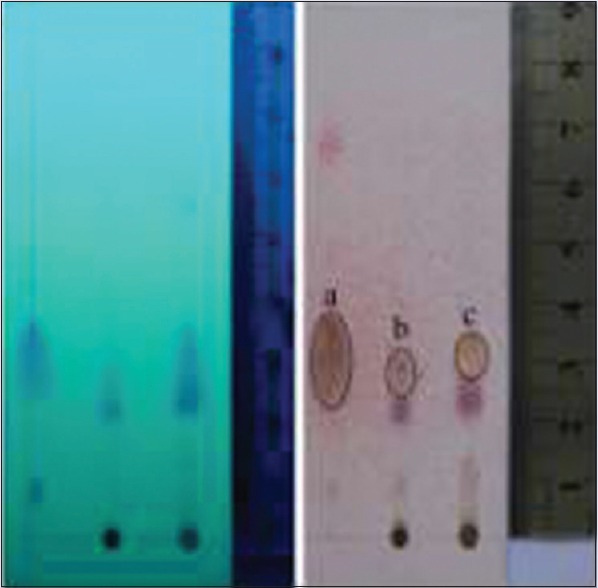
Thin-layer chromatography profile of purified massoia oil and extract by using silica gel F254 aluminum precoated plate; toluene: EtOAc = 93:7 v/v; observation under UV254 (left) and under visible light after sprayed with annisaldehyde H2SO4 (right). (a) C-10 massoia lactone purified massoia oil, (b) C-10 Massoia lactone on extract (solid phase), (c) C-10 massoia lactone on extract (oily phase)
Figure 2.
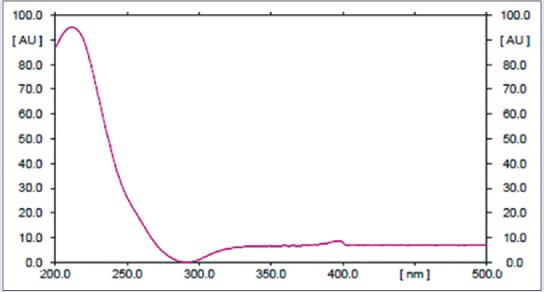
Profile of the C-10 massoia lactone UV-spectrum showing a maximum wavelength at 211 nm
Table 1.
Content of C-10 massoia lactone in massoia oils and massoia extracts
Effect of massoia lactone and massoia extracts on Candida albicans hyphal growth
The effect of different concentration of tested compounds on C. albicans hyphal growth on solid media was observed by cultivating fungal cells and observing colony morphology on Spider agar and RPMI agar at 37°C. It was found that until the lowest concentration tested (62.5 μg/mL for massoia solid extract and 62.5 μg/mL for massoia oily extract) growth of C. albicans hypha was inhibited by the extracts. On the contrary, up to highest concentration tested of C-10 ML (100 µg/mL), the compound was found failed to abrogate C. albicans filamentation. This finding can be interpreted that other constituents other than the ML were responsible for the hyphae development inhibition.
Effect of massoia lactone and massoia extact on Candida albicans planktonic growth
Candida planktonic growth was inhibited depending on the concentration of the sample [Figure 3]. C-10 ML inhibition on the planktonic growth was showed as IC50 of 0.167 µg/mL. While the calculated IC50 of massoia solid and oily extracts to inhibit Candida planktonic growth were determined at 6.40 µg/mL and 0.017% v/v, respectively.
Figure 3.
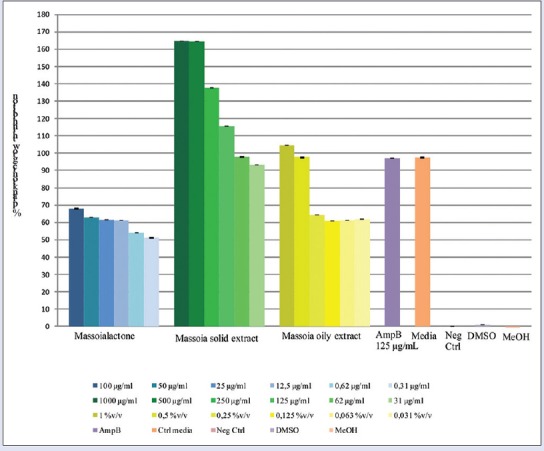
Inhibition of Candida albicans planktonic growth by C-10 massoia lactone and massoia extracts
Effect of massoia lactone and massoia extract on Candida albicans biofilm inhibition
It is interesting to note that despite the lower concentration of C-10 ML in the solid extract, the biofilm inhibition activity was comparable to the oily suggesting that there are other active constituents present in the solid extract contributing to the activity [Figure 4]. Concentration-dependent inhibitions were observed. A comparison effect between the C-10 ML and the oily extracts exhibited no significant different, except for the lowest concentration tested.
Figure 4.
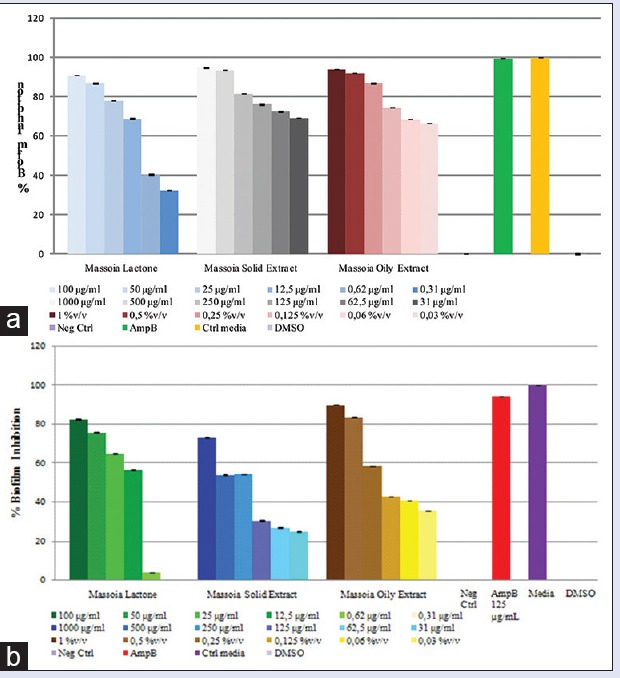
(a) Biofilm formation inhibition after sample application (24 h). (b) Biofilm formation inhibition after sample application (48 h)
C. albicans biofilm formation is preceded in two distinct developmental phases: Intermediate (12–30 h) and mature (38–72 h).[9] Massoia liquid (oily) extract at concentration of 1–0.03% v/v, C-10 ML at concentration of 100–0.31 µg/mL and massoia solid extract at concentration of 1.000–30 μg/mL were tested against C. albicans adherent cells populations at different stages of biofilm development.
The result of this experiment, as shown in Figure 4, demonstrated that all inhibition of C. albicans intermediate and mature biofilms occurred in the presence of compound tested. Until the concentration of 12.5 µg/mL, C-10 ML exhibited up to 50% inhibition of C. albicans biofilm intermediate phase formation, but higher concentration needed to exhibit up to 50% inhibition of the mature phase. The calculated IC50 of C-10 ML application to inhibit formation Candida biofilm was 1.63 µg/mL and 8.92 µg/mL toward intermediate and mature biofilm, respectively. Similar patterns were also showed by massoia oily and solid extracts, which partially inhibited C. albicans intermediate biofilm (IC50 was 0.006% v/v and 3.43 µg/mL, respectively), but needed a higher concentration to disturb the mature phase biofilm.
Effect of massoia lactone and massoia extracts on fungal established biofilms
The phenomena in biofilm inhibition were also shown in the established biofilm but in less effect [Figure 5]. The extracts showed slightly less inhibitory effects in comparison to the oil. The calculated IC50 values resulted from the C-10 ML application to degrade Candida biofilm were 0.026 µg/mL and 0.43 µg/mL toward intermediate and mature biofilm, respectively. Similar patterns were also showed by the massoia oily and solid extract, which partially degraded the C. albicans intermediate biofilm at concentration of 0.06% v/v and 250 µg/mL (calculated IC50 was 0.074% v/v and 271 µg/mL, respectively), but a higher concentration (0.12% v/v and 500 µg/mL) was required to disrupt the mature phase biofilm (calculated IC50 was 0.16% v/v and 427 µg/mL).
Figure 5.
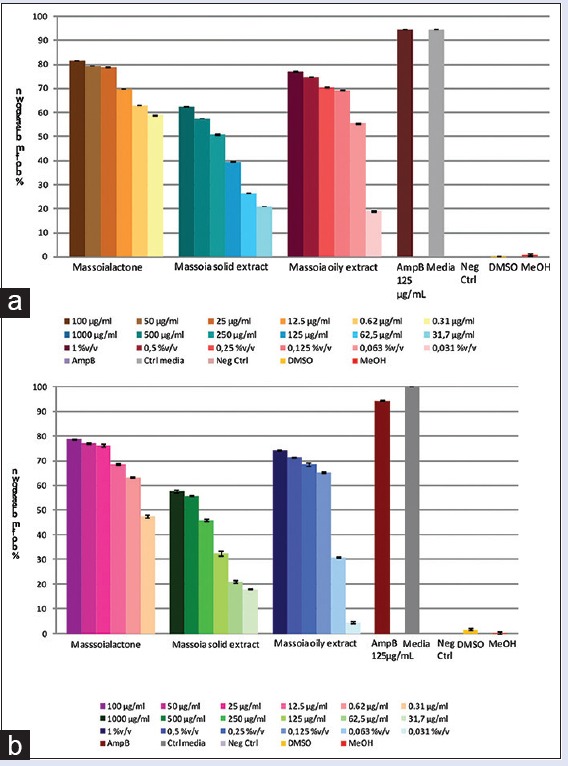
Inhibition of preformed Candida albicans biofilm by C-10 massoia lactone and massoia extracts against: (a) intermediate (24 h) biofilm (b) mature (48 h) biofilm
Anti-biofilm activity observed by confocal laser scanning microscope
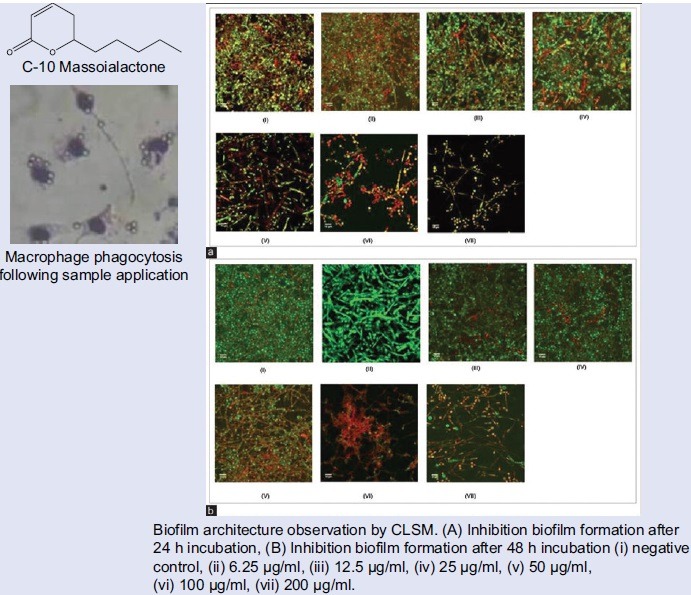
The anti-biofilm activity in the microtiter plate assay was confirmed by confocal laser scanning microscope (CLSM) analysis of C. albicans biofilm. The biofilms were grown on the surface of coverslips and stained with SYTO9 and PI for live/dead cells monitoring. We observed a preventive activity on biofilm formation when we inoculated C. albicans with different concentration of samples. Growth suppression of C. albicans biofilm by essential oils was clearly seen by CLSM with the live/dead stain, compared with dense biofilm growth of the control.
The architecture of C. albicans biofilm in the presence of tested oils in biofilm formation inhibition was shown in Figure 6. The estimated three-dimensional view of the biofilm refers to the total area in the x-y-z dimension, where x and y are the coordinates of the pixel positioning and z is the intensity collected using ImageJ. Live cells are labeled in green (SYT09), and dead cells are labeled in red (PI).
Figure 6.
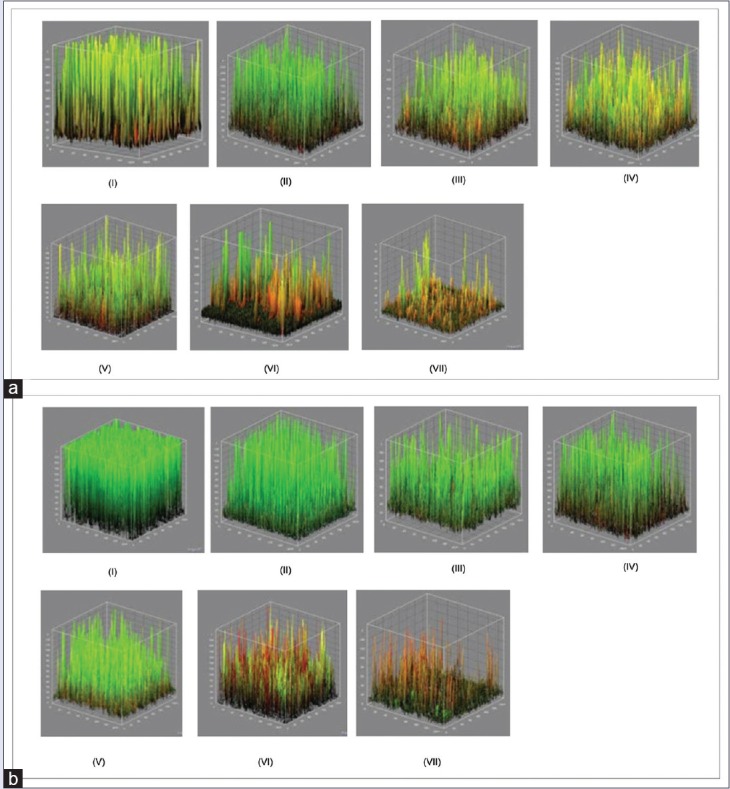
Estimated three-dimensional view of biofilm refers to the total area of the dimensions xyz, where x and y are the coordinates of the pixel position and z is the intensity of the collected using ImageJ. Living cells are labeled green (SYT09), and dead cells are labeled red (propidium iodide). (a) at 24 h, (b) at 48 h. (i) negative control, (ii) 6.25 μg/ml, (iii) 12.5 μg/ml, (iv) 25 μg/ml, (v) 50 μg/ml, (vi) 100 μg/ml, (vii) 200 μg/ml
PI is a nonpermeant fluorescent nucleic acid stain that enters nonviable cells by binding to double-stranded nucleic acids through intercalation between base pairs with no preference to purine or pyrimidine base pairs.[25] Figure 7 shows intense green fluorescence resulting from membrane permeant SYT09 binding to DNA of C. albicans intact cells while PI stains the cells red. Thus, areas of red fluorescence represent dead cells, and green fluorescent indicates healthy cells. The yellow color (formed as a result of overlapping of red and green images which were the representative of SYTO9 and PI colocalization due to the presence of dead cells or eDNA.
Figure 7.
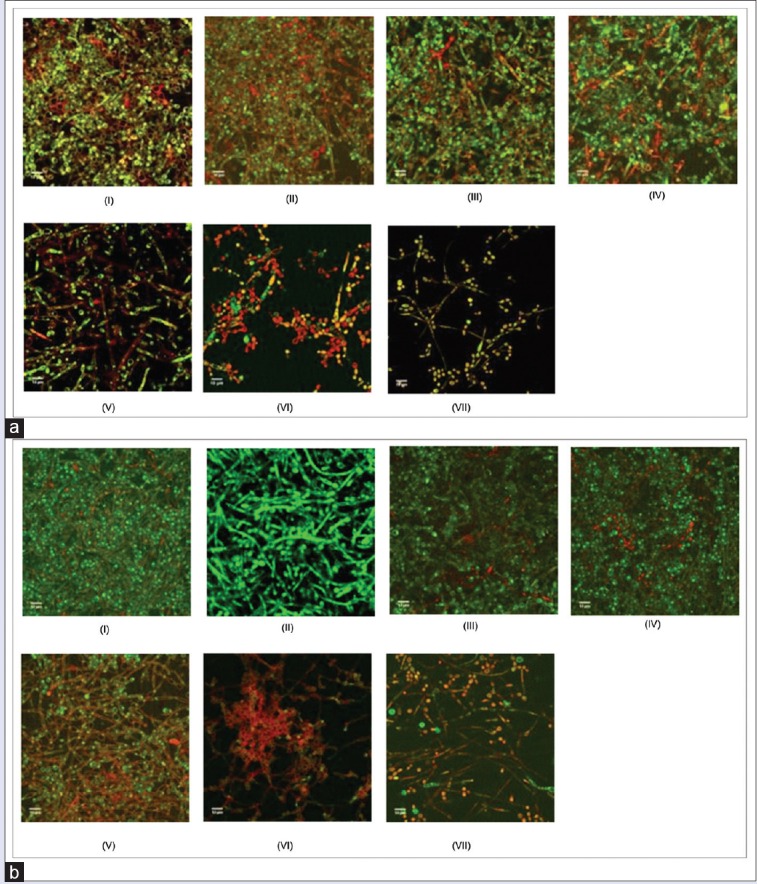
Biofilm architecture observation by confocal laser scanning microscope. (a) Inhibition biofilm formation after 24 h incubation, (b) Inhibition biofilm formation after 48 h incubation (i) negative control, (ii) 6.25 μg/ml, (iii) 12.5 μg/ml, (iv) 25 μg/ml, (v) 50 μg/ml, (vi) 100 μg/ml, (vii) 200 μg/ml
The result of this experiment, as assessed by colorimetric assay and microscopy, demonstrated that negative control (biofilm formed in the absence of samples) exhibited typical biofilm architecture. At intermediate phase of biofilm development, C. albicans biofilm was found to be composed of yeast cells, elongated pseudohyphae and hyphae. At mature phase, C. albicans biofilms composed mainly of intertwining mycelia structures, and a basal layer of blastospores [Figure 6].
Pretreatment of C. albicans biofilm with various C-10 ML concentrations showed that there was a dose-dependent effect on biofilm formation. CLSM study shows that the number of viable cells (green) decreased when the concentration of extract exposure increased. Figure 6v showed that in the presence of compound tested (concentration of 50 μg/mL), a significant decrease of C. albicans biofilm biomass in comparison to the negative control was evident. When the concentration was decreased, pseudohyphae and mycelia were observed [Figure 7]. This finding suggests that the anti-biofilm activity may be due to the antifungal activity of the essential oils and not caused by disruption in hypha development. Further evaluation was performed by examining the samples’ effect on the hyphal growth.
Effect of massoia lactone, massoia extracts, and massoia oil on phagocytosis activity of mice macrophages
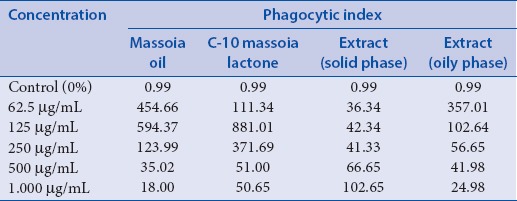
Effect of C-10 ML, massoia extracts, and massoia oil on the immune system was observed through the influence on the phagocytosis activity of mice macrophages. Phagocytosis activity was evaluated by counting phagocytic index. As much as 300 macrophages were observed for each treatment, revealed that the highest phagocytic index were achieved at concentration 125 μg/ml for massoia oil and C-10 ML, 1000 μg/ml for oil extract and 62.5 μg/ml for solid extract. However, the greatest value of the phagocytic index was indicated in the presence of C-10 ML suggesting the main contribution of the compound to the activity [Table 2]. As described previously, massoia extract has the fewest content of C-10 ML which may explain the low value of phagocytic index. Nevertheless, increase massoia extract concentration could not increase the phagocytic index, which may happen due to the poor solubility in the media. This was also the case in the application of C-10 ML and massoia oil at concentrations of 500 and 1.000 μg/ml.
Table 2.
Phagocytic index of macrophage treated with massoia oil, C-10 massoia lactone and massoia extract
CONCLUSION
Massoia bark extracts (oily and solid phase) and C-10 ML exhibited promising activities as anti-biofilm against C. albicans at IC50 are 0.074% v/v, 271 µg/mL and 0.026 µg/mL, respectively. The major constituent, C-10 ML (C10H16O2) plays major role in exerting anti candida activity and potentially acts as an immunomodulator as well. Phagocytosis stimulation effect on mice macrophage was shown in a sequence from the highest to the lowest phagocytic index as follows: C-10 ML, massoia oil, massoia extract (solid phase), and massoia extract (oily phase). The C-10 ML did not inhibit the hyphae development at the concentration tested; however, the extracts showed inhibition at 62.5 μg/mL (oily extract) and 62.5 μg/mL (solid extract).
Financial support and sponsorship
The Indonesia Directorate of Higher Education under grant Nr. LPPM-UGM/993/LIT/2014.
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHOR

Dr. Triana Hertiani
Dr. Triana Hertiani graduated from the Pharmaceutical Biology and Biotechnology Department, Heinrich-heine University, Duesseldorf, Germany in 2007. She works as associate Professor in the Pharmaceutical Biology Department, Faculty of Pharmacy, Gadjah Mada University, Indonesia. Her research interest is in the field of Pharmaceutical Biology, particularly in natural product chemistry. Antiifective from natural resources is her main interest of research.
Acknowledgment
We are thankful for Prof. C.A.M.J.J. Van den Hondel and Institute Biology Leiden, Leiden University for research collaboration. This research was funded by “International Collaboration Grant Research” LPPM-UGM, 2014 under Nr. LPPM-UGM/993/LIT/2014.
REFERENCES
- 1.Rali T, Wossa SW, Leach DN. Comparative chemical analysis of the essential oil constituents in the bark, heartwood and fruits of Cryptocarya massoy (Oken) Kosterm. (Lauraceae) from Papua New Guinea. Molecules. 2007;12:149–54. doi: 10.3390/12020149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barros ME, Freitas JC, Oliveira JM, da Cruz CH, da Silva PB, de Araújo LC, et al. Synthesis and evaluation of (-)-Massoialactone and analogues as potential anticancer and anti-inflammatory agents. Eur J Med Chem. 2014;76:291–300. doi: 10.1016/j.ejmech.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro RS, Robbins N, Cowen LE. Regulatory circuitry governing fungal development, drug resistance, and disease. Microbiol Mol Biol Rev. 2011;75:213–67. doi: 10.1128/MMBR.00045-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bandara A, Fraser S, Chambers PJ, Stanley GA. Trehalose promotes the survival of Saccharomyces cerevisiae during lethal ethanol stress, but does not influence growth under sublethal ethanol stress. FEMS Yeast Res. 2009;9:1208–16. doi: 10.1111/j.1567-1364.2009.00569.x. [DOI] [PubMed] [Google Scholar]
- 5.Lal S, Sandanaraj E, Jada SR, Kong MC, Lee LH, Goh BC, et al. Influence of APOE genotypes and VKORC1 haplotypes on warfarin dose requirements in Asian patients. Br J Clin Pharmacol. 2008;65:260–4. doi: 10.1111/j.1365-2125.2007.03053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kojic EM, Darouiche RO. Candida infections of medical devices. Clin Microbiol Rev. 2004;17:255–67. doi: 10.1128/CMR.17.2.255-267.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donlan RM. Biofilms: Microbial life on surfaces. Emerg Infect Dis. 2002;8:881–90. doi: 10.3201/eid0809.020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agarwal V, Tanwar C, Pruthi V. Quebec, Canada: American Society for Microbiology; 2007. CLSM Studies of Candida albicans Biofilm on Biomaterials. ASM Conference-Biofilm. [Google Scholar]
- 9.Lewis K. Riddle of biofilm resistance. Antimicrob Agents Chemother. 2001;45:999–1007. doi: 10.1128/AAC.45.4.999-1007.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jabra-Rizk MA, Falkler WA, Meiller TF. Fungal biofilms and drug resistance. Emerg Infect Dis. 2004;10:14–9. doi: 10.3201/eid1001.030119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pratiwi SU, Lagendijk EL, de Weert S, Hertiani T, Idroes R, Hondel CA. Effect of Cinnamomum burmannii Nees ex Bl. and Massoia aromatica Becc. Essential oils on planktonic growth and biofilm formation of Pseudomonas aeruginosa and Staphylococcus aureus In vitro. Int J Appl Res Nat Prod. 2015;8:1–13. [Google Scholar]
- 12.Khajanchi BK, Kirtley ML, Brackman SM, Chopra AK. Immunomodulatory and protective roles of quorum-sensing signaling molecules N-acyl homoserine lactones during infection of mice with Aeromonas hydrophila. Infect Immun. 2011;79:2646–57. doi: 10.1128/IAI.00096-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tauber SC, Nau R. Immunomodulatory properties of antibiotics. Curr Mol Pharmacol. 2008;1:68–79. [PubMed] [Google Scholar]
- 14.Niphade SR, Asad M, Chandrakala GK, Toppo E, Deshmukh P. Immunomodulatory activity of Cinnamomum zeylanicum bark. Pharm Biol. 2009;47:1168–73. [Google Scholar]
- 15.Roshan N, Savitri P. Review on chemical constituents and parts of plants as immunomodulators. Res J Pharm Biol Chem Sci. 2013;4:76–89. [Google Scholar]
- 16.Wagner H, Blast S. London, New York: Springer Science and Business Media; 1996. Plant Drug Analysis: A Thin Layer Chromatography Atlas; p. 373. [Google Scholar]
- 17.Horváth G, Szabó LG, Lemberkovics É, Botz L, Kocsis B. Characterization and TLC-bioautographic detection of essential oils from some thymus taxa. Determination of the Activity of the oils and their components against plant pathogenic bacteria. J Planar Chromatogr Mod TLC. 2004;17:300–4. [Google Scholar]
- 18.Faria NC, Kim JH, Gonçalves LA, Martins Mde L, Chan KL, Campbell BC. Enhanced activity of antifungal drugs using natural phenolics against yeast strains of Candida and Cryptococcus. Lett Appl Microbiol. 2011;52:506–13. doi: 10.1111/j.1472-765X.2011.03032.x. [DOI] [PubMed] [Google Scholar]
- 19.Pierce CG, Uppuluri P, Tristan AR, Wormley FL, Jr, Mowat E, Ramage G, et al. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat Protoc. 2008;3:1494–500. doi: 10.1038/nport.2008.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramage G, Saville SP, Wickes BL, López-Ribot JL. Inhibition of Candida albicans biofilm formation by farnesol, a quorum-sensing molecule. Appl Environ Microbiol. 2002;68:5459–63. doi: 10.1128/AEM.68.11.5459-5463.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin Y, Zhang T, Samaranayake YH, Fang HH, Yip HK, Samaranayake LP. The use of new probes and stains for improved assessment of cell viability and extracellular polymeric substances in Candida albicans biofilms. Mycopathologia. 2005;159:353–60. doi: 10.1007/s11046-004-6987-7. [DOI] [PubMed] [Google Scholar]
- 22.Dusane DH, Dam S, Nancharaiah YV, Kumar AR, Venugopalan VP, Zinjarde SS. Disruption of Yarrowia lipolytica biofilms by rhamnolipid biosurfactant. Aquat Biosyst. 2012;8:17. doi: 10.1186/2046-9063-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Syamsudin, Darmono, Kusmardi The Effect of Garcinia parvifolia Miq (Active Fraction) on phagocytosis by peritoneal macrophages during Plasmodium berghei Infection in Mice. Res J Immunol. 2008;1:16–20. [Google Scholar]
- 24.Tatarczuch L, Bischof RJ, Philip CJ, Lee CS. Phagocytic capacity of leucocytes in sheep mammary secretions following weaning. J Anat. 2002;201:351–61. doi: 10.1046/j.0021-8782.2002.00104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stocks SM. Mechanism and use of the commercially available viability stain, BacLight. Cytometry A. 2004;61:189–95. doi: 10.1002/cyto.a.20069. [DOI] [PubMed] [Google Scholar]


