Abstract
Steroids are key factors in a myriad of mammalian biological systems, including the brain, kidney, heart, bones, and gonads. While alternative potential steroid receptors have been described, the majority of biologically relevant steroid responses appear to be mediated by classical steroid receptors that are located in all parts of the cell, from the plasma membrane to the nucleus. Interestingly, these classical steroid receptors modulate different signals depending upon their location. For example, receptors in the plasma membrane interact with membrane signaling molecules, including G proteins and kinases. In contrast, receptors in the nucleus interact with nuclear signaling molecules, including transcriptional co-regulators. These extranuclear and intranuclear signals function together in an integrated fashion to regulate important biological functions. While most studies on extranuclear steroid signaling have focused on estrogens, recent work has demonstrated that nongenomic androgen signaling is equally important and that these two steroids modulate similar signaling pathways. In fact, by taking advantage of a simple model system whereby a physiologically relevant androgen-mediated process is regulated completely independent of transcription (Xenopus laevis oocyte maturation), many novel and conserved concepts in nongenomic steroid signaling have been uncovered and characterized.
Keywords: androgen, nongenomic, extranuclear, kinase, paxillin
Introduction
Androgens regulate a number of important biological processes, including male [1] and female reproduction [2–4] prostate growth and development [5, 6], prostate cancer proliferation [7, 8], and bone metabolism [9]. In all of these examples, androgens are believed to mediate their effects primarily through classical androgen receptors (ARs)1. While ARs are traditionally thought to mediate their effects primarily through the regulation of transcription in the nucleus, studies performed over the past 10 years have demonstrated that, in fact, ARs also mediate extranuclear (nongenomic) signaling pathways in response to androgens. These AR-mediated extranuclear pathways, that include G protein and kinase signaling, function synergistically with both AR-dependent and AR-independent transcriptional signals to regulate the aforementioned physiologic processes. Here we provide an overview of extranuclear androgen actions, focusing on the remarkable conservation of nongenomic androgen-mediated signaling pathways from frog germ cell development to human prostate cancer proliferation.
Oocyte maturation: A biologically relevant model of nongenomic androgen signaling
Although great strides have been made in the identification and characterization of a number of extranuclear steroid signals, the physiological significance of many of these nongenomic actions remains elusive [10]. Since most steroid-induced processes involve both genomic and nongenomic effects, delineating the relative importance of nongenomic steroid actions in a biologically relevant process is a daunting task. To circumvent this problem, oocyte maturation in frogs has served as an ideal model system to study nongenomic steroid signaling in a physiologically important system [10–12]. Oocyte maturation refers to the resumption of meiosis. In all animals, oocytes are arrested in prophase I of meiosis (termed “immature” oocytes) until shortly before ovulation, when gonadotropins trigger meiotic re-entry, or maturation. Oocytes progress through meiosis to metaphase II, where they again arrest (termed “mature” oocytes). They remain in this stage until after ovulation and fertilization, when meiosis is finally completed [10, 13– 15]. Importantly, it is universally accepted that, not only do steroids induce this maturation process in oocytes from the frog Xenopus laevis, but that they do so completely independent of transcription [16, 17].
Historically, progesterone was considered the physiological mediator of Xenopus oocyte maturation [16–20]. However, gonadotropins stimulate more than 10 times more androgen than progesterone production at the time of ovulation, and androgens are equally or more potent promoters of oocyte maturation in-vitro [12, 21, 22]. Furthermore, inhibition of androgen but not progesterone production in-vivo almost completely blocks gonadotropin-induced oocyte maturation and ovulation [23]. Together, these observations prove that, in fact, androgens rather than progesterone are the physiologic regulators of oocyte maturation and release.
Androgen-induced Xenopus oocyte maturation is mediated by classical ARs, as both the androgen receptor antagonist flutamide [21] and AR knockdown by siRNA or antisense oligonucleotides [24] abrogates androgen-triggered maturation. Based on immunohistochemistry and biochemical studies, classical ARs are expressed throughout the cell, with approximately 5% found in the plasma membrane [24]. These membrane-localized ARs are presumed to be the regulators of androgen-mediated maturation, in part because testosterone coupled to BSA triggers oocyte maturation as well as free steroid; however, definitive proof of their importance has yet to be demonstrated.
How do androgens trigger Xenopus oocyte maturation? Most studies implicate a “release of inhibition” mechanism whereby oocytes are held in meiotic arrest by constitutive inhibitory Gαs [25] and Gβγ signaling [24, 26–28]. These inhibitory G protein signaling are mediated at least in part via the constitutively activated G protein-coupled receptor called GPR3 [29–31]. Combined Gαs and Gβγ signaling stimulate adenylyl cyclase to elevate intracellular cAMP levels [25, 32], which then prevents meiotic progression through mechanisms that are not well understood [33, 34] but may involve the scaffold protein named Modulator of Nongenomic steroid Responses (MNAR), or proline, glutamic acid, and leucine rich protein 1 (PELP1) [35]. In somatic cells, MNAR/PELP1 acts as a scaffold that links steroid receptors to Src and other signaling molecules [36]. In Xenopus oocytes, MNAR/PELP1 directly interacts with Gβ and AR to enhance Gβγ-mediated stimulation of adenylyl cyclase [10, 15, 35]. Following gonadotropin stimulation, testosterone binding to ARs might cause a conformational change in the AR-PELP1-Gβγ complex that suppresses G-protein mediated signaling, leading to decreased intracellular cAMP levels and subsequent oocyte maturation [15, 35].
Once cAMP levels drop, downstream kinases are activated, starting with the germ cell specific Raf homolog called MOS. In immature Xenopus oocytes, though there is sufficient Mos mRNA, little is translated into MOS protein [37–41]. When cAMP levels drop, Mos mRNA becomes polyadenylated, resulting in a small increase in MOS protein expression. MOS in turn activates the MEK-Erk pathway [42]. Androgen-induced expression of MOS and subsequent Erk activation requires the scaffolding protein called paxillin [43]. Paxillin is a 68kDa focal adhesion protein that, in somatic cells, acts as a multi-domain adaptor and/or scaffold molecule to integrate many signals from integrins, cell surface receptors and growth factors [44]. Interestingly, in oocytes, after paxillin assists in androgen-triggered MOS and Erk activation, Erk phosphorylates paxillin on serine residues, which in turn leads to increased MOS protein expression and more Erk activation. Thus, paxillin functions both upstream and downstream of Erk, and this positive feedback loop ultimately leads to activation of cyclin dependent kinase CDK1 and subsequent meiotic resumption [43, 45–48].
Androgens are also capable of promoting mammalian oocyte maturation. Studies using mouse [10, 15, 49] and porcine [50, 51] oocytes show that testosterone induces oocyte maturation in a transcription independent manner that involves activation of MAPK and CDK1 signaling. In fact, androgen-induced maturation of mouse oocytes is blocked by the AR antagonist flutamide [49] and no longer occurs in oocytes lacking androgen receptors [4], providing pharmacologic and genetic evidence that, as in frogs, androgen-triggered oocyte maturation requires the classical AR. However, unlike in frog oocytes, the physiologic role of androgens (and progestins) in regulating mammalian oocyte maturation is still uncertain.
Extranuclear androgen signaling regulates transcription in the testes
While the physiologic role of androgens in murine oocyte development remains obscure, the role of testosterone and ARs in spermatogenesis is well established [52, 53]. Surprisingly, although ARs are expressed in Sertoli cells [54] and testosterone is known to promote germ cell development [55, 56], microarray studies of Sertoli cells in AR knockout mice reveal few transcriptional targets for testosterone. Furthermore, relatively few genes expressed in Sertoli cells are known to have AR binding elements (AREs) [57– 60], although evidence in other tissues suggests that AREs might be located further from promoters than initially assumed [61]. Nonetheless, together, these observations suggest that extranuclear, or nongenomic, androgen signaling might be important for spermatogenesis [62].
In Sertoli cells, androgen-induced extranuclear signaling involves trans-activation of the epidermal growth factor receptor (EGFR) [56]. Testosterone binds to classical ARs [56, 62–64] located at or near the plasma membrane, leading to activation of Src [65–67]. Src in turn promotes phosphorylation of the EGFR either directly or through factors yet to be identified, resulting in activation of the MAPK pathway (Raf-MEK-Erk) [67]. Once Erk is phosphorylated, it activates the kinase p90RSK, which promotes phosphorylation of the cAMP Response Element Binding (CREB) protein. CREB then acts as a transcriptional co-activator upon binding to cAMP Response Elements (CREs) [62, 65, 67, 68]. This pathway highlights the concept that, outside the nucleus, androgens function similarly to growth factors by activating extranuclear kinases. These androgen-triggered extranuclear kinases can then affect both AR-dependent and AR-independent intranuclear transcription. This concept of extranuclear androgen signaling regulating intranuclear transcription will be further discussed later in this review.
In addition to promoting kinase signaling, testosterone in Sertoli cells also stimulates rapid and transient calcium influx through L-type calcium channels [69–71]. This testosterone-mediated increase in intra-cellular calcium level then appears to induce a number of secondary messengers such as calmodulin kinase, or PKC [65], the physiological significance of which is yet to be determined.
Nongenomic androgen signaling pathways are conserved in prostate cancer cells
In addition to regulating gonadal function, both intranuclear and extranuclear androgen signaling is also required for normal differentiation and development of the prostate [72, 73], as well as for early prostate cancer (PCa) proliferation and development [74]. While testosterone is the androgen regulating Sertoli cell function during spermatogenesis, the more potent androgen dihydrotestosterone (DHT) regulates prostate growth and development [73]. DHT promotes these processes by regulating AR-mediated transcription of a number of specific genes that include prostate-specific antigen (PSA) as well as critical growth regulatory proteins [75]. However, as in Sertoli cells, androgens also trigger cytoplasmic kinase cascades in prostate cancer cells that can then regulate downstream nuclear signals [76].
Interestingly, prostate cancer is usually androgen-dependent to start; thus prostate cancer is initially highly responsive to androgen deprivation (castration). However, with time, prostate cancer cells become castration resistant [77]. Despite early belief that advanced prostate cancers lose their sensitivity to androgens, it has become clear that the cells remain dependent on AR function [78]. Castration resistance is suggested to occur through a variety of molecular mechanisms, including AR amplification/mutation that allows a response to lower levels of androgens, ligand-independent AR activation, or perhaps de novo biosynthesis of androgens by the tumor cells themselves [79, 80].
Nongenomic androgen signaling has been widely studied in both androgen-responsive and androgen unresponsive cell lines derived from human prostate cancers (e.g., LnCAP and PC3 cells, respectively). As described in Sertoli cells, androgens trigger extranuclear signals via extranuclear classical androgen receptors and EGFR transactivation. Some studies suggest that the classical AR directly binds to the SH3 domain of Src, perhaps in a complex with estrogen receptor β (ERβ), to activate kinase signaling and subsequent proliferation [81–83]. In contrast, more recent data demonstrate that androgen-induced transactivation of the EGFR occurs via matrix metalloproteinse (MMP)-mediated release of EGFR ligands, as the MMP inhibitor Galardin abrogates DHT-induced release of these ligands, as well as EGFR transactivation and subsequent Erk phosphorylation [84]. Of note, the orphan G protein-coupled receptor GPR6A has recently been reported to regulate extranuclear androgen-induced kinase signaling in prostate and other cells, perhaps through Gαi signaling; however, the biological importance of this receptor in androgen signaling has yet to be determined [85].
Interestingly, siRNA-mediated knockdown of paxillin in LnCAP cells abrogates DHT-mediated activation of Erk, indicating that, as in Xenopus oocytes, paxillin is required for steroid-triggered Erk signaling in prostate cancer cells. In fact, in addition to blocking DHT-induced Erk activation, paxillin knockdown similarly blocks EGF-induced Erk activation, indicating that paxillin functions downstream of the EGFR to regulate Erk activation in response to both direct (EGF) and indirect (DHT) EGFR activation [84]. Furthermore, EGFR-mediated Erk activation requires Src-dependent phosphorylation of tyrosine residues on paxillin, but expression of constitutively activated Raf stimulates Erk independent of paxillin. Together, these data place paxillin downstream of EGFR and Src, but upstream of Raf in the MAPK pathway. Most importantly, these data highlight the conserved function of paxillin from frog oocytes to human prostate cells, with paxillin serving as a regulator of MAPK signaling at the level of Raf in prostate cells and of MOS in oocytes.
In addition to its conserved function as an activator of Raf/MOS, paxillin function downstream of Erk is also conserved from frog oocytes to human prostate cancer, as Erk-mediated phosphorylation of serine residues on paxillin is necessary to promote downstream Erk-mediated processes in prostate cancer cells such as proliferation and migration. In fact, paxillin regulates these Erk-mediated processes by modulating Erk-mediated transcription of both AR-dependent and AR-independent genes, suggesting that, in PCa cells, paxillin serves as a liaison between extranuclear Erk signaling and intranuclear transcriptional signaling.
Besides paxillin function being conserved from the frog oocyte to the human prostate cancer cell, MNAR/PELP1 function also appears to be conserved in nongenomic AR signaling. MNAR/PELP1 expression has been found to be up-regulated in high grade prostate tumors [86]. Furthermore, through its polyproline and LXXLL motifs, MNAR/PELP1 appears to facilitate the interactions between extranuclear AR and Src to promote kinase signaling in PCa cells [87, 88]. In fact, DHT triggers the formation of a PELP1/Src/AR complex in androgen-dependent LnCap cells, in response to DHT. In contrast, when LnCAP cells are made androgen-independent, this complex is present in the absence of androgen, suggesting that MNAR/PELP1 deregulation might play a role in the transition of PCa tumors to a castration resistant type [87].
Finally, as seen in Xenopus oocytes, work in prostate cancer cells reveals a subpopulation of classic ARs that are localized to the membrane. In PCa cells, these ARs are found in caveolae [89]. Caveolin-1 (Cav-1), a small integral membrane protein found in caveolae, is upregulated in PCa cells and may be a regulator of AR actions in the membrane [90, 91]. In fact, AR and EGFR co-immunoprecipitate with Cav-1 in some PCa cells [92], suggesting that Cav-1 might regulate the aforementioned androgentriggered trans-activation of the EGFR. Furthermore, over-expression of Cav-1 in PCa cells enhances AR-mediated transcription and protection against apoptosis [64, 93], while downregulation of Cav-1 expression abrogates pro-survival effects of androgens in LnCAP cells [94]. These observations are again consistent with extranuclear AR-mediated kinase signaling being an enhancer of intranucelar AR-mediated transcription.
Extranuclear androgen signaling in muscle
Other targets for androgen actions include both skeletal and smooth muscles. For example, androgens regulate myogenesis during embryonic development [95] and determine muscle mass and strength later in life [96]. As in the testes, testosterone increases intracellular Ca2+ concentration in skeletal and smooth muscles within seconds [97, 98]. The mechanisms of androgen-induced Ca2+ elevation are divided into AR-dependent and AR-independent pathways. In guinea pig cardiomyocytes, testosterone induces shortening of the action potential duration within 5 minutes, mainly due to enhancement of slowly activating potassium currents and suppression of L-type Ca2+ currents [99]. In this study, the AR antagonist, nilutamide, completely blocks the testosterone effect on action potential duration shortening, indicating an AR-dependent pathway. In contrast, testosterone and nandrolone (an AR agonist) increase intracellular Ca2+ levels within seconds and activation of Erk within minutes, in cultured rat myotubules [100]. These effects are inhibited by PLC, IP3 and G-protein inhibitors but not by an AR-antagonist, suggesting that, as seen in oocytes, androgen signaling can alter G protein signaling in muscle, though in this case perhaps independent of classical ARs [100]. The same pathway has been detected in rat cardiac myocytes, where testosterone coupled to BSA induces a rapid increase in intracellular calcium levels through activation of a plasma membrane receptor coupled to Pertussis toxin-sensitive G protein [98]. Finally, a recent study demonstrates a rapid action of DHT in regulating force production in isolated intact mouse skeletal muscle fibers that is unaffected by AR inhibitors, but abolished by MEK and EGFR inhibitors [101], again demonstrating the importance of androgen-triggered kinase signaling, either via AR or a yet unidentified receptor.
Of note, androgens have also been shown to stimulate mouse colonic smooth muscle cells in an AR-dependent fashion that involves rapid induction of calcium sensitization pathways [102].
Nongenomic androgen signaling in bone
Physiological effects of nongenomic androgen signaling have also been demonstrated in the bone [9, 103], where androgens exert anti-apoptotic effects in osteoblasts and osteocyts through activation of the Src/Shc/Erk signaling cascade and inhibition of the JNK pathway [104]. These nongenomic actions of androgens are mediated via a ligand-binding region of the classical AR that is distinct from the one responsible for its genomic actions. Moreover, as described earlier in other systems, nongenomic sex steroid signaling modulates transcription of several transcription factors in the bone, such as Elk-1, cJun/cFos, CREB and CCAAT enhancer binding protein-β (C/EBPβ) [103, 105]. Based on these and other data, it has been proposed that the nongenomic anti-apoptotic actions of steroids on osteoblasts and osteocytes help balance bone formation and resorption [9]. Recently [106], it has been shown the PKCβ/p66shc/NF-κB signaling cascade is an important mediator of oxidative stress induced apoptosis in osteoblastic cells that is prevented by the nongenomic effects of androgens and estrogens. Steroid-induced activation of Erk inhibits PKC activation, thereby preventing the phosphorylation of p66shc that is essential for oxidative stress-stimulated osteoblastic cell apoptosis. Thus, this antiapoptotic effect of androgens and estrogens is exclusively mediated through its nongenomic actions and might not require any “classical” genomic actions [106].
Nongenomic androgen signaling in the immune system
Despite a lack of detectable AR [107] in macrophages, and a very low level of AR in T cells [108], nongenomic pathways mediated by testosterone have been detected and are associated with a suppressive effect of the immune response [109, 110]. Similar to its effect in the testis and the muscles, testosterone rapidly increases intracellular Ca2+ concentration in immune cells such T-cells and macrophages [111–113]. However, the mechanisms of testosterone-induced intracellular Ca2+ elevation may be cell type- as well as tissue-specific. In peritoneal macrophage cell lines, intracellular Ca2+ elevation is reached by the release of intracellular store in response to GPCR and PLC activation [107]. In T-cells, as well as in murine bone marrow-derived macrophages, the increase in intracellular Ca2+ concentration is by influx of extracellular Ca2+, predominantly via non-voltage-gated Ni2+ calcium channels [108, 114]. However, both in T cells and in macrophages, testosterone effects do not appear to be mediated by the classical AR, as AR antagonists had no effect on rapid testosterone signaling. Additional investigation will be required in order to fully understand both the significance and mechanism of testosterone-mediated effects in the immune system.
Conclusions
In summary, through the use of diverse model systems, many novel concepts regarding the integration of extranuclear and intranuclear steroid signaling have been discovered over the past decade. Important concepts include: 1) Extranuclear androgen-induced signaling pathways are very well conserved from lower to higher vertebrates. 2) Androgens signaling outside the nucleus are similar to growth factors, functioning to activate cytoplasmic kinases; 3) By activating cytoplasmic kinase cascades, extranuclear androgen signaling modulates intranuclear transcription; 4) Paxillin may act as a liaison between extranuclear kinase signaling and intranuclear transcription.
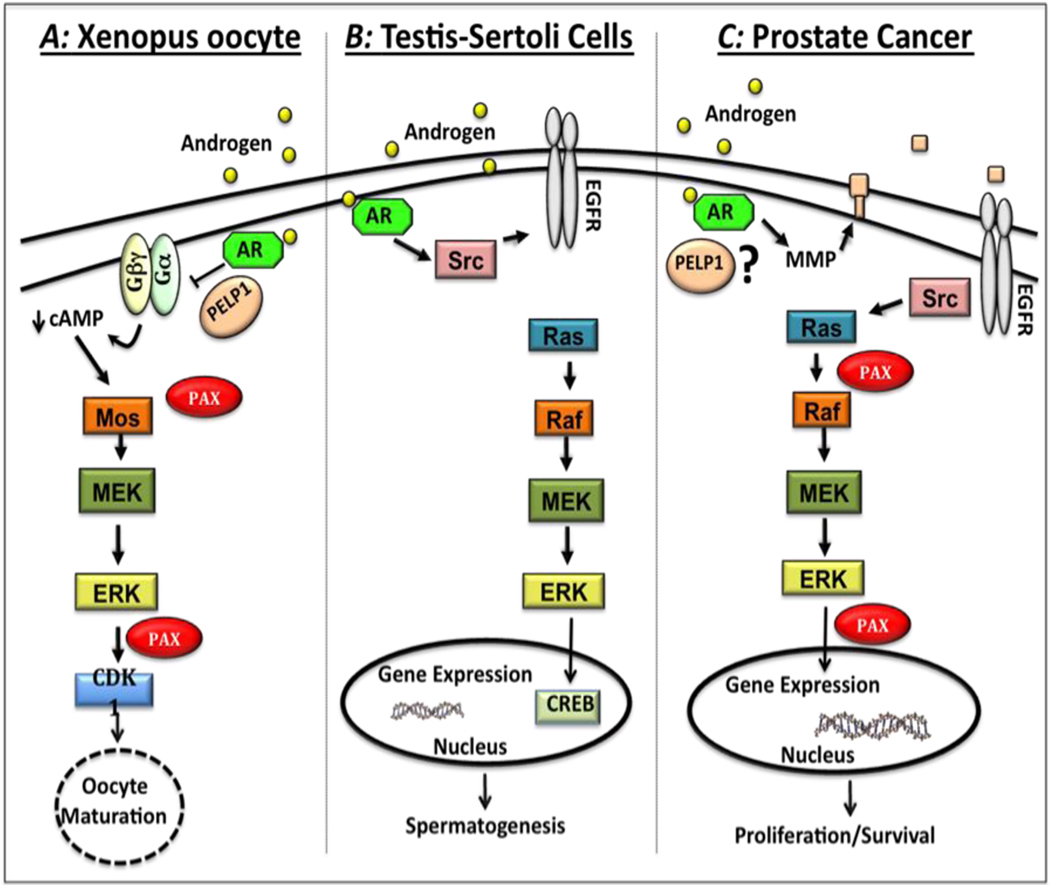
Figure 1. Comparison of androgen-induced signaling pathways in Xenopus oocytes, Sertoli cells, and prostate cancer cells.
A) In Xenopus oocytes, testosterone binding to classical androgen receptors (ARs) cause a conformational change in the AR-PELP1-Gβγ complex that suppresses G-protein mediated constitutive signaling, leading to decreased intracellular cAMP levels. Once cAMP levels drop, downstream kinases are activated, starting with a small increase in MOS protein expression. MOS in turn activates the MEK-Erk pathway. Activated Erk phosphorylates paxillin, which in turn leads to increased MOS protein expression and more Erk activation. This positive feedback loop ultimately leads to activation of cyclin dependent kinase CDK1 and subsequent oocyte maturation. B) In Sertoli cells, testosterone binds to classical ARs leading to activation of Src that promotes phosphorylation of the epidermal growth factor receptor (EGFR), resulting in activation of the MAPK pathway (Raf-MEK-Erk). Once Erk is phosphorylated, it activates the kinase p90RSK, which promotes phosphorylation of the cAMP Response Element Binding (CREB) protein leading to increased transcription. C) In prostate cancer cells, androgen induces transactivation of the EGFR via matrix metalloproteinase (MMP)-mediated release of membrane-bound EGFR ligands. EGFR activation leads to Src-mediated phosphorylation of paxillin that is essential for subsequent activation of Ras/Raf/MEK/Erk pathway. Activated Erk in turn phosphorylates paxillin that regulates Erk-mediated downstream processes such as intranuclear transcription, proliferation, cell survival and migration.
Table 1.
Nongenomic androgen signaling and physiological functions in different tissues.
| Tissue | Physiology | Signaling Pathway |
Specific Signaling Proteins |
Downstream Signaling Mechanism |
|---|---|---|---|---|
| Oocytes | Oocyte maturation | Gβγ; cAMP; MAPK | Mos, PELP-1, Paxillin, Erk, CDK1 |
All-or-none positive feedback; CDK1 activation |
|
Sertoli cells |
Spermatogenesis | EGFR; MAPK | Src, Erk, CREB | p90RSK; CREB-mediated transcription |
|
Prostate Cancer |
Proliferation/survival migration |
EGFR; MAPK | PELP-1, Src, Paxillin, Caveolin-1, Erk |
Cytoplasmic-nuclear cross talk; transcription |
| Bones | Bone turnover | MAPK | Src/Shc/Erk | PCKβ/p66shc/NF-κB; apoptosis |
| Muscles | Unknown | Ca2+; MAPK | Ca2+/K+ channels, PLC,IP3R |
Transient Ca2+ release |
Acknowledgements
Work discussed in this review was funded by R01DK059913.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AR, androgen receptor; Modulator of Nongenomic steroid Responses, MNAR; proline, glutamic acid, and leucine rich protein 1, PELP1; epidermal growth factor receptor, EGFR
References
- 1.Quigley CA, et al. Androgen receptor defects: historical, clinical, and molecular perspectives. Endocr Rev. 1995;16(3):271–321. doi: 10.1210/edrv-16-3-271. [DOI] [PubMed] [Google Scholar]
- 2.Hu YC, et al. Subfertility and defective folliculogenesis in female mice lacking androgen receptor. Proc Natl Acad Sci U S A. 2004;101(31):11209–11214. doi: 10.1073/pnas.0404372101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shiina H, et al. Premature ovarian failure in androgen receptor-deficient mice. Proc Natl Acad Sci U S A. 2006;103(1):224–229. doi: 10.1073/pnas.0506736102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sen A, Hammes SR. Granulosa Cell-Specific Androgen Receptors Are Critical Regulators of Ovarian Development and Function. Mol Endocrinol. 2010;24(7):1393–1403. doi: 10.1210/me.2010-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marker PC, et al. Hormonal, cellular, and molecular control of prostatic development. Dev Biol. 2003;253(2):165–174. doi: 10.1016/s0012-1606(02)00031-3. [DOI] [PubMed] [Google Scholar]
- 6.Hayward SW, Cunha GR. The prostate: development and physiology. Radiol Clin North Am. 2000;38(1):1–14. doi: 10.1016/s0033-8389(05)70146-9. [DOI] [PubMed] [Google Scholar]
- 7.Xu Y, et al. Androgens induce prostate cancer cell proliferation through mammalian target of rapamycin activation and post-transcriptional increases in cyclin D proteins. Cancer Res. 2006;66(15):7783–7792. doi: 10.1158/0008-5472.CAN-05-4472. [DOI] [PubMed] [Google Scholar]
- 8.Lange CA, et al. Integration of rapid signaling events with steroid hormone receptor action in breast and prostate cancer. Annu Rev Physiol. 2007;69:171–199. doi: 10.1146/annurev.physiol.69.031905.160319. [DOI] [PubMed] [Google Scholar]
- 9.Manolagas SC, Kousteni S, Jilka RL. Sex steroids and bone. Recent Prog Horm Res. 2002;57:385–409. doi: 10.1210/rp.57.1.385. [DOI] [PubMed] [Google Scholar]
- 10.Hammes SR, Levin ER. Extranuclear steroid receptors: nature and actions. Endocr Rev. 2007;28(7):726–741. doi: 10.1210/er.2007-0022. [DOI] [PubMed] [Google Scholar]
- 11.Hammes SR. The further redefining of steroid-mediated signaling. Proc Natl Acad Sci U S A. 2003;100(5):2168–2170. doi: 10.1073/pnas.0530224100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hammes SR. Steroids and oocyte maturation--a new look at an old story. Mol Endocrinol. 2004;18(4):769–775. doi: 10.1210/me.2003-0317. [DOI] [PubMed] [Google Scholar]
- 13.Albertini DF, Carabatsos MJ. Comparative aspects of meiotic cell cycle control in mammals. J Mol Med. 1998;76(12):795–799. doi: 10.1007/s001090050283. [DOI] [PubMed] [Google Scholar]
- 14.Albertini DF, et al. Cellular basis for paracrine regulation of ovarian follicle development. Reproduction. 2001;121(5):647–653. doi: 10.1530/rep.0.1210647. [DOI] [PubMed] [Google Scholar]
- 15.Deng J, et al. Nongenomic steroid-triggered oocyte maturation: of mice and frogs. Steroids. 2009;74(7):595–601. doi: 10.1016/j.steroids.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maller JL, Krebs EG. Regulation of oocyte maturation. Curr Top Cell Regul. 1980;16:271–311. doi: 10.1016/b978-0-12-152816-4.50012-1. [DOI] [PubMed] [Google Scholar]
- 17.Smith LD, Ecker RE. The interaction of steroids with Rana pipiens Oocytes in the induction of maturation. Dev Biol. 1971;25(2):232–247. doi: 10.1016/0012-1606(71)90029-7. [DOI] [PubMed] [Google Scholar]
- 18.Newport JW, Kirschner MW. Regulation of the cell cycle during early Xenopus development. Cell. 1984;37(3):731–742. doi: 10.1016/0092-8674(84)90409-4. [DOI] [PubMed] [Google Scholar]
- 19.Tian J, et al. Identification of XPR-1, a progesterone receptor required for Xenopus oocyte activation. Proc Natl Acad Sci U S A. 2000;97(26):14358–14363. doi: 10.1073/pnas.250492197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bayaa M, et al. The classical progesterone receptor mediates Xenopus oocyte maturation through a nongenomic mechanism. Proc Natl Acad Sci U S A. 2000;97(23):12607–12612. doi: 10.1073/pnas.220302597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lutz LB, et al. Evidence that androgens are the primary steroids produced by Xenopus laevis ovaries and may signal through the classical androgen receptor to promote oocyte maturation. Proc Natl Acad Sci U S A. 2001;98(24):13728–13733. doi: 10.1073/pnas.241471598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang WH, Lutz LB, Hammes SR. Xenopus laevis ovarian CYP17 is a highly potent enzyme expressed exclusively in oocytes. Evidence that oocytes play a critical role in Xenopus ovarian androgen production. J Biol Chem. 2003;278(11):9552–9559. doi: 10.1074/jbc.M212027200. [DOI] [PubMed] [Google Scholar]
- 23.White SN, et al. Specific modulation of nongenomic androgen signaling in the ovary. Steroids. 2005;70(5–7):352–360. doi: 10.1016/j.steroids.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 24.Lutz LB, et al. Selective modulation of genomic and nongenomic androgen responses by androgen receptor ligands. Mol Endocrinol. 2003;17(6):1106–1116. doi: 10.1210/me.2003-0032. [DOI] [PubMed] [Google Scholar]
- 25.Gallo CJ, et al. Stimulation of Xenopus oocyte maturation by inhibition of the G-protein alpha S subunit, a component of the plasma membrane and yolk platelet membranes. J Cell Biol. 1995;130(2):275–284. doi: 10.1083/jcb.130.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Evaul K, et al. Testosterone and progesterone rapidly attenuate plasma membrane Gbetagamma-mediated signaling in Xenopus laevis oocytes by signaling through classical steroid receptors. Mol Endocrinol. 2007;21(1):186–196. doi: 10.1210/me.2006-0301. [DOI] [PubMed] [Google Scholar]
- 27.Lutz LB, et al. G protein beta gamma subunits inhibit nongenomic progesterone-induced signaling and maturation in Xenopus laevis oocytes. Evidence for a release of inhibition mechanism for cell cycle progression. J Biol Chem. 2000;275(52):41512–41520. doi: 10.1074/jbc.M006757200. [DOI] [PubMed] [Google Scholar]
- 28.Sheng Y, Montplaisir V, Liu XJ. Co-operation of Gsalpha and Gbetagamma in maintaining G2 arrest in Xenopus oocytes. J Cell Physiol. 2005;202(1):32–40. doi: 10.1002/jcp.20084. [DOI] [PubMed] [Google Scholar]
- 29.Ferrell JE, Jr, Machleder EM. The biochemical basis of an all-or-none cell fate switch in Xenopus oocytes. Science. 1998;280(5365):895–898. doi: 10.1126/science.280.5365.895. [DOI] [PubMed] [Google Scholar]
- 30.Guadagno TM, Ferrell JE., Jr Requirement for MAPK activation for normal mitotic progression in Xenopus egg extracts. Science. 1998;282(5392):1312–1315. doi: 10.1126/science.282.5392.1312. [DOI] [PubMed] [Google Scholar]
- 31.Deng J, et al. The Xenopus laevis isoform of G protein-coupled receptor 3 (GPR3) is a constitutively active cell surface receptor that participates in maintaining meiotic arrest in X. laevis oocytes. Mol Endocrinol. 2008;22(8):1853–1865. doi: 10.1210/me.2008-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheng Y, et al. Regulation of Xenopus oocyte meiosis arrest by G protein betagamma subunits. Curr Biol. 2001;11(6):405–416. doi: 10.1016/s0960-9822(01)00123-3. [DOI] [PubMed] [Google Scholar]
- 33.Sadler SE, Maller JL. Progesterone inhibits adenylate cyclase in Xenopus oocytes. Action on the guanine nucleotide regulatory protein. J Biol Chem. 1981;256(12):6368–6373. [PubMed] [Google Scholar]
- 34.Conti M, et al. Role of cyclic nucleotide signaling in oocyte maturation. Mol Cell Endocrinol. 2002;187(1–2):153–159. doi: 10.1016/s0303-7207(01)00686-4. [DOI] [PubMed] [Google Scholar]
- 35.Haas D, et al. The modulator of nongenomic actions of the estrogen receptor (MNAR) regulates transcription-independent androgen receptor-mediated signaling: evidence that MNAR participates in G protein-regulated meiosis in Xenopus laevis oocytes. Mol Endocrinol. 2005;19(8):2035–2046. doi: 10.1210/me.2004-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vadlamudi RK, Kumar R. Functional and biological properties of the nuclear receptor coregulator PELP1/MNAR. Nucl Recept Signal. 2007;5:e004. doi: 10.1621/nrs.05004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gebauer F, et al. Translational control by cytoplasmic polyadenylation of cmos mRNA is necessary for oocyte maturation in the mouse. EMBO J. 1994;13(23):5712–5720. doi: 10.1002/j.1460-2075.1994.tb06909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stebbins-Boaz B, Hake LE, Richter JD. CPEB controls the cytoplasmic polyadenylation of cyclin, Cdk2 and c-mos mRNAs and is necessary for oocyte maturation in Xenopus. EMBO J. 1996;15(10):2582–2592. [PMC free article] [PubMed] [Google Scholar]
- 39.de Moor CH, Richter JD. The Mos pathway regulates cytoplasmic polyadenylation in Xenopus oocytes. Mol Cell Biol. 1997;17(11):6419–6426. doi: 10.1128/mcb.17.11.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mendez R, et al. Phosphorylation of CPE binding factor by Eg2 regulates translation of c-mos mRNA. Nature. 2000;404(6775):302–307. doi: 10.1038/35005126. [DOI] [PubMed] [Google Scholar]
- 41.Martinez SE, et al. XGef mediates early CPEB phosphorylation during Xenopus oocyte meiotic maturation. Mol Biol Cell. 2005;16(3):1152–1164. doi: 10.1091/mbc.E04-07-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Resing KA, et al. Determination of v-Mos-catalyzed phosphorylation sites and autophosphorylation sites on MAP kinase kinase by ESI/MS. Biochemistry. 1995;34(8):2610–2620. doi: 10.1021/bi00008a027. [DOI] [PubMed] [Google Scholar]
- 43.Rasar M, DeFranco DB, Hammes SR. Paxillin regulates steroid-triggered meiotic resumption in oocytes by enhancing an all-or-none positive feedback kinase loop. J Biol Chem. 2006;281(51):39455–39464. doi: 10.1074/jbc.M608959200. [DOI] [PubMed] [Google Scholar]
- 44.Brown MC, Turner CE. Paxillin: adapting to change. Physiol Rev. 2004;84(4):1315–1339. doi: 10.1152/physrev.00002.2004. [DOI] [PubMed] [Google Scholar]
- 45.Rasar MA, Hammes SR. The physiology of the Xenopus laevis ovary. Methods Mol Biol. 2006;322:17–30. doi: 10.1007/978-1-59745-000-3_2. [DOI] [PubMed] [Google Scholar]
- 46.Gotoh Y, et al. Initiation of Xenopus oocyte maturation by activation of the mitogen-activated protein kinase cascade. J Biol Chem. 1995;270(43):25898–25904. doi: 10.1074/jbc.270.43.25898. [DOI] [PubMed] [Google Scholar]
- 47.Matten WT, et al. Positive feedback between MAP kinase and Mos during Xenopus oocyte maturation. Dev Biol. 1996;179(2):485–492. doi: 10.1006/dbio.1996.0277. [DOI] [PubMed] [Google Scholar]
- 48.Roy LM, et al. Mos proto-oncogene function during oocyte maturation in Xenopus. Oncogene. 1996;12(10):2203–2211. [PubMed] [Google Scholar]
- 49.Gill A, Jamnongjit M, Hammes SR. Androgens promote maturation and signaling in mouse oocytes independent of transcription: a release of inhibition model for mammalian oocyte meiosis. Mol Endocrinol. 2004;18(1):97–104. doi: 10.1210/me.2003-0326. [DOI] [PubMed] [Google Scholar]
- 50.Li M, et al. Testosterone potentially triggers meiotic resumption by activation of intra-oocyte SRC and MAPK in porcine oocytes. Biol Reprod. 2008;79(5):897–905. doi: 10.1095/biolreprod.108.069245. [DOI] [PubMed] [Google Scholar]
- 51.Li M, Schatten H, Sun QY. Androgen receptor's destiny in mammalian oocytes: a new hypothesis. Mol Hum Reprod. 2009;15(3):149–154. doi: 10.1093/molehr/gap006. [DOI] [PubMed] [Google Scholar]
- 52.Neill JD. In: Knobil and Neill's Physiology of Reproduction. 3rd ed. Challis JRG, et al., editors. Vol. 1. Elsevier Academic Press; 2006. [Google Scholar]
- 53.McLachlan RI, et al. Identification of specific sites of hormonal regulation in spermatogenesis in rats, monkeys, and man. Recent Prog Horm Res. 2002;57:149–179. doi: 10.1210/rp.57.1.149. [DOI] [PubMed] [Google Scholar]
- 54.Lyon MF, Glenister PH, Lamoreux ML. Normal spermatozoa from androgen-resistant germ cells of chimaeric mice and the role of androgen in spermatogenesis. Nature. 1975;258(5536):620–622. doi: 10.1038/258620a0. [DOI] [PubMed] [Google Scholar]
- 55.Griswold MD. The central role of Sertoli cells in spermatogenesis. Semin Cell Dev Biol. 1998;9(4):411–416. doi: 10.1006/scdb.1998.0203. [DOI] [PubMed] [Google Scholar]
- 56.Walker WH. Molecular mechanisms of testosterone action in spermatogenesis. Steroids. 2009;74(7):602–607. doi: 10.1016/j.steroids.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 57.Eacker SM, et al. Transcriptional profiling of androgen receptor (AR) mutants suggests instructive and permissive roles of AR signaling in germ cell development. Mol Endocrinol. 2007;21(4):895–907. doi: 10.1210/me.2006-0113. [DOI] [PubMed] [Google Scholar]
- 58.Lindsey JS, Wilkinson MF. Pem: a testosterone- and LH-regulated homeobox gene expressed in mouse Sertoli cells and epididymis. Dev Biol. 1996;179(2):471–484. doi: 10.1006/dbio.1996.0276. [DOI] [PubMed] [Google Scholar]
- 59.Lim K, et al. Testosterone regulation of proto-oncogene c-myc expression in primary Sertoli cell cultures from prepubertal rats. J Androl. 1994;15(6):543–550. [PubMed] [Google Scholar]
- 60.Walker WH. Molecular mechanisms controlling Sertoli cell proliferation and differentiation. Endocrinology. 2003;144(9):3719–3721. doi: 10.1210/en.2003-0765. [DOI] [PubMed] [Google Scholar]
- 61.Shang Y, Myers M, Brown M. Formation of the androgen receptor transcription complex. Mol Cell. 2002;9(3):601–610. doi: 10.1016/s1097-2765(02)00471-9. [DOI] [PubMed] [Google Scholar]
- 62.Fix C, et al. Testosterone activates mitogen-activated protein kinase and the cAMP response element binding protein transcription factor in Sertoli cells. Proc Natl Acad Sci U S A. 2004;101(30):10919–10924. doi: 10.1073/pnas.0404278101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pedram A, et al. A conserved mechanism for steroid receptor translocation to the plasma membrane. J Biol Chem. 2007;282(31):22278–22288. doi: 10.1074/jbc.M611877200. [DOI] [PubMed] [Google Scholar]
- 64.Lu ML, et al. Caveolin-1 interacts with androgen receptor. A positive modulator of androgen receptor mediated transactivation. J Biol Chem. 2001;276(16):13442–13451. doi: 10.1074/jbc.M006598200. [DOI] [PubMed] [Google Scholar]
- 65.Walker WH, Cheng J. FSH and testosterone signaling in Sertoli cells. Reproduction. 2005;130(1):15–28. doi: 10.1530/rep.1.00358. [DOI] [PubMed] [Google Scholar]
- 66.Walker WH. Nongenomic actions of androgen in Sertoli cells. Curr Top Dev Biol. 2003;56:25–53. doi: 10.1016/s0070-2153(03)01006-8. [DOI] [PubMed] [Google Scholar]
- 67.Cheng J, Watkins SC, Walker WH. Testosterone activates mitogen-activated protein kinase via Src kinase and the epidermal growth factor receptor in sertoli cells. Endocrinology. 2007;148(5):2066–2074. doi: 10.1210/en.2006-1465. [DOI] [PubMed] [Google Scholar]
- 68.Scobey M, et al. Delivery of a cyclic adenosine 3',5'-monophosphate response element-binding protein (creb) mutant to seminiferous tubules results in impaired spermatogenesis. Endocrinology. 2001;142(2):948–954. doi: 10.1210/endo.142.2.7948. [DOI] [PubMed] [Google Scholar]
- 69.Lyng FM, Jones GR, Rommerts FF. Rapid androgen actions on calcium signaling in rat sertoli cells and two human prostatic cell lines: similar biphasic responses between 1 picomolar and 100 nanomolar concentrations. Biol Reprod. 2000;63(3):736–747. doi: 10.1095/biolreprod63.3.736. [DOI] [PubMed] [Google Scholar]
- 70.Loss ES, et al. Testosterone modulates K(+)ATP channels in Sertoli cell membrane via the PLC-PIP2 pathway. Horm Metab Res. 2004;36(8):519–525. doi: 10.1055/s-2004-825753. [DOI] [PubMed] [Google Scholar]
- 71.Gorczynska E, Handelsman DJ. Androgens rapidly increase the cytosolic calcium concentration in Sertoli cells. Endocrinology. 1995;136(5):2052–2059. doi: 10.1210/endo.136.5.7720654. [DOI] [PubMed] [Google Scholar]
- 72.Radmayr C, et al. 5-alpha-reductase and the development of the human prostate. Indian J Urol. 2008;24(3):309–312. doi: 10.4103/0970-1591.42610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carson C, 3rd, Rittmaster R. The role of dihydrotestosterone in benign prostatic hyperplasia. Urology. 2003;61(4 Suppl 1):2–7. doi: 10.1016/s0090-4295(03)00045-1. [DOI] [PubMed] [Google Scholar]
- 74.Berger R, et al. Androgen-induced differentiation and tumorigenicity of human prostate epithelial cells. Cancer Res. 2004;64(24):8867–8875. doi: 10.1158/0008-5472.CAN-04-2938. [DOI] [PubMed] [Google Scholar]
- 75.Gioeli D, et al. Androgen receptor phosphorylation. Regulation and identification of the phosphorylation sites. J Biol Chem. 2002;277(32):29304–29314. doi: 10.1074/jbc.M204131200. [DOI] [PubMed] [Google Scholar]
- 76.Foradori CD, Weiser MJ, Handa RJ. Non-genomic actions of androgens. Front Neuroendocrinol. 2008;29(2):169–181. doi: 10.1016/j.yfrne.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Taplin ME, Balk SP. Androgen receptor: a key molecule in the progression of prostate cancer to hormone independence. J Cell Biochem. 2004;91(3):483–490. doi: 10.1002/jcb.10653. [DOI] [PubMed] [Google Scholar]
- 78.Watson PA, et al. Constitutively active androgen receptor splice variants expressed in castration-resistant prostate cancer require full-length androgen receptor. Proc Natl Acad Sci U S A. 2010;107(39):16759–16765. doi: 10.1073/pnas.1012443107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hoimes CJ, Kelly WK. Redefining hormone resistance in prostate cancer. Ther Adv Med Oncol. 2010;2(2):107–123. doi: 10.1177/1758834009356433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shen MM, Abate-Shen C. Molecular genetics of prostate cancer: new prospects for old challenges. Genes Dev. 2010;24(18):1967–2000. doi: 10.1101/gad.1965810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Migliaccio A, et al. Steroid-induced androgen receptor-oestradiol receptor beta-Src complex triggers prostate cancer cell proliferation. EMBO J. 2000;19(20):5406–5417. doi: 10.1093/emboj/19.20.5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Migliaccio A, et al. Inhibition of the SH3 domain-mediated binding of Src to the androgen receptor and its effect on tumor growth. Oncogene. 2007;26(46):6619–6629. doi: 10.1038/sj.onc.1210487. [DOI] [PubMed] [Google Scholar]
- 83.Migliaccio A, et al. Crosstalk between EGFR and extranuclear steroid receptors. Ann N Y Acad Sci. 2006;1089:194–200. doi: 10.1196/annals.1386.006. [DOI] [PubMed] [Google Scholar]
- 84.Sen A, et al. Paxillin regulates androgen- and epidermal growth factor-induced MAPK signaling and cell proliferation in prostate cancer cells. J Biol Chem. 2010;285(37):28787–28795. doi: 10.1074/jbc.M110.134064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pi M, Parrill AL, Quarles LD. GPRC6A mediates the non-genomic effects of steroids. J Biol Chem. 2010;285(51):39953–39964. doi: 10.1074/jbc.M110.158063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nair SS, et al. Proline-, glutamic acid-, and leucine-rich protein-1/modulator of nongenomic activity of estrogen receptor enhances androgen receptor functions through LIM-only coactivator, four-and-a-half LIM-only protein 2. Mol Endocrinol. 2007;21(3):613–624. doi: 10.1210/me.2006-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Unni E, et al. Changes in androgen receptor nongenotropic signaling correlate with transition of LNCaP cells to androgen independence. Cancer Res. 2004;64(19):7156–7168. doi: 10.1158/0008-5472.CAN-04-1121. [DOI] [PubMed] [Google Scholar]
- 88.Barletta F, et al. Characterization of the interactions of estrogen receptor and MNAR in the activation of cSrc. Mol Endocrinol. 2004;18(5):1096–1108. doi: 10.1210/me.2003-0335. [DOI] [PubMed] [Google Scholar]
- 89.Bennett N, et al. Androgen receptor and caveolin-1 in prostate cancer. IUBMB Life. 2009;61(10):961–970. doi: 10.1002/iub.244. [DOI] [PubMed] [Google Scholar]
- 90.Yang G, et al. Elevated caveolin-1 levels in African-American versus white-American prostate cancer. Clin Cancer Res. 2000;6(9):3430–3433. [PubMed] [Google Scholar]
- 91.Mouraviev V, et al. The role of caveolin-1 in androgen insensitive prostate cancer. J Urol. 2002;168(4 Pt 1):1589–1596. doi: 10.1016/S0022-5347(05)64526-0. [DOI] [PubMed] [Google Scholar]
- 92.Bonaccorsi L, et al. Prostate cancer: a model of integration of genomic and non-genomic effects of the androgen receptor in cell lines model. Steroids. 2008;73(9–10):1030–1037. doi: 10.1016/j.steroids.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 93.Timme TL, et al. Caveolin-1 is regulated by c-myc and suppresses c-myc-induced apoptosis. Oncogene. 2000;19(29):3256–3265. doi: 10.1038/sj.onc.1203654. [DOI] [PubMed] [Google Scholar]
- 94.Li L, et al. Caveolin-1 mediates testosterone-stimulated survival/clonal growth and promotes metastatic activities in prostate cancer cells. Cancer Res. 2001;61(11):4386–4392. [PubMed] [Google Scholar]
- 95.Vlahopoulos S, et al. Recruitment of the androgen receptor via serum response factor facilitates expression of a myogenic gene. J Biol Chem. 2005;280(9):7786–7792. doi: 10.1074/jbc.M413992200. [DOI] [PubMed] [Google Scholar]
- 96.Sinha-Hikim I, et al. Testosterone-induced increase in muscle size in healthy young men is associated with muscle fiber hypertrophy. Am J Physiol Endocrinol Metab. 2002;283(1):E154–E164. doi: 10.1152/ajpendo.00502.2001. [DOI] [PubMed] [Google Scholar]
- 97.Hall J, et al. Selective inhibition of L-type Ca2+ channels in A7r5 cells by physiological levels of testosterone. Endocrinology. 2006;147(6):2675–2680. doi: 10.1210/en.2005-1243. [DOI] [PubMed] [Google Scholar]
- 98.Vicencio JM, et al. Testosterone induces an intracellular calcium increase by a nongenomic mechanism in cultured rat cardiac myocytes. Endocrinology. 2006;147(3):1386–1395. doi: 10.1210/en.2005-1139. [DOI] [PubMed] [Google Scholar]
- 99.Bai CX, et al. Nontranscriptional regulation of cardiac repolarization currents by testosterone. Circulation. 2005;112(12):1701–1710. doi: 10.1161/CIRCULATIONAHA.104.523217. [DOI] [PubMed] [Google Scholar]
- 100.Estrada M, et al. Testosterone stimulates intracellular calcium release and mitogen-activated protein kinases via a G protein-coupled receptor in skeletal muscle cells. Endocrinology. 2003;144(8):3586–3597. doi: 10.1210/en.2002-0164. [DOI] [PubMed] [Google Scholar]
- 101.Hamdi MM, Mutungi G. Dihydrotestosterone activates the MAPK pathway and modulates maximum isometric force through the EGF receptor in isolated intact mouse skeletal muscle fibres. J Physiol. 2010;588(Pt 3):511–525. doi: 10.1113/jphysiol.2009.182162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gonzalez-Montelongo MC, et al. Androgens induce nongenomic stimulation of colonic contractile activity through induction of calcium sensitization and phosphorylation of LC20 and CPI-17. Mol Endocrinol. 2010;24(5):1007–1023. doi: 10.1210/me.2009-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vanderschueren D, et al. Androgens and bone. Endocr Rev. 2004;25(3):389–425. doi: 10.1210/er.2003-0003. [DOI] [PubMed] [Google Scholar]
- 104.Kousteni S, et al. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell. 2001;104(5):719–730. [PubMed] [Google Scholar]
- 105.Kousteni S, et al. Reversal of bone loss in mice by nongenotropic signaling of sex steroids. Science. 2002;298(5594):843–846. doi: 10.1126/science.1074935. [DOI] [PubMed] [Google Scholar]
- 106.Almeida M, et al. Oxidative stress stimulates apoptosis and activates NF-kappaB in osteoblastic cells via a PKCbeta/p66shc signaling cascade: counter regulation by estrogens or androgens. Mol Endocrinol. 2010;24(10):2030–2037. doi: 10.1210/me.2010-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Benten WP, et al. Testosterone signaling through internalizable surface receptors in androgen receptor-free macrophages. Mol Biol Cell. 1999;10(10):3113–3123. doi: 10.1091/mbc.10.10.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Benten WP, et al. Functional testosterone receptors in plasma membranes of T cells. FASEB J. 1999;13(1):123–133. doi: 10.1096/fasebj.13.1.123. [DOI] [PubMed] [Google Scholar]
- 109.Rife SU, et al. The effect of testosterone on the immune response. 1. Mechanism of action on antibody-forming cells. Immunol Invest. 1990;19(3):259–270. doi: 10.3109/08820139009041841. [DOI] [PubMed] [Google Scholar]
- 110.Benten WP, Wunderlich F, Mossmann H. Testosterone-induced suppression of self-healing Plasmodium chabaudi malaria: an effect not mediated by androgen receptors? J Endocrinol. 1992;135(3):407–413. doi: 10.1677/joe.0.1350407. [DOI] [PubMed] [Google Scholar]
- 111.Wunderlich F, et al. Testosterone signaling in T cells and macrophages. Steroids. 2002;67(6):535–538. doi: 10.1016/s0039-128x(01)00175-1. [DOI] [PubMed] [Google Scholar]
- 112.Guo Z, et al. Nongenomic testosterone calcium signaling. Genotropic actions in androgen receptor-free macrophages. J Biol Chem. 2002;277(33):29600–29607. doi: 10.1074/jbc.M202997200. [DOI] [PubMed] [Google Scholar]
- 113.Benten WP, et al. Testosterone induces Ca2+ influx via non-genomic surface receptors in activated T cells. FEBS Lett. 1997;407(2):211–214. doi: 10.1016/s0014-5793(97)00346-3. [DOI] [PubMed] [Google Scholar]
- 114.Liu L, et al. Testosterone induced Ca2+ influx in bone marrow-derived macrophages via surface binding sites. Methods Find Exp Clin Pharmacol. 2005;27(9):623–628. doi: 10.1358/mf.2005.27.9.939336. [DOI] [PubMed] [Google Scholar]