Abstract
The telomerase ribonucleoprotein is a specialized reverse transcriptase required to maintain protective chromosome end-capping structures called telomeres. In most cells, telomerase is not active and the natural shortening of telomeres with each round of DNA replication ultimately triggers cell growth arrest. In contrast, the presence of telomerase confers a high level of renewal capacity upon rapidly dividing cells. Telomerase is aberrantly activated in 90% of human cancers and thus represents an important target for anticancer therapeutics. However, the naturally low abundance of telomerase has hampered efforts to obtain high-resolution models for telomerase structure and function. To circumvent these challenges, single molecule techniques have recently been employed to investigate telomerase assembly, structure, and catalysis.
Introduction
The ends of linear chromosomes must be differentiated from sites of double-stranded DNA breaks to avoid detection by DNA damage repair machinery and to prevent chromosomal fusion events. This problem is solved through the action of telomerase, an RNA-dependent DNA polymerase that catalyzes the addition of telomere DNA repeats onto chromosome ends. The long stretches of telomere DNA (5–15 kilobases in humans) specifically recruit a host of telomere-specific DNA binding proteins that protect the end of the chromosome from nucleolytic degradation and processing [1]. Although the Nobel Prize in Medicine was recently awarded for the discovery of telomeres and telomerase, the determination of high-resolution structural models for telomerase structure and function remains a substantial challenge in the field.
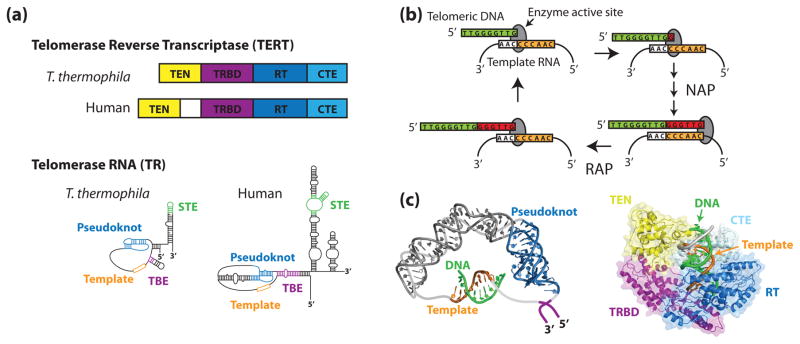
Telomerase is comprised of the telomerase reverse transcriptase (TERT) protein, telomerase RNA (TR), and several additional species-specific protein subunits [2–4]. TERT extends 3′ ends of telomeric DNA by reverse transcribing a small template region of TR into DNA. Single-molecule studies of telomerase have focused on the human enzyme and the Tetrahymena thermophila model system, which share a similar organization of TERT and TR functional elements (Figure 1a). The telomerase catalytic cycle can be divided into two separable activities (Figure 1b). In nucleotide addition processivity (NAP), individual nucleotides are reverse transcribed from the template RNA into telomeric DNA. When the end of the template is reached, the DNA-RNA duplex must be denatured and the template RNA is repositioned for the addition of another repeat, a process known as repeat addition processivity (RAP).
Figure 1. The telomerase ribonucleoprotein complex.
(a) Tetrahymena thermophila and human TERTs and TRs share a similar domain organization. In both species, TERT is composed of an N-terminal (TEN) domain, an RNA-binding domain (TRBD), a reverse transcriptase domain (RT), and a C-terminal extension (CTE). Conserved RNA structural features include an RNA pseudoknot fold, template, template boundary element (TBE), and a stem terminal element (STE). (b) During the telomerase catalytic cycle, telomeric DNA is positioned in the enzyme active site by basepairing alignment with the template RNA. Individual nucleotides are reverse transcribed off of the template RNA in a process known as nucleotide addition processivity (NAP). When the end of the template is reached, the DNA-RNA duplex is denatured and the RNA is re-positioned to add another repeat, a process known as repeat addition processivity (RAP). (c) In silico modeling of human TR (left) and human TERT (right) (adapted from [1,2] with permission). Zhang and co-workers used residual dipolar coupling orientation constraints to model the core region of human TR using existing NMR structures. Steczkiewicz et al. used homology modeling and structure prediction algorithms to model human TERT based on solved structures of the Tribolium castaneum TERT and the Tetrahymena thermophila TEN and TRBD domains, and used molecular docking to predict the position of the TEN domain within the enzyme.
X-ray crystallography and NMR spectroscopy have provided insights into the structures of both TERT and TR. TERT consists of four protein domains, a conserved N-terminal domain (TEN), an RNA binding domain (TRBD), a reverse transcriptase domain (RT), and a C-terminal extension (CTE). A recent crystal structure of the Tribolium castaneum TERT details the orientation of the TRBD, RT, and CTE domains in this species [5]. The structure reveals that TERT resembles viral reverse transcriptases, forming a ring-like structure to grip the primer-template hybrid [6]. In addition, structures of the Tetrahymena TEN and TRBD domains have been determined individually by X-ray crystallography [7,8].
TRs contain many conserved structural elements that have been implicated in telomerase activity (Figure 1a). Core structural features of TRs include a pseudoknot, template, and template boundary element (TBE, a region of protein interaction which precludes reverse transcription of RNA other than the template) [9–12]. Structures for many important RNA elements have been solved [13–19]. Of particular interest are the human RNA pseudoknot [20,21] and the adjacent human RNA sequences [22]. However, there is no high-resolution structure for the complete RNA. In the absence of further high-resolution structures, in silico modeling techniques have been employed to model both TR and TERT [22–24] (Figure 1c). While forming a useful point of reference, validation of in silico models for TR and TERT is critical and represents a natural target for future single molecule investigation.
In this review, we describe several exciting examples of the application of single molecule techniques to telomerase research. First, we begin by describing the specific single molecule techniques that have been employed in telomerase research. Second, we discuss efforts to characterize the folding and structure of TR using single molecule methods. Third, we examine several experiments where single molecule analysis has provided unique insight into the assembly and composition of the telomerase complex. Finally, we review efforts to develop novel telomerase activity assays using single molecule approaches.
Single Molecule Techniques Applied to Telomerase Research
Single molecule techniques permit the direct characterization of structural dynamics intrinsic to the function of virtually all biological macromolecules and allow researchers to reconstruct distributions of molecular properties from heterogeneous populations. These unique properties, together with the requirement for very small amounts of biological sample, make single molecule approaches particularly attractive for telomerase studies.
The first single molecule approach to be employed in telomerase studies is a fluorescence method developed by the Klenerman and Balasubramanian groups called two-color coincidence detection (TCCD) [25,26] (Figure 2a). The TCCD technique consists of two partially overlapping confocal volumes, detection optics to separate the two different emission wavelengths, and a pair of avalanche photodiodes (APD) to detect the signal from individual fluorophore-labeled molecules diffusing through the overlapping excitation volume. TCCD employs ratiometric analysis of different color fluorescence intensities to extract information about sub-populations and to establish stoichiometries of multi-subunit complexes. The sensitivity of TCCD was initially demonstrated using model DNA fragments [26], but was soon thereafter applied to multiple aspects of telomerase structure and function as described in the following sections [27–30].
Figure 2. Single molecule approaches to telomerase research.
(a) Two-color coincidence detection (TCCD). In the TCCD approach, two partially overlapping red and blue lasers are focused through a confocal microscope and very dilute biological macromolecules labeled with either a blue or red dye are studied. Transient diffusion of dye-labeled molecules through the confocal volume gives rise to fluorescence bursts (lower panel), and the ratio of red and blue fluorescence intensities provides information about the composition and stoichiometry of individual complexes. (b) Single molecule FRET measured by prism-type total internal reflection fluorescence (TIRF) microscopy. A molecule of interest is labeled with a FRET donor (green) and acceptor (red) dye and surface immobilized via a biotin-streptavidin linkage onto a microscope slide. The sample is illuminated by the evanescent wave generated by TIRF microscopy, which suppresses fluorescence background to levels that permit prolonged detection of individual FRET pairs. Typically, FRET is measured as the ratio of the fluorescence intensity of the acceptor dye divided by the sum of the donor plus acceptor intensities. (c) Force-measuring optical trap. The RNA molecule of interest is attached to a micron-scale bead held on a glass micropipette as well as a second bead held in the optical trap. Displacement of the micropipette away from the optical trap results in a stretching force applied to the RNA molecule, and both the applied force and molecular displacements are measured in real time.
A second single molecule method used in telomerase studies is Förster resonance energy transfer (FRET) [31,32]. Single molecule FRET (smFRET) measures molecular scale distances (2–8nm) as the efficiency of energy transfer between a directly excited donor dye and a nearby acceptor dye (Figure 2b). To date, single molecule FRET (smFRET) studies of telomerase have employed prism-type total internal reflection fluorescence (TIRF) microscopy [33], wherein the molecule of interest is immobilized onto a microscope slide and illuminated by an evanescent wave generated at the interface of the slide and the aqueous medium of the experiment. As described in the following sections, smFRET has been used to study various aspects of telomerase structure and assembly.
Another class of single molecule techniques, the micromanipulation methods, permits researchers to apply precise amounts of tension to biological macromolecules, facilitating the study of folding/unfolding reactions under near-physiological solutions and temperatures. The Tinoco laboratory recently employed a force-measuring laser tweezers apparatus to explore the folding pathway of a conserved RNA structural motif within the human telomerase RNA subunit (see below) [34]. In this approach, the RNA of interest is covalently ligated between DNA handles and attached to one bead fixed on a glass micropipette and a second bead held in an optical trap (Figure 2c). For more detailed description of optical tweezers and their application to RNA folding studies the reader is referred to several excellent reviews [35–37].
The design and synthesis of molecular constructs for single-molecule analysis varies with the specific requirements for each method. In the case of both TCCD and smFRET-based telomerase studies, fluorescence probes were site specifically attached to telomerase RNA using chemically synthesized RNA fragments which were then incorporated into larger RNA fragments using DNA-splinted RNA ligation techniques ([30,38–41]). For telomerase protein labeling, genetically encoded fluorescent proteins have been employed [28]. Single molecule methods which require surface immobilization involve additional modifications to the bio-molecule of interest. In the case of smFRET experiments, telomerase RNA molecules or telomere DNA substrates have been surface immobilized on streptavidin coated surfaces via a biotin moiety ([30,38–41]). Force spectroscopy methods have employed the use of long DNA handles covalently attached to the RNA molecule of interest to serve as spacers between two different micron-scale polystyrene beads that are used to apply and measure force. Specificity of the attachments between DNA handles and beads was controlled through the use of small chemically modified DNA linker fragments, generated using the polymerase chain reaction (PCR) in the presence of modified (either biotin- or digoxigenin-modified) dNTPS. The modified DNA linker fragments were digested with a restriction endonuclease, specifically ligated to the ends of the DNA handles, and immobilized onto streptavidin or anti-digoxigenin coupled polystyrene beads, respectively.
Single-Molecule Studies of Telomerase RNA Structure
RNA can adopt a complex array of tertiary structures which often demonstrate structural dynamics related to their biological function. Thus, probing the structural properties of individual RNA molecules was a logical launching point for seminal single molecule experiments using either smFRET or optical trapping methods to directly elucidate folding stabilities and pathways for several model RNA systems [42,43]. Since these early studies, single molecule approaches have been broadly applied to a myriad of RNA systems, including telomerase RNA.
A number of studies suggest that human telomerase may act as a higher-order complex in vivo, most likely a dimer [44]. In an early study, Ren et al. employed TCCD to directly identify a novel dimerization site within hTR at the J7b/8a junction of the conserved region 7 (CR7) domain (Figure 3a) [27]. An hTR construct containing residues 380–444 was 5′ end labeled with either a blue (Alexa 488) or red (Alexa 647) fluorophore. Dilute concentrations (typically around 100pM) were used to facilitate detection of single complexes carrying both dyes, while homodimers were excluded from analysis. Mutations within this region of hTR that give rise to the premature aging syndrome Dyskeratosis Congenita (DKC) abrogated this novel RNA-RNA interaction, supporting the notion that telomerase dimerization may be physiologically important.
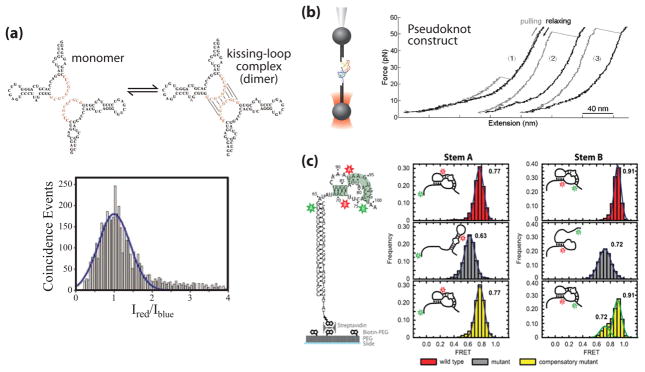
Figure 3. Single molecule studies of telomerase RNA structure.
(a) Proposed dimerization interaction in the J7b/8a region of the CR7 region of hTR (adapted from [3] with permission). RNA is 5′ end labeled with either Alexa 488 or Alexa 647 in a 1:1 ratio and diluted to a total concentration of 100 pM. A histogram of the log ratio of red to blue fluorescence for single molecules diffusing through the excitation volume was indicative of a single distribution centered at a 1:1 ratio, corresponding to the hTR-Alexa 488: hTR-Alexa 647 dimer. (b) Mechanical folding/unfolding of the hTR pseudoknot domain RNA (adapted from [4] with permission). The pseudoknot construct used in this study was oriented between two DNA handles and held in an optical trap. The RNA molecule is pulled (grey line) and relaxed (black line) at a constant rate of 100 nm/sec as the resultant force is measured. The rip at ~24 pN (trace 1) corresponds to the unfolding force of an alternative hairpin conformation observed in a subset of traces. The rips at ~50 pN (traces 2 & 3) correspond to the unfolding force of the complete pseudoknot, resulting in a rip size of ~36 nm which corresponds to the dimensions of the pseudoknot. (c) Single molecule FRET (smFRET) studies of the Tetrahymena TR (tTR) pseudoknot domain RNA (adapted from [5] with permission). Constructs were labeled with a donor dye (green) at residue U63 and an acceptor dye (red) at U92 to measure folding of stem A or labeled with a donor dye at U73 and an acceptor dye at U99 to measure folding of stem B. smFRET histograms were obtained with the stem A and stem B labeling sites for the wild-type RNA sequence (red), a mutant pseudoknot designed to disrupt basepairing in stem A (grey), and a compensatory mutant designed to restore basepairing (yellow).
Perhaps the most notable conserved RNA structure within TR is a pseudoknot motif found downstream of the RNA template (Figure 1a). Mutations to this region of hTR abrogate telomerase activity in vitro and are associated with telomerase-related disease in vivo [45,46]. Using the optical trapping system described above, Chen and coworkers analyzed the mechanical unfolding/refolding of a minimal hTR pseudoknot characterized previously by NMR (Figure 3b) [20,21,34]. These studies revealed two distinct unfolding forces for the pseudoknot construct: a low rupture force which the authors assigned to the unfolding of an RNA hairpin intermediate, and a high rupture force which was attributed to one step unfolding of the fully folded pseudoknot domain [34]. The direct observation of an RNA hairpin intermediate is in good agreement with prior NMR studies of hTR pseudoknot folding [46]. Furthermore, this work employed a constant force feedback mode of the optical trap to reveal previously unidentified non-native RNA conformations during hTR pseudoknot folding.
In a separate study of the hTR pseudoknot domain, Yeoman et al employed a variation of the TCCD technique called TCCD-1ex in which FRET efficiencies can be extracted [25,30]. The authors analyzed the hTR pseudoknot domain within the functional RNA in the presence and absence of the TERT protein. Their results indicate the hTR pseudoknot domain forms two predominant conformations in the absence of TERT, and the addition of TERT introduces a third RNA conformation. Although the authors make the conclusion that TERT may be acting to stabilize the pseudoknot conformation in the minority of complexes that are properly assembled, additional studies will be required to properly assign each FRET state observed in this work to particular RNA conformations.
In addition to the hTR pseudoknot domain, recent work from Mihalusova et al investigated the folding dynamics of the Tetrahymena telomerase RNA (tTR) pseudoknot by smFRET (Figure 3c) [39]. This work initially utilized a minimal construct to establish the FRET signature of a folded pseudoknot, and used a series of mutations to demonstrate the observed FRET signature is strictly dependent on pseudoknot-specific RNA basepairing. Next, the folding behavior of the tTR pseudoknot was examined within the context of the full-length tTR, as well as in the catalytically active in vitro reconstituted telomerase RNP. The authors demonstrated the minimal tTR pseudoknot was stably folded; however, the same structure positioned with the full-length tTR sequence displayed a reduced efficiency of pseudoknot folding. Interestingly, reconstitution of the full-length RNA into a functional RNP complex including the TERT protein restored the folding of the tTR pseudoknot domain. This elegant set of single molecule experiments demonstrates the importance of telomerase proteins in stabilizing the native RNA pseudoknot structure.
Telomerase Ribonucleoprotein Assembly and Composition
The faithful assembly of TR with the telomerase reverse transcriptase (TERT) and additional protein subunits is essential for telomerase function. In Tetrahymena, the assembly of tTR and tTERT requires a holoenzyme subunit called p65 [47,48]. To explore the mechanism of p65-mediated telomerase RNP assembly, Stone et al employed smFRET to directly visualize tTR folding upon p65 and TERT binding (Figure 4a) [40]. These experiments revealed a hierarchical assembly pathway for the Tetrahymena telomerase RNP: p65 alters the conformation of tTR, which then facilitates the binding of the TERT subunit. In addition, these studies employed RNA mutagenesis to show that an evolutionarily conserved GA-bulge motif within tTR is essential for p65-mediated RNP assembly.
Figure 4. Single molecule analysis of telomerase RNP assembly, composition, and activity.
(a) Schematic diagram of proposed p65-mediated Tetrahymena telomerase RNP assembly pathway. For smFRET measurements, donor (green) and acceptor (red) dyes were placed on tTR to monitor RNA folding during the RNP assembly process. A representative smFRET trace displays a FRET value of 0.29 in the absence of proteins. Upon addition of p65 and TERT (dashed line), the RNA undergoes stepwise folding transitions (black arrows) to 0.46 FRET and 0.65 FRET, corresponding to the sequential binding of p65 and TERT, respectively (adapted from [6] with permission). (b) Characterization of telomerase composition by TCCD. TCCD histograms for human telomerase RNP complexes harboring blue and red dyes on either: hTERT and hTR (left); hTERT and DNA substrate (middle); or hTR and DNA substrate (right) are consistent with a 1:1:1 stoichiometry of hTERT:hTR:DNA substrate within the active telomerase complex (adapted from [7] with permission). (c) Human telomerase activity detection by TCCD. Reconstituted human telomerase is incubated with telomeric DNA primers labeled with a blue reference dye and reacted in the presence dATP coupled to a red dye. One dATP is incorporated for each telomeric repeat, thus the ratio of blue and red fluorescence intensities provides a direct measure of the number of repeats added to each DNA substrate (adapted from [8] with permission). (d) smFRET-based Tetrahymena telomerase structure-function assay. Telomeric DNA primers were surface immobilized and incubated with RNP complexes reconstituted with tTR harboring FRET donor and acceptor dyes. Individual smFRET measurements were made during transient telomerase binding events. During the activity detection phase, DNA primers were labeled in situ with a new FRET donor (green star) and telomerase activity was detected by measuring the hybridization kinetics of an acceptor (red star) labeled detection oligo (DO) (adapted from [9] with permission).
In a separate study, Alves et al. used TCCD to examine the composition of the in vitro reconstituted human telomerase RNP complex. By introducing different color dye molecules on each component of the telomerase complex, the authors determined an absolute stoichiometry of hTERT:hTR:DNA substrate of 1:1:1 within the catalytically functional complex [28]. These studies indicate the human telomerase RNP is functional as a monomer in vitro, leaving open the question of the biological significance of higher order telomerase complexes observed in vivo.
Single molecule approaches to Telomerase Activity Detection
Current methods for detecting telomerase activity include a direct primer extension assay and the telomere repeat amplification protocol (TRAP) assay [49,50]. The TRAP assay is a PCR-based method capable of detecting very small amounts of enzymatic activity. The method is incredibly sensitive; however one drawback of this approach is that some information on enzyme processivity is lost during the amplification process. The direct primer extension assay monitors telomerase activity as the incorporation of radiolabeled dNTPs into DNA primer substrates, which are then resolved on a denaturing polyacrylamide gel. Although less sensitive than the TRAP assay, the direct primer extension technique is a more reliable way to measure catalytic properties of telomerase such as processivity.
Ren and coworkers reported a single molecule method for detecting human telomerase activity using the TCCD approach described above (Fig. 4C) [29]. This method directly measures telomerase activity as a ratio between the fluorescence intensity of a reference dye coupled to the DNA primer and the intensity of dye-modified dATP substrates incorporated into the primer during the telomerase reaction. This approach has the advantages of eliminating the need for product amplification or gel separation and does not require the use of radioactivity. The authors demonstrated the sensitivity of their technique by detecting the products generated by attomole amounts of telomerase enzyme and recovered processivity values in close quantitative agreement with results from the direct primer extension assay. They further validated their approach by re-characterizing a telomerase mutant with reduced processivity, demonstrating the single molecule method may be utilized in future studies to investigate the mechanism of telomerase function.
A consistent problem with probing telomerase structure is that standard reconstitution techniques produce large amounts of mis-assembled telomerase RNP complexes. To circumvent this problem, Wu et al developed a smFRET-based strategy that uses single molecule analysis to separate out only the smFRET signals associated with active telomerase. During the enzyme binding phase of the experiment, a blue reference dye coupled to the DNA primer was used to localize DNA substrates, and telomerase binding was observed as an abrupt onset of FRET between the donor and acceptor dyes at the site of a DNA primer. Wu et al then used oligonucleotide hybridization probes to identify which telomerase RNPs successfully extended an immobilized telomeric DNA substrate. This approach permitted the statistically significant categorization of each telomerase binding event as either no extension, single base extension, or processive extension. The assay has thus far been applied to make a variety of two point distance measurements throughout tTR, which can be used to place constraints on models for tTR within the active RNP complex [39,41]. In the future, we anticipate this assay will be particularly valuable in identifying transient conformational rearrangements associated with telomerase activity.
Conclusions and Future Outlook
In this review, we have illustrated how single molecule methods can be applied to the complex problem of studying telomerase structure and function. Moving forward, we anticipate single molecule approaches will play an important role in establishing and validating models of telomerase structure and assembly. Furthermore, once improved structural models for telomerase function have been obtained, single molecule methods are uniquely poised to directly characterize the protein and RNA conformational rearrangements required for the unique catalytic activity of telomerase.
Acknowledgments
We acknowledge the support of the National Institutes of Health (R01GM095850) grant to M.D.S. M.H. is supported by a German Research Foundation fellowship (DFG HE 6181/1-1), and B.M.A. is supported by a National Institutes of Health training grant (T32 GM8646).
References
- 1.Palm W, de Lange T. How shelterin protects mammalian telomeres. Annu Rev Genet. 2008;42:301–334. doi: 10.1146/annurev.genet.41.110306.130350. [DOI] [PubMed] [Google Scholar]
- 2.Greider CW, Blackburn EH. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature. 1989;337:331–337. doi: 10.1038/337331a0. [DOI] [PubMed] [Google Scholar]
- 3.Lingner J, Hughes TR, Shevchenko A, Mann M, Lundblad V, Cech TR. Reverse transcriptase motifs in the catalytic subunit of telomerase. Science. 1997;276:561–567. doi: 10.1126/science.276.5312.561. [DOI] [PubMed] [Google Scholar]
- 4.Collins K. The biogenesis and regulation of telomerase holoenzymes. Nat Rev Mol Cell Biol. 2006;7:484–494. doi: 10.1038/nrm1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gillis AJ, Schuller AP, Skordalakes E. Structure of the Tribolium castaneum telomerase catalytic subunit TERT. Nature. 2008;455:633–637. doi: 10.1038/nature07283. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell M, Gillis A, Futahashi M, Fujiwara H, Skordalakes E. Structural basis for telomerase catalytic subunit TERT binding to RNA template and telomeric DNA. Nat Struct Mol Biol. 2010;17:513–518. doi: 10.1038/nsmb.1777. [DOI] [PubMed] [Google Scholar]
- 7.Jacobs SA, Podell ER, Cech TR. Crystal structure of the essential N-terminal domain of telomerase reverse transcriptase. Nat Struct Mol Biol. 2006;13:218–225. doi: 10.1038/nsmb1054. [DOI] [PubMed] [Google Scholar]
- 8.Rouda S, Skordalakes E. Structure of the RNA-binding domain of telomerase implications for RNA recognition and binding. Structure. 2007;15:1403–1412. doi: 10.1016/j.str.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Lin J, Ly H, Hussain A, Abraham M, Pearl S, Tzfati Y, Parslow TG, Blackburn EH. A universal telomerase RNA core structure includes structured motifs required for binding the telomerase reverse transcriptase protein. Proc Natl Acad Sci U S A. 2004;101:14713–14718. doi: 10.1073/pnas.0405879101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ten Dam E, van Belkum A, Pleij K. A conserved pseudoknot in telomerase RNA. Nucleic Acids Res. 1991;19:6951. doi: 10.1093/nar/19.24.6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen JL, Blasco MA, Greider CW. Secondary structure of vertebrate telomerase RNA. Cell. 2000;100:503–514. doi: 10.1016/s0092-8674(00)80687-x. [DOI] [PubMed] [Google Scholar]
- 12.Chen JL, Greider CW. Template boundary definition in mammalian telomerase. Genes Dev. 2003;17:2747–2752. doi: 10.1101/gad.1140303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim NK, Theimer CA, Mitchell JR, Collins K, Feigon J. Effect of pseudouridylation on the structure and activity of the catalytically essential P6.1 hairpin in human telomerase RNA. Nucleic Acids Res. 2010;38:6746–6756. doi: 10.1093/nar/gkq525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Theimer CA, Jady BE, Chim N, Richard P, Breece KE, Kiss T, Feigon J. Structural and functional characterization of human telomerase RNA processing and cajal body localization signals. Mol Cell. 2007;27:869–881. doi: 10.1016/j.molcel.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 15.Richards RJ, Wu H, Trantirek L, O’Connor CM, Collins K, Feigon J. Structural study of elements of Tetrahymena telomerase RNA stem-loop IV domain important for function. RNA. 2006;12:1475–1485. doi: 10.1261/rna.112306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richards RJ, Theimer CA, Finger LD, Feigon J. Structure of the Tetrahymena thermophila telomerase RNA helix II template boundary element. Nucleic Acids Res. 2006;34:816–825. doi: 10.1093/nar/gkj481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y, Fender J, Legassie JD, Jarstfer MB, Bryan TM, Varani G. Structure of stem-loop IV of Tetrahymena telomerase RNA. EMBO J. 2006;25:3156–3166. doi: 10.1038/sj.emboj.7601195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leeper T, Leulliot N, Varani G. The solution structure of an essential stem-loop of human telomerase RNA. Nucleic Acids Res. 2003;31:2614–2621. doi: 10.1093/nar/gkg351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leeper TC, Varani G. The structure of an enzyme-activating fragment of human telomerase RNA. RNA. 2005;11:394–403. doi: 10.1261/rna.7222505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Theimer CA, Blois CA, Feigon J. Structure of the human telomerase RNA pseudoknot reveals conserved tertiary interactions essential for function. Mol Cell. 2005;17:671–682. doi: 10.1016/j.molcel.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 21.Kim NK, Zhang Q, Zhou J, Theimer CA, Peterson RD, Feigon J. Solution structure and dynamics of the wild-type pseudoknot of human telomerase RNA. J Mol Biol. 2008;384:1249–1261. doi: 10.1016/j.jmb.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Q, Kim NK, Peterson RD, Wang Z, Feigon J. Structurally conserved five nucleotide bulge determines the overall topology of the core domain of human telomerase RNA. Proc Natl Acad Sci U S A. 2010;107:18761–18768. doi: 10.1073/pnas.1013269107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gavory G, Symmons MF, Krishnan Ghosh Y, Klenerman D, Balasubramanian S. Structural analysis of the catalytic core of human telomerase RNA by FRET and molecular modeling. Biochemistry. 2006;45:13304–13311. doi: 10.1021/bi061150a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steczkiewicz K, Zimmermann MT, Kurcinski M, Lewis BA, Dobbs D, Kloczkowski A, Jernigan RL, Kolinski A, Ginalski K. Human telomerase model shows the role of the TEN domain in advancing the double helix for the next polymerization step. Proc Natl Acad Sci U S A. 2011;108:9443–9448. doi: 10.1073/pnas.1015399108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orte A, Clarke R, Balasubramanian S, Klenerman D. Determination of the fraction and stoichiometry of femtomolar levels of biomolecular complexes in an excess of monomer using single-molecule, two-color coincidence detection. Anal Chem. 2006;78:7707–7715. doi: 10.1021/ac061122y. [DOI] [PubMed] [Google Scholar]
- 26.Li H, Ying L, Green JJ, Balasubramanian S, Klenerman D. Ultrasensitive coincidence fluorescence detection of single DNA molecules. Anal Chem. 2003;75:1664–1670. doi: 10.1021/ac026367z. [DOI] [PubMed] [Google Scholar]
- 27.Ren X, Gavory G, Li H, Ying L, Klenerman D, Balasubramanian S. Identification of a new RNA.RNA interaction site for human telomerase RNA (hTR). structural implications for hTR accumulation and a dyskeratosis congenita point mutation. Nucleic Acids Res. 2003;31:6509–6515. doi: 10.1093/nar/gkg871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alves D, Li H, Codrington R, Orte A, Ren X, Klenerman D, Balasubramanian S. Single-molecule analysis of human telomerase monomer. Nat Chem Biol. 2008;4:287–289. doi: 10.1038/nchembio.82. [DOI] [PubMed] [Google Scholar]
- 29.Ren X, Li H, Clarke RW, Alves DA, Ying L, Klenerman D, Balasubramanian S. Analysis of human telomerase activity and function by two color single molecule coincidence fluorescence spectroscopy. J Am Chem Soc. 2006;128:4992–5000. doi: 10.1021/ja056613z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeoman JA, Orte A, Ashbridge B, Klenerman D, Balasubramanian S. RNA conformation in catalytically active human telomerase. J Am Chem Soc. 2010;132:2852–2853. doi: 10.1021/ja909383n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roy R, Hohng S, Ha T. A practical guide to single-molecule FRET. Nat Methods. 2008;5:507–516. doi: 10.1038/nmeth.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ha T. Single-molecule fluorescence resonance energy transfer. Methods. 2001;25:78–86. doi: 10.1006/meth.2001.1217. [DOI] [PubMed] [Google Scholar]
- 33.Axelrod D, Thompson NL, Burghardt TP. Total internal inflection fluorescent microscopy. J Microsc. 1983;129:19–28. doi: 10.1111/j.1365-2818.1983.tb04158.x. [DOI] [PubMed] [Google Scholar]
- 34.Chen G, Wen JD, Tinoco I., Jr Single-molecule mechanical unfolding and folding of a pseudoknot in human telomerase RNA. RNA. 2007;13:2175–2188. doi: 10.1261/rna.676707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tinoco I., Jr Force as a useful variable in reactions. unfolding RNA. Annu Rev Biophys Biomol Struct. 2004;33:363–385. doi: 10.1146/annurev.biophys.33.110502.140418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moffitt JR, Chemla YR, Smith SB, Bustamante C. Recent advances in optical tweezers. Annu Rev Biochem. 2008;77:205–228. doi: 10.1146/annurev.biochem.77.043007.090225. [DOI] [PubMed] [Google Scholar]
- 37.Woodside MT, Garcia-Garcia C, Block SM. Folding and unfolding single RNA molecules under tension. Curr Opin Chem Biol. 2008;12:640–646. doi: 10.1016/j.cbpa.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akiyama BM, Stone MD. Assembly of complex RNAs by splinted ligation. Methods Enzymol. 2009;469:27–46. doi: 10.1016/S0076-6879(09)69002-9. [DOI] [PubMed] [Google Scholar]
- 39.Mihalusova M, Wu JY, Zhuang X. Telomerase and Retrotransposons. Reverse Transcriptases That Shaped Genomes Sackler Special Feature. Functional importance of telomerase pseudoknot revealed by single-molecule analysis. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1017686108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stone MD, Mihalusova M, O’Connor CM, Prathapam R, Collins K, Zhuang X. Stepwise protein-mediated RNA folding directs assembly of telomerase ribonucleoprotein. Nature. 2007;446:458–461. doi: 10.1038/nature05600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu JY, Stone MD, Zhuang X. A single-molecule assay for telomerase structure-function analysis. Nucleic Acids Res. 2009 doi: 10.1093/nar/gkp1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhuang X, Bartley LE, Babcock HP, Russell R, Ha T, Herschlag D, Chu S. A single-molecule study of RNA catalysis and folding. Science. 2000;288:2048–2051. doi: 10.1126/science.288.5473.2048. [DOI] [PubMed] [Google Scholar]
- 43.Liphardt J, Onoa B, Smith SB, Tinoco I, Jr, Bustamante C. Reversible unfolding of single RNA molecules by mechanical force. Science. 2001;292:733–737. doi: 10.1126/science.1058498. [DOI] [PubMed] [Google Scholar]
- 44.Wenz C, Enenkel B, Amacker M, Kelleher C, Damm K, Lingner J. Human telomerase contains two cooperating telomerase RNA molecules. EMBO J. 2001;20:3526–3534. doi: 10.1093/emboj/20.13.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Theimer CA, Feigon J. Structure and function of telomerase RNA. Curr Opin Struct Biol. 2006;16:307–318. doi: 10.1016/j.sbi.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 46.Theimer CA, Finger LD, Trantirek L, Feigon J. Mutations linked to dyskeratosis congenita cause changes in the structural equilibrium in telomerase RNA. Proc Natl Acad Sci U S A. 2003;100:449–454. doi: 10.1073/pnas.242720799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prathapam R, Witkin KL, O’Connor CM, Collins K. A telomerase holoenzyme protein enhances telomerase RNA assembly with telomerase reverse transcriptase. Nat Struct Mol Biol. 2005;12:252–257. doi: 10.1038/nsmb900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Witkin KL, Collins K. Holoenzyme proteins required for the physiological assembly and activity of telomerase. Genes Dev. 2004;18:1107–1118. doi: 10.1101/gad.1201704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 50.Xin H. Telomeric repeat amplification protocol measuring the activity of the telomerase. Methods Mol Biol. 2011;735:107–111. doi: 10.1007/978-1-61779-092-8_10. [DOI] [PubMed] [Google Scholar]