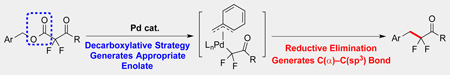
Abstract
SN1 and SN2-like alkylation reactions of ketone enolates with sp3-based electrophiles are essential transformations for generating α-substituted ketone products. However, alkylation reactions of weakly nucleophilic α,α-difluoroketone enolates with sp3-hybridized electrophiles remain nearly unexplored, even for activated benzylic electrophiles. To generate the key C(α)–C(sp3) bond, we report a Pd-catalyzed decarboxylative benzylation reaction of α,α-difluoroketone enolates, in which this bond is generated by reductive elimination from a Pd(II) intermediate. The transformation provides convergent access to α-benzyl-α,α-difluoroketone-based products, and should be useful for accessing biological probes.
Keywords: benzylation; palladium; chemoselectivity; α,α-difluoroketone; synthetic methods
Graphical Abstract
Alkylation reactions of α,α-difluoroketone enolates with sp3-based electrophiles have been underdeveloped, because of intrinsically weak nucleophilicity and chemoselective formation associated with this enolate. Herein, we report a Pd-catalyzed decarboxylative benzylation of α,α-difluoroketone enolates, in which the external α,α-difluoroenolate formed in situ underwent reductive elimination from a Pd(II) intermediate to generate the key C(α)–C(sp3) bond.
The α,α-difluoroketone is a privileged substructure in medicinal chemistry.[1] For this substructure, the electron-withdrawing fluorine atoms encourage rehybridization of the sp2-hybridized C=O to form sp3-hybridized hydrates or hemihydrates,[2] which can then interact with aspartyl proteases via non-covalent H-bonding networks involving water molecules, and with serine proteases via reversible covalent interactions.[1] In addition, the subset of α-benzyl-α,α-difluoroketone derivatives also have demonstrated cholesterol-lowering,[3] analgesic,[4] anxiolytic,[5] and pro-inflammatory[6] activities (Figure 1). Thus, convergent and mild strategies for accessing α-benzyl-α,α-difluoroketone-based substructures should be useful for developing new therapeutic candidates and biological probes.
Figure 1.
α-Benzyl-α,α-Difluoroketone Motifs in Bioactive Molecules.
A convergent preparation of this substructure would involve a transformation capable of generating the C(α)–C(sp3) bond, presumably by reacting a nucleophilic α,α-difluoroketone enolate with an sp3-hybridized benzylic electrophile (Figure 2A). Alkylation of ketone enolates with sp3-based electrophiles is a fundamental transformation for accessing a broad spectrum of α-functionalized ketones.[7] However, nucleophilic substitution reactions of α,α-difluorinated enolates with sp3-based electrophiles have not been generally developed, because of two problems. First, chemoselective formation of α,α-difluoroketone enolates presents challenges, because deprotonation of α,α-difluoromethyl ketones produces enolates at the non-fluorinated position under both thermodynamic and kinetic conditions (Figure 2B),[8] which upon trapping, cannot afford α-functionalized-α,α-difluoroketones. Second, α,α-difluoroenolates possess unique physicochemical properties that preclude formation of the C(α)–C(sp3) bond. Specifically, the strong inductive effect of the two fluorine atoms[9a] decreases the charge density of an enolate at the α-position,[9b] which reduces the nucleophilicity of the anion and disfavors reactions with sp3-based electrophiles (Figure 2C). As a result, α,α-difluoroketone enolates react via SN2 reactions at the O atom to generate difluorovinyl ethers, instead of at C(α) (Figure 2D).[10] Because of these two factors, only two manuscripts describe SN1- or SN2-like alkylation reactions of α,α-difluoroketones, both of which require stoichiometric metal reagents to promote the reactions (Figure 2E).[11]
Figure 2.
Underdeveloped Reactions of α,α-Difluoroketone Enolates with sp3-Hybridized Electrophiles.
Because of this intrinsically poor reactivity, several alternative strategies for accessing α-benzyl-α,α-difluoroketones have been developed, including: 1) deoxyfluorination of α-ketoesters using strong fluorinating reagents, followed by addition of organolithium or Grignard reagents to the resulting α,α-difluoroester, for which the strong bases and harsh reagents destroy many functional groups;[3] 2) 1,2-addition of α-lithio-β,β-difluorovinyl ethers to aldehydes followed by deoxygenation (or cyclization) of the resulting alcohol, which only accesses a small subset of products;[12] 3) a single radical addition reaction of an aldehyde to a (2,2-difluorovinyl)benzene;[13] and 4) a late-stage electrophilic difluorination of prefunctionalized imines using Selectfluor/NFSI followed by acid-mediated hydrolysis, which is not a convergent strategy, and which generates a mixture of fluorinated products for substrates bearing two sites capable of undergoing imine-enamine isomerization.[14] However, none of these reactions convergently generate the key C(α)–C(sp3) bond.
To complement these known strategies, a desirable and convergent alternative for accessing α-benzyl-α,α-difluoroketones involves the net reaction of an α,α-difluoroketone enolate with an sp3-based electrophile (Figure 2A). Although recently reported reactions have coupled α,α-difluoroketone enolates with aryl[15] and allyl electrophiles,[16] never has a Pd-based catalytic system effectively promoted the benzylation reaction of α,α-difluoroketone enolates (Table 1A). In this reaction, a decarboxylative strategy would chemoselectively generate the appropriate α,α-difluoroenolate, and the critical C(α)–C(sp3) bond would form by reductive elimination from a high-energy LnPd(benzyl)(α, α-difluoroenolate) intermediate (Table 1B, A). Herein, we report such a Pd-catalyzed process that accomplishes a net benzylation of α,α-difluoroketone enolates to provide α-benzyl-α,α-difluoroketones.
Table 1.
Pd-Catalyst Promotes Decarboxylative Benzylation Reaction of α,α-Difluoroenolates.[a]
 | ||||
|---|---|---|---|---|
| entry | X1, X2 | conversion (%) | NMR yield (%)[b] | yield (%) |
| 1 | F, F (1a) | 100% | 90% | 80% |
| 2 | H, F (1a') | 8% | 0% | 0% |
| 3 | H, H (1a") | 0% | 0% | 0% |
Standard reaction conditions: 1a–1a″ (1.0 equiv), Pd(PPh3)4 (2.5 mol%), o-xylene (0.05 M), 120 °C, 15 h. The reported data represents an average of two independent experiments.
The yields were determined by 19F NMR using α,α,α-trifluorotoluene and fluorobenzene (1a and 1a') and by 1H NMR using CH2Br2 (1a″) as an internal standard.
Thorough and systematic screening of Pd-based catalysts and precatalysts, and P-based ligands identified Pd(PPh3)4 as an efficient catalyst for the present reaction.[17] Additionally, certain biarylmonophosphine-based ligands derived from S-Phos, X-Phos and Ru-Phos scaffolds[18] also generated the coupled product.[17] After optimization, the final system [2.5% Pd(PPh3)4/o-xylene/120 °C] readily generated the desired α-benzyl-α,α-difluoroketone (Table 1B, entry 1), confirming our hypothesis that transition metal catalysis should form the critical C(α)–C(sp3) bond.
However, this catalyst system only coupled the α,α-difluorinated substrate, while the mono- and non-fluorinated substrates did not provide the expected products (Table 1B, entries 2–3). This dramatic fluorine effect facilitated the present reaction with neutral and even electron-deficient benzyl esters (vide infra), while some other transformations involving oxidative addition of Pd(PPh3)4 into non-fluorinated benzyl esters typically require an extended conjugated system or an electron-rich benzylic moiety.[19] This phenomenon likely reflects the strong σ-withdrawing inductive effect of the two fluorine atoms, which increases the electrophilicity of the substrate, and accelerates the oxidative addition step to generate the high-energy dearomatized π-benzyl intermediate (A, Table 1B).[20] In contrast, we believe that the fluorine substituents likely do not accelerate the decarboxylation step of the reaction. Despite the increased stability of the α,α-difluorinated enolate (ketone–CF2H pKa = 20.2; ketone–CFH2 pKa = 21.7; ketone–CH3 pKa = 24.7),[21], rehybridization of α,α-difluorinated enolate carbanions from C(sp3) to C(sp2) actually occurs more slowly than non-fluorinated enolates,[22] which contradicts the trend observed (Table 1B).
A variety of substrates bearing electron-rich, -neutral, and -deficient benzylic moieties underwent the decarboxylative reaction to generate α-benzyl-α,α-difluoroketones (Table 2). Generally, the optimized conditions converted electron-rich, -neutral, and -weakly deficient substrates into products 4a–h in high yields. However, moderately electron-deficient substrates required higher catalyst loading and/or reaction temperatures to provide good yields of products 4i–j. Further, substrates bearing strong electron-withdrawing groups were less active, and generated products 4k–l in lower yields, even after optimization. This trend implicates the intermediacy of Pd-(π-benzyl)(α,α-difluoroenolate) (Table 1B, A),[20] as electron-donating groups stabilize the intermediate and facilitate the reaction, and electron-withdrawing groups destabilize the intermediate and retard the reaction. While the electronic nature of substrates affected the outcome of the reaction, steric effects did not impede the reaction; reactions of ortho-substituted benzyl esters afforded products in comparably high yields to the analogous para-substituted substrates (4m–n vs. 4a–b). Further, N-containing heterobenzyl substrates also tolerated the present reaction conditions, and provided the corresponding products in modest-to-high yields (4o–p).
Table 2.
Decarboxylative Difluorobenzylation of Substrates Bearing Distinct Benzyl Moieties.[a]
Standard reaction conditions: 3a–p (1.0 equiv), Pd(PPh3)4 (2.5 mol%), o-xylene (0.05 M), 120 °C, 15 h. 19F NMR Yields were determined using α,α,α-trifluorotoluene as an internal standard. The value in parentheses indicates the isolated yield.
24 h.
Pd(PPh3)4 (5.0 mol%).
Pd(PPh3)4 (5.0 mol%), 140 °C.
Pd(PPh3)4 (20 mol%), 140 °C, o-xylene (0.01 M), 24 h.
Pd(PPh3)4 (5.0 mol%), 140 °C, 24 h.
140 °C, 16 h.
PdCp(η3-1-Ph-C3H4) (2.0 mol%), PhXPhos (4.0 mol %), 155 °C, 15 h.
The decarboxylative reaction also successfully produced products bearing a variety of aryl- and alkyl α,α-difluoroketone moieties (Table 3). Reactions of substrates bearing electron-rich, -neutral and -withdrawing aryl α,α-difluoroketones provided the corresponding products 6a–c in high yields under the standard conditions. Further, both S- and N-containing heteroaryl α,α-difluoroketone moieties were tolerated (6d–e), and at an increased temperature, the reaction of an aliphatic α,α-difluoroketone substrate afforded product (6f) in reasonable yield.
Table 3.
Decarboxylative Difluorobenzylation of Substrates Bearing Distinct Ketone Moieties.[a]
Standard reaction conditions: 5a–f (1.0 equiv), Pd(PPh3)4 (2.5 mol%), o-xylene (0.05 M), 120 °C, 15 h. 19F NMR Yields were determined using α,α,α-trifluorotoluene as an internal standard. The value in parentheses indicates the isolated yield.
130 °C, 24 h.
24 h.
As previously noted, alkylation reactions of α,α-difluoroketone enolates suffer from two classical problems, namely, generation of the appropriate enolate[8] and alkylation at C(α) instead of at O.[10] Although examples 4a–p and 6a–f confirm the ability of the Pd-catalyzed system to generate the C(α)–C(sp3) bond, most of the substrates do not bear enolizable H-atoms at the non-fluorinated α-position of the ketone, and therefore cannot form a non-fluorinated enolate. As such, these substrates do not confirm whether the Pd-catalyzed decarboxylative protocol would selectively generate the fluorinated enolate. To address this concern, we explored the reaction of aliphatic substrate, which could theoretically decarboxylate and isomerize to generate the undesired enolate 8a at non-fluorinated position (Scheme 1). Subjection of 7 to Pd(PPh3)4 at 140 °C, generated product 9 in 53% isolated yield, with no detectable products arising from alkylation at the non-fluorinated position. This reaction, combined with the reaction of substrate 6f, likely proceeds via exclusive participation of Pd-bound external enolate 8b, and does not isomerize to generate the internal enolate 8a. Thus, the present decarboxylative reaction overcomes both previously presented challenges associated with alkylation reactions of α,α-difluoroketone enolates.
Scheme 1.
Decarboxylative Strategy Chemoselectively Generates the Desired Enolate.
In conclusion, a Pd-catalyzed decarboxylative coupling reaction generated an unfavourable enolate and formed a key C(α)–C(sp3) bond. This method facilitated the preparation of α-benzyl-α,α-difluoroketones under neutral conditions, and provided access to molecules bearing sensitive functional groups and N-containing heterocycles. We envision that this strategy should not only provide a straightforward route to access biologically important α-benzyl-α,α-difluoroketone-based compounds, but also enable the development of additional transition metal-catalyzed coupling reactions of functionalized fluoroalkyl anions with sp3-based electrophiles.
Supplementary Material
Acknowledgments
We thank the donors of the Herman Frasch Foundation for Chemical Research (701-HF12), and the National Science Foundation (CHE-1455163) for supporting this work. Additional financial support from the University of Kansas Office of the Provost, Department of Medicinal Chemistry, and General Research Fund (2506008) is gratefully acknowledged. Support for the NMR instrumentation was provided by the NSF Academic Research Infrastructure Grant (9512331), the NSF Major Research Instrumentation Grant (9977422), and the NIH Center Grant (P20 GM103418).
Contributor Information
Ming-Hsiu Yang, Department of Medicinal Chemistry, the University of Kansas, 1251 Wescoe Hall Drive, Lawrence, Kansas 66045, United States.
Jordan R. Hunt, Department of Medicinal Chemistry, the University of Kansas, 1251 Wescoe Hall Drive, Lawrence, Kansas 66045, United States
Dr. Niusha Sharifi, Department of Medicinal Chemistry, Faculty of Pharmacy, Tehran University of Medical Science, 16 Azar St., Tehran 1417614411, Iran
Prof. Dr. Ryan A. Altman, Email: raaltman@ku.edu, Department of Medicinal Chemistry, the University of Kansas, 1251 Wescoe Hall Drive, Lawrence, Kansas 66045, United States.
References
- 1.(a) Bégué J-P, Bonnet-Delpon D. Bioorganic and Medicinal Chemistry of Fluorine. Chapter 7. Hoboken, NJ: John Wiley & Sons, Inc.; 2008. Inhibition of Enzymes by Fluorinated Compounds; pp. 246–256. [Google Scholar]; (b) Ojima I, editor. Fluorine in Medicinal Chemistry and Chemical Biology. West Sussex, UK: Wiley; 2009. pp. 29–31. [Google Scholar]
- 2.(a) Guthrie JP. Can. J. Chem. 1975;53:898–905. [Google Scholar]; (b) Braendlin HP, McBee ET. In: Advances in Fluorine Chemistry. Stacey M, Tatlow JC, Sharp AG, editors. Vol. 3. London: Butterworths; 1993. p. 1. [Google Scholar]
- 3.Dreyer GB, Metcalf BW. Tetrahedron Lett. 1988;29:6885–6888. [Google Scholar]
- 4.Hamer RL, Freed B, Allen RC. U.S. 5006563. Hoechst-Roussel Pharmaceuticals. 1991
- 5.Han C, Salyer AE, Kim EH, Jiang X, Jarrard RE, Powers MS, Kirchhoff AM, Salvador TK, Chester JA, Hockerman GH, Colby DA. Med. Chem. 2013;56:2456–2465. doi: 10.1021/jm301805e. [DOI] [PubMed] [Google Scholar]
- 6.Banville J, Remillard R, Balasubramanian N, Bouthillier G, Martel A. U.S. 20020037875A1. Bristol-Myers Squibb. 2002
- 7.(a) Stolz D, Kazmaier U. Metal Enolates as Synthons in Organic Chemistry. In: Zabicky J, editor. Chemistry of Metal Enolates. Chapter 7. London, UK: John Wiley & Sons, Ltd.; 2009. pp. 355–409. [Google Scholar]; (b) Stoltz BM, Bennett NB, Duquette DC, Goldberg AFG, Liu Y, Loewinger MB, Reeve CM. In: Comprehensive Organic Synthesis II (Second Edition) Knochel P, Molander GA, editors. Vol. 3. Elsevier, English; 2014. pp. 1–55. [Google Scholar]
- 8.Yamana M, Ishihara T, Ando T. Tetrahedron Lett. 1983;24:507–510. [Google Scholar]; (b) Kuroboshi M, Ishihara T. Bull. Chem. Soc. Jpn. 1990;63:428–437. [Google Scholar]; (c) Liu Y-L, Zhou J. Chem. Commun. 2012;48:1919–1921. doi: 10.1039/c2cc17140f. [DOI] [PubMed] [Google Scholar]
- 9.(a) Uneyama K. Organofluorine Chemistry. Chapter 1. Oxford, UK: Blackwell; 2006. Fundamentals in Organic Fluorine Chemistry; p. 10. [Google Scholar]; (b) Qian C-P, Nakai T. J Am. Chem. Soc. 1990;112:4602–4604. [Google Scholar]
- 10.Qian C-P, Nakai T. Tetrahedron Lett. 1988;29:4119–4122. [Google Scholar]
- 11.(a) Brigaud T, Doussot P, Portella C. J Chem. Soc., Chem. Commun. 1994:2117–2118. [Google Scholar]; (b) Lefebvre O, Brigaud T, Portella C. Tetrahedron. 1999;55:7233–7242. [Google Scholar]; (c) Kobayashi S, Tanaka H, Amii H, Uneyama K. Tetrahedron. 2003;59:1547–1552. [Google Scholar]
- 12.Harrington PE, Li L, Tius MA. J Org. Chem. 1999;64:4025. [Google Scholar]
- 13.Suda M. Tetrahedron Lett. 1981;22:2395–2396. [Google Scholar]
- 14.Pravst I, Zupan M, Stavber S. Synthesis. 2005:3140–3146. [Google Scholar]; (b) Ying W, DesMarteau DD, Gotoh Y. Tetrahedron. 1996;52:15–22. [Google Scholar]
- 15.(a) Guo Y, Shreeve JM. Chem. Commun. 2007:3583–3585. doi: 10.1039/b705137a. [DOI] [PubMed] [Google Scholar]; (b) Guo C, Wang R-W, Qing F-L. J Fluorine Chem. 2012;143:135–142. [Google Scholar]; (c) Ge S, Chaladaj W, Hartwig JF. J Am. Chem. Soc. 2014;136:4149–4152. doi: 10.1021/ja501117v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang M-H, Orsi DL, Altman RA. Angew. Chem. Int. Ed. 2015;54:2361–2365. doi: 10.1002/anie.201410039. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2015;127:2391–2395. [Google Scholar]
- 17.For the detailed screening conditions, please see the supporting information.
- 18.Surry DS, Buchwald SL. Chem. Sci. 2011;2:27. doi: 10.1039/C0SC00331J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.(a) Legros JY, Toffano M, Fiaud JC. Tetrahedron. 1995;51:3235–3246. [Google Scholar]; (b) Torregrosa RP, Ariyarathna Y, Chattopadhyay K, Tunge JA. Am. Chem. Soc. 2010;132:9280–9282. doi: 10.1021/ja1035557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.(a) Weaver JD, Recio A, III, Grenning AJ, Tunge JA. Chem. Rev. 2011;111:1846–1913. doi: 10.1021/cr1002744. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Trost BM, Czabaniuk LC. Angew. Chem. Int. Ed. 2014;53:2826–2851. doi: 10.1002/anie.201305972. [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2014;126:2868–2895. [Google Scholar]
- 21.Bordwell FG. Acc. Chem. Res. 1988;21:456. [Google Scholar]
- 22.Hine J, Mahone LG, Liotta CL. J Am. Chem. Soc. 1967;89:5911. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.