Abstract
Coronary artery bypass grafting surgery effectively relieves signs and symptoms of myocardial ischemia. The left internal thoracic artery (LITA) graft is the gold standard having 90–95% patency rate at 10 years, whereas only 50% of saphenous vein (SV) grafts are patent at 10 years. However, there is a novel “no touch” technique in order to harvest an SV complete with its cushion of surrounding tissue, thus maintaining its endothelium-intact. Significantly superior short- and long-term graft patency rates comparable to LITA grafts can be achieved. Consequently, the SV may be revived as an important conduit in coronary artery bypass surgery.
Keywords: Coronary artery bypass grafting, Graft patency, Myocardial revascularization, Saphenous vein, Venous grafts
INTRODUCTION
Coronary artery bypass grafting effectively relieves signs and symptoms of myocardial ischemia.[1] However, the patency of grafts used is a sine qua non for its long-term success.[2] The left internal mammary artery (LIMA) is the gold standard among the conduits used for coronary artery bypass surgery (CABG).[3,4] However, the most commonly used vessel is the saphenous vein (SV).[5] There is an obvious superiority of LIMAs as 90–95% of LIMA grafts are patent at 10 years, whereas only 50% of SV grafts are patent at 10 years.[6,7] More surprisingly, up to 15% of vein grafts are early occluded, just in the 1st month,[8,9] and another 15–30% of them are occluded in the first post-CABG year.[8,10] But why does such high early occlusion rates happen? Technical surgical factors and damage to the endothelium during SV harvesting can be the reasons why this happens.[11] The adventitial layer of the vein is commonly extracted, so vascular spasm takes place. Consequently, high-pressure distention is required to dilate the vessel leading to extensive damage to the endothelium of the vein wall.[12] On the other hand, Souza[13] proposed an innovative “no touch (NT)” technique for the harvesting of SV in 1996. No touch technique is an atraumatic approach to remove the SV complete with its cushion of surrounding tissue without touching the vessel at all.[13] This novel method contributes to better preservation of endothelial integrity and luminal nitric oxide synthase (NOS).[14,15] Fat, elastic fibers, nerves, and vasa vasorum supplying nutrients and oxygen to the SV wall are the components of this tissue surrounding the SV.[16] Moreover, the surrounding fat tissue of the vein is a source of several vasoactive factors.[17] When the SV is harvested using the “NT” technique, surgical instruments do not touch at all the vessel itself, so no spasm occurs making the distension of the vein graft unnecessary,[18] thus further minimizing the endothelial damage caused.[14,19] Therefore, “NT” technique is related to superior early and long-term graft patency when compared to the conventional technique.[2,18] In this study, we will make a review of the literature in terms of the advantages of NT SV harvesting technique and its impact on long-term SV patency.
NO TOUCH TECHNIQUE PROCEDURE
A classic incision is made longitudinally on the skin of the leg. The incision can be either continuous[2,18,20] or on the calf and on the thigh separately in order to avoid the multibranched SV segment of the poor quality of the knee.[21] A plane is created around the vein using scissors. The SV is covered by a thin layer of adherent tissue anteriorly and posteriorly. Perivascular fat of 0.5-cm on either side is included in the plane.[21] The SV is extracted from its bed with its perivascular fat pedicle and all its side branches are ligated.[2,18,20,21] No venous spasm occurs as it is the perivascular tissue and not the vessel itself that it is handled by surgical instruments.[21] As a result, neither flushing nor manual dilation is required.[2,18] Sponges soaked in pure saline solution are then used to cover the vessel and when it is totally extracted, blood from the aortic cannula serves as the storage solution.[2,18,20,21] Finally, interrupted or continuous sutures are used to close the leg wounds[21] [Figure 1].
Figure 1.
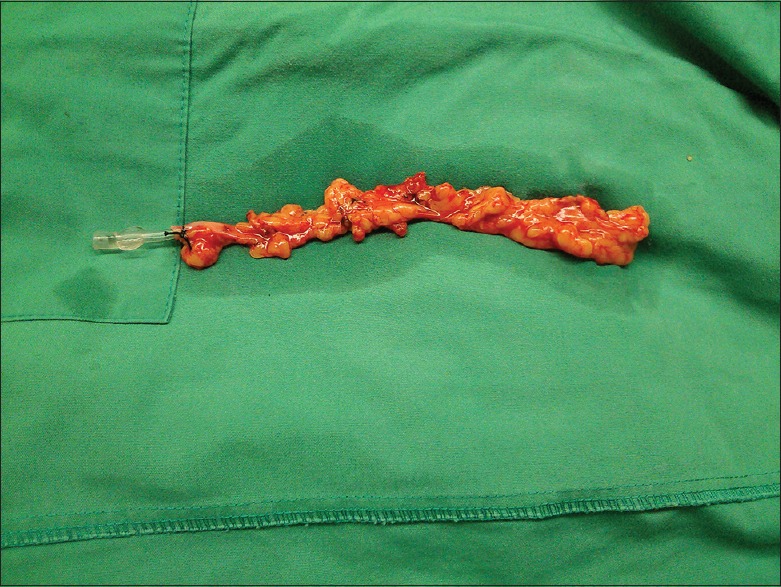
Saphenous vein graft harvested complete with its surrounding fat by no touch technique
PATHOGENESIS OF GRAFT OCCLUSION: WHY IS THE VEIN DAMAGED?
Vein graft failure is the result of three main causes depending on the time after surgery: Acute thrombosis, intimal hyperplasia, and atherosclerosis.[1] During the 1st postoperative month, acute thrombosis is the main cause of graft failure.[1,19] Acute thrombosis can occur due to technical factors such as small size of the target vessel leading to poor distal runoff and size mismatch between the graft and the target vessel resulting in turbulent flow. Graft ischemia and endothelial layer removal because of mechanical trauma and manual distention can also cause acute thrombosis. Platelet adhesion and thrombosis are induced by the removal of the endothelial layer. Furthermore, nitric oxide (NO) levels are decreased leading to vasospasm.[1] Distension with normal saline to overcome spasm causes more damage to the media and intima layers of the graft.[21]
From 1 to 12 months after surgery (the subacute period), intimal hyperplasia is responsible for graft failure.[1,19] Both hemodynamic and nonhemodynamic factors, such as mechanical stretch and vascular injury, provoke the activation of vascular smooth muscle cells (VSMCs) which contribute to neointimal hyperplasia.[22,23] Higher arterial pressures make smooth muscle cells proliferate and then migrate into the intima, where proliferation goes on.[1] This proliferation does not significantly differ between the uninjured vein and the ITA,[24] but the damaged vein endothelium results in more intense proliferation.[20] Vein wall ischemia due to loss of vasa vasorum blood supply is another contributing factor leading to intimal hyperplasia.[25]
Atheroma development following intimal hyperplasia is responsible for graft failure after the 1st postoperative year.[19] Vein atheromas can rupture and cause thrombotic occlusion of the graft just like coronary artery atheromas.[26] However, vein atheromas characteristics make them more prone to rupture. They are more diffuse and concentric, less calcified and have poorly developed or no fibrous caps.[27]
The deleterious effects of endothelial damage of SV during its preparation[28] appeared immediately after its introduction in CABG in the 1960s.[29] Injuries happening during surgery lead to intimal loss and consequently to biochemical and functional changes of the graft.[30] During the removal of the perivascular SV tissue when the conventional method is used, the adventitia of the vein is damaged and vasospasm of the vessel takes place.[31] Subsequent distention is responsible for the reduction of endothelial NOS (eNOS) concentration in the endothelium and in the medial layer, and stripping is associated with the removal of eNOS from the adventitia.[20] All vessel layers and their cells are affected by the harvesting approach.[28] For example, deformed, flattened, and polymorphic endothelial cells containing an abundance of cytoplasmic vesicles[32] and VSMC with altered medial morphology and signs of nuclear division indicating VSMC proliferation[33] are observed when conventional SV harvesting is adopted.
However, the NT SV harvesting approach prevents endothelial damage and subsequent intimal hyperplasia which result in graft failure.[1] The major advantages of NT method are as follows:
Vein trauma is limited during vein harvesting.[13] Electron microscopy proves that NT technique preserves the endothelium to the maximum extent.[14,34] A continuous endothelium attached to the basal membrane in veins received with the NT technique is recognized by the electron microscopy, whereas veins prepared with the conventional technique have not a continuous endothelium and the endothelial cells are separated from the basal membrane.[13,14,33] As a result, an intact adventitial layer with microvessels is recognized by immunohistochemistry after the NT technique, whereas the conventional technique is not associated with such an observation.[20] An intact vasa vasorum channel contributes to the improved vein graft patency rate.[16] Dreifaldt et al.[16] observed a significant reduction of vasa vasorum in the media (P = 0.007) and adventitia (P = 0.014) of SVs harvested by the conventional technique in comparison with those prepared with the NT technique. A continuous endothelium and an intact adventitial collagen layer after NT technique are also confirmed by an ultrastructural analysis.[35] Hence, intact structure, preserved endothelial function, preserved levels of eNOS, and reduced neutrophil adhesion are typical of the NT SV harvesting technique.[22,23,32] Slower progression of atherosclerosis is also observed resulting in improved graft patency rate[36]
The vein wall is maintained intact, so no spasm occurs.[36] As a result, there is no need for high-pressure distension to overcome venous spasm[13,37]
The perivascular adipose vein pedicle is preserved.[13] This perivascular fat serves as an “external biological stent”[28,37] which is consisted of numerous collagen fibers that prevent from the deleterious effects of aortic pressure to the vein wall.[20] This external stent has been shown effective in reducing early thrombotic occlusions, intimal, and medial hyperplasia.[38,39,40] In addition, the surrounding tissue of the vein is a source of NO and vasodilatory adipokines such as leptin,[41] and eNOS activity is recognized in all three layers of the vein wall after NT technique, whereas stripping and distention used during the conventional technique lead to the reduction of eNOS.[20,35] eNOS activity preservation offers increased thromboresistance of the vein, improves its vasorelaxation and prevents venospasm.[13,14,15,19]
GRAFT PATENCY
Harvesting the SV graft by the conventional technique leads to graft occlusion within the 1st month in up to 15% of SV grafts.[8,9] Another 15–30% of SV grafts occlude during the 1st year.[8,10] When the conventional technique is used, handling the vein with surgical instruments, stripping and dilation in order to overcome spasm injure the vein wall.[42] According to Souza et al.,[2] the harvesting technique of the SV for CABG, which is directly related to the preoperative quality of the vein,[2,43] constitutes the most significant factor for graft patency.[2] According to this study by Souza et al., the NT technique not only provides a significantly better SV patency rate in comparison with the conventional technique (P = 0.007) but also SV patency is similar to that of the left internal thoracic artery (LITA).[2] According to an angiography assessment by Souza et al.[44] comparing 52 patients whose veins were harvested by the NT technique with 52 patients submitted to the conventional technique, graft patency of veins harvested with the conventional technique was 89% versus 95% for the NT technique at 18 months postoperatively.[44] Same results were reported by Rueda et al.[20] who compared NT technique with the conventional one and with an intermediate harvesting technique. The angiography assessment at a mean of 18 months showed that 118 out of the 124 veins were patent in the NT Group (95.4%) which was significantly higher than the 88.9% (113/127) patency rate of the conventional group and the 86.2% (100/116) patency rate of the intermediate group (P = 0.025). Even ITA patency rate (91.5%) was lower than the NT SV patency rate in both studies.[20,44] At 8.5 years postoperatively, there was a clear difference between the two groups. NT Group presented a 90% SV graft patency whereas the patency rate of the veins prepared with the conventional technique was only 76% (P = 0.01).[2] Furthermore, the NT technique was associated with an impressively higher SV graft patency rate than the conventional technique in veins of poor quality having either varicose or fibrotic changes during surgery. The patency rate of veins of poor quality before harvesting at 8.5 years was 88.9% versus 36.4%, respectively (P = 0.002).[2] It is important to mention that the overall long-term LITA patency was 90% at 8.5 years.[2] In a subsequent study, the same group compared long-term graft patency rates of an SV harvested by the NT technique with a radial artery graft.[45] If harvested by the conventional technique, an SV graft has a lower long-term patency rate compared to a radial artery.[46] However, the patency rate of the NT SV graft at 3 years was 94% which was significantly higher than the 82% patency rate of the radial artery graft (P = 0.01).[45] In another randomized study, 49 patients in NT Group were compared with 44 patients in the conventional group with regard to graft occlusion rates at 8.5 years on average. NT Group was superior to conventional group (24.3% vs. 43.2%) (P = 0.14)[47] [Table 1].
Table 1.
Harvesting the saphenous vein with surrounding tissue provides high short- and long-term patency rates comparable to the left internal thoracic artery
| Study | Early patency rate for C SV (18 months), % | Early patency rate for NT SV (18 months), % | Pe | Late patency rate for C SV (8.5 years), % | Late patency rate for NT SV (8.5 years), % | Pl | Early patency rate for LITA (18 months), % | Late patency rate for LITA (8.5 years), % |
|---|---|---|---|---|---|---|---|---|
| Souza et al.[44] | 88.9 (113/127) | 95.4 (118/124) | 0.10 | - | - | - | 91.5 (108/118) | - |
| Souza et al.[2] | - | - | - | 76 (77/101) | 90 (91/101) | 0.01 | - | 90 (63/70) |
| Rueda et al.[20] | 88.9 (113/127) | 95.4 (118/124) | 0.025 | - | - | - | 91.5 (108/118) | - |
| Johansson et al.[36] | 75 (84/112) | 89 (105/118) | 0.006 | 84.4 | 92.3 | 0.14 | - | - |
| Johansson et al.[47] | - | - | - | 75.7 | 66.8 | 0.14 | - | - |
C: Conventional, SV: Saphenous vein, NT: No touch, Pe: P value for statistical significance between the two groups for early patency rate, Pl: P value for statistical significance between the two groups for late patency rate, LITA: Left internal thoracic artery
Similarly, Johansson et al.[36] compared graft patency rates with a focus on early atherosclerotic changes between veins harvested by the NT technique and by the conventional technique in a short term at 18 months and in a long term at 8.5 years study. In the short-term study, graft patency was 89% for the NT Group, whereas it was 75% for the conventional group (P = 0.006). A bolus of 12,500 IU of heparin intravenous and 0.2 mg nitroglycerin was administered and then intravascular ultrasonography was performed. If the diameter of the target coronary artery was over 2 mm forming a favorable angle with the SV graft, the ultrasound catheter was advanced into the coronary artery, or else it was advanced close to the junction of the graft with the coronary vessel.[36] Intravascular ultrasonography showed less intimal thickness (P = 0.03), less grafts with considerable intimal hyperplasia (P = 0.011), and larger graft lumen volumes (P = 0.07) in the NT Group. In the long-term study (8.5 years), the NT technique was associated with a 92.3% patency rate which was superior to the 84.4% patency rate of the conventional technique (P = 0.14).[36] Intravascular ultrasonography also revealed much more intense atherosclerotic changes in the conventional group at 8.5 years. In overall, fewer patients in the NT Group had grafts containing plaque compared to the conventional group (50% vs. 80%, P = 0.13). In detail, comparing the NT group to the conventional one, there were significantly fewer grafts containing multiple plaques in the former (14.8% vs. 50%, P = 0.008), significantly less advanced plaque with lipid (11.8% vs. 63.9%, P = 0.0004) and significantly less maximal plaque thickness (1.04 mm vs. 1.32 mm, P = 0.02). Finally, lumen volumes continued to be significantly larger in the NT Group (P = 0.03)[36] [Table 1].
COMPLICATIONS
It is hypothesized that wound complications following the NT method happen more often than when using the conventional approach due to the removal of the pedicle of perivascular tissue during the former.[48,49] Rueda et al.[20] compared three groups of patients: A group of NT SV grafts (NT Group), another of veins prepared with the conventional technique (C Group), and the third one including veins prepared with the conventional technique but not distended (I Group). About 10% of patients in each group developed cellulites and superficial infection of the leg wound. In another study comparing same groups with the aforementioned, two of 46 patients (4.3%) in C Group, three of 41 patients (7.3%) in I Group, and five of 45 patients (11.1%) in NT Group suffered superficial infection or cellulitis.[21,44] Verma et al.[49] observed leg wound infections only in diabetic patients submitted to NT harvesting technique. Nonetheless, there was no significant difference with regard to functional recovery of the leg between those treated by the NT method and those treated by the conventional one at the 1st postoperative year.[49] In addition, an endoscopic method for SV harvesting has been adopted by many cardiac surgical centers as it is related to a lower rate of harvest site complications.[50] Mannion et al.[48] compared 87 patients whose SVs were harvested NT with 123 patients whose veins were prepared endoscopically during 2 years. Harvest site complications were significantly higher in the NT Group. Eighteen percent of the patients submitted to NT approach required vacuum-assisted wound closure or intravenous antibiotics, whereas only 2% of the endoscopically treated patients did (P < 0.0001). However, NT technique was related to a superior vein graft patency than the endoscopic approach (94% vs. 27%, P < 0.02).[48] Therefore, an NT endoscopic approach would be an advantageous technique. To do so, a device which would allow one to remove the vein intact together with its perivascular tissue is required.[2]
In another study, patients treated by the NT method complained about mild-to-severe numbness and swelling. Greater swelling seems to be reasonable as more venous and lymphatic vessels are injured. However, this effect is minor and it is resolved over time.[49] On the other hand, numbness sensation may be explained by the fact that the saphenous nerve is inevitably extracted within the perivascular SV pedicle during NT approach, whereas it is usually preserved during conventional approach.[49] Souza et al.[18] observed that although the saphenous nerve was damaged in the NT technique, no severe neurological symptoms were observed. The sensory reduction at harvesting site was the most common neurological finding. However, in another study, similar neurological symptoms were also observed in most patients submitted to the conventional technique due to the saphenous nerve innervations.[51]
Kinking is another possible complication after the removal of the surrounding tissue of the vein when the graft is too long. Kinking leads to later functional impairment. Consequently, when the conventional technique is used, graft size has to be carefully adjusted to avoid the risk of kinking.[20] Nevertheless, in the NT technique, the perivascular tissue of the vein is preserved thus supporting the graft and safely preventing from kinking no matter the length of the graft.[2,18,20]
MORBIDITY AND MORTALITY
Neither mortality nor morbidity is increased by NT technique compared to conventional technique. No perioperative myocardial infarction or death was occurred in 52 patients who underwent coronary artery bypass grafting with NT SVs according to a Souza et al. study in 2001.[18] Rueda et al.[20] compared three groups of patients: A group of NT SV grafts (NT Group), another of veins prepared with the conventional technique (C Group), and the third one including veins prepared with the conventional technique but not distended (I Group). Mortality rate was 0% in all three groups. Two patients in the C Group suffered perioperative acute myocardial infarction and one patient in the NT Group was reoperated due to bleeding from the internal mammary artery. In another study, comparing same groups with the aforementioned, no major complication requiring surgery was occurred in either of the three groups. Mortality was also zero in all three groups, whereas two patients of the Group C suffered a myocardial infarction preoperatively.[21] Similar results were also observed by Souza et al. in 2006. No perioperative death was observed in either group and two patients from Group C suffered a perioperative myocardial infarction.[2] In another randomized study by Johansson et al.[47] comparing 49 patients in group NT to 44 patients in Group C at 8.5 years postoperatively, no cardiac death was found in group NT, whereas three patients died in Group C. Moreover, there was a statistically significant advantage for the NT patients as 67.3% of them were asymptomatic-free from angina and in New York Heart Association Class I versus 43.2% of C patients (P = 0.02). Trends toward fewer cardiac deaths and myocardial infarctions (3.8 vs. 13.4%; P = 0.16) as well as toward more patients free from angina (75.5 vs. 63.6%; P = 0.26) were also observed.[47]
Limitations of the studies
In spite of the encouraging results, there is an absence of clinical outcomes in most studies.[2,16,19,20,28,32,36] Outcomes extracted by intravascular ultrasonography were reported by Johansson et al.[36] Angiography assessment results were reported by Rueda et al.[20] and by Souza et al.[2] Morphometric analysis of the vessel wall and ultrastructural analysis was the basis on which other studies extracted their results,[16,19,20,28,32] In Dreifaldt et al.‘s[16] study, veins harvested from a limited number of patients were examined to extract their results with regard to vasa vasorum of the veins. A relatively high variability in measurements was also existed due to the individual variations among patients, the variation of vessel wall thickness within the same vein, and the variation related to stripping the adventitia during the conventional technique.[16] According to Verma et al.‘s[49] study limitations, there cannot be a secure conclusion by their protocol if the VSMC changes are secondary to the harvesting technique, no need for distension or the intact surrounding adipose tissue. They also mention that ex vivo time prior to fixation was not estimated and NT veins were usually harvested prior to the conventional veins leading to greater activation in NT veins. Moreover, NT veins were preserved ex vivo in heparinized blood solutions, whereas conventional veins were kept ex vivo in normal saline solutions. In addition, all measurements were not made in the same sample due to the limited amount of tissue collected. Finally, the absence of clinical outcomes and complications due to insufficient sample size was another limitation of this study.[49]
CONCLUSIONS
In conclusion, the conventional technique of SV harvesting includes the removal of SV without its surrounding tissue and the distension of the vein at high-pressure after harvesting in order to abolish the potential spasm of the graft. In contrast to this, during the NT technique, the perivascular tissue of the vein is prepared together with the vessel in order to prevent any spasm of the graft.[35] The preservation of the surrounding tissue of the vein keeps the endothelium of the vein intact. As a result, the endothelium maintains its structure and its functionality,[18] sources of NO[28] and vasa vasorum of the vein[16] are also preserved, atherosclerosis progresses slower[36] and therefore, a better graft patency rate is achieved.[16,18,28,36] In contrast, when the SV is stripped off its surrounding tissue, spasm of the graft happens and mechanical distension is necessary in order to overcome it. As a result, endothelial, medial, and adventitial integrity is compromised and both short- and long-term venous graft patency are influenced.[21] In addition, a long vein graft without the threat of kinking can be achieved when the surrounding tissue is maintained.[21] In overall, the “NT” SV harvesting technique can produce significantly superior short- and long-term graft patency than the conventional technique can, and more importantly, the patency rates of the grafts harvested by the NT technique are even comparable to that of the LITA graft.[2,35] Consequently, the SV is revived as an important conduit in CABG surgery. The novel NT technique of SV harvesting provides better structural, functional, and mechanical protection of the vein wall. As a result, a nonthrombogenic graft is produced and less intimal and medial hyperplasia take place.[2] However, more studies with clinical outcomes are required in order to confirm these encouraging observations. Perfecting the technique of this harvesting method and more reliable future studies may reveal a graft comparable to arterial grafts in terms of quality and long-term patency.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors had full control of the design of the study, methods used, outcome parameters and results, analysis of data, and production of the written report.
REFERENCES
- 1.Parang P, Arora R. Coronary vein graft disease: Pathogenesis and prevention. Can J Cardiol. 2009;25:e57–62. doi: 10.1016/s0828-282x(09)70486-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Souza DS, Johansson B, Bojö L, Karlsson R, Geijer H, Filbey D, et al. Harvesting the saphenous vein with surrounding tissue for CABG provides long-term graft patency comparable to the left internal thoracic artery: Results of a randomized longitudinal trial. J Thorac Cardiovasc Surg. 2006;132:373–8. doi: 10.1016/j.jtcvs.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Grondin CM, Campeau L, Lesperance J, Enjalbert M, Bourassa M. Comparison of late changes in internal mammary artery and saphenous vein in two consecutive series of patients 1 year after operation. Circulation. 1984;70:1208–12. [PubMed] [Google Scholar]
- 4.Loop FD, Lytle BW, Cosgrove DM, Stewart RW, Goormastic M, Williams GW, et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med. 1986;314:1–6. doi: 10.1056/NEJM198601023140101. [DOI] [PubMed] [Google Scholar]
- 5.Tsui JC, Dashwood MR. Recent strategies to reduce vein graft occlusion: A need to limit the effect of vascular damage. Eur J Vasc Endovasc Surg. 2002;23:202–8. doi: 10.1053/ejvs.2002.1600. [DOI] [PubMed] [Google Scholar]
- 6.Galbut DL, Traad EA, Dorman MJ, DeWitt PL, Larsen PB, Kurlansky PA, et al. Seventeen-year experience with bilateral internal mammary artery grafts. Ann Thorac Surg. 1990;49:195–201. doi: 10.1016/0003-4975(90)90138-v. [DOI] [PubMed] [Google Scholar]
- 7.Campeau L, Enjalbert M, Lespérance J, Vaislic C, Grondin CM, Bourassa MG. Atherosclerosis and late closure of aortocoronary saphenous vein grafts: Sequential angiographic studies at 2 weeks, 1 year, 5 to 7 years, and 10 to 12 years after surgery. Circulation. 1983;68(3 Pt 2):II1–7. [PubMed] [Google Scholar]
- 8.Mehta D, Izzat MB, Bryan AJ, Angelini GD. Towards the prevention of vein graft failure. Int J Cardiol. 1997;62(Suppl 1):S55–63. doi: 10.1016/s0167-5273(97)00214-3. [DOI] [PubMed] [Google Scholar]
- 9.Manninen HI, Jaakkola P, Suhonen M, Rehnberg S, Vuorenniemi R, Matsi PJ. Angiographic predictors of graft patency and disease progression after coronary artery bypass grafting with arterial and venous grafts. Ann Thorac Surg. 1998;66:1289–94. doi: 10.1016/s0003-4975(98)00757-7. [DOI] [PubMed] [Google Scholar]
- 10.Izzat MB, West RR, Bryan AJ, Angelini GD. Coronary artery bypass surgery: Current practice in the United Kingdom. Br Heart J. 1994;71:382–5. doi: 10.1136/hrt.71.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malone JM, Kischer CW, Moore WS. Changes in venous endothelial fibrinolytic activity and histology with in vitro venous distention and arterial implantation. Am J Surg. 1981;142:178–82. doi: 10.1016/0002-9610(81)90271-3. [DOI] [PubMed] [Google Scholar]
- 12.Angelini GD, Passani SL, Breckenridge IM, Newby AC. Nature and pressure dependence of damage induced by distension of human saphenous vein coronary artery bypass grafts. Cardiovasc Res. 1987;21:902–7. doi: 10.1093/cvr/21.12.902. [DOI] [PubMed] [Google Scholar]
- 13.Souza D. A new no-touch preparation technique. Technical notes. Scand J Thorac Cardiovasc Surg. 1996;30:41–4. doi: 10.3109/14017439609107239. [DOI] [PubMed] [Google Scholar]
- 14.Souza DS, Christofferson RH, Bomfim V, Filbey D. “No-touch” technique using saphenous vein harvested with its surrounding tissue for coronary artery bypass grafting maintains an intact endothelium. Scand Cardiovasc J. 1999;33:323–9. doi: 10.1080/14017439950141362. [DOI] [PubMed] [Google Scholar]
- 15.Tsui J, Souza DS, Filbey D, Bomfim V, Dashwood MR. Preserved endothelial integrity and nitric oxide synthase in human saphenous vein harvested by a novel ‘no-touch’ technique. Br J Surg. 2001;88:1209–15. doi: 10.1046/j.0007-1323.2001.01855.x. [DOI] [PubMed] [Google Scholar]
- 16.Dreifaldt M, Souza DS, Loesch A, Muddle JR, Karlsson MG, Filbey D, et al. The “no-touch” harvesting technique for vein grafts in coronary artery bypass surgery preserves an intact vasa vasorum. J Thorac Cardiovasc Surg. 2011;141:145–50. doi: 10.1016/j.jtcvs.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Gollasch M, Dubrovska G. Paracrine role for periadventitial adipose tissue in the regulation of arterial tone. Trends Pharmacol Sci. 2004;25:647–53. doi: 10.1016/j.tips.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Souza DS, Bomfim V, Skoglund H, Dashwood MR, Borowiec JW, Bodin L, et al. High early patency of saphenous vein graft for coronary artery bypass harvested with surrounding tissue. Ann Thorac Surg. 2001;71:797–800. doi: 10.1016/s0003-4975(00)02508-x. [DOI] [PubMed] [Google Scholar]
- 19.Tsui JC, Souza DS, Filbey D, Karlsson MG, Dashwood MR. Localization of nitric oxide synthase in saphenous vein grafts harvested with a novel “no-touch” technique: Potential role of nitric oxide contribution to improved early graft patency rates. J Vasc Surg. 2002;35:356–62. doi: 10.1067/mva.2002.121072. [DOI] [PubMed] [Google Scholar]
- 20.Rueda Fd, Souza D, Lima Rde C, Menezes A, Johansson B, Dashwood M, et al. Novel no-touch technique of harvesting the saphenous vein for coronary artery bypass grafting. Arq Bras Cardiol. 2008;90:356–62. [Google Scholar]
- 21.Souza DS, Arbeus M, Botelho Pinheiro B, Filbey D. The no-touch technique of harvesting the saphenous vein for coronary artery bypass grafting surgery. Multimed Man Cardiothorac Surg 2009. 2009 doi: 10.1510/mmcts.2008.003624. MMCTS 2009 (0731):mmcts.2008.003624. [DOI] [PubMed] [Google Scholar]
- 22.Khaleel MS, Dorheim TA, Duryee MJ, Durbin HE, Jr, Bussey WD, Garvin RP, et al. High-pressure distention of the saphenous vein during preparation results in increased markers of inflammation: A potential mechanism for graft failure. Ann Thorac Surg. 2012;93:552–8. doi: 10.1016/j.athoracsur.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 23.Nolte A, Secker S, Walker T, Greiner TO, Neumann B, Simon P, et al. Veins are no arteries: Even moderate arterial pressure induces significant adhesion molecule expression of vein grafts in an ex vivo circulation model. J Cardiovasc Surg (Torino) 2011;52:251–9. [PubMed] [Google Scholar]
- 24.Holt CM, Francis SE, Newby AC, Rogers S, Gadsdon PA, Taylor T, et al. Comparison of response to injury in organ culture of human saphenous vein and internal mammary artery. Ann Thorac Surg. 1993;55:1522–8. doi: 10.1016/0003-4975(93)91103-t. [DOI] [PubMed] [Google Scholar]
- 25.Barker SG, Talbert A, Cottam S, Baskerville PA, Martin JF. Arterial intimal hyperplasia after occlusion of the adventitial vasa vasorum in the pig. Arterioscler Thromb. 1993;13:70–7. doi: 10.1161/01.atv.13.1.70. [DOI] [PubMed] [Google Scholar]
- 26.Cox JL, Chiasson DA, Gotlieb AI. Stranger in a strange land: The pathogenesis of saphenous vein graft stenosis with emphasis on structural and functional differences between veins and arteries. Prog Cardiovasc Dis. 1991;34:45–68. doi: 10.1016/0033-0620(91)90019-i. [DOI] [PubMed] [Google Scholar]
- 27.Motwani JG, Topol EJ. Aortocoronary saphenous vein graft disease: Pathogenesis, predisposition, and prevention. Circulation. 1998;97:916–31. doi: 10.1161/01.cir.97.9.916. [DOI] [PubMed] [Google Scholar]
- 28.Dashwood MR, Savage K, Dooley A, Shi-Wen X, Abraham DJ, Souza DS. Effect of vein graft harvesting on endothelial nitric oxide synthase and nitric oxide production. Ann Thorac Surg. 2005;80:939–44. doi: 10.1016/j.athoracsur.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 29.Favaloro RG. Saphenous vein graft in the surgical treatment of coronary artery disease. Operative technique. J Thorac Cardiovasc Surg. 1969;58:178–85. [PubMed] [Google Scholar]
- 30.Mills NL, Everson CT. Vein graft failure. Curr Opin Cardiol. 1995;10:562–8. doi: 10.1097/00001573-199511000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Mann JM, McIntosh CL, Roberts WC. Spasm of saphenous veins used as conduits for aortocoronary bypass grafting. Am J Cardiol. 1987;59:1000–2. doi: 10.1016/0002-9149(87)91146-5. [DOI] [PubMed] [Google Scholar]
- 32.Dashwood MR, Savage K, Tsui JC, Dooley A, Shaw SG, Fernández Alfonso MS, et al. Retaining perivascular tissue of human saphenous vein grafts protects against surgical and distension-induced damage and preserves endothelial nitric oxide synthase and nitric oxide synthase activity. J Thorac Cardiovasc Surg. 2009;138:334–40. doi: 10.1016/j.jtcvs.2008.11.060. [DOI] [PubMed] [Google Scholar]
- 33.Ahmed SR, Johansson BL, Karlsson MG, Souza DS, Dashwood MR, Loesch A. Human saphenous vein and coronary bypass surgery: Ultrastructural aspects of conventional and “no-touch” vein graft preparations. Histol Histopathol. 2004;19:421–33. doi: 10.14670/HH-19.421. [DOI] [PubMed] [Google Scholar]
- 34.Souza DS, Dashwood MR, Tonazi A, Johansson B, Buffalo E, Bomfim V, et al. Preparation of VS in coronary artery bypass surgery: A new technique - “No- touch” - That keeps the vein wall full and provides high immediate patency. Rev Bras Cir Cardiovasc. 2003;18:303–11. [Google Scholar]
- 35.Sepehripour AH, Jarral OA, Shipolini AR, McCormack DJ. Does a ‘no-touch’ technique result in better vein patency? Interact Cardiovasc Thorac Surg. 2011;13:626–30. doi: 10.1510/icvts.2011.281998. [DOI] [PubMed] [Google Scholar]
- 36.Johansson BL, Souza DS, Bodin L, Filbey D, Loesch A, Geijer H, et al. Slower progression of atherosclerosis in vein grafts harvested with ‘no touch’ technique compared with conventional harvesting technique in coronary artery bypass grafting: An angiographic and intravascular ultrasound study. Eur J Cardiothorac Surg. 2010;38:414–9. doi: 10.1016/j.ejcts.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 37.Souza DS. ‘No touch’ technique harvesting saphenous vein with its surrounding tissue for coronary artery bypass surgery provides an intact endothelium and a high mid-term patency rate. In: Rubanyi GM, Dzau VJ, Cooke JP, editors. Vascular Protection: Molecular Mechanisms, Novel Therapeutic Principles and Clinical Application. London: Taylor and Francis; 2002. pp. 97–106. [Google Scholar]
- 38.Vijayan V, Shukla N, Johnson JL, Gadsdon P, Angelini GD, Smith FC, et al. Long-term reduction of medial and intimal thickening in porcine saphenous vein grafts with a polyglactin biodegradable external sheath. J Vasc Surg. 2004;40:1011–9. doi: 10.1016/j.jvs.2004.08.047. [DOI] [PubMed] [Google Scholar]
- 39.Zurbrügg HR, Wied M, Angelini GD, Hetzer R. Reduction of intimal and medial thickening in sheathed vein grafts. Ann Thorac Surg. 1999;68:79–83. doi: 10.1016/s0003-4975(99)00452-x. [DOI] [PubMed] [Google Scholar]
- 40.Izzat MB, Mehta D, Bryan AJ, Reeves B, Newby AC, Angelini GD. Influence of external stent size on early medial and neointimal thickening in a pig model of saphenous vein bypass grafting. Circulation. 1996;94:1741–5. doi: 10.1161/01.cir.94.7.1741. [DOI] [PubMed] [Google Scholar]
- 41.Hinokiyama K, Valen G, Tokuno S, Vedin JB, Vaage J. Vein graft harvesting induces inflammation and impairs vessel reactivity. Ann Thorac Surg. 2006;82:1458–64. doi: 10.1016/j.athoracsur.2006.05.038. [DOI] [PubMed] [Google Scholar]
- 42.Quist WC, Haudenschild CC, LoGerfo FW. Qualitative microscopy of implanted vein grafts. Effects of graft integrity on morphologic fate. J Thorac Cardiovasc Surg. 1992;103:671–7. [PubMed] [Google Scholar]
- 43.Friedl R, Li J, Schumacher B, Hanke H, Waltenberger J, Hannekum A, et al. Intimal hyperplasia and expression of transforming growth factor-beta1 in saphenous veins and internal mammary arteries before coronary artery surgery. Ann Thorac Surg. 2004;78:1312–8. doi: 10.1016/j.athoracsur.2004.02.066. [DOI] [PubMed] [Google Scholar]
- 44.Souza DS, Dashwood MR, Tsui JC, Filbey D, Bodin L, Johansson B, et al. Improved patency in vein grafts harvested with surrounding tissue: Results of a randomized study using three harvesting techniques. Ann Thorac Surg. 2002;73:1189–95. doi: 10.1016/s0003-4975(02)03425-2. [DOI] [PubMed] [Google Scholar]
- 45.Dreifaldt M, Mannion JD, Bodin L, Olsson H, Zagozdzon L, Souza D. The no-touch saphenous vein as the preferred second conduit for coronary artery bypass grafting. Ann Thorac Surg. 2013;96:105–11. doi: 10.1016/j.athoracsur.2013.01.102. [DOI] [PubMed] [Google Scholar]
- 46.Deb S, Cohen EA, Singh SK, Une D, Laupacis A, Fremes SE. RAPS Investigators. Radial artery and saphenous vein patency more than 5 years after coronary artery bypass surgery: Results from RAPS (Radial Artery Patency Study) J Am Coll Cardiol. 2012;60:28–35. doi: 10.1016/j.jacc.2012.03.037. [DOI] [PubMed] [Google Scholar]
- 47.Johansson BL, Souza DS, Bodin L, Filbey D, Bojö L. No touch vein harvesting technique for CABG improves the long-term clinical outcome. Scand Cardiovasc J. 2009;43:63–8. doi: 10.1080/14017430802140104. [DOI] [PubMed] [Google Scholar]
- 48.Mannion JD, Marelli D, Brandt T, Stallings M, Cirks J, Dreifaldt M, et al. “No-touch” versus “endo” vein harvest: Early patency on symptom-directed catheterization and harvest site complications. Innovations (Phila) 2014;9:306–11. doi: 10.1097/IMI.0000000000000084. [DOI] [PubMed] [Google Scholar]
- 49.Verma S, Lovren F, Pan Y, Yanagawa B, Deb S, Karkhanis R, et al. Pedicled no-touch saphenous vein graft harvest limits vascular smooth muscle cell activation: The PATENT saphenous vein graft study. Eur J Cardiothorac Surg. 2014;45:717–25. doi: 10.1093/ejcts/ezt560. [DOI] [PubMed] [Google Scholar]
- 50.Sastry P, Rivinius R, Harvey R, Parker RA, Rahm AK, Thomas D, et al. The influence of endoscopic vein harvesting on outcomes after coronary bypass grafting: A meta-analysis of 267,525 patients. Eur J Cardiothorac Surg. 2013;44:980–9. doi: 10.1093/ejcts/ezt121. [DOI] [PubMed] [Google Scholar]
- 51.Mountney J, Wilkinson GA. Saphenous neuralgia after coronary artery bypass grafting. Eur J Cardiothorac Surg. 1999;16:440–3. doi: 10.1016/s1010-7940(99)00294-8. [DOI] [PubMed] [Google Scholar]


