Abstract
Background: After lung and prostate cancers, colorectal cancer (CRC) is the third most common cancer in men and the second most common cancer in women after breast cancer worldwide. Every year, more than one million people are diagnosed with colorectal cancer worldwide and half of these patients die from this disease, making it the fourth leading cause of death in the world. This systematic review aimed to assess the effectiveness of the two colorectal diagnostic tests of FOBT (fecal occult blood test) and FIT (fecal immunochemical test)) in terms of technical performance.
Methods: To retrieve the relevant evidence, appropriate medical databases such as Cochrane library, NHSEED, Scopus and Google scholar were searched from February 2013 to July 2014, using free-texts and Mesh. In this study, inclusion/exclusion criteria of the papers, randomized controlled trials, economic evaluations, systematic reviews, meta-analyses and meta-syntheses of the effectiveness of FIT versus FOBT tests in moderate-risk populations (age: 50 to 70 years), which had reported the least of such outcomes as sensitivity, specificity and clinical outcomes were reviewed. The analyses of the effectiveness outcomes were performed in the form of meta-analysis.
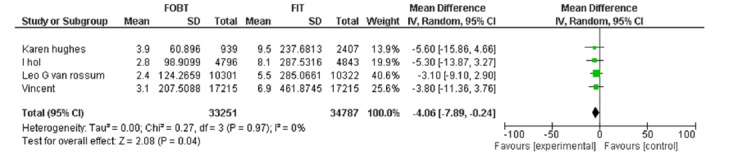
Results: Five papers were eligible to be included in the final phase of the study for synthesis. FIT showed a better performance in participation and positivity rate. Moreover, in terms of false positive and negative rate, FIT showed fewer rates compared to FOBT (RR:-4.06; 95% CI (-7.89-0.24), and NN-scope (Number need to scope) (2.2% vs. 1.6%), and NN-screen (Number need to screen) (84% vs. 31-49% in different cut off levels) showed significant differences in FOBT vs. FIT, respectively.
Conclusion: In the five included studies (3, 11-14), the acceptability of FIT was more than FOBT. However, in our meta-analysis, no difference was found between the two tests. FIT was significant in positivity rate and had a better performance in participation rate, and a fewer false negative numbers compared to FOBT.
Keywords: Neoplasm, FOBT, FIT
Introduction
After lung and prostate cancers, colorectal cancer (CRC) is the third most common cancer in men and the second most common cancer in women after breast cancer worldwide. Every year, more than one million people are diagnosed with colorectal cancer worldwide and half of these patients die from this disease, making it the fourth leading cause of death in the world (1). An appropriate population-based screening program in the early stages of precancerous lesions including early detection and removal of polyps and adenoma will reduce and prevent the incidence and mortality of CRC (2). According to the medical guidelines of the Western countries for screening people at average risk, the first-line screening stool-based method is recommended because of its cost effectiveness, non-invasive nature, good accessibility and patient compatibility (3). Gaiac (FOBT) and safety-chemical method (FIT) are two types of routine stool tests used for initial screening (4). Among the available options, screening with guaiac-based fecal occult blood test (g-FOBT) was associated with a 13–18% CRC-mortality reduction in major randomized studies (5). This mortality reduction was primarily resulted from detecting CRC in early stages (6-7). The immunochemical fecal occult blood test (FIT) has a better sensitivity than g FOBT and a similar specificity to g-FOBT for detecting advanced neoplasia and it specifically uses human hemoglobin for detection(8). Fecal tests have the advantages of being relatively simple and safe screening tests, suitable for a mass screening programs. However, because of the poor sensitivity for premalignant lesions, fecal test needs to be repeated every 1–2 years. Therefore, a high compliance to repeat testing is required to achieve long-term effectiveness with fecal tests (5). The effectiveness of the screening tests depends not only on the sensitivity for colorectal neoplasia, but also on population attendance. Low participation rates of CRC screening tests resulted in weakening of the true efficiency of the test and reducing the overall productivity for advanced neoplasia in the community. The impact of adherence on the eventual effectiveness of any screening strategy has been confirmed by simulation modeling in which showing apparently large differences in efficacy is reversed by small gradients in adherence rates (9-10). Therefore, a societal decision maker confronted with the choice of alternative tests to prevent the CRC incidence and/or mortality, and stated that one should choose the strategy with the most efficient compromise between adherence and efficacy, i.e., the highest effectiveness. Uncertainty in this choice will appear to be mainly related to the technical and procedural differences among the available tests, primarily between the fecal tests on the one hand and endoscopic strategies on the other.
Research Question
Which one of these tests (FOBT vs. FIT) is effective in term of different diagnostic validity indexes?
Study Objectives
This study aimed to assess the effectiveness of colorectal diagnostic tests (FOBT versus FIT) in terms of technical performance and to examine the ethical, organizational, social and legal aspects of this technology in those Iranians at moderate-risk of colorectal cancer.
Methods
Literature Search
This was a systematic review of the literature. Fourteen electronic reference databases were searched. Most major search sites in appropriate medical databases such as Cochrane library, NHSEED, Scopus and Google scholar were searched by proper keywords such as "neoplasm", fecal occult blood test", fecal immunochemical test" from February 2013 to July 2014 using free-texts and Mesh (Appendix 2). Gray literatures were searched via Google, the web sites of the Trial Registers Current Controlled Trial, the National Research Register, and Clinicaltrials.gov. In addition, references of all the included papers were searched to identify any additional relevant studies. Studies without control groups and non-English language studies were excluded. The titles and abstracts of the identified papers were checked to exclude non-relevant studies. The full texts of the remaining articles were checked against the inclusion-exclusion criteria. Papers were controlled independently by two reviewers. The risk of bias was checked by two reviewers independently.
The two reviewers independently screened the articles by title, abstract and full text and they then extracted the full texts of the articles, using a standard data extraction form and consulted a third investigator in cases of any disagreement. Data were extracted by identifying the formation of articles, the study objectives, study design, inclusion and exclusion criteria of the studies, the intervention and control groups, joint interventions, covert interventions, method of randomization, blinding, potential confounding, outcome of the study, statistical analysis, baseline characteristics of patients and outcome events.
Scope
In this study, the inclusion/exclusion criteria of the papers, randomized controlled trials, economic evaluations, systematic reviews, meta-analyses and meta-syntheses of the effectiveness of FIT versus FOBT were reviewed.
PICO Question
Which of the two stool-based colorectal cancer screening tests (FOBT vs. FIT) is more effective to be used for an average-risk population (age: 50 to70 years) in terms of technical performance rates?
Comparators: FOBT or FIT
Study Design: A Meta- analysis
Outcomes: The performance rate of the diagnostic test (sensitivity, specificity, positive predictive value, negative predictive value).
Quality Appraisal Method
The quality appraisal of the included RCTs was performed using JADAD checklist to evaluate the confounding factors used for RCTs apart from the clinical trials (Oxford quality scoring system, and independent evaluation of methodological quality of clinical trials). The analysis of technical performance outcomes was performed in the form of meta-analysis via the Rev Man software (Version 5.3).
Results
In the first phase, 1,737 papers were retrieved; of them, 333 were duplicated, so they were excluded. From the 1,404 remaining papers, after checking the titles and abstracts, 1,339 articles did not meet the inclusion/exclusion criteria, so only 65 papers remained. After reviewing the full texts, 44 articles were excluded because of their poor study design and inadequate control groups, and 21 papers remained in the final phase. All of the 21 articles were assessed with jaded checklist and 16 articles were excluded after reviewing their full texts because their quality was unclear due to not reporting the outcomes and existence of deficiencies both in sequence generation and allocation concealment. Finally, five articles were selected for the final analysis (3,11-14) (Fig. 1) (Appendix 3).
Fig. 1 .
Literature Search Results with PRISMA Flowchart
Four papers got 5 points and one study got 3 based on JADAD items (randomization, blinding and adequate sample size of patients) (Table 1). The five selected studies have been conducted from 2005 to 2013 in the Netherlands, Australia, Germany and France. In all the included studies, population-based screening was performed on the basis of FOBT and FIT kits in men and women in the age range of 75-50 years. Some studies reported test positive if at least one of the six kits had a color change (each panel contains two cards). FIT was positive at different cut off levels of fecal hemoglobin concentration per ml of sample buffer in case the color changed (in response to the hemoglobin molecule present in fecal samples). Most of the studies used FOBT in the form of hem occult non-rehydrated type (Beckman Coulter Inc. USA), and different brands were used for FIT.
Table 1. Quality Appraisal of the Included RCTs .
| Title |
Study Design |
Screening Test | Comparator | JADAD Checklist | |||
| Randomization 2 | Blinding 2 |
An account of all patients 1 |
score | ||||
| Screening for colorectal cancer: Random comp-arison of guaiac and immunochemical faecal occult blood testing at different cut-off levels | Diagnostic (RCT) | Hem occult (Beckman coulter ,Inc .Fullerton, CA, USA) non re hydration | OC-sensor (micro e liken chemical co, Tokyo. Japan) | √ | √ | √ | 5 |
| Guaiac versus immunochemical tests: faecal occult blood test screening for colorectal cancer in a rural community | Diagnostic (RCT) | Hem occult(Beckman coulter, Inc. Fullerton, CA, USA) non re hydration | !form(enter ix) | √ | X | √ | 3 |
| Superior diagnostic performance of faecal immunochemical tests for haemoglobin in a head-to-head comparison with guaiac based faecal occult blood test among 2235 participants of screening colonoscopy | Diagnostic (RCT) | Hem occult, Beckman coulter, Krefeld, Germany) |
1) Rid a screen haemo globin 2) Rid a screen haemo-/ hapto globin complex, R- Biopharm AG, Darmstadt, Germany 3) OC SENSOR,Tokyo, Japan |
√ | √ | √ | 5 |
| Random comparison of guaiac and immuno-chemical fecal occult blood tests for colorectal cancer in a screening population | Diagnostic (RCT) | Hem occult l I (Beckman coulter) | OC- sensor (e liken chemical Co.) | √ | √ | √ | 5 |
| Immunochemical faecal occult blood tests are superior to guaiac-based tests for Detection of colorectal neoplasms | Diagnostic (RCT) | Hem occult II. beck man coulter inc ,fuller ton CA.USA (without rehydration) | Instant. view, alpha scientific designs, Poway, CA, USA | √ | √ | √ | 5 |
A) Analysis of Common Indicators in Final Studies with the Rev Man Software
In this analysis, the significance cut off point was set at 0.05 for the p-value, and normal distribution and random effects model were also assumed.
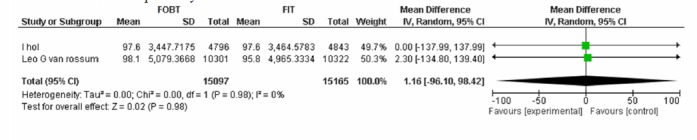
A-1) In the comparative approach of FOBT vs. FIT at hemoglobin cut off level of 50Ng/ml, three studies were included in which the specificity of the two tests was not significantly different in ruling out colorectal cancer(Appendix 4).
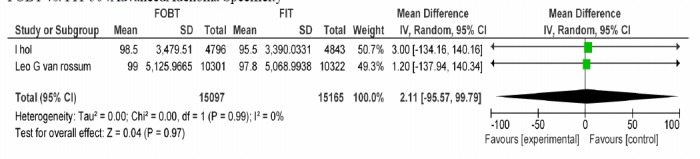
A-2) In the comparative approach of FOBT vs. FIT at hemoglobin cut off level of 50Ng/ml, two studies were included in which specificity of the two tests was not significantly different in ruling out advanced adenoma (Appendix 4).
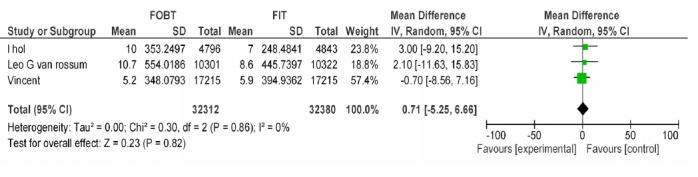
A-3) In the comparative approach of FOBT vs. FIT at hemoglobin cut off level of 50Ng/ml, three studies were included in which the positive predictive value of the two tests was not significantly different in existence of colorectal cancer (Appendix 4).
A-4) In the comparative approach of FOBT vs. FIT at hemoglobin cut off level of 50Ng/ml, three studies were included in which the positive predictive value of the two tests was not significantly different in the existence of advanced adenoma (Appendix 4).
A-5) In the comparative approach of FOBT vs. FIT at hemoglobin cut off level of 50Ng/ml, two studies were included in which the positive predictive value of two tests was not significantly different in the existence of non-advanced adenoma (Appendix 4).
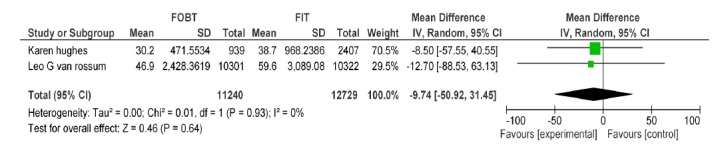
A-6) In the comparative approach of FOBT vs. FIT at hemoglobin cut off level of 50Ng/ml, two studies were included in which participation rates of the two tests were not significantly different (Appendix 4).
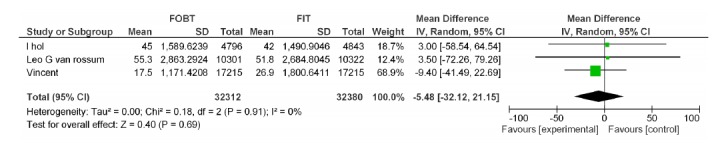
A-7) In the comparative approach of FOBT vs. FIT at hemoglobin cut off level of 50Ng/ml, four studies were included in which the positivity rate of the two tests with heterogeneity tau²=0.00 (p=0.04, MD=-4.06, 95% CI: -7.89, -0.24) was significantly different (Appendix 4).
A-8) In the comparative approach of FOBT vs. FIT at hemoglobin cut off level of 50Ng/ml, three studies were included in which detection rates of the two tests were not significantly different for colorectal cancer (Appendix 4).
A-9) In the comparative approach of FOBT vs. FIT at hemoglobin cut off level of 50Ng/ml, three studies were included in which the detection rates of the two tests were not significantly different for advanced adenoma (Appendix 4).
A-10) In the comparative approach of FOBT vs. FIT at hemoglobin cut off level of 100ng/ml, two studies were included in which the specificity of the two tests was not significantly different for colorectal cancer (Appendix 4).
A-11) In the comparative approach of FOBT vs. FIT at hemoglobin cut off level of 100Ng/ml, two studies were included in which the specificity of the two tests was not significantly different for advanced adenoma (Appendix 4).
A-12) In the comparative approach of FOBT vs. FIT at hemoglobin cut off level of 100Ng/ml, two studies were included in which the positive predictive value of the two tests was not significantly different for colorectal cancer (Appendix 4).
A-13) In the comparative approach of FOBT vs. FIT at hemoglobin cut off level of 100Ng/ml, two studies were included in which the positive predictive value of the two tests was not significantly different for advanced adenoma (Appendix 4).
Discussion
Screening status for moderate-risk groups in Iran is unclear, and the trend of cancer occurrence is seen in a population of younger than40 years of age. Therefore, this study focused on comparing the performance of diagnostic tests to detect CRC based on fecal FOBT and FIT in the first line of treatment. According to the included studies, FIT compared to FOBT, has a better performance in specificity, positivity rate, NN-scope and NN-screening. The results of the five large trials were meaningful in measuring the performance of FOBT and FIT tests (3,11,12,14,15). Interpretation of the test results according to the manufacturers’ instructions may have been affected by the results of each RCT. In some studies, dietary restriction did not apply, or only a sample of three stool samples were used for analysis, and this may give rise to false positive rates and may impose costs and mental burden on the patients. However, some of the references listed in the dietary restrictions lead to a reduction in the amount of positive FOBT test and increase the specificity of FOBT (15). According to the manufacturers’ instructions and the restrictions imposed by The Food and Drug Organization, dietary restrictions impact on the sensitivity and specificity had been reported in various studies, and so the comparison with other studies were difficult. Since the samples were collected in plastic containers, freezed and then defrosted again to be prepared for the analysis process, it was impossible to compare the performance of the two tests for multiple stool samples. In addition, according to some studies, when colonoscopy capacity was limited, FOBT could reduce the demand by limiting the range and extensive screening intervals. Moreover, when colonoscopy capacity was unlimited, the best strategy to screen people at the age of 45 to 80 years was FIT with cutoff 50Ng/cc, and 50 Ng/cc cut off level was recommended for colonoscopy follow- ups for all people with positive adenoma. However, when faced with limited capacity of colonoscopy, the optimal strategy was using FIT with cutoff of 200nm/cc at the range of 50 to 75 years; and consequently, by reducing the follow up rounds of colonoscopy, the demand will decrease (16). The low hemoglobin cut off levels provided a higher detection of advanced neoplasia and it also reduced the number of false-positives, and those that were not in a priority for performing colonoscopy. False-positive results may impose concerns and additional costs for accurate diagnosis. An increase in hemoglobin cut off levels leads to reduction in the detection rate and thus reduces sensitivity. Thereby, increasing the false negatives can progress to metastatic diseases, making it more difficult to be treated and leading to higher costs of the treatment. In one of the included studies, the detection rate of FIT in the cut off of 75Ng/cc was two times higher than FOBT, suggesting that this cut- off point is more favorable in assessing the performance rate. However, the general conclusion based on the meta-analysis was not different between the two tests in terms of detection rate or sensitivity. Detection rate and false positive rate can be considered as an indicator of sensitivity and specificity. Therefore, in our study, no differences were observed between the two indicators. Since most studies used the 75ng/cc cut-off to assess FIT versus FOBT, it can be stated that the results of our study have a good validity for generalization. FIT, compared to FOBT, was more sensitive in detecting CRC, advanced and non-advanced adenomas, considering that it also may contain false positive numbers. Therefore, FIT may be less specific than FOBT. This result was similar to that of the study conducted by Dancourt et al. in 2008. Based on their results, the performance of FIT (sensitivity, specificity, PPV, NPV and likelihood ratios)was better than FOBT (3). In the five included studies (3,11-14), the acceptability of FIT was more than FOBT. However, in our meta-analysis, we detected no difference between the two tests. FIT, compared to FOBT, had fewer false negative rates, and NN-scope (Number need to scope) and NN-screen (Number need to screen) were significantly different between the two tests, but in the final meta-analysis, no significant difference was found between NN-screen and NN-scope.
Conclusion
FIT detected more positive results compared to FOBT and showed fewer false negatives. Also, FIT was a more acceptable test for the participants because it was easy to take because of short sampling times and no food restrictions.
Acknowledgements
This article was derived from a thesis titled: "The Effectiveness of FOBT vs. FIT, which is appropriate: A Meta-Analysis on colorectal cancer screening test" by Maryam Mousavinezhad supervised by Dr AliAkbari Sari and Dr RezaMajdzadeh, that was submitted to the Graduate Studies Office in partial fulfillment of the requirements for the degree of Master in Health Technology Assessment. This study was financially supported by the Vice Chancellor for Research at Tehran University of Medical Sciences (Contract no 240/1548).
Conflict of Interest
There was no conflict of interest.
Appendix
Appendix 1. Characteristics of the Included Trials .
| Study | Country | Population | Range age | Year | Setting | Intervention | Comparator | Outcome | (FIT vs. FOBT) | Jaded |
| Wilschut et al (11) | LH Netherlands | 10011 | 50-75 years | 2009 | At home | FIT | GFOBT | Specificity PPV NNscope¹ NNscreen² Positivity rate Detection rate | Specificity 97.6% (cut off 50ng/cc) 92.9 % (cut off 75ng/cc) VS. FOBT 97.6% (p7lt;0.05) Positivity rate 8.1 (cut off 50 ng/cc) 5.7 (cut off 75 ng/cc) 4.8 (100 ng/cc) 4.1(125ng/cc) 4 (150ng/cc) 3.6 (175 ng/cc) 3.5 (200ng/cc) VS. FOBT 2.8% (p<0.05) | 5 |
| Hughes K et al (12) | Australia | 3358 | 50-75 year | 2005 | At home | FIT | GFOBT | Sensitivity Participation rate Positivity rate | No difference | 3 |
| Brenner H et al (13) | Germany | 2414 | 50-75 year | 2013 | At home | FIT | GFOBT | Specificity Sensitivity PPV NPV | No difference | 5 |
| Vanrossom LG et al (14) | Netherlands | 20623 | 50-75 year | 2008 | At home | FIT | GFOBT | Specificity PPV Intention to screen Participation rate | Intention to screen 5 FIT 0.4% VS. FOBT 0.2% P<0.01 (95% CI 0.3-0.5) | 5 |
| Dancourt V et al (3) | France | 17215 | 50-75 year | 2008 | At home | FIT | GFOBT | Positivity rate Detection rate PPV Positivity rate | No difference | 5 |
Appendix 2. The Search Strategies .
|
Search (((((((colorectal screening [Title/Abstract]) AND fecal occult blood[Title/Abstract]) OR fecal occult[Title/Abstract]) AND fecal immunochemical[Title/Abstract]) OR fecal occult blood test[Title/Abstract]) OR colorectal cancer screening[Title/Abstract]) OR FOBT[Title/Abstract]) OR fecal immonochemical test[Title/Abstract] Search fecal occult blood test[MeSH Terms] Search fecal immonochemical test [MeSH Terms] Search (((fecal immunochemical test) AND fecal occult blood test) AND ((((("Colorectal Neoplasms"[Mesh] OR "Colorectal Neoplasms, Hereditary Nonpolyposis"[Mesh] OR "Lynch Syndrome II"[Mesh])) OR colorectal cancer) OR colorectal neoplasia) OR colorectal neoplasms)) AND ((FIT ) OR "FOBT"[Mesh]) Sort by: Title Search (((("Colorectal Neoplasms"[Mesh] OR "Colorectal Neoplasms, Hereditary Nonpolyposis"[Mesh] OR "Lynch Syndrome II"[Mesh])) OR colorectal cancer) OR colorectal neoplasia) OR colorectal neoplasms |
Appendix 3. Excluded Articles in the Final Step .
| Reason for Exclusion | Address of the Articles in References |
| Descriptive study | 16-17-18-19 |
| Review article | 31-32-33-34-35 |
| Mismatch with PICOD | 42-43-44-45-46-47-48 |
A) Appendix 4. Meta-analysis of Common Indicators in Final Studies with the RevMan Software .
| Study | Strategy | Validity Index | MD. 95% CI | p |
| Wilschut LH Vanrossom LG | FOBT, FIT 50ng/dl | CRC specifity | 1.16[-96.10,98.42] | 0.98 |
| Wilschut LH Vanrossom LG | FOBT, FIT 50ng/dl | Advanced adenoma specifity | 2.11[-95.57,99.79] | 0.97 |
| Wilschut LH Vanrossom LG Dancourt V | FOBT, FIT 50ng/dl | CRC PPV | 0.71[-5.25,6.66] | 0.82 |
| Wilschut LH Vanrossom LG Dancourt V | FOBT, FIT 50ng/dl | Advanced adenoma PPV | -5.48[-32.12,21.15] | 0.69 |
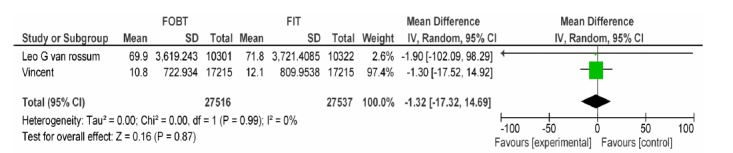
| Vanrossom LG Dancourt V | FOBT, FIT 50ng/dl | Non-Advanced adenoma PPV | -1.32[-17.32,14.69] | 0.87 |
| Hughes k Vanrossom LG | FOBT,FIT 50ng/dl | Participation rate | -9.74[-50.92,31.45] | 0.64 |
| Hughes k Vanrossom LG Wilschut LH Dancourt V | FOBT, FIT 50ng/dl | Positivity rate | -4.06[-7.89,-0.24] | 0.04 |
| Vanrossom LG Wilschut LH | FOBT, FIT 50ng/dl | CRC detection rate | -0.99[-2.08,0.09] | 0.07 |
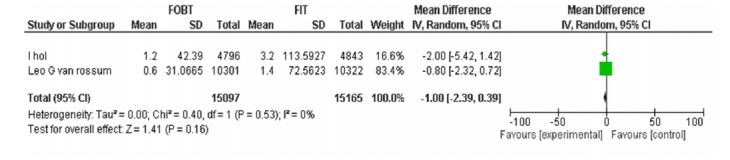
| Vanrossom LG Wilschut LH | FOBT, FIT 50ng/dl Advanced adenoma | -1.00[-2.39,0.39] | 0.16 | |
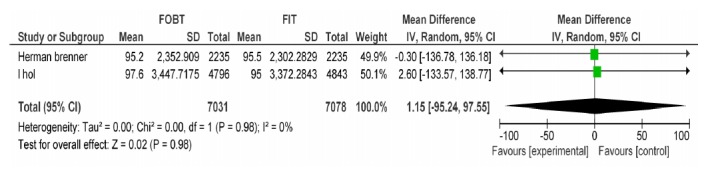
| Brenner H Wilschut LH | FOBT, FIT 100ng/dl | CRC specifity | 1.15[-95.24, 96.55] | 0.99 |
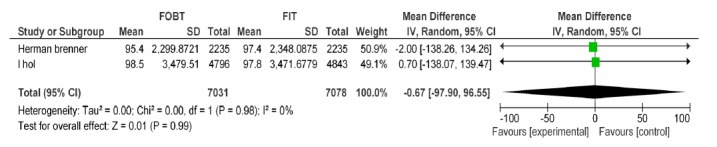
| Brenner H Wilschut LH | FOBT, FIT 100ng/dl | Advanced adenoma specifity | -0.67[-97.90, 96.55] | 0.99 |
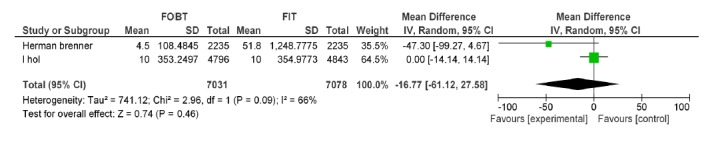
| Brenner H Wilschut LH | FOBT, FIT 100ng/dl | CRC PPV | -16.77[-61.12, 27.58] | 0.46 |
| Brenner H Wilschut LH | FOBT, FIT 100ng/dl | Advanced adenoma PPV | -24.53[-67.43, 18.37] | 0.26 |
FOBTvs.FIT 50: CRC Specificity.
FOBT vs. FIT 50 :AdvancedAdenoma Specificity.
FOBTvs. FIT 50:CRC PPV.
FOBTvs.FIT 50 :Advanced Adenoma PPV.
FOBTvs.FIT 50:Non advanced Adenoma PPV.
FOBTvs.FIT 50:Participation Rate.
FOBT vs. FIT 50: Positivity Rate.
FOBT ǃ FIT 50:CRC Detection Rate.
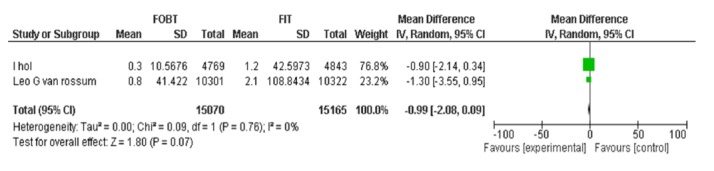
FOBTvs.FIT 50: Advanced Adenoma Detection Rate.
FOBT vs. FIT 100: CRC Specificity.
FOBT vs. FIT 100: Advanced Adenoma Specificity.
FOBT vs. FIT 100: CRC PPV.
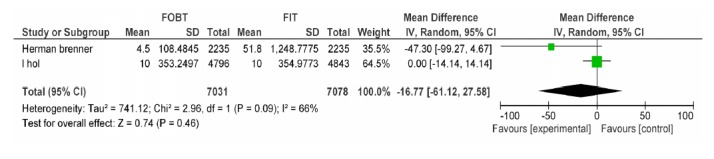
FOBT vs. FIT 100: Advanced Adenoma PPV.
Cite this article as: Mousavinezhad M, Majdzadeh R, Akbari Sari A, Delavari A, Mohtasham F. The effectiveness of FOBT vs. FIT: A meta-analysis on colorectal cancer screening test. Med J Islam Repub Iran 2016 (9 May). Vol. 30:366.
References
- 1.Increased use of colorectal cancer tests -United States, 2002 and 2004. Morbidity and Mortality Weekly Report. 2006;55(11):308–11. [PubMed] [Google Scholar]
- 2.Barouni M, Ghaderi H, Shahmoradi MK. The economic evaluation of screening for colorectal cancer: case of Iran. Clin Lab. 2013;59(5-6):667–74. doi: 10.7754/clin.lab.2012.120812. [DOI] [PubMed] [Google Scholar]
- 3.Dancourt V, Lejeune C, Lepage C, Gailliard MC, Meny B, Faivre J. Immunochemical faecal occult blood tests are superior to guaiac-based tests for the detection of colorectal neoplasms. European Journal of Cancer. 2008;44(15):2254–8. doi: 10.1016/j.ejca.2008.06.041. [DOI] [PubMed] [Google Scholar]
- 4.Davila RE, Rajan E, Baron TH. ASGE guideline: colorectal cancer screening and surveillance. Gastrointestinal endoscopy. 2006;63(4):546–57. doi: 10.1016/j.gie.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Hassan C, Rossi PG, Camilloni L, Rex D, Jimenez‐Cendales B, Ferroni E. et al. Meta-analysis: adherence to colorectal cancer screening and the detection rate for advanced neoplasia, according to the type of screening test. Alimentary pharmacology & therapeutics. 2012;36(10):929–40. doi: 10.1111/apt.12071. [DOI] [PubMed] [Google Scholar]
- 6.Mandel JS, Church TR, Ederer F, Bond JH. Colorectal cancer mortality: effectiveness of biennial screening for fecal occult blood. Journal of the National Cancer Institute. 1999;91(5):434–7. doi: 10.1093/jnci/91.5.434. [DOI] [PubMed] [Google Scholar]
- 7.Hardcastle JD, Chamberlain JO, Robinson MH, Moss SM, Amar SS, Balfour TW. et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. The Lancet. 1996;348(9040):1472–7. doi: 10.1016/S0140-6736(96)03386-7. [DOI] [PubMed] [Google Scholar]
- 8.Allison JE, Tekawa IS, Ransom LJ, Adrain AL. A comparison of fecal occult-blood tests for colorectal-cancer screening. New England Journal of Medicine. 1996;334(3):155–60. doi: 10.1056/NEJM199601183340304. [DOI] [PubMed] [Google Scholar]
- 9.Hassan C, Hunink MM, Laghi A, Pickhardt PJ, Zullo A, Kim DH. et al. Value-of-Information Analysis to Guide Future Research in Colorectal Cancer Screening 1. Radiology. 2009;253(3):745–52. doi: 10.1148/radiol.2533090234. [DOI] [PubMed] [Google Scholar]
- 10.Knudsen AB, Lansdorp-Vogelaar I, Rutter CM, Savarino JE, van Ballegooijen M, Kuntz KM. et al. Cost-effectiveness of computed tomographic colonography screening for colorectal cancer in the medicare population. Journal of the National Cancer Institute. 2010;102(16):1238–52. doi: 10.1093/jnci/djq242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hol L, Wilschut J, van Ballegooijen M, Van Vuuren A, Van Der Valk H, Reijerink J. et al. Screening for colorectal cancer: random comparison of guaiac and immunochemical faecal occult blood testing at different cut-off levels. British journal of cancer. 2009;100(7):1103–10. doi: 10.1038/sj.bjc.6604961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hughes K, Leggett B, Mar CD, Croese J, Fairley S, Masson J. et al. Guaiac versus immunochemical tests: faecal occult blood test screening for colorectal cancer in a rural community. Australian and New Zealand Journal of Public Health. 2005;29(4):358–64. doi: 10.1111/j.1467-842x.2005.tb00207.x. [DOI] [PubMed] [Google Scholar]
- 13.Brenner H, Altenhofen L, Tao S. Matching of controls may lead to biased estimates of specificity in the evaluation of cancer screening tests. J Clin Epidemiol. 2013 Feb;66(2):202–8. doi: 10.1016/j.jclinepi.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 14.van Rossum LG, van Rijn AF, Laheij RJ, van Oijen MG, Fockens P, van Krieken HH. et al. Random comparison of guaiac and immunochemical fecal occult blood tests for colorectal cancer in a screening population. Gastroenterology. 2008 Jul;135(1):82–90. doi: 10.1053/j.gastro.2008.03.040. [DOI] [PubMed] [Google Scholar]
- 15.Brenner H, Tao S. Superior diagnostic performance of faecal immunochemical tests for haemoglobin in a head-to-head comparison with guaiac based faecal occult blood test among 2235 participants of screening colonoscopy. European Journal of Cancer. 2013;49(14):3049–54. doi: 10.1016/j.ejca.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 16.Wilschut JA, Habbema JD, van Leerdam ME, Hol L, Lansdorp-Vogelaar I, Kuipers EJ. et al. Fecal occult blood testing when colonoscopy capacity is limited. J Natl Cancer Inst . 2011 Dec 7;103(23):1741–51. doi: 10.1093/jnci/djr385. [DOI] [PubMed] [Google Scholar]
- 17.Harford WV. Colorectal cancer screening and surveillance. Surgical Oncology Clinics of North America. 2006;15(1):1–20. doi: 10.1016/j.soc.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Stegeman I, de Wijkerslooth TR, Mallant-Hent RC, de Groot K, Stroobants AK, Fockens P. et al. Implementation of population screening for colorectal cancer by repeated Fecal Immunochemical Test (FIT): third round. BMC Gastroenterol. 2012;12:73. doi: 10.1186/1471-230X-12-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Provenzale D. Cost-effectiveness of screening the average-risk population for colorectal cancer. Gastrointestinal Endoscopy Clinics of North America. 2002;12(1):93–109. doi: 10.1016/s1052-5157(03)00061-8. [DOI] [PubMed] [Google Scholar]
- 20.Bretthauer M, Thiis-Evensen E, Huppertz-Hauss G, Gisselsson L, Grotmol T, Skovlund E. et al. NORCCAP (Norwegian colorectal cancer prevention): a randomised trial to assess the safety and efficacy of carbon dioxide versus air insufflation in colonoscopy. Gut. 2002;50(5):604–7. doi: 10.1136/gut.50.5.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choe JW, Chang HS, Yang SK, Myung SJ, Byeon JS, Lee D. et al. Screening colonoscopy in asymptomatic average‐risk Koreans: Analysis in relation to age and sex. Journal of Gastroenterology and Hepatology. 2007;22(7):1003–8. doi: 10.1111/j.1440-1746.2006.04774.x. [DOI] [PubMed] [Google Scholar]
- 22.Ko CW, Dominitz JA, Nguyen TD. Fecal occult blood testing in a general medical clinic: comparison between guaiac-based and immunochemical-based tests. Am J Med. 2003 Aug 1;115(2):111–4. doi: 10.1016/s0002-9343(03)00294-8. [DOI] [PubMed] [Google Scholar]
- 23.Young GP, St John DJ, Winawer SJ, Rozen P. Choice of fecal occult blood tests for colorectal cancer screening: recommendations based on performance characteristics in population studies: a WHO (World Health Organization) and OMED (World Organization for Digestive Endoscopy) report. Am J Gastroenterol. 2002 Oct;97(10):2499–507. doi: 10.1111/j.1572-0241.2002.06046.x. [DOI] [PubMed] [Google Scholar]
- 24.Mandel JS. Screening of patients at average risk for colon cancer. Medical Clinics of North America. 2005;89(1):43–59. doi: 10.1016/j.mcna.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 25.Lieberman D. Screening for Colorectal Cancer in Average-Risk Populations. American Journal of Medicine. 2006;119(9):728–35. doi: 10.1016/j.amjmed.2006.03.037. [DOI] [PubMed] [Google Scholar]
- 26.Ustun C, Ceber E. Ethical issues for cancer screenings Five countries - Four types of cancer. Preventive medicine. 2004;39(2):223–9. doi: 10.1016/j.ypmed.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 27.Zauber AG, Lansdorp-Vogelaar I, Knudsen AB, Wilschut J, van Ballegooijen M, Kuntz KM. Evaluating test strategies for colorectal cancer screening: a decision analysis for the US Preventive Services Task Force. Ann Intern Med. 2008 Nov 4;149(9):659–69. doi: 10.7326/0003-4819-149-9-200811040-00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lieberman D. Colon cancer screening and surveillance controversies. Curr Opin Gastroenterol. 2009 Sep;25(5):422–7. doi: 10.1097/MOG.0b013e32832d1e2a. [DOI] [PubMed] [Google Scholar]
- 29.Lee JH. Screening Test for Colorectal Cancer: Colonoscopy and Fecal Immunochemical Test. The Korean Journal of Gastroenterology. 2012;59(5):389–91. [Google Scholar]
- 30.Segnan N, Senore C, Andreoni B, Azzoni A, Bisanti L, Cardelli A. et al. Comparing attendance and detection rate of colonoscopy with sigmoidoscopy and FIT for colorectal cancer screening. Gastroenterology. 2007;132(7):2304–12. doi: 10.1053/j.gastro.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 31.Nicholson F, Barro J, Atkin W, Lilford R, Patnick J, Williams C. et al. Review article: population screening for colorectal cancer. Alimentary pharmacology & therapeutics. 2005;22(11-12):1069–77. doi: 10.1111/j.1365-2036.2005.02695.x. [DOI] [PubMed] [Google Scholar]
- 32.Carballo F, Munoz-Navas M. Prevention or cure in times of crisis: the case of screening for colorectal cancer. Rev Esp Enferm Dig. 2012 Oct-Nov;104(10):537–45. doi: 10.4321/s1130-01082012001000006. [DOI] [PubMed] [Google Scholar]
- 33.Allen E, Nicolaidis C, Helfand M. The evaluation of rectal bleeding in adults: A cost-effectiveness analysis comparing four diagnostic strategies. Journal of General Internal Medicine. 2005;20(1):81–90. doi: 10.1111/j.1525-1497.2005.40077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chartier L, Arthurs E, Sewitch MJ. Patient satisfaction with colonoscopy: A literature review and pilot study. Canadian Journal of Gastroenterology. 2009;23(3):203–9. doi: 10.1155/2009/903545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pignone M. Is population screening for colorectal cancer cost-effective? Nature Clinical Practice Gastroenterology and Hepatology. 2005;2(7):288–9. doi: 10.1038/ncpgasthep0216. [DOI] [PubMed] [Google Scholar]
- 36.Harewood GC, Lawlor GO, Larson MV. Incident rates of colonic neoplasia in older patients: When should we stop screening? Journal of Gastroenterology and Hepatology. 2006;21(6):1021–5. doi: 10.1111/j.1440-1746.2006.04218.x. [DOI] [PubMed] [Google Scholar]
- 37.Lau P, Sung J. Screening for colorectal cancer. Chinese Journal of Digestive Diseases. 2004;5(3):87–92. doi: 10.1111/j.1443-9573.2004.00153.x. [DOI] [PubMed] [Google Scholar]
- 38.Jin KN, Lee JM, Kim SH, Shin KS, Lee JY, Han JK. et al. The diagnostic value of multiplanar reconstruction on MDCT colonography for the preoperative staging of colorectal cancer. European radiology. 2006;16(10):2284–91. doi: 10.1007/s00330-006-0316-0. [DOI] [PubMed] [Google Scholar]
- 39.Cummings LC, Cooper GS. Editorial: Detection of small polyps: Much ado about nothing. American Journal of Gastroenterology. 2010;105(12):2586–7. doi: 10.1038/ajg.2010.365. [DOI] [PubMed] [Google Scholar]
- 40.Mandel JS, Church TR, Bond JH, Ederer F, Geisser MS, Mongin SJ. et al. The effect of fecal occult-blood screening on the incidence of colorectal cancer. New England Journal of Medicine. 2000;343(22):1603–7. doi: 10.1056/NEJM200011303432203. [DOI] [PubMed] [Google Scholar]
- 41.O’Mahony JF, van Rosmalen J, Zauber AG, van Ballegooijen M. Multicohort Models in Cost-Effectiveness Analysis Why Aggregating Estimates over Multiple Cohorts Can Hide Useful Information. Medical Decision Making. 2013;33(3):407–14. doi: 10.1177/0272989X12453503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heitman SJ. Fecal occult blood testing while waiting for screening colonoscopy in average-risk individuals: Durable option or short-term solution? Canadian Journal of Gastroenterology. 2011;25(5):247. doi: 10.1155/2011/680178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mandel JS. Colorectal cancer screening. Cancer Metastasis Rev. 1997 Sep-Dec;16(3-4):263–79. doi: 10.1023/a:1005848110521. [DOI] [PubMed] [Google Scholar]
- 44.Morikawa T, Kato J, Yamaji Y, Wada R, Mitsushima T, Shiratori Y. A comparison of the immunochemical fecal occult blood test and total colonoscopy in the asymptomatic population. Gastroenterology. 2005 Aug;129(2):422–8. doi: 10.1016/j.gastro.2005.05.056. [DOI] [PubMed] [Google Scholar]
- 45.Woo HY, Mok RS, Park YN, Park DI, Sung IK, Sohn CI. et al. A prospective study of a new immunochemical fecal occult blood test in Korean patients referred for colonoscopy. Clin Biochem. 2005 Apr;38(4):395–9. doi: 10.1016/j.clinbiochem.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 46.Scholefield J, Moss S, Sufi F, Mangham C, Hardcastle J. Effect of faecal occult blood screening on mortality from colorectal cancer: results from a randomised controlled trial. Gut. 2002;50(6):840–4. doi: 10.1136/gut.50.6.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Charlton ME, Mengeling MA, Halfdanarson TR, Makki NM, Malhotra A, Klutts JS. et al. Evaluation of a Home‐Based Colorectal Cancer Screening Intervention in a Rural State. The Journal of Rural Health. 2013 doi: 10.1111/jrh.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheng TI, Wong JM, Hong CF, Cheng SH, Cheng TJ, Shieh MJ. et al. Colorectal cancer screening in asymptomatic adults: comparison of colonoscopy, sigmoidoscopy and fecal occult blood tests. Journal of the Formosan Medical Association. 2002;101(10):685–90. [PubMed] [Google Scholar]
- 49.Pasetto LM, Monfardini S. Colorectal cancer screening in elderly patients: When should be more useful? Cancer Treatment Reviews. 2007;33(6):528–32. doi: 10.1016/j.ctrv.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 50.O'leary BA, Olynyk JK, Neville AM, Platell CF. Cost-effectiveness of colorectal cancer screening: Comparison of community‐based flexible sigmoidoscopy with fecal occult blood testing and colonoscopy. Journal of Gastroenterology and Hepatology. 2004;19(1):38–47. doi: 10.1111/j.1440-1746.2004.03177.x. [DOI] [PubMed] [Google Scholar]
- 51.Rozen P, Knaani J, Samuel Z. Comparative screening with a sensitive guaiac and specific immunochemical occult blood test in an endoscopic study. Cancer . 2000 Jul 1;89(1):46–52. [PubMed] [Google Scholar]
- 52.Smith A, Young GP, Cole SR, Bampton P. Comparison of a brush‐sampling fecal immunochemical test for hemoglobin with a sensitive guaiac‐based fecal occult blood test in detection of colorectal neoplasia. Cancer. 2006;107(9):2152–9. doi: 10.1002/cncr.22230. [DOI] [PubMed] [Google Scholar]
- 53.Sung J, Chan F, Leung WK, Wu J, Lau J, Ching J. et al. Screening for colorectal cancer in Chinese: comparison of fecal occult blood test, flexible sigmoidoscopy, and colonoscopy. Gastroenterology. 2003;124(3):608. doi: 10.1053/gast.2003.50090. [DOI] [PubMed] [Google Scholar]
- 54.Kerr J, Day P, Broadstock M, Weir R, Bidwell S. Systematic review of the effectiveness of population screening for colorectal cancer. New Zealand Medical Journal. 2007;120(1258) [PubMed] [Google Scholar]
- 55.Taupin D, Chambers SL, Corbett M, Shadbolt B. Colonoscopic screening for colorectal cancer improves quality of life measures: a population-based screening study. Health and Quality of life outcomes. 2006;4(1):82. doi: 10.1186/1477-7525-4-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Frommer D. What's new in colorectal cancer screening? Journal of Gastroenterology and Hepatology. 1998;13(5):528–33. doi: 10.1111/j.1440-1746.1998.tb00681.x. [DOI] [PubMed] [Google Scholar]
- 57.Michalek A, Freedman A, Baroni M, Mink I, Rodriguez-Bigas M. Immunochemical versus guaiac occult blood stool tests: results of a community-based screening program. Surgical oncology. 1994;3(1):27–36. doi: 10.1016/0960-7404(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 58.Young GP. Population-based screening for colorectal cancer: Australian research and implementation. J Gastroenterol Hepatol. 2009 Oct;24 Suppl 3:S33–42. doi: 10.1111/j.1440-1746.2009.06069.x. [DOI] [PubMed] [Google Scholar]
- 59.Clavarino AM, Janda M, Hughes KL, Del Mar C, Tong S, Stanton WR. et al. The view from two sides: A qualitative study of community and medical perspectives on screening for colorectal cancer using FOBT. Preventive medicine. 2004;39(3):482–90. doi: 10.1016/j.ypmed.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 60.Wong CS, Sung JY, Tsoi KF, Chan KL, Choi YP, Griffiths SM. A Comparison of the Acceptance of FIT and Colonoscopy in Colorectal Cancer Screening: A Prospective Study Among Chinese. Alimentary Pharmacology and Therapeutics. 2010;32(1):74–82. doi: 10.1111/j.1365-2036.2010.04312.x. [DOI] [PubMed] [Google Scholar]
- 61.Walsh JME, Terdiman JP. Colorectal Cancer Screening: Clinical Applications. Journal of the American Medical Association. 2003;289(10):1297–302. doi: 10.1001/jama.289.10.1297. [DOI] [PubMed] [Google Scholar]
- 62.Anwar S, Hall C, Elder JB. Screening for colorectal cancer: Present, past and future. European Journal of Surgical Oncology. 1998;24(6):477–86. doi: 10.1016/s0748-7983(98)93176-6. [DOI] [PubMed] [Google Scholar]
- 63.Quintero E, Gimeno-García AZ, Salido E. Blood tests for early detection of colorectal cancer. Current Colorectal Cancer Reports. 2010;6(1):30–7. [Google Scholar]
- 64.Federici A, Giorgi Rossi P, Bartolozzi F, Farchi S, Borgia P, Guastcchi G. Survey on colorectal cancer screening knowledge, attitudes, and practices of general practice physicians in Lazio, Italy. Preventive medicine. 2005;41(1):30–5. doi: 10.1016/j.ypmed.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 65.Lansdorp-Vogelaar I, Knudsen AB, Brenner H. Cost-effectiveness of colorectal cancer screening: An overview. Clinical Gastroenterology. 2010;24(4):439, 49. doi: 10.1016/j.bpg.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lansdorp-Vogelaar I, Knudsen AB, Brenner H. Cost-effectiveness of colorectal cancer screening. Epidemiologic reviews. 2011;33(1):88–100. doi: 10.1093/epirev/mxr004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Allison JE, Tekawa IS, Ransom LJ, Adrain AL. A comparison of fecal occult-blood tests for colorectal-cancer screening. N Engl J Med . 1996 Jan 18;334(3):155–9. doi: 10.1056/NEJM199601183340304. [DOI] [PubMed] [Google Scholar]
- 68.Frazier AL, Colditz GA, Fuchs CS, Kuntz KM. Cost-effectiveness of screening for colorectal cancer in the general population. Jama. 2000;284(15):1954–61. doi: 10.1001/jama.284.15.1954. [DOI] [PubMed] [Google Scholar]
- 69.Maciosek MV, Coffield AB, Edwards NM, Flottemesch TJ, Goodman MJ, Solberg LI. Priorities among effective clinical preventive services: results of a systematic review and analysis. American journal of preventive medicine. 2006;31(1):52–61. doi: 10.1016/j.amepre.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 70.Heitman SJ, Hilsden RJ, Au F, Dowden S, Manns BJ. Colorectal cancer screening for average-risk North Americans: an economic evaluation. PLoS Med. 2010;7(11):e1000370. doi: 10.1371/journal.pmed.1000370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guittet L, Bouvier V, Mariotte N, Vallee JP, Levillain R, Tichet J. et al. Comparison of a guaiac and an immunochemical faecal occult blood test for the detection of colonic lesions according to lesion type and location. Br J Cancer . 2009 Apr 21;100(8):1230–5. doi: 10.1038/sj.bjc.6604996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Park DI, Ryu S, Kim YH, Lee SH, Lee CK, Eun CS. et al. Comparison of guaiac-based and quantitative immunochemical fecal occult blood testing in a population at average risk undergoing colorectal cancer screening. Am J Gastroenterol. 2010 Sep;105(9):2017–25. doi: 10.1038/ajg.2010.179. [DOI] [PubMed] [Google Scholar]
- 73.Zhu MM, Xu XT, Nie F, Tong JL, Xiao SD, Ran ZH. Comparison of immunochemical and guaiac-based fecal occult blood test in screening and surveillance for advanced colorectal neoplasms: a meta-analysis. J Dig Dis. 2010 Jun;11(3):148–60. doi: 10.1111/j.1751-2980.2010.00430.x. [DOI] [PubMed] [Google Scholar]