Abstract
Computer simulations can come in handy to train medical personnel with necessary skills to face the clinical scenarios involving various coagulopathies. Now a days, point of care (POC) devices such as thromboelastography, Sonoclot analyzer and newly approved rotational thromboelastometry (ROTEM) with faster results to assess coagulopathies are available on bedside of patients. ROTEM is emerging as a quick, portable, and well-validated device to evaluate coagulopathy in critical care and perioperative setup. A novel platelet-aggregometry integrated module enables simultaneous analysis of platelets as well as coagulation tests on the same screen. The entire gamut of POC signature curves obtained with different coagulation defects can be learned with graphical simulations. These simulations can be a valuable strategy to elucidate latent conditions, for which simulation interventions can then be designed to mimic different clinical scenarios.
Keywords: Coagulation, Graphical simulation, Platelet-aggregometry, Reagents, Rotational thromboelastometry, Thromboelastometer-graphs
INTRODUCTION
The use of computer simulation in medical field has increased the quality of training and safety in terms of patient care. These simulators can come in handy to train medical personnel with necessary skills to face the clinical scenarios involving various coagulopathies. Coagulopathy in critically ill and in patients undergoing major surgery is a concerned cause of morbidity and mortality. The management of these patients was earlier guided by conventional laboratory coagulations tests such as platelet count, prothrombin time, activated partial thromboplastin time, and serum fibrinogen levels.[1,2] The lengthy turnaround time of these lab tests was a cause of undue delay and improper treatment.[3] Now a days, point of care (POC) devices such as thromboelastography (TEG), Sonoclot analyzer, and newly Food and Drug Administration approved rotational thromboelastometry (ROTEM) with faster results to assess coagulopathies are available on bedside of patients. These viscoelastic POC devices are used to measure the time until clot formation, the dynamics of clot formation, and the stability of clots over time.[4,5]
The entire gamut of POC signature curves obtained with different coagulation defects can be learned with graphical simulations. These simulations can be a valuable strategy to elucidate latent conditions, for which simulation interventions can then be designed to mimic different clinical scenarios.
ROTATIONAL THROMBOELASTOMETER
ROTEM is an advancement of TEG and differs in the way of analyzing the coagulation process: In the TEG, the cup is slowly oscillated and changes in viscoelasticity are measured by a pin attached to a suspended torsion wire; whereas, in ROTEM, the cup remains fixed while the pin is oscillated. Moreover, the improved design of ROTEM allows it to be used as a portable device at bedside of the patient. The TEG device has the limitation of inability to distinguish hypofibrinogenemia from thrombocytopenia. However, ROTEM is more suitable as fibrin-specific clot formation (FIBTEM) can be analyzed by inhibiting platelet-fibrinogen interaction using cytochalasin D.
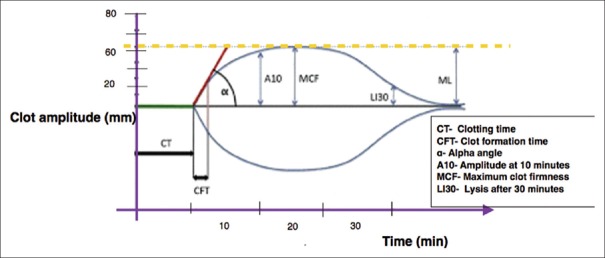
The ROTEM device has four independent analyzer channels which enable the simultaneous independent tests of all parameters [Figure 1]. The various tests of ROTEM are available by use of different activated reagents[6] [Table 1]. These different tests are used simultaneously to derive various ROTEM variables through thromboelastometer graph [Figure 2]. The graphical plot of the clot amplitude versus time displayed on the ROTEM screen is called a “TEMogram” [Figure 3]. The onset of clot is represented by clot time while the rate of fibrin polymerization is represented by clot formation time (CFT) and alpha angle. The maximum clot strength can be judged by maximum clot firmness; whereas, premature lysis or hyperfibrinolysis can be diagnosed by lysis index [Table 2]. In a multicenter study,[7] ROTEM thromboelastometry was shown to yield consistent reference values of these variables among various centers [Table 3].
Figure 1.
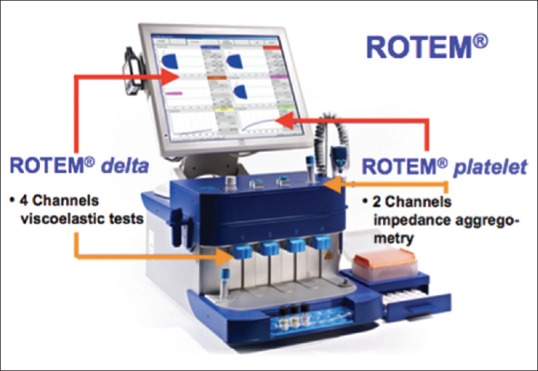
ROTEM device with integrated platelet-aggregometry module. ROTEM device containing 4 independendent channels in lower pannel for carrying out simultaneous different ROTEM tests (INTEM/EXTEM/FIBTEM/HEPTEM) and 2 channels in upper pannel for impedence aggregometry tests. ROTEM platelet assays (ARATEM/ADPTEM/TRAPTEM) are used to study effect on various platelet inhibior drugs. ROTEM: Rotational thromboelastometry
Table 1.
Various rotational thromboelastometry tests with different reagents
| ROTEM tests | Reagents used | Description |
|---|---|---|
| EXTEM | Tissue factor activator | Coagulation is activated by small amount of tissue factor to monitor coagulation via extrinsic pathway |
| INTEM | Ellagicacid/phospholipid activator | Coagulation is activated by contact phase to monitor coagulation via intrinsic pathway |
| HEPTEM | Heparinase in combination with INTEM | Comparison of HEPTEM with INTEM parameters can help in detection of heparinrelated coagulation disturbances |
| FIBTEM | Cytochalasin D in combination with EXTEM | Coagulation is activated as in EXTEM after neglecting platelet contribution to the clot firmness |
| APTEM | Aprotinin, fibrinolysis inhibitor in combination with EXTEM | Coagulation is activated as in EXTEM to monitor the clot firmness after blocking hyperfibrinolysis by aprotinin |
| ECATEM | Ecarin (prothrombin activator) | Diagnose direct thrombin inhibitor |
| NATEM | Activated by recalcification only | Sensitive to detect endogenous activators like tissue factor expression on monocytes cells in sepsis, extracorporeal device use, cirrhosis |
ROTEM: Rotational thromboelastometry
Figure 2.
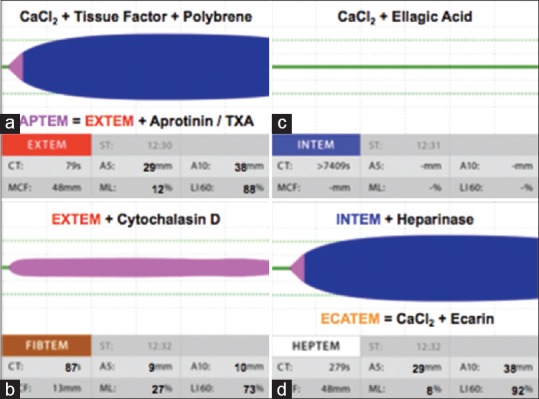
Different ROTEM graphs with various reagents. Various ROTEM tests like (a) EXTEM (activator, tissue factor with polybrene); APTEM (EXTEM plus activator, aprotinin, or tranexamic acid) (b) FIBTEM (EXTEM plus activator, cytochalasin D) (c) INTEM (activator, ellagic-acid) (d) HEPTEM (INTEM plus activator heparinase) and ECATEM (activator, ecarin) are possible simultaneously with the use of different reagents. ROTEM: Rotational thromboelastometry
Figure 3.
Variables of ROTEM in TEMogram. The graphical representation (TEMogram) of clot amplitude (mm) against time (min) showing various ROTEM variables. ROTEM: Rotational thromboelastometry. TEMogram: Thromboelastometer-graphs
Table 2.
Rotational thromboelastometry variables derived from thromboelstometer graph
| ROTEM variable | Description |
|---|---|
| CT: Clotting time (s) | Time until the recognizable start of clot formation (2 mm amplitude) |
| CFT: Clot formation time (s) | Time until amplitude of 20 mm is reached. Represents the clot formation dynamics |
| Angle: Alpha-angle (°) | Angle between central line and a tangent to the curve through the 2 mm amplitude point. Represents the kinetics of clot formation |
| MCF: Maximum clot firmness (mm) | Maximum firmness (amplitude) that the clot achieves during the measurement |
| A10-A30: Amplitude at 10-30 min after CT (mm) | Describes the clot firmness at 10-30 min after CT |
| CL30: Clot lysis index at 30 min after CT (%) | Ratio between the MCF and the amplitude 30-60 min after CT. Describes the progress of fibrinolysis |
| ML: Maximal clot lysis (%) | Ratio of the lowest amplitude detected after achieving MCF to the MCF |
Table 3.
The reference values of thromboelastometer variables with differents rotational thromboelastometry tests in a multicentre study[7]
| CT (s) | CFT (s) | MCF (mm) | A10 (mm) | CL 30 (%) | ML (%) | |
|---|---|---|---|---|---|---|
| INTEM | 137-246 | 40-100 | 52-72 | 44-68 | 94-100 | 0-12 |
| EXTEM | 42-74 | 46-148 | 49-71 | 43-65 | 95-100 | 0-18 |
| FIBTEM | 43-69 | - | 9-25 | 9-24 | - | - |
CT: Clot time, CFT: Clot formation time, MCF: Maximum clot firmness, A10: Amplitude 10 min after CT, CL30: Clot lysis index 30 min after CT, ML: Maximal clot lysis
ROTATIONAL THROMBOELASTOMETRY AND HAEMOSTATIC MANAGEMENT
The rotational thromboelastometer has been used for successfully for POC management in various clinical settings.[8,9] Weber et al. revealed that the hemostatic management based on POC devices (ROTEM) can reduce the patient exposure to blood products and provides significant benefits with respect to clinical outcomes in cardiac surgery patients.[10] Similarly, Fayed et al. observed that ROTEM can predict preoperative transfusion requirements in adult living donor transplant recipients, which may allow better preparation and less cross-matching before surgery.[11]
ROTATIONAL THROMBOELASTOMETRY AND CONVENTIONAL LABORATORY TESTS
The ability of ROTEM to predict thrombocytopenia and hypofibrinogenemia has been tested. Olde Engberink et al. evaluated the ROTEM device to predict thrombocytopenia and hypofibrinogenemia in cardiac surgery patients using the clot amplitude after 5 min. The authors observed that the turnaround time for ROTEM tests (12 min), was comparable with emergency requests for platelet count, (13 min) and shorter than emergency requests for fibrinogen levels (37 min).[12] The ROTEM parameters have been compared and correlated with conventional laboratory coagulation tests. Ogawa et al. conducted a prospective observational study to compare different ROTEM parameters with standard coagulation tests in elective cardiac surgery patients. The author observed strong correlations between FIBTEM-amplitude at 10 min (A10) and fibrinogen level (r = 0.87; P < 0.001) and between EXTEM/INTEM-A10 variables and platelet count (r = 0.72 and 0.67, respectively; P < 0.001). Receiver operating characteristic analysis demonstrated that EXTEM-A10 and INTEM- A10 are predictive of thrombocytopenia below 80 × 109/L (area under the curve [AUC], 0.83 and 0.82, respectively), and FIBTEM-A10 was highly predictive of fibrinogen level below 200 mg/dl (AUC, 0.96).[13]
ROTATIONAL THROMBOELASTOMETRY AND POSTOPERATIVE BLEEDING PREDICTABILITY
The diagnostic ability of ROTEM to predict postoperative bleeding is a matter of controversy. Reinhöfer et al. studied the value of ROTEM to monitor disturbed hemostasis and bleeding risk in patients with cardiopulmonary bypass. The author observed that the positive predictive value and specificity of ROTEM variables such as CFT (71%, 94%) and FIBTEM (73%, 95%) to predict postoperative bleeding was significantly higher than conventional laboratory tests such as aPTT (56%, 72%) and prothrombin time (43%, 5%).[14]
Contrary to this, Davidson et al. observed that ROTEM thromboelastometry has poor positive predictive value (14.8%) to identify patients who bleed more than 200 mL/h in the early postoperative period after cardiac surgery. However, its negative predictive value was found to be good (100%). The authors realized that their study was not adequately powered to confirm its poor positive predictive value as the prevalence of bleeding in the early postoperative period was small (13.7%).[15]
ROTATIONAL THROMBOELASTOMETRY VERSUS OTHER POINT OF CARE DEVICES
The results of ROTEM analysis have significant less inter- and intra-operator variability as compared to TEG results. Hence, ROTEM is more suitable for use at multiple places as compared to TEG.[16] Recently, a study has compared ROTEM, TEG and Sonoclot analyzer in small number of patients undergoing adult cardiac surgery. The authors observed significant correlations between plasma fibrinogen levels, platelet counts, and variables from the three instruments. They concluded that TEG and ROTEM could be used to detect changes in hemostasis following cardiac surgery. However, Sonoclot seems to be less suitable to detect such changes.[17]
ROTATIONAL THROMBOELASTOMETRY PLATELET ASSAYS
The effect of various antiplatelet medications can be studied in ROTEM by integrating a novel aggregometry module in ROTEM system.[18] The various platelet assays can be performed with the use of different activators based on impedance aggregometry [Table 4]. This enables simultaneous analysis of platelet-aggregometry as well as coagulation tests on the same screen [Figure 1].[19]
Table 4.
Various rotational thromboelastometry platelet assays[18]
| ROTEM platelet assays | Reagents used | Description |
|---|---|---|
| ARATEM | Arachidonic acid | Studies COX-1 and GP IIb/IIIa receptors inhibition |
| ADPTEM | ADP | Studies ADP and GP IIb/IIIa receptors inhibition |
| TRAPTEM | Thrombin receptor activating peptide-6 | Studies Thrombin and GPII/bIIIa receptors inhibition |
COX: Cyclooxygenase, GP: Glycoprotein, ADP: Adenosine diphosphate, ROTEM: Rotational thromboelastometry, TEM: Thromboelastometry
CONCLUSION
ROTEM is emerging as a quick, portable, and well-validated device to assess coagulopathy in critical care and perioperative setup. The routine use of this bedside device can help in timely, hemostatic management of patients prone to bleeding. The designing of computer simulation, mimicking various coagulopathies can prepare the health-care providers to manage hemostasis in the clinical set-up. This POC management can guide the proper usage of coagulation products and reduce the unnecessary blood transfusion and morbidity associated with bleeding and transfusion.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Levi M, Opal SM. Coagulation abnormalities in critically ill patients. Crit Care. 2006;10:222. doi: 10.1186/cc4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy GJ, Reeves BC, Rogers CA, Rizvi SI, Culliford L, Angelini GD. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation. 2007;116:2544–52. doi: 10.1161/CIRCULATIONAHA.107.698977. [DOI] [PubMed] [Google Scholar]
- 3.Avidan MS, Alcock EL, Da Fonseca J, Ponte J, Desai JB, Despotis GJ, et al. Comparison of structured use of routine laboratory tests or near-patient assessment with clinical judgement in the management of bleeding after cardiac surgery. Br J Anaesth. 2004;92:178–86. doi: 10.1093/bja/aeh037. [DOI] [PubMed] [Google Scholar]
- 4.Meybohm P, Zacharowski K, Weber CF. Point-of-care coagulation management in intensive care medicine. Crit Care. 2013;17:218. doi: 10.1186/cc12527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mishra PK, Thekkudan J, Sahajanandan R, Gravenor M, Lakshmanan S, Fayaz KM, et al. The role of point-of-care assessment of platelet function in predicting postoperative bleeding and transfusion requirement after coronary artery bypass grafting. Ann Card Anaesth. 2015;18:45–51. doi: 10.4103/0971-9784.148321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ganter MT, Hofer CK. Coagulation monitoring: Current techniques and clinical use of viscoelastic point-of-care coagulation devices. Anesth Analg. 2008;106:1366–75. doi: 10.1213/ane.0b013e318168b367. [DOI] [PubMed] [Google Scholar]
- 7.Lang T, Bauters A, Braun SL, Pötzsch B, von Pape KW, Kolde HJ, et al. Multi-centre investigation on reference ranges for ROTEM thromboelastometry. Blood Coagul Fibrinolysis. 2005;16:301–10. doi: 10.1097/01.mbc.0000169225.31173.19. [DOI] [PubMed] [Google Scholar]
- 8.Haas T, Görlinger K, Grassetto A, Agostini V, Simioni P, Nardi G, et al. Thromboelastometry for guiding bleeding management of the critically ill patient: A systematic review of the literature. Minerva Anestesiol. 2014;80:1320–35. [PubMed] [Google Scholar]
- 9.Görlinger K, Shore-Lesserson L, Dirkmann D, Hanke AA, Rahe-Meyer N, Tanaka KA. Management of hemorrhage in cardiothoracic surgery. J Cardiothorac Vasc Anesth. 2013;27(4 Suppl):S20–34. doi: 10.1053/j.jvca.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 10.Weber CF, Görlinger K, Meininger D, Herrmann E, Bingold T, Moritz A, et al. Point-of-care testing: A prospective, randomized clinical trial of efficacy in coagulopathic cardiac surgery patients. Anesthesiology. 2012;117:531–47. doi: 10.1097/ALN.0b013e318264c644. [DOI] [PubMed] [Google Scholar]
- 11.Fayed N, Mourad W, Yassen K, Görlinger K. Preoperative thromboelastometry as a predictor of transfusion requirements during adult living donor liver transplantation. Transfus Med Hemother. 2015;42:99–108. doi: 10.1159/000381733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olde Engberink RH, Kuiper GJ, Wetzels RJ, Nelemans PJ, Lance MD, Beckers EA, et al. Rapid and correct prediction of thrombocytopenia and hypofibrinogenemia with rotational thromboelastometry in cardiac surgery. J Cardiothorac Vasc Anesth. 2014;28:210–6. doi: 10.1053/j.jvca.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Ogawa S, Szlam F, Chen EP, Nishimura T, Kim H, Roback JD, et al. A comparative evaluation of rotation thromboelastometry and standard coagulation tests in hemodilution-induced coagulation changes after cardiac surgery. Transfusion. 2012;52:14–22. doi: 10.1111/j.1537-2995.2011.03241.x. [DOI] [PubMed] [Google Scholar]
- 14.Reinhöfer M, Brauer M, Franke U, Barz D, Marx G, Lösche W. The value of rotation thromboelastometry to monitor disturbed perioperative haemostasis and bleeding risk in patients with cardiopulmonary bypass. Blood Coagul Fibrinolysis. 2008;19:212–9. doi: 10.1097/MBC.0b013e3282f3f9d4. [DOI] [PubMed] [Google Scholar]
- 15.Davidson SJ, McGrowder D, Roughton M, Kelleher AA. Can ROTEM thromboelastometry predict postoperative bleeding after cardiac surgery? J Cardiothorac Vasc Anesth. 2008;22:655–61. doi: 10.1053/j.jvca.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Anderson L, Quasim I, Steven M, Moise SF, Shelley B, Schraag S, et al. Interoperator and intraoperator variability of whole blood coagulation assays: A comparison of thromboelastography and rotational thromboelastometry. J Cardiothorac Vasc Anesth. 2014;28:1550–7. doi: 10.1053/j.jvca.2014.05.023. [DOI] [PubMed] [Google Scholar]
- 17.Espinosa A, Stenseth R, Videm V, Pleym H. Comparison of three point-of-care testing devices to detect hemostatic changes in adult elective cardiac surgery: A prospective observational study. BMC Anesthesiol. 2014;14:80. doi: 10.1186/1471-2253-14-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorlinger K, Dirkmann D, Hanke AA. Rotational thromboelastometry. In: Gonzalez E, Moore HB, Moore EE, editors. Trauma Induced Coagulopathy. Switzerland: Springer International Publishing; 2016. pp. 267–300. [Google Scholar]
- 19.Sartorius D, Waeber JL, Pavlovic G, Frei A, Diaper A, Myers P, et al. Goal-directed hemostatic therapy using the rotational thromboelastometry in patients requiring emergent cardiovascular surgery. Annals of Cardiac Anaesthesia. 2014;17:100–8. doi: 10.4103/0971-9784.129829. [DOI] [PubMed] [Google Scholar]