Abstract
Introduction:
Etomidate is usually preferred in the induction of cardiac compromised patients due to its relative cardiovascular stability. However, the use of this drug has been limited as etomidate induces suppression of cortisol biosynthesis as a result of blockade of 11-beta-hydroxylation in the adrenal gland, mediated by the imidazole radical of etomidate. This study was carried out to observe the effect of Vitamin C on adrenal suppression after etomidate induction in patients undergoing cardiac surgery.
Materials and Methods:
A total of 78 patients were randomly distributed into two groups. Group-I received oral Vitamin C (500 mg) twice daily and Group-II received antacid tablet as placebo twice daily instead of Vitamin C for 7 consecutive days prior to surgery till morning of surgery. Patients of both the groups induced with etomidate (0.1–0.3 mg/kg). Blood cortisol was estimated at different points of time till 24th postinduction hour/blood lactate, glucose, hemodynamic parameters, and perioperative outcomes were assessed.
Results:
Data of seventy patients (n = 35 in each group) were finally analyzed. Cortisol level is statistically significantly higher in Group-I (69.51 ± 7.65) as compared to Group-II (27.74 ± 4.72) (P < 0.05) in the 1st postinduction hour. In Group-II, cortisol was consistently lower for 1st 24 postinduction hour. Total adrenaline requirement was statistically significantly high in Group-II. Time of extubation, length of Intensive Care Unit stay arrhythmia was similar in both the groups.
Conclusion:
Vitamin C effectively inhibits etomidate-induced adrenal suppression in cardiac patients, thereby etomidate can be used as a safe alternative for induction in cardiac surgery under cardiopulmonary bypass when pretreated with Vitamin C.
Keywords: Adrenal suppression, Cardiac surgery, Cortisol, Etomidate, Vitamin C
INTRODUCTION
Etomidate is a short acting hypnotic anesthetic agent usually used as induction agent in cardiac patients. It is preferred over other induction agents in patients with heart disease due to its relative cardiovascular stability.[1] However, etomidate inhibits cortisol synthesis due to reversible adrenal suppression.[2] This limits the use of etomidate as an induction agent despite having so many favorable effects on hemodynamics in cardiac patients as compared to other conventional agents for the induction of anesthesia.
It has been proved that etomidate causes a dose-dependent inhibition of the enzyme 11ß-hydroxylase which converts 11-deoxycortisol to cortisol, thereby decreasing cortisol level. It is well known that even a single dose of etomidate can suppress adrenal function for up to 24 h[3] and can decrease serum cortisol level even after cardiac surgery which provides high level of stimulation to release endogenous stress hormones.[4]
Now, for safe use of etomidate, a drug is to be searched for which will prevent this reversible adrenal insufficiency, so that etomidate can be used as an induction agent mainly in cardiac compromised patient without its undesired side effects on the adrenal gland. It is very important in cardiac compromised patients with poor cardiopulmonary reserve where smooth induction is the prime goal to all cardiac anesthesiologists. Moreover, adrenal dysfunction associated with etomidate may contribute to acute respiratory distress syndrome.[5]
Vitamin C (ascorbic acid) is a water soluble vitamin that has a role in the synthesis of cortisol. This drug can inhibit the suppression of cortisol formation after etomidate injection. Vitamin C helps to maintain a normal adrenal function and helps in the formation of cortisol specifically in the terminal step of the conversion of 11-deoxycortisol to cortisol.[6] Boidin et al. introduce Vitamin C as a treatment option to decrease etomidate-induced adrenal insufficiency. But many studies reported a controversial result whether Vitamin C can promote cortisol formation in patient having etomidate-induced cortical suppression.[7] There is a paucity of evidence which has kept this area sill under research.
This study has been proposed to observe the effect of Vitamin C on adrenal suppression after etomidate induction in patients undergoing cardiac surgery.
We hypothesize that after etomidate induction, adrenocortical suppression will be less in patients pretreated with Vitamin C as compared to those not receiving the drug preoperatively.
MATERIALS AND METHODS
After obtaining the Institutional Ethical Committee clearance and written informed consent from all patients, the study was conducted.
Inclusion criteria
Patients of either sex, free of any endocrine disease, aged 25–60 years with New York Heart Association status < IV, scheduled for elective cardiac surgery were included in this study.
Exclusion criteria
Patients with Parsonnet score > 10, on steroid, with history of diabetes mellitus, alcohol abuse, smoker, pregnant, suffering from epilepsy, hematological disease, impaired hepatic and renal function, patients having hypersensitivity to any of the study drugs and a patient requiring cross clamp time of more than 2 h were excluded from the study.
During preanesthetic check-up, all patients were explained the procedure, and informed written consent was obtained. Angiotensin-converting enzyme inhibitors were stopped 24 h before surgery. However, calcium-channel antagonists and β-adrenergic blocking drugs were continued till the day of surgery. Group-I received oral Vitamin C (500 mg) twice daily and Group-II received antacid tablet as placebo (aluminum hydroxide and magnesium hydroxide) twice daily instead of Vitamin C for 7 consecutive days prior to surgery till morning of surgery. The drug was administered according to a computer-generated randomization chart by an anesthesiologist who was not involved in the study.
After proper preanesthetic optimization, all patients were examined again the day before surgery. Patients of both the groups were premedicated with lorazepam 1 mg, ranitidine 150 mg, and metoclopramide 10 mg orally, the night before surgery. Patients were wheeled into operating room and standard American Society of Anesthesiologists monitors (pulse oxymeter, noninvasive blood pressure till the invasive blood pressure were done, electrocardiogram) were attached. An intravenous (i.v.) line was inserted in all patients in right dorsum or forearm. Left radial arterial cannulation was done after local anesthesia. All patients received i.v. piperacillin and tazobactam (4.5 g).
Induction of anesthesia was performed with injection midazolam (0.01 mg/kg), fentanyl (2–3 μg/kg), and etomidate (0.1–0.3 mg/kg) till the loss of consciousness. Neuromuscular blockade was achieved by 1 mg/kg of rocuronium to facilitate endotracheal intubation. Intraoperatively, neuromuscular relaxation was maintained with rocuronium. The lungs were ventilated with nitrous oxide in oxygen (50:50). End-tidal CO2 was maintained between 35 and 45 mmHg. Anesthesia was maintained with isoflurane at 0.8–1.0 minimal alveolar concentration. Pulmonary artery catheterization and central venous cannulation were performed thereafter and surgery was started. All the drugs were diluted/dissolved in normal saline. In both groups, serum cortisol and lactate level were checked preoperatively 10 min before induction and was evaluated 3 min after laryngoscopy, after sternotomy, at 1 h of induction, after cross clamp application, and after cross clamp release and then at 6th, 12th, and 24th h after induction. Arterial blood gas analysis was done hourly to check acid base imbalance and electrolytes and was corrected accordingly. Blood glucose was estimated hourly and insulin infusion was started to maintain < 180 mg/dl in the intraoperative and postoperative period. Hemodynamic parameters were monitored continuously and recorded at different point of time. Urine output was monitored. Data were collected by a resident blinded to the study. Heparin (400 units/kg) was administered before the placement of aortic cannulation to achieve an activated clotting time of > 480 s. The cardiopulmonary bypass (CPB) circuit was primed with lactated Ringer's solution, sodium bicarbonate, mannitol, and heparin. Hematocrit was maintained at 21–28% during CPB. Myocardial protection was achieved by antegrade cold cardioplegia (at 4°C, St. Thomas’ solution-based crystalloid-blood cardioplegic solution PLEGIOCARD as 1:4 ratio) after aortic cross clamp and the cardioplegia was repeated every 20 min. The cardiac repair or valve replacement was carried out under CPB with mild hypothermia using standard extracorporeal techniques. Nitroglycerine infusion 0.5 μg/kg/min, adrenaline 0.05 μg/kg/min, and dopamine 5 μg/kg/min were started at the onset of rewarming as per the institutional protocol. Infusion of milrinone, noradrenaline, and amiodarone was also initiated according to the need to maintain mean arterial pressure > 70 mmHg, pulmonary artery pressure below 30 mmHg, and normal sinus rhythm. All the patients were rewarmed to 37°C. Serum potassium levels were optimized to 4–4.5 mEq/L throughout surgery. Ventricular fibrillation (VF) or ventricular tachycardia (VT) was treated with Xylocard, MgSO4, amiodarone as appropriate, and in cases refractory to medical management internal defibrillation with 20–50 J was administered. i.v. protamine sulfate 1.3 times the heparin dose was used to reverse the effect of heparin when patient was weaned off CPB support to return the activated clotting time to within 20% of the preoperative value. In all cases, epsilon-aminocaproic acid was administered before going on pump, on pump, and postpump at the dose of 100 mg/kg.
After surgical closure, all patients were transferred to postoperative Intensive Care Unit (ICU) where they were monitored closely for the first few postoperative days. Any adverse events such as new onset arrhythmia, hypo- or hyper-glycemia, electrolyte imbalance, bleeding, infections, and acute renal failure in the perioperative period were recorded and treated accordingly.
Statistics
For the purpose of sample size calculation, serum cortisol level at 1 h following etomidate administration was taken as the primary outcome measure. It is calculated that 35 subjects will be required per group in order to detect the difference of 0.5 μg/dl in serum cortisol level between the two groups with 80% power and 5% probability of type I error. This calculation assumes two-sided testing and 0.75 μg/dl as a standard deviation for serum cortisol level. We enrolled a total of 78 patients considering dropout. Patients were randomized by a computerized randomization chart on the day of first preanesthetic check-up into two groups; Group-I (n = 35) and Group-II (n = 35) to receive either oral Vitamin C (500 mg) twice daily and antacid tablet (aluminum hydroxide and magnesium hydroxide) as placebo twice daily instead of Vitamin C for 7 consecutive days prior to surgery, respectively.
In this study, multivariate regression model, especially a difference in difference method has been used to figure out whether there is any difference of cortisol secretion between individuals belonging in the Group-I and the Group-II. Other numerical variables were compared between groups by Student's unpaired t-test if normally distributed or a Mann–Whitney U-test as appropriate. Fisher's exact test was employed for inter group comparison of categorical variables. A two-tailed P < 0.05 was considered as statistically significant.
RESULTS
A total of 78 patients were assessed initially for the eligibility for inclusion in this prospective parallel group, double-blinded, randomized controlled trial. In the beginning, six patients were excluded as they refused to participate. The study was started with 72 patients, who were randomized to be divided into two groups (n = 36) equal in numbers as per a computerized randomization chart. During operation, two patients - one from each group was excluded from the study as the aortic cross clamp was applied more than 2 h. Hence, data of 35 patients of each group were finally analyzed.
Cortisol level at different points of time in between the two groups was assessed, and it shows clearly that cortisol level is much lower in Group-II (27.74 ± 4.72) as compared to Group-I (69.51 ± 7.65) in the first postinduction hour (P = 0.000) [Figure 1]. It has been also strongly established that cortisol suppression in Group-II is statistically significantly higher as compared to Group-I. In Group-II cortisol level is lower not only at the first postinduction hour but also throughout the first 24 postinduction hour. On the contrary, patients of Group-I have higher cortisol level throughout the first 24 postinduction hour indicating the positive effect of Vitamin C.
Figure 1.
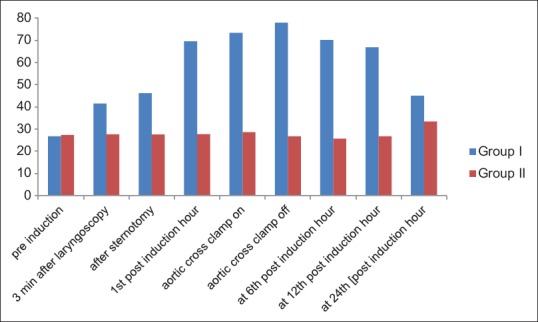
Difference in cortisol secretion at different points of time in between two groups
Eight different regression equation has been used here to calculate the difference in cortisol level at eight different points of time (considering the first observation t = 0). The regression equation for time point t is
CSidt = a+b.Dd+αΓt+δ.Dd.Γt+θXt+uit
XT is the vector of controls. Γt is a time specific dummy variable which takes values 1 if time is t and 0 for the initial time point. The coefficient vector β captures the effect of control. We have considered the proxies of stress variable in X. uit is the error term, following the usual classical linear regression model assumption (Woldridge 2005). Now, at time point 0 (first observation), the difference in average cortisol secretion between Groups-I and II captured the differences of interperson differences [Table 1].
Table 1.
Difference in difference estimators before and after treatments
| Time 1 | Time 2 | Time 3 | Time 4 | Time 5 | Time 6 | Time 7 | Time 8 | |
|---|---|---|---|---|---|---|---|---|
| 1.t | 1.69 (1.22) | 0.40 (0.18) | 0.07 (0.02) | 5.98 (1.87) | −1.16 (−0.39) | −3.03 (−0.83) | −5.98 (−1.54) | 10.81*** (3.40) |
| 1.D | −0.59 (−0.54) | −0.58 (−0.56) | −0.58 (−0.46) | −0.59 (−0.52) | −0.58 (−0.51) | −0.58 (−0.56) | −0.58 (−0.57) | −0.59 (−0.51) |
| Delta | 14.88*** (9.42) | 19.14*** (11.97) | 42.15*** (16.30) | 48.39*** (19.05) | 51.16*** (17.49) | 44.03*** (15.60) | 38.11*** (16.78) | 13.87*** (7.16) |
| Lactate | −3.14 (−1.67) | −0.08 (−0.06) | 0.17 (0.11) | −2.16 (−1.57) | 0.19 (0.21) | 0.39 (0.40) | 1.55 (1.44) | −2.42 (−1.59) |
| Constant | 30.58*** (14.71) | 27.43*** (17.36) | 27.18*** (14.39) | 29.57*** (18.21) | 27.16*** (22.63) | 26.95*** (21.89) | 25.77*** (19.58) | 29.84*** (16.95) |
| Observations | 140 | 140 | 140 | 140 | 140 | 140 | 140 | 140 |
| R2 | 0.65 | 0.79 | 0.93 | 0.95 | 0.96 | 0.95 | 0.94 | 0.71 |
| Adjusted R2 | 0.643 | 0.781 | 0.923 | 0.946 | 0.956 | 0.949 | 0.943 | 0.699 |
t-statistics in parentheses *P<0.05, **P<0.01, ***P<0.001. t: Time interval, D: Day
The result of the model corresponding to the regression equation estimated the model separately with or without including controls for the lactate [Table 2]. It is clearly observable that the results remain consistent with the previous finding [Table 1].
Table 2.
Difference in difference estimators in a panel data framework
| Cortisol | Cortisol | |
|---|---|---|
| 1.t | 0.289 (0.23) | 0.309 (0.24) |
| 2.t | 0.283 (0.23) | 0.351 (0.24) |
| 3.t | 0.386 (0.31) | 0.469 (0.30) |
| 4.t | 1.289 (1.04) | 1.387 (0.83) |
| 5.t | −0.574 (−0.46) | −0.430 (−0.21) |
| 6.t | −1.623 (−1.31) | −1.457 (−0.65) |
| 7.t | −0.591 (−0.48) | −0.432 (−0.20) |
| 8.t | 6.080*** (4.90) | 6.169*** (3.86) |
| 1.D | −0.583 (−0.47) | −0.583 (−0.47) |
| Time 1 | 14.45*** (8.23) | 14.45*** (8.22) |
| Time 2 | 19.11*** (10.88) | 19.13*** (10.78) |
| Time 3 | 42.35*** (24.12) | 42.40*** (22.86) |
| Time 4 | 45.29*** (25.80) | 45.36*** (23.80) |
| Time 5 | 51.68*** (29.44) | 51.81*** (22.75) |
| Time 6 | 45.00*** (25.63) | 45.11*** (20.68) |
| Time 7 | 40.64*** (23.15) | 40.72*** (20.89) |
| Time 8 | 12.20*** (6.95) | 12.23*** (6.82) |
| Lactate | −0.0456 (−0.09) | |
| Constant | 27.35*** (31.16) | 27.40*** (26.72) |
| Observations | 630 | 630 |
| R2 | 0.932 | 0.932 |
| Adjusted R2 | 0.930 | 0.930 |
t-statistics in parentheses. *P<0.05, **P<0.01, ***P<0.001, t: Time interval, D: Day
Total requirement of adrenaline is higher in Group-II (3.4 ± 1.09) as compared to Group-I (2.83 ± 1.27), which is statistically significant [P = 0.047, Table 3]. Though noradrenaline dose was higher in Group-II as compared to Group-I, it is not statistically significant (P = 0.064) though it has a positive impact on the study result.
Table 3.
Requirements of infusion of cardiac drugs
| Total requirement of | Group I (n=35) | Group II (n=35) | P | Total requirement of | U | Z | P |
|---|---|---|---|---|---|---|---|
| Adrenaline | 2.8±1.27 | 3.4±1.09 | 0.047* | Noradrenaline | 455.00 | −1.850 | 0.064 |
| Dopamine | 169.43±40.36 | 159.43±38.26 | 0.291 | Milrinone | 609.50 | −0.035 | 0.972 |
| NTG | 31.34±13.29 | 30.03±12.42 | 0.670 | Amiodarone | 580.50 | −0.375 | 0.707 |
| Student’s unpaired t-test has been done | Mann–Whitney U-test has been done | ||||||
Values represent mean±SD. *P<0.05: Statistically significant, NTG: Nitroglycerine, SD: Standard deviation
The demographic parameters and patient profiles are similar in both the groups [Table 4]. The total requirement of insulin was statistically insignificant (P = 0.126) in between two groups (Group-I/Group-II: 34.23 ± 7.23/31.34 ± 8.32). The perioperative events such as incidence of atrial fibrillation, ectopic beats, VF/tachycardia, perioperative myocardial infarction, incidence of hypoglycemia, reintubation, and mortality up to 30 days after operation did not show any statistically significant difference between the two groups [Table 5].
Table 4.
Patient profile
| Parameters | Group I (n=35) | Group II (n=35) | P |
|---|---|---|---|
| Age (year) | 48.14±6.46 | 45.23±11.47 | 0.195 |
| Mean arterial pressure (mmHg) | 65.09±4.93 | 66.03±4.96 | 0.428 |
| Ejection fraction (%) | 56.17±5.86 | 56.14±6.15 | 0.984 |
| Duration of surgery (h) | 6.83±1.45 | 6.47±1.26 | 0.280 |
| CPB time (min) | 93.63±19.03 | 93.60±22.20 | 0.995 |
| Duration of aortic cross clamp (min) | 71.46±20.36 | 65.52±23.53 | 0.263 |
| NYHA (II/III) | 22/13 | 20/15 | 0.808 |
| History of previous cardiac intervention (yes/no) | 4/31 | 2/33 | 0.673 |
Values represent mean±SD. P<0.05 is statistically significant; NYHA: New York Heart Association functional classification, SD: Standard deviation, CPB: Cardiopulmonary bypass
Table 5.
Perioperative events
| Parameters | Group I (n=35) | Group II (n=35) | P |
|---|---|---|---|
| Incidence of hypoglycemia (yes/no) | 4/31 | 3/32 | 1.000 |
| Incidence of atrial fibrillation (yes/no) | 5/30 | 7/28 | 0.752 |
| Incidence of ventricular tachycardia (yes/no) | 3/32 | 5/30 | 0.710 |
| Incidence of ectopic beats (yes/no) | 6/29 | 3/32 | 0.477 |
| 30 days mortality (yes/no) | 2/33 | 1/34 | 1.000 |
| Incidence of reintubation (yes/no) | 4/31 | 5/30 | 1.000 |
| Incidence of preoperative MI (yes/no) | 3/32 | 2/33 | 1.000 |
| Total Insulin requirement (unit) | 34.23±7.23 | 31.34±8.32 | 0.126 |
Values represent mean±SD. P<0.05 is statistically significant. MI: Myocardial infarction, SD: Standard deviation
DISCUSSION
The properties of etomidate include hemodynamic stability, minimal respiratory depression, cerebral protection, and pharmacokinetics enabling rapid recovery after a single dose. In cardiac patients, etomidate results in very stable hemodynamics with almost no change in heart rate, mean arterial pressure, mean pulmonary artery pressure, pulmonary capillary wedge pressure, central venous pressure, stroke volume, cardiac index, or pulmonary and systemic vascular resistance.[8] In cardiac surgery, where all patients are suffering from poor cardiac function, thereby compromised cardiac reserve where now a days etomidate is increasingly used. A recent study of etomidate in pediatric patients with intracardiac shunt lesions showed that induction with 0.3 mg/kg produced minimal changes in hemodynamics or shunt fraction.[9] Though many studies have reported its advantages over other induction agent in cardiac compromised patients, its safe use is still controversial rather restricted due its unwanted adverse effect on adrenal gland. It is already well established that etomidate causes reversible adrenal insufficiency for at least 24 h even after a single bolus dose.[10]
To overcome such problem, this study has evaluated the effect of Vitamin C on etomidate-induced adrenal suppression in patients undergoing cardiac surgery under CPB.
In present study, adrenal suppression by etomidate was statistically significantly less in Group-I as compared to Group-II which is reflected by a higher level of cortisol in patients who received Vitamin C. This is statistically significant throughout the first 24 postinduction hour. The total requirement of adrenaline dose was also statistically significantly higher in Group-II (3.40 ± 1.09) than Group-I (2.83 ± 1.27) with P = 0.047 that also signifies the positive correlation of inhibitory effect of Vitamin C on adrenal suppression by etomidate.
Etomidate inhibits 11 β-hydroxylase (CYP11B1), 11 β- and 18-hydroxylase (CYP11B2), and cholesterol side-chain cleavage enzyme system (CYP11A) with decreasing effectiveness. Decreased CYP11B1 activity will lead to lower levels of cortisol and increased levels of the upstream precursor 11-deoxycortisol. Decreased CYP11B2 will lead to lower aldosterone and higher 11-deoxycorticosterone levels, whereas decreased CYP11A will lead to generally decreased steroidogenesis.[11,12]
Supplementation with ascorbic acid, a major source of nicotinamide adenine dinucleotide phosphate, inhibits adrenal suppression by etomidate by promoting the turnover rate of 11- β -hydroxylase, thereby increasing the cortisol formation. The blockade of 11 β-hydroxylase seems to be related to the free imidazole radical of etomidate-binding cytochrome P-450.[13] This results in the inhibition of ascorbic acid resynthesis, which is required for steroid production in humans. Blockade of the cytochrome P-450 dependent enzyme 11 β-hydroxylase results in decreased mineralocorticoid production in critically ill patients.[14] Therefore, Vitamin C supplementation restores cortisol levels to normal after the use of etomidate.
Duthie et al. showed that patients undergoing minor peripheral surgery, plasma cortisol levels were slightly depressed from the preinduction levels for 1 h postoperatively and the nadir of mean cortisol levels did not fall out of the normal range.[15] However, high-stress surgery can induce a clinically significant level of cortisol deficiency and adrenocortical suppression. Other studies showed no evidence of a clinically relevant attenuating effect of ascorbic acid or xylitol on etomidate-induced adrenocortical suppression.[16,17,18]
In this study, oral Vitamin C rather than i.v. was selected as it is less invasive and easy with effective blood concentration when taken 500 mg twice daily. Daily dose is recommended as maintaining optimal levels of Vitamin C is difficult because it is water soluble and cannot be stored in the body especially with regard to chronic illness.[19]
Iribarren et al. examined a prospective cohort of 120 patients and found etomidate to be a risk factor for relative adrenal insufficiency and higher vasopressor requirements after surgery up to 4 h of ICU admission.[20] In the present study, total requirement of adrenaline was statistically significantly higher in patients who did not receive Vitamin C that indicates adrenal suppression is significant though noradrenaline dose were similar in both the groups in this study. This may be due to variation in type of surgery. There are few studies which showed no difference in vasopressor requirements in between groups with or without etomidate. This variation may be due to different in patient population and different stress response to various cardiac surgeries.
The perioperative adverse events such as arrhythmia, VF, VT, blood loss, and incidence of blood transfusion were consistent in both the groups.
In this study, there is no clinically or statistically significant difference in length of ICU stay, 30 days mortality or incidence of infection rates in between the two groups though relative adrenal insufficiency is associated with increased mortality and morbidity in seriously hemodynamic compromised patients. The retrospective analysis of the corticosteroid therapy of septic shock (CORTICUS) study suggested that etomidate use was associated with a 1.8-fold increased risk of mortality although the 95% confidence interval ranged from 0.5 to 6.4.[20,21] The CORTICUS cohort suggest that patients receiving etomidate before enrollment had 28 day mortality significantly higher than other patients in the trial.[21,22,23]
No large studies have evaluated the effect of etomidate use on prolonged mechanical ventilation or hospital length of stay. This study suggests that Vitamin C administration provided no added advantage in length of ICU stay and early extubation. Though it is an indirect evidence that lower requirements of inotropes in the perioperative period in Group-I enhances early recovery, there is no statistically significant difference in extubation time and length of ICU stay in between the two groups. This finding is consistent with one of the largest retrospective studies in sepsis patients where etomidate use is not associated with longer mechanical ventilation or hospital length of stay.[24]
The present study did not show any statistically significant difference in total dose of insulin required to maintain blood glucose level < 200 mg/dl throughout the study period. Though a recent study has shown that oral Vitamin C 1000 mg daily decreases HbA1c in diabetic patient.[25] This contrary is probably due to the selection of nondiabetic patients in our study and further research is still needed.
To avoid the confounding effect of circadian rhythm on hormone levels, all operations were performed in the morning between 8:30 and 9:00 am. We have used the advanced statistics (multivariate regression model), so that an unmeasured variable cannot influence the evidence of relation between etomidate use with Vitamin C pretreatment and perioperative outcomes.
Limitation of the study
We did not assess adrenal function preoperatively rather just clinically excluded any endocrinal disease. A large study sample would be better to evaluate the exact result. This result may not be similar in pediatric population. Hence, further research is needed.
CONCLUSION
Oral Vitamin C 500 mg twice daily for 7 days preoperatively can inhibit adrenal suppression by etomidate induction in cardiac patients who are posted for elective cardiac surgery under CPB. Hence, etomidate may be an acceptable option for the induction of anesthesia in cardiac compromised patients with pretreatment of Vitamin C.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors thank Dr. Amit Mandal, (MS G and O), MRCOG 1, Senior Resident, M.R. Bangur Hospital, Kolkata, for their support.
REFERENCES
- 1.Gooding JM, Weng JT, Smith RA, Berninger GT, Kirby RR. Cardiovascular and pulmonary responses following etomidate induction of anesthesia in patients with demonstrated cardiac disease. Anesth Analg. 1979;58:40–1. doi: 10.1213/00000539-197901000-00016. [DOI] [PubMed] [Google Scholar]
- 2.Sprung J, Ogletree-Hughes ML, Moravec CS. The effects of etomidate on the contractility of failing and nonfailing human heart muscle. Anesth Analg. 2000;91:68–75. doi: 10.1097/00000539-200007000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Absalom A, Pledger D, Kong A. Adrenocortical function in critically ill patients 24 h after a single dose of etomidate. Anaesthesia. 1999;54:861–7. doi: 10.1046/j.1365-2044.1999.01003.x. [DOI] [PubMed] [Google Scholar]
- 4.Hoda MR, El-Achkar H, Schmitz E, Scheffold T, Vetter HO, De Simone R. Systemic stress hormone response in patients undergoing open heart surgery with or without cardiopulmonary bypass. Ann Thorac Surg. 2006;82:2179–86. doi: 10.1016/j.athoracsur.2006.06.087. [DOI] [PubMed] [Google Scholar]
- 5.Warner KJ, Cuschieri J, Jurkovich GJ, Bulger EM. Single-dose etomidate for rapid sequence intubation may impact outcome after severe injury. J Trauma. 2009;67:45–50. doi: 10.1097/TA.0b013e3181a92a70. [DOI] [PubMed] [Google Scholar]
- 6.Jenkins JS. The effect of ascorbic acid on adrenal steroid synthesis in vitro. Endocrinology. 1962;70:267–71. doi: 10.1210/endo-70-2-267. [DOI] [PubMed] [Google Scholar]
- 7.Seravalli L, Pralong F, Revelly JP, Que YA, Chollet M, Chioléro R. Adrenal function after induction of cardiac surgery patients with an etomidate bolus: A retrospective study. Ann Fr Anesth Reanim. 2009;28:743–7. doi: 10.1016/j.annfar.2009.07.074. [DOI] [PubMed] [Google Scholar]
- 8.Reves JG, Peter SA, Lubarsky DA, Mc Evoy MD, Martinez-Ruiz R. Intravenous anesthetics. In: Miller RD, editor. Miller's Anesthesia. 7th ed. Ch 26. Philadelphia: Elsevier Churchill Livingstone; 2009. pp. 747–51. [Google Scholar]
- 9.Dhawan N, Chauhan S, Kothari SS, Kiran U, Das S, Makhija N, et al. Hemodynamic responses to etomidate in pediatric patients with congenital cardiac shunt lesions. J Cardiothorac Vasc Anesth. 2010;24:802–7. doi: 10.1053/j.jvca.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 10.den Brinker M, Hokken-Koelega AC, Hazelzet JA, de Jong FH, Hop WC, Joosten KF. One single dose of etomidate negatively influences adrenocortical performance for at least 24 h in children with meningococcal sepsis. Intensive Care Med. 2008;34:163–8. doi: 10.1007/s00134-007-0836-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fraser R, Watt I, Gray CE, Ledingham IM, Lever AF. The effect of etomidate on adrenocortical function in dogs before and during hemorrhagic shock. Endocrinology. 1984;115:2266–70. doi: 10.1210/endo-115-6-2266. [DOI] [PubMed] [Google Scholar]
- 12.Boidin MP. Modification of corticosteroid synthesis by etomidate/fentanyl and air anesthesia. Acta Anaesthesiol Belg. 1986;37:213–8. [PubMed] [Google Scholar]
- 13.Boidin MP, Erdmann WE, Faithfull NS. The role of ascorbic acid in etomidate toxicity. Eur J Anaesthesiol. 1986;3:417–22. [PubMed] [Google Scholar]
- 14.Malerba G, Romano-Girard F, Cravoisy A, Dousset B, Nace L, Lévy B, et al. Risk factors of relative adrenocortical deficiency in intensive care patients needing mechanical ventilation. Intensive Care Med. 2005;31:388–92. doi: 10.1007/s00134-004-2550-8. [DOI] [PubMed] [Google Scholar]
- 15.Duthie DJ, Fraser R, Nimmo WS. Effect of induction of anaesthesia with etomidate on corticosteroid synthesis in man. Br J Anaesth. 1985;57:156–9. doi: 10.1093/bja/57.2.156. [DOI] [PubMed] [Google Scholar]
- 16.Schraag S, Pawlik M, Mohl U, Böhm BO, Georgieff M. The role of ascorbic acid and xylitol in etomidate-induced adrenocortical suppression in humans. Eur J Anaesthesiol. 1996;13:346–51. doi: 10.1046/j.1365-2346.1996.00985.x. [DOI] [PubMed] [Google Scholar]
- 17.Dabbagh A, Sa’adat N, Heidari Z. Etomidate infusion in the critical care setting for suppressing the acute phase of Cushing's syndrome. Anesth Analg. 2009;108:238–9. doi: 10.1213/ane.0b013e318187ed37. [DOI] [PubMed] [Google Scholar]
- 18.Jacob RA, Sotoudeh G. Vitamin C function and status in chronic disease. Nutr Clin Care. 2002;5:66–74. doi: 10.1046/j.1523-5408.2002.00005.x. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Schellhorn HE. New developments and novel therapeutic perspectives for Vitamin C. J Nutr. 2007;137:2171–84. doi: 10.1093/jn/137.10.2171. [DOI] [PubMed] [Google Scholar]
- 20.Iribarren JL, Jiménez JJ, Hernández D, Lorenzo L, Brouard M, Milena A, et al. Relative adrenal insufficiency and hemodynamic status in cardiopulmonary bypass surgery patients. A prospective cohort study. J Cardiothorac Surg. 2010;5:26. doi: 10.1186/1749-8090-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sprung CL, Annane D, Keh D, Moreno R, Singer M, Freivogel K, et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358:111–24. doi: 10.1056/NEJMoa071366. [DOI] [PubMed] [Google Scholar]
- 22.Lipiner-Friedman D, Sprung CL, Laterre PF, Weiss Y, Goodman SV, Vogeser M, et al. Adrenal function in sepsis: The retrospective Corticus cohort study. Crit Care Med. 2007;35:1012–8. doi: 10.1097/01.CCM.0000259465.92018.6E. [DOI] [PubMed] [Google Scholar]
- 23.Cuthbertson BH, Sprung CL, Annane D, Chevret S, Garfield M, Goodman S, et al. The effects of etomidate on adrenal responsiveness and mortality in patients with septic shock. Intensive Care Med. 2009;35:1868–76. doi: 10.1007/s00134-009-1603-4. [DOI] [PubMed] [Google Scholar]
- 24.McPhee LC, Badawi O, Fraser GL, Lerwick PA, Riker RR, Zuckerman IH, et al. Single-dose etomidate is not associated with increased mortality in ICU patients with sepsis: Analysis of a large electronic ICU database. Crit Care Med. 2013;41:774–83. doi: 10.1097/CCM.0b013e318274190d. [DOI] [PubMed] [Google Scholar]
- 25.Afkhami-Ardekani M, Shojaoddiny-Ardekani A. Effect of Vitamin C on blood glucose, serum lipids & serum insulin in type 2 diabetes patients. Indian J Med Res. 2007;126:471–4. [PubMed] [Google Scholar]


