Abstract
In the realm of natural product chemistry, few isolates have risen to the level of fame justifiably accorded to Taxol™ (1) and its chemical siblings. This report describes the most concise route to date for accessing the highly oxidized members of this family. As representative members of taxanes containing five oxygen atoms, decinnamoyltaxinine E (2) and taxabaccatin III (3) have succumbed to enantioselective total synthesis for the first time in only 18 steps from a simple olefin starting material. The route to these natural products is enabled by a strategy that holistically mimics Nature's approach (two-phase synthesis) and features a carefully choreographed sequence of stereoselective oxidations and a remarkable redox-isomerization to set the key trans-diol present in 2 and 3. This work lays the critical groundwork necessary to access even higher oxidized taxanes such as 1 in a more practical fashion thus empowering a medicinal chemistry campaign that is not wedded to semi-synthesis.
Graphical abstract
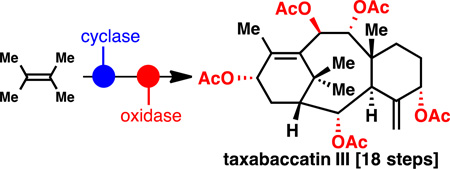
Terpene synthesis, take two… Starting from a simple feedstock olefin, highly oxidized natural taxanes were constructed using two-phase terpene synthesis.
The spectacular clinical success of taxanes combined with their stunning complexity has motivated and inspired scores of chemists over the past several decades1–3. Indeed, Taxol™ (1, Figure 1) can be treated as a barometer to measure progress and advances in the science of chemical synthesis. This iconic natural product has been prepared on ten separate occasions (seven total syntheses4–12 and three formal syntheses13–16), elegantly demonstrating the feasibility of its reconstitution by purely chemical means. Notwithstanding the beauty of these landmark accomplishments, they are 8–9 orders of magnitude less efficient than biological production. To be sure, mere milligrams were produced all together by total synthesis whereas metric tons of Taxol™ are accessed every year through a plant cell culture process developed by Bristol-Myers Squibb and Phyton Biotech, Inc. This gap in efficiency is therefore an exciting opportunity for invention and exploration in chemical synthesis17. As a first step for addressing this profound challenge, a strategy for emulating the two-phase biosynthesis18 of terpenes in the laboratory was delineated and demonstrated in the context of simple eudesmane terpenes19. Since then, this strategy has enabled simplified approaches to representative members of the germacrene20–21, steroid22–24, and ingenane terpene families25. The two-phase approach to ingenanes has been particularly significant from an industrial standpoint as it enabled the scalable preparation of not only the parent natural product but also analogs with improved biological profiles that are unavailable through semi-synthesis or synthetic biology approaches26.
Figure 1.
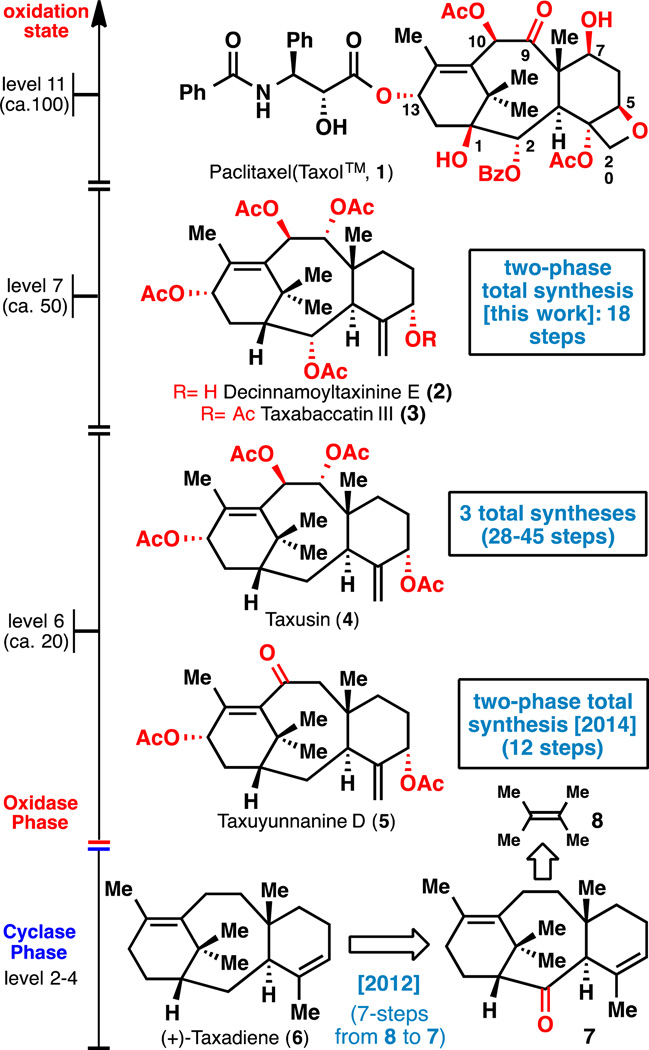
Taxol™ (1) and taxane family members ordered in sequential oxidation levels.
Two-phase terpene synthesis27 is critically reliant on two strategic choices: (1) the selection of an oxidized hydrocarbon bearing the minimum oxidation pattern required to effectively build the carbon skeleton of a terpene family (cyclase phase) and (2) a plan which maximizes divergent access to a broad array of oxidized natural products in the family by the deliberate use of C–H functionalization logic (oxidase phase). In the case of the taxanes, of which hundreds are known28, this approach simplifies the retrosynthetic blueprint as family members can be simply abbreviated as mathematical sets rather than discreet targets. As illustrated in Figure 1, Taxol™ can be viewed as a "level 11" taxane due to the presence of 9 oxidized carbon atoms and two degrees of unsaturation; over 100 natural products at this oxidation state have been isolated. Similarly, decinnamoyltaxinine E (2)29, taxabaccatin III (3)30, taxusin (4)31–36, taxuyunnanine D (5)37,38, and taxadiene (6)39–41 can be viewed as level 3–5 taxanes and represent over 100 additional natural products. In this report, the first enantioselective total syntheses of decinnamoyltaxinine E (2) and taxabaccatin III (3) are presented. As the most advanced embodiment of two-phase chemical synthesis to date, this route builds highly complex taxanes containing five oxidized carbon atoms and two degrees of unsaturation in only 18 steps from a simple olefin feedstock (tetramethylethylene 8).
The chemical cyclase phase of taxane synthesis was reported in 2010 and featured a highly efficient, triply convergent, enantioselective, and scalable approach that was amenable to outsourcing42. Ready access to the taxane skeleton enabled the first simple access to synthetic taxadiene (6)41 from taxadienone (7). Whereas the former is Nature's cyclase phase endpoint, the latter served as our laboratory's departure point for highly oxidized members of the family38,41. Notably, synthetic taxadiene (6) has since been dispersed to multiple laboratories for use in their independent studies. Of the eight oxygen atoms adorning the Taxol skeleton, three of them are derived from oxidation of weak allylic C–H bonds (C–5,10,13). Thus, initial efforts focused on deciphering the exact reagent choice and oxidation choreography needed for their installation. This resulted in the first total synthesis of a level 6 taxane, taxuyunnanine D (5)38 in 2014 in only five steps from 7 (12 steps from tetramethylethylene 8). Level 7 taxanes were targeted next, such as decinnamoyltaxinine E (2) and taxabaccatin III (3), neither of which have been prepared before. To put this challenge in the proper context, it is worth briefly reviewing the elegant total syntheses of the related level 6 taxane, taxusin (4). This simpler natural product has been the subject of numerous studies culminating in three total syntheses from the Holton (49 steps, chiral pool)32, Paquette (40 steps, chiral pool)35,36, and Kuwajima (27 steps, racemic; 28 steps, enantioselective)33,34 groups. These target–oriented approaches demonstrated the feasibility of chemically accessing a single taxane but would not be viable blueprints for a medicinal chemistry program that minimizes steps and maximizes divergency. This might be due to reliance on a retrosynthetic strategy oriented on specific strategic bond disconnections triggered by specific oxidation patterns found in a specific target. In stark contrast, we outline below an enantioselective pathway to 2 and 3 that represents a step-change in convenience to access highly functionalised taxanes because it targets the entire family in a unified fashion.
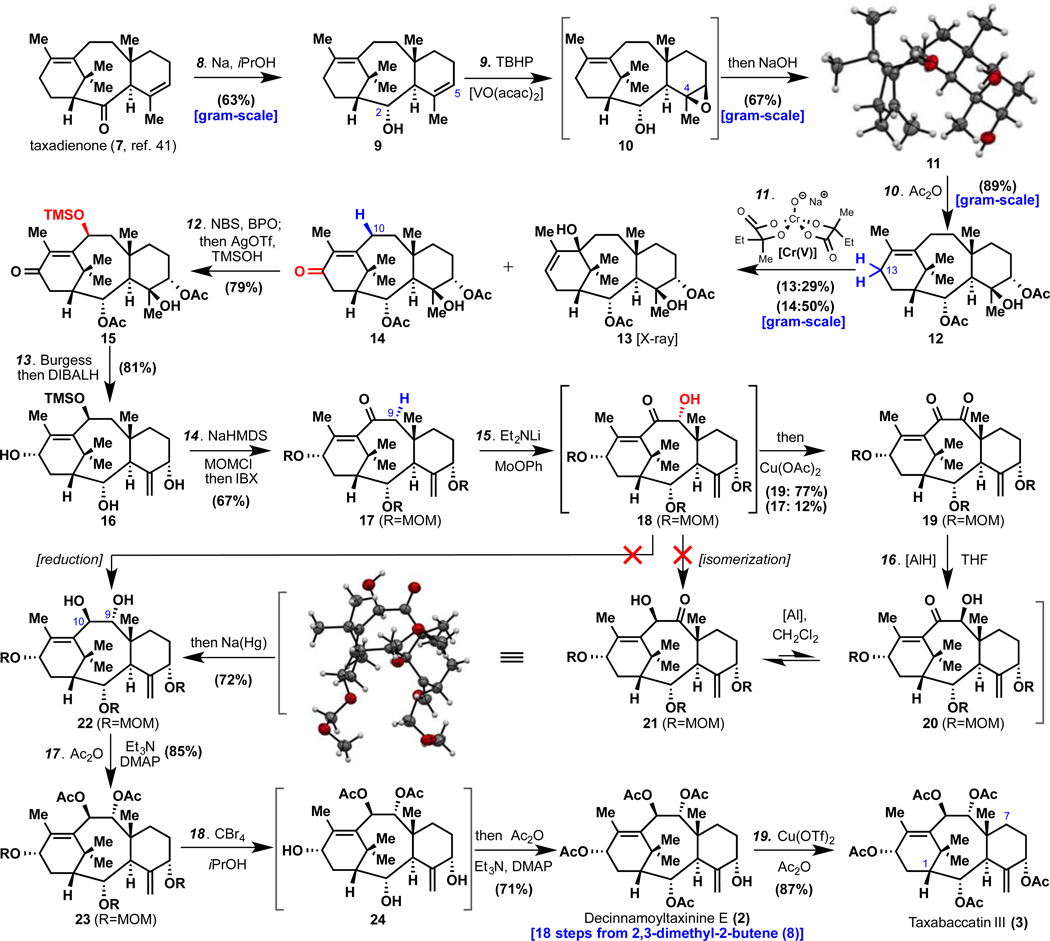
Commencing with taxadienone (7), Bouveault-Blanc reduction (Na, iPrOH) selectively afforded alcohol 9 in 63% yield (gram-scale). Based on prior studies38, it was expected that Pd-catalysed allylic oxidation would take place smoothly to install the C–5 oxygenation. Instead, the C–2 alcohol interfered and produced a cyclic ether (SI-10) that could not be utilized further in the synthesis (see SI for details). A simple workaround approach was pursued via Sharpless V-catalysed epoxidation to deliver 10 with immediate exposure to hydroxide, affording the crystalline triol 11 in 67% yield (gram-scale, structure confirmed by X–ray crystallography). Acetylation of the secondary alcohols (Ac2O, 89%, gram-scale) set the stage of 12 for Cr(V)-mediated installation of the C–13 oxygenation. This C–H oxidation, invented specifically for this oxidation but finding utility in related contexts26, could be used to robustly access enone 14 (50% yield, gram-scale) along with 13 (29%). As with the synthesis of 14, this reagent43 proved essential and was unique in its ability to enable access to C–13 oxidized taxane intermediates.
Installation of the C–10 oxidation relied on a radical-based C–H functionalization as described previously38. Thus, exposure of 14 to NBS in the presence of benzoyl peroxide furnished an allylic bromide that could be smoothly converted to silyl-ether 15 via solvolysis with AgOTf and TMSOH (79% yield, one-pot). The free tertiary alcohol 15 that served as a bystander to two C–H oxidations could now be eliminated using Burgess reagent and the crude olefin was subjected to a strategic reduction using DIBALH to set the proper stereochemistry of 16 at C–13 (81% yield, one-pot). In preparation for installation of the challenging C–9 oxygen atom, the triol was shielded as the tri-MOM ether and the allylic silyl ether was selectively oxidized directly using IBX (85% yield, one-pot) to afford 17. The net conversion of enone 17 to trans-diol 22 proved to be an incredibly challenging undertaking (vide infra) with a surprisingly simple solution. First, subjection of 17 to Et2NLi followed by Vedejs reagent (MoOPh)44 delivered α-hydroxy enone 18 which was directly exposed to Cu(OAc)245 to form dione 19 in 77% yield (along with 12% recovered enone 17). The unusual Al-based reducing agent LiAlH(OtBu)sBu246 (2.5 equiv) was then employed to stereo- and site-selectively produce 20. Over a short period of time in CH2Cl2 this compound fortuitously isomerized into α-hydroxy ketone 21; a species that could be isolated, and crystallized (structure verified by X-ray crystallography). In the optimized route 21 was carried forward in the same flask and exposed to sodium amalgam47 at room temperature to produce the coveted trans-diol 22 in 72% yield from 19. It is worth reflecting on the fact that this taxane 22, bearing an additional layer of complexity (C–2 oxidation) relative to taxusin (4), could be procured in only 16 steps from olefin 8. With all of the proper stereochemistry and oxygenation in place all that remained was to remove the sturdy MOM ethers and replace them with acetates. To this end, acetylation of the 9,10-diol using Ac2O (85% yield) delivered 23. Mild MOM ether removal using CBr448 produced a triol 24 that could be acetylated to produce (+)–2 (71% yield, +23°, CHCl3, c = 0.15). Decinnamoyltaxinine E (2) can be further acetylated in the presence of Cu(OTf)2 and Ac2O49 to afford (+)–3 (87% yield, +14°, CDCl3, c = 0.14) thus completing the first synthesis of these complex, level-7 taxanes.
It is instructive to reflect on some of the challenges encountered in this total synthesis. The very first step, reduction of 7 to 9, was strategically timed as the stereochemistry was difficult to set at any other stage stereoselectively (see SI for details). As mentioned above, C–5 oxidation necessitated the use of a diol as a redox-mask for an allylic alcohol. Next, C–H oxidation at C–13 using our Cr(V)-based reagent was successful only on a handful of substrates. For example, the MOM-protected version of 12 failed to deliver more than 5% oxidized product. For a complete summary of the substrates evaluated in order to identify the correct choreography for this C–H oxidation, see the Supplementary Information.
Puzzlingly, and in contrast to studies on the C–2 deoxy series38, if the tertiary alcohol at C–4 was dehydrated, the resulting allylic acetate could not be oxidized at either C–13 (using Cr) or C–10 (radical based systems). The choice of MOM ethers 17 for shielding the C–2, 5, and 13 alcohols was based solely on the stability of downstream intermediates and execution of the key C–9 oxidation (Bz, silyl, and unprotected variants all failed). Oxygenation of the enolate derived from ketone 17 proved quite challenging (MoOPh is the only oxidant that succeeded) and required the use of a non-hindered base (Et2NLi) and precise control of temperature (for the >40 conditions attempted see SI). As illustrated in the Supporting Information, direct reduction of 18 to the trans-diol 22 was unworkable after trying over 60 different reduction conditions. Isomerization of 18 to 21 was also fruitless despite literature precedent for such transformations on related systems using KOtBu6,7,13. Oxidation to the dione 19 followed by controlled reduction to 20 led directly to 21 after isomerization. The choice of reducing agent (LiAlH(OtBu)sBu2) was critical as reagents such as DIBALH led to overreduction and NaBH4 did not react. The residual aluminium salts present after addition of water accelerated the isomerization of 20 to 21 (as monitored by NMR, see SI for illustration).
Dozens of reducing agents screened on both 20 and 21 delivered either only the undesired cis-diol or led to decomposition. The pivotal reduction of 21 to trans-diol 22 could only be achieved using sodium amalgam (see SI for list of conditions). Finally, gentle removal of the MOM ethers required the use of CBr4/iPrOH, presumably as a means to slowly release HBr, as conventional reagents (Brønsted acid, BBr3, catechol borane bromide, TMSI) all led to undesired outcomes.
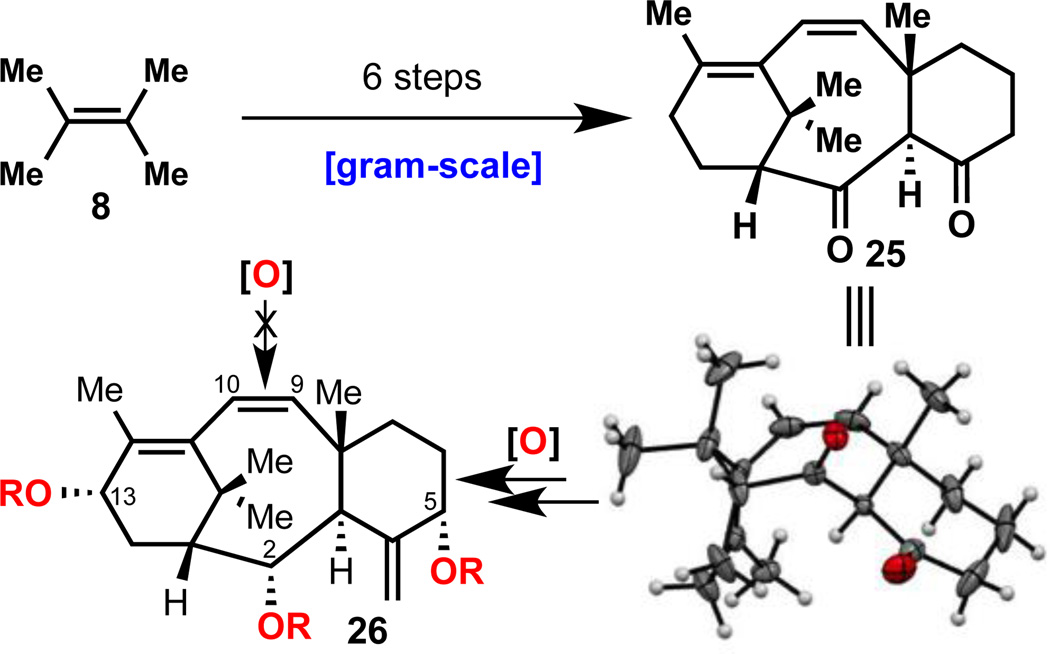
As this overarching strategy brings us closer to 1 it is worth reflecting on the inherent wisdom of selecting 7 (level-4 taxane) as a synthetic cyclase phase end point. Since introduction of the C–9,10 alcohols proved so challenging, a revised cyclase phase endpoint harboring an additional point of unsaturation was also explored. Thus, as depicted in Figure 3, the unsaturated dione 25 was targeted and constructed in only 6 steps from olefin 8 (see SI for preparation). The structure and the extremely hindered nature of the C9,10 olefin was verified by X-ray crystallography. Despite several hundred experiments on 25 and derivatives thereof we were unable to oxidize this olefin in the presence or absence of oxygen at either C–2, C–13 or C–5 (26). While frustrating, this spectacular failure bolsters the original design of 7 as the ideal synthetic cyclase phase endpoint.
Figure 3.
Exploration of an alternative cyclase phase endpoint for taxane synthesis.
With the completion of short total syntheses of two representative level-7 taxanes a light can be seen at the end of the tunnel: a scalable total synthesis of 1. While much remains to be learned and more challenges await prior to its eventual completion it is tempting to speculate how it will take place. It is clear that two unactivated C–H bonds remain to be functionalized: C–1 and C–7. Although precedent exists for the oxidation of C–1 using an electrophilic dioxirane50, the latter C–H bond is a significant hurdle to overcome. Meanwhile, the two-phase strategy for the preparation of complex terpenes has enabled access to taxanes never before accessible through synthesis. Since most of the members of this family differ only by the identity and location of ester side chains this route will permit access to dozens of natural and unnatural cogeners. This synthesis represents a short step count relative to the myriad of previous studies, and will thus hopefully contribute to the budding mindset that even the most complex natural products can be the subject of modern medicinal chemistry efforts via total synthesis.
Supplementary Material
Figure 2.
Conditions: 8. Na (5 × 7 equiv., each portion added every 30 min), iPrOH, 80 °C, 3 h, 63% (11% epi-9, see SI); 9. VO(acac)2 (0.05 equiv.), TBHP (1.1 equiv.), DCE, 0→23 °C, 2 h; then aq. NaOH (3M, 8.3 equiv.), DMSO, 140 °C (sealed tube), 4 h, 67%; 10. Ac2O (6 equiv.), Et3N (8 equiv.), DMAP (0.2 equiv.), THF, 60 °C, 10 h, 89%; 11. Cr(V) (4 equiv.), 15-crown-5 (5 equiv.), MnO2 (20 equiv.), tBuOMe, 90 °C (sealed tube), 36 h, 14 50%, 13 29%; 12. NBS (1.05 equiv.), BPO (0.2 equiv.), CCl4, 80 °C, 15 min; then TMSOH (xs.), 2,6-tert-butylpyridine (5 equiv.), AgOTf (5 equiv.), toluene, 23 °C, 1.5 h, 79%; 13. Burgess reagent (2 equiv.), toluene, 80 °C, 1 h; then DIBALH (15 equiv.), toluene, −78→23 °C, 15 min, 81%; 14. KHMDS (12 equiv.), MOMCl (12 equiv.), THF, −78→23 °C, 30 min; then IBX (26 equiv.), DMSO, 80 °C, 20 h, 67%; 15. Et2NLi (2 equiv.), MoOPh (4 equiv.), THF, 23→−20→23 °C, 20 min; then Cu(OAc)2 (40 equiv.), MeOH, 23 °C, 5 h, 19 77%, 17 12%; 16. LiAlH(OtBu)sBu2 (2.5 equiv.), THF, 23 °C, 1 min; then H2O (66 equiv.), evaporated to dryness and CH2Cl2, 23 °C, 8 h; Na (Hg) (2.2 equiv.), MeOH, 23 °C, 30 min, 72%; 17. Ac2O (10 equiv.), Et3N (10 equiv.), DMAP (1 equiv.), THF, 60 °C, 9 h, 85%; 18. CBr4 (50 equiv.), iPrOH, 82 °C, 8 min; then Ac2O (360 equiv.), Et3N (96 equiv.), DMAP (100 equiv.), 23 °C, 30 min.
Acknowledgments
We are grateful to Dr. Yoshihiro Ishihara and Dr. Minetaka Isomura for helpful discussions and Ms. Lily Maloney for experimental assistance. We thank Dr. D.-H. Huang and Dr. L. Pasternack for NMR spectroscopic assistance, Professor A. Rheingold (UCSD) for X-ray crystallographic analysis. Financial support for this work was provided by the NIH/NIGMS (GM-097444), Bristol-Myers Squibb (unrestricted research support) and Bestsyn Technologies (postdoctoral fellowship to Y. J.).
References
- 1.Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. J. Am. Chem. Soc. 1971;93:2325–2327. doi: 10.1021/ja00738a045. [DOI] [PubMed] [Google Scholar]
- 2.Suffness M, editor. TAXOL®: Science and Applications. CRC Press; 1995. [Google Scholar]
- 3.Itokawa H, Lee L-H, editors. Taxus: The Genus Taxus. Taylor & Francis; 2003. [Google Scholar]
- 4.Nicolaou KC, Yang Z, Liu JJ, Ueno H, Nantermet PG, Guy RK, Claiborne CF, Renaud J, Couladouros EA, Paulvannan K, Sorensen EJ. Nature. 1994;367:630–634. doi: 10.1038/367630a0. [DOI] [PubMed] [Google Scholar]
- 5.Holton RA, Somoza C, Kim HB, Liang F, Biediger RJ, Boatman PD, Shindo M, Smith CC, Kim S. J. Am. Chem. Soc. 1994;116:1597–1598. [Google Scholar]
- 6.Holton RA, Kim HB, Somoza C, Liang F, Biediger RJ, Boatman PD, Shindo M, Smith CC, Kim S. J. Am. Chem. Soc. 1994;116:1599–1600. [Google Scholar]
- 7.Masters JJ, Link JT, Snyder LB, Young WB, Danishefsky SJ. Angew. Chem. Int. Ed. 1995;34:1723–1726. [Google Scholar]
- 8.Wender PA, Badham NF, Conway SP, Floreancig PE, Glass TE, Gränicher C, Houze JB, Jänichen J, Lee D, Marquess DG, McGrane PL, Meng W, Mucciaro TP, Mühlebach M, Natchus MG, Paulsen H, Rawlins DB, Satkofsky J, Shuker AJ, Sutton JC, Taylor RE, Tomooka K. J. Am. Chem. Soc. 1997;119:2755–2756. [Google Scholar]
- 9.Wender PA, Badham NF, Conway SP, Floreancig PE, Glass TE, Houze JB, Krauss NE, Lee D, Marquess DG, McGrane PL, Meng W, Natchus MG, Shuker AJ, Sutton JC, Taylor RE. J. Am. Chem. Soc. 1997;119:2757–2758. [Google Scholar]
- 10.Shiina I, Saitoh K, Fréchard-Ortuno I, Mukaiyama T T. Chem. Lett. 1998;27:3–4. [Google Scholar]
- 11.Morihira K, Hara R, Kawahara S, Nishimori T, Nakamura N, Kusama H, Kuwajima I. J. Am. Chem. Soc. 1998;120:12980–12981. [Google Scholar]
- 12.Lim J. PhD thesis. Harvard University; 2000. A Total Synthesis of Taxol. [Google Scholar]
- 13.Doi T, Fuse S, Miyamoto S, Nakai K, Sasuga D, Takahashi T. Chem. Asian J. 2006;1:370–383. doi: 10.1002/asia.200600156. [DOI] [PubMed] [Google Scholar]
- 14.Hirai S, Utsugi M, Iwamoto M, Nakada M. Chem. Eur. J. 2015;21:355–359. doi: 10.1002/chem.201404295. [DOI] [PubMed] [Google Scholar]
- 15.Fukaya K, Tanaka Y, Sato AC, Kodama K, Yamazaki H, Ishimoto T, Nozaki Y, Iwaki YM, Yuki Y, Umei K, Sugai T, Yamaguchi Y, Watanabe A, Oishi T, Sato T, Chida N. Org. Lett. 2015;17:2570–2573. doi: 10.1021/acs.orglett.5b01173. [DOI] [PubMed] [Google Scholar]
- 16.Fukaya K, Kodama K, Tanaka Y, Yamazaki H, Sugai T, Yamaguchi Y, Watanabe A, Oishi T, Sato T, Chida N. Org. Lett. 2015;17:2573–2577. doi: 10.1021/acs.orglett.5b01174. [DOI] [PubMed] [Google Scholar]
- 17.Hudlicky T, Reed JW. The Way of Synthesis: Evolution of Design and Methods for Natural Products 1st edn. Wiley-VCH; 2007. p. 165. [Google Scholar]
- 18.Davis EM, Croteau R. Top. Curr. Chem. 2000;209:53–95. [Google Scholar]
- 19.Chen K, Baran PS. Nature. 2009;459:824–828. doi: 10.1038/nature08043. [DOI] [PubMed] [Google Scholar]
- 20.Holte D, Götz DCG, Baran PS. J. Org. Chem. 2012;77:825–842. doi: 10.1021/jo202314a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foo K, Usui I, Götz DCG, Werner EW, Holte D, Baran PS. Angew. Chem. Int. Ed. 2012;51:11491–11495. doi: 10.1002/anie.201206904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cherney EC, Lopchuk JM, Green JC, Baran PS. J. Am. Chem. Soc. 2014;136:12592–12595. doi: 10.1021/ja507321j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosen BR, Simke LR, Thuy-Boun PS, Dixon DD, Yu J-Q, Baran PS. Angew. Chem. Int. Ed. 2013;52:7317–7320. doi: 10.1002/anie.201303838. [DOI] [PubMed] [Google Scholar]
- 24.Renata H, Zhou Q, Baran PS. Science. 2013;339:59–63. doi: 10.1126/science.1230631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jørgensen L, McKerrall SJ, Kuttruff CA, Ungeheuer F, Felding J, Baran PS. Science. 2013;341:878–882. doi: 10.1126/science.1241606. [DOI] [PubMed] [Google Scholar]
- 26.Jin Y, Yeh C-H, Kuttruff CA, Jørgensen L, Dünstl G, Felding J, Natarajan SR, Baran PS. Angew. Chem. Int. Ed. 2015;54:14044–14048. doi: 10.1002/anie.201507977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishihara Y, Baran PS. Synlett. 2010:1733–1745. [Google Scholar]
- 28.Shi Q-W, Kiyota H. Chem. Biodiversity. 2005;2:1597–1623. doi: 10.1002/cbdv.200590131. [DOI] [PubMed] [Google Scholar]
- 29.Chen Y-J, Chen C-Y, Shen Y-C. J. Nat. Prod. 1999;62:149–151. doi: 10.1021/np9802365. [DOI] [PubMed] [Google Scholar]
- 30.Marcano DPDCD, Halsall TG. J. Chem. Soc. D. Chem. Comm. 1969;21:1282–1283. [Google Scholar]
- 31.Miyazaki M, Shimizu K, Mishimma H, Kurabayashi M. Chem. Pharm. Bull. 1968;16:546–548. [Google Scholar]
- 32.Holton RA, Juo RR, Kim HB, Williams AD, Harusawa S, Lowenthal RE, Yogai S. J. Am. Chem. Soc. 1988;110:6558–6560. [Google Scholar]
- 33.Hara R, Furukawa T, Horiguchi Y, Kuwajima I. J. Am. Chem. Soc. 1996;118:9186–9187. [Google Scholar]
- 34.Hara R, Furukawa T, Kashima H, Kusama H, Horiguchi Y, Kuwajima I. J. Am. Chem. Soc. 1999;121:3072–3082. [Google Scholar]
- 35.Paquette LA, Zhao M. J. Am. Chem. Soc. 1998;120:5203–5212. [Google Scholar]
- 36.Paquette LA, Wang H-L, Su Z, Zhao M. J. Am. Chem. Soc. 1998;120:5213–5225. [Google Scholar]
- 37.Zhang HJ, Takeda Y, Minami Y, Yoshida K, Matsumoto T, Xiang W, Mu O, Sun HD. Chem. Lett. 1994;23:957–960. [Google Scholar]
- 38.Wilde NC, Isomura M, Mendoza A, Baran PS PS. J. Am. Chem. Soc. 2014;136:4909–4912. doi: 10.1021/ja501782r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ajikumar PK, Xiao W-H, Tyo KEJ, Wang Y, Simeon F, Leonard E, Mucha O, Phon TH, Pfeifer B, Stephanopoulos G. Science. 2010;330:70–74. doi: 10.1126/science.1191652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rubenstein SM, Williams RM. J. Org. Chem. 1995;60:7215–7223. [Google Scholar]
- 41.Mendoza A, Ishihara Y, Baran PS. Nat. Chem. 2012;4:21–25. doi: 10.1038/nchem.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krasutsky SG, Jacobo SH, Tweedie SR, Krishnamoorthy R, Filatov AS. Org. Process Res. Dev. 2015;19:284–289. [Google Scholar]
- 43.Krumpolic M, Roeek J. Inorg. Chem. 1985;24:617–621. [Google Scholar]
- 44.Vedejs E. J. Am. Chem. Soc. 1974;96:5944–5946. [Google Scholar]
- 45.Jin Y, Qiu GF. Org. Biomol. Chem. 2012;10:5452–5455. doi: 10.1039/c2ob25940k. [DOI] [PubMed] [Google Scholar]
- 46.Kim MS, Mi Y, An DK. Tetrahedron Lett. 2007;48:5061–5064. [Google Scholar]
- 47.Bachmann WE. J. Am. Chem. Soc. 1933;55:770–774. [Google Scholar]
- 48.Lee AS-Y, Hu Y-J, Chu S-F S-F. Tetrahedron. 2001;57:2121–2126. [Google Scholar]
- 49.Chandra KL, Saravanan P, Singh RK, Singh VK VK. Tetrahedron Lett. 2002;58:1369–1374. [Google Scholar]
- 50.Horiguchi T, Cheng Q, Oritani T T. Tetrahedron Lett. 2000;41:3907–3910. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.