Abstract
Many factors including genetic, environmental, and acquired are involved in breast cancer development across various societies. Among all of these factors in families with a history of breast cancer throughout several generations, genetics, like predisposing genes to develop this disease, should be considered more. Early detection of mutation carriers in these genes, in turn, can play an important role in its prevention. Because this disease has a high prevalence in half of the global population, female screening of reported mutations in predisposing genes, which have been seen in breast cancer patients, seems necessary. In this review, a number of mutations in two predisposing genes (BRCA1 and BRCA2) that occurred in patients with a family history was investigated. We studied published articles about mutations in genes predisposed to breast cancer between 2000 and 2015. We then summarized and classified reported mutations in these two genes to recommend some exons which have a high potential to mutate. According to previous studies, exons have been reported as most mutated exons presented in this article. Considering the large size and high cost of screening all exons in these two genes in patients with a family history, especially in developing countries, the results of this review article can be beneficial and helpful in the selection of exon to screen for patients with this disease.
Keywords: BRCA1 gene, BRCA2 gene, Mutations, Breast cancer
Introduction
Breast cancer is not only the most common malignancy in women throughout the world and constitutes 22.9% of cancer in women, but it is also one of the major causes of death (1-18). The incidence rate varies according to geographic location (5). Studies demonstrate that breast cancer therapeutic course in the younger population is worse than the older one(4). Affected people have a 29% risk until age 50 and a 44% risk until age 70 to be affected by ovarian cancer (3). The major course of breast cancer incidence rate occurs in women after age 50; however, 5% to 12% of this cancer is seen in women under 45 years, is genetic, and proceeds from a mutation in genes predisposed to breast cancer (5,19,20). Hereditary breast cancer is characterized by the following:
Early-onset disease, 2- high incidence of bilateral disease, 3- repetitive correlation with ovarian cancer (21).
Early-onset breast cancer is a hallmark for the existence of genetic predisposing factor (1). Breast cancer molecularly divides to subgroups on the base of cell surface receptors, which are components of human epidermal growth factors and include estrogen receptor (ER), progesterone receptors (PR), and HER-2.
Table 1. In this section, all results of conducted studies, which are investigated in this review article, are classified on the basis of mutation type and location, and being novel or reported.
| BRCA2 mutations | |||
| Novelty | Mutation location |
Mutation type |
References |
| ND | Exon 2 UTR | ND | (2) |
| Novel | Exon2 | SPLICING | (2) |
| Novel | Exon 3 | ND | (43) |
| Novel | Exon 3 | MISSENSE | (42) |
| Novel | Exon7 | ND | (43) |
| ND | Exon10 | ND | (5) |
| Novel | Exon11 | DELETION | (10) |
| Novel | Exon11 | DELETION | (10) |
| Novel | Exon11 | ND | (10) |
| Novel | Exon11 | MISSENSE | (71) |
| ND | Exon11 | ND | (4) |
| ND | Exon11 | ND | (5) |
| ND | Exon11 | ND | (10) |
| Novel | Exon11 | DELETION | (37) |
| Novel | Exon11 | DELETION | (37) |
| Novel | Exon11 | DELETION | (37) |
| Novel | Exon11 | DELETION | (41) |
| Novel | Exon11 | DELETION | (71) |
| Novel | Exon11 | INSERTION | (72) |
| Novel | Exon11 | DUPLICATION | (21) |
| Novel | Exon11 | ND | (10) |
| Novel | Exon11 | ND | (10) |
| Novel | Exon11 | ND | (2) |
| Has been reported | Exon11 | ND | (37) |
| Novel | Exon11 | DELETION | (21) |
| ND | Exon11 | ND | (2) |
| ND | Exon11 | ND | (2) |
| ND | Exon11 | ND | (2) |
| ND | Exon13 | ND | (5) |
| Novel | Exon18 | ND | (43) |
| Novel | Exon23 | DELETION | (2) |
| Novel | Exon11 | DELETION | (7) |
| ND | Exon24 | ND | (5) |
| ND | Exon25 | ND | (5) |
| ND | Exon27 | ND | (5) |
| ND | Exon2 | ND | (73) |
| ND | Exon2 | DELETION | (74) |
| ND | Exons2 and 3 | DELETION | (75) |
| Novel | Exon3 | DELETION AND SPLICING | (10) |
| ND | Exons3 to7 | DELETION | (73) |
| ND | Exon8 | ND | (73) |
| Novel | Exon10 | INSERTION | (41) |
| Novel | Exon10 | INSERTION | (72) |
| Novel | Exon10 | MISSENSE | (72) |
| Novel | Exon11 | ND | (25) |
| Has been reported | Exon11 | ND | (25) |
| ND | Exon11 | ND | (4) |
| ND | Exon11 | ND | (5) |
| Novel | Exon11 | ND | (25) |
| Novel | Exon11 | DELETION | (41) |
| Novel | Exon11 | DELETION | (76) |
| Novel | Exon11 | DELETION | (71) |
| Novel | Exon11 | SPLICING | (71) |
| Novel | Exon11 | SPLICING | (71) |
| Novel | Exon11 | INSERTION | (71) |
| Novel | Exon11 | NONSENSE | (21) |
| Novel | Exon14 | DELETION | (74) |
| ND | Exon16 | ND | (5) |
| Novel | Intron18 | DELETION AND SPLICING | (76) |
| ND | Exons18 and19 | DUPLICATION | (73) |
| ND | Exon19 | INSERTION | (74) |
| Novel | Exon21 | ND | (3) |
| Novel | Intron21 | SPLICING | (25) |
| Has been reported | Exon22 | ND | (25) |
| Novel |
Exon24 |
ND | (25) |
ND: Not Determined
Molecular subtypes of breast cancer
Molecular subtypes of breast cancer may be effective to determine the treatment plan and new therapies. Therefore, many studies have been conducted in this field. Some of the more common subtypes of breast cancer are shown in Figure 1.
Fig. 1 .
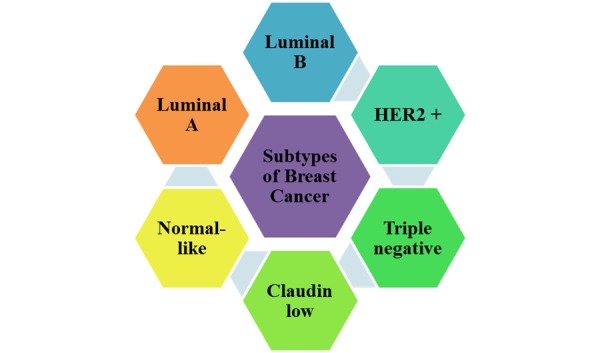
Breast cancer division to 6 major subgroups
Most studies divide breast cancer into six main molecular subgroups that include:
Luminal A, Luminal B, Triple negative (basal-like), HER-2 type, Claudin-low, and normal-like.
Luminal A: Luminal tumor cells are most likely breast cancer cells with an onset in the inner cells lining the breast ducts. Luminal A tumor cells tend to be positive for ER and PR but negative for HER-2, and are present in tumors with grade 1 and 2. Luminal A tumors have the best prognosis, high survival rates, and low recurrence rates.
Luminal B: Contrary to luminal A cells, luminal B tumors tend to be positive for ER and/or PR. Most of these types of tumors are HER-2 positive. It is interesting that compared to luminal A tumors, luminal B tumors are detected at a younger age in women, but due to lower grade tumor, larger tumor size, and lymph node involvement, they have a poorer prognosis.
Triple negative/basal-like: Triple negative breast cancers are negative for the PR, ER, and HER-2, and are therefore called triple negative. There are several categories of this type of breast cancer tumor. One of these categories is called basal-like because this tumor cell type has features similar to outer cells (basal) lining the breast ducts. Approximately 15% to 20% of breast cancers are triple negative or basal-like. These tumors tend to occur in younger and African-American women. It is noteworthy that the majority of breast cancer associated with BRCA1 gene, which is one of the predisposing genes to breast cancer development, is triple negative and basal-like as well. Triple negative/basal-like tumors often are aggressive and have a poorer prognosis.
HER-2 type: HER-2 type tumors tend to be negative for ER and PR, and also accompany lymph node involvement and lower tumor grade. Approximately 10% to 15% of breast cancers are in this subgroup. HER-2 type tumors have a poor prognosis and are prone to repetitive and early recurrence and metastasis. HER-2 type tumors are seemingly detected in women at a younger age than are luminal A or luminal B tumors.
Claudin-low: This type of tumor often is triple negative, but its differences are that in claudin-low tumors, expression of cell-cell adhesion proteins such as E-cadherin is reduced, and lymphocyte infiltration is seen frequently. This type of tumor also has features of mesenchymal cells and stem cells.
Normal-like: Approximately 6% to 10% of all cases of breast cancer are classified as normal-like tumors. These tumors are usually small and prone to have a good prognosis.
Predisposing factors for breast cancer
Ethnic and geographical differences in the incidence of breast cancer demonstrate the effect of environmental conditions and lifestyle. Epidemiological studies have known many risk factors for breast cancer. These non-genetic factors include: early menopause, alcohol and tobacco, exposure to radiation, obesity, decreased physical activity, urbanization, sedentary lifestyle, high fat diet, frequent spontaneous miscarriages, lack of breast-feeding, hormone replacement therapy, aging, geographical location, socio-economic conditions, reproduction events, exogenous hormones, breast density, and family history of breast cancer or other cancers (2,4,22-32). Among the risk factors for breast cancer, family history is the most important (9,33). Therefore, genetic factors are known as the major causes of breast cancer (2,4), and the hereditary risk factor is responsible for 3% to 10% of all breast cancer cases and up to 30% of all early-onset breast cancer (4,32). According to another study, in most western countries, 5% to 10% of all breast cancer cases are due to a main genetic cause (1). Germ-line mutations in BRCA1 and BRCA2 genes are the main part of genetic and hereditary factors for breast and ovarian cancers (6). Generally, BRCA1 and BRCA2 genes are the strongest susceptibility genes for breast cancer (Seong, Cho et al. 2009). Therefore, mutations in BRCA1 and BRCA2 genes are so effective in the increased risk for developing early-onset breast cancer and familial ovarian cancer, that mutations in these two genes are not only responsible for 90% of hereditary breast cancer cases and but also for the majority of hereditary ovarian cancer (4,25,34-39). It is also noteworthy that increased cases of early-onset breast cancer depend on disease-causing mutation in society (7). This type of breast cancer follows autosomal dominant inheritance pattern and tends to occur as an early-onset, high intensity, and bilateral form of the disease. BRCA1 and BRCA2 gene mutation carriers have a higher risk of developing this disease and other cancers, especially ovarian cancer. These mutations are frequently seen in people with a family history (8,21,40-44). In addition to an increased risk of breast cancer, carriers of the mutation in either BRCA1 or BRCA2 genes have an increased risk of other cancers like colon, prostate, pancreatic, melanoma, and gastric cancers (38,45). Therefore, according to the conducted studies, screening for BRCA1 and BRCA2 genes should be proposed for all breast or ovarian cancer patients with a family history of the disease (44).
Global prevalence
Breast cancer is the most common malignancy in women worldwide. This disease constitutes 22.9% of all cancers in women and is one of the main causes of death in women (1-18). 1,000,000 women are affected by this disease annually, and another 300,000 women die annually as a result (1,2,4). Although the incidence rate is different in various geographical areas (5), the risk in the general population is on average 1/10 (1,16,37). The rate of breast cancer among women in developed and developing countries is 1/12 and 1/22, respectively (2). Breast cancer is the most common cancer in Iranian women, constituting 24.4% of all neoplasm cases in Iran. The age at which women face the greatest risk of developing this disease is in their forties for Asian women and in their sixties for American and European women (9,46). Thus, breast cancer is classified on the base of early-onset status in Asia (10). The incidence rate among women younger than 25 and older than 79 is 1/20,000 and 1/9, respectively. However, the effect of ethnical and geographical differences in breast cancer incidence indicates the effect of environmental conditions and lifestyle, which is shown in Figure 2.
Fig. 2 .
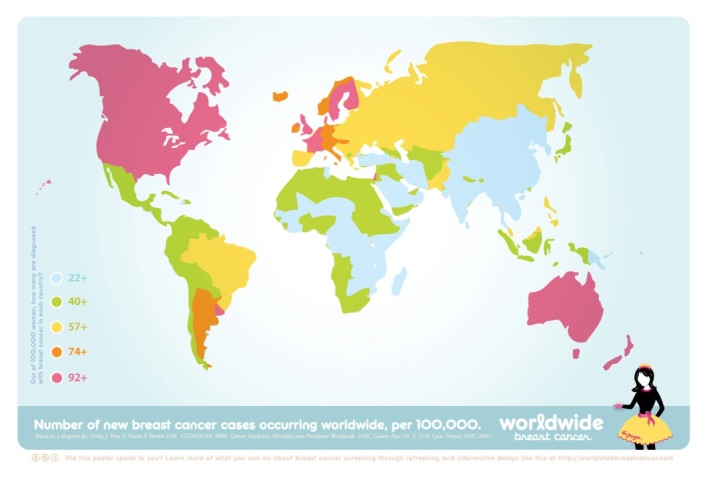
Breast cancer prevalence around the world
BRCA1 and BRCA2 genes
BRCA1 and BRCA2 genes are the two most common genes in autosomal dominant and high penetrance form of breast cancer and ovarian cancer (2). BRCA1 and BRCA2 genes produce Tumor Suppressor Gene (TSG) proteins so that two genes are called as TSGs. BRCA1 gene is located on chr17q, and any changes or mutations in this gene can lead to an increased risk of developing breast, ovarian, and prostate cancer. BRCA2 gene is located on chr13q, which is one of the acrocentric chromosomes in men, and any changes or mutations in this gene can lead to an increased risk of developing breast, ovarian, and prostate cancer. Figure 3 shows the location of these two genes on their chromosomes.
Fig. 3 .
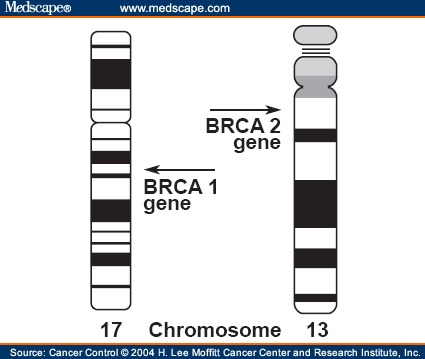
Tow breast cancer predisposing genes location on human chromosomes
These two genes act as cell growth suppressors and produce TSG proteins. BRCA1 protein has 1863 amino acids, and 300 disease-causing mutations have been reported in this gene thus far. BRCA2 protein has 3418 amino acids. These proteins are also called anti-oncogene and help the cell repair damaged DNA and ensure the genetic material preservation. Therefore, if either one of these two genes is damaged, damaged DNA will not be repaired, which can lead to more changes and more mutations in cell DNA and eventually lead to cancer. An event that can occur in these two genes as TSGs is Loss of Heterozygosity (LOH) phenomenon. This phenomenon will occur in a person who has one specific TSG allele in trouble; in other words, this person is heterozygous for this gene. LOH in this person can arise and lead to another healthy allele mutated. According to Nadson two-hit hypothesis, the TSG performance will be impaired, and the cell will be prone to develop a tumor and eventually lead to cancer. BRCA2 gene was discovered as the second breast cancer predisposing gene. This gene is important in intact double-strand DNA break repair and transcription regulation. BRCA2 gene in healthy cells would ensure DNA cell stability and controlled cell growth (2,47-49). BRCA1 gene mutation causes a possibility of 60% to 80% breast cancer in women as well as an increased possibility of developing ovarian cancer in women and prostate cancer in men. BRCA2 germ-line mutations are seen in approximately 35% of families with early-onset breast cancer in women and also cause an increased risk of ovarian cancer in women and breast cancer in men (2).
BRCA1 and BRCA2 genes involvement measurement in disease
Genetic factors are known as main disease-causing factors (2,4), such that hereditary risk factor is responsible for 3% to 10% of all breast cancer cases and up to 30% of all early-onset breast cancer (4,32). According to another study, in most western countries, 5% to 10% of all breast cancer cases are due to a main genetic cause (1). Hereditary breast cancer, which is usually caused by a mutation in BRCA1 and BRCA2 genes, is responsible for 5% to 10% of all these cancer cases as well as 10% to 15% of ovarian cancer cases. This type of breast cancer follows autosomal dominant pattern inheritance and tends to occur as an early-onset, high intensity, and bilateral form of the disease. BRCA1 and BRCA2 genes mutation carriers have a higher risk of developing this disease and other cancers, especially ovarian cancer. These mutations are frequently seen in people with a family history and those affected by multiple site forms of this disease. Approximately 30% of all breast and ovarian cancer cases are caused by mutations in BRCA1 or BRCA2 genes(8,21,40-44,50). 5% to 10% of breast cancer cases are caused by rare deleterious mutations in predisposing and high penetrance like BRCA1 and BRCA2 genes (7,8,25,51-54). As noted, germ-line mutations in these two genes are the most important causes of hereditary breast and ovarian cancer. Hence, detection of these two genes’ mutations is important in counseling for targeted family members and in reducing the incidence of breast cancer (9,33). In counseling for selecting prone women for the screening of germ-line mutations in BRCA1 gene, it is important to combine information about family history, diagnosis age, and tumor morphology (55,56).
As shown in Figure 4, BRCA1 and BRCA2 genes are responsible for 52% and 32% of families with breast cancer, respectively(3). However, the prevalence of mutations in BRCA1 and BRCA2 genes are different in various ethnic groups and may be affected by fundamental mutations (9). For example, in the Jewish female population, BRCA1 gene is the dominant gene involved in breast cancer development, but in the Italian female population, BRCA2 gene is the dominant gene (4). Approximately 20% to 25% of breast cancer risk is due to BRCA1 and BRCA2 genes (9,33). The risk of breast cancer in women with a mutation in BRCA1 or BRCA2 genes is estimated at 60% to 85%, and somewhere else it is estimated at 80% to 90%; however, other studies and articles have reported various numbers and possibilities (5,37,39,40,57-60). In the same way the risk of ovarian cancer in these women is estimated at 15% to 40% and somewhere else it is estimated at 40% to 60%; however other studies and articles have reported various numbers and possibilities (37,39,40,57-60). Generally, mutations in BRCA1 and BRCA2 genes as well as in genes that are necessary to preserve genome intact, such as STK11, ATM, CHEK2, P53, and CDH1 genes, lead to 50% of all familial breast cancer cases and 1/3 (one third) of all breast cancer cases (1,61). BRCA1 and BRCA2 genes are two of the most common genes to create autosomal dominant form and high penetrance forms of breast and ovarian cancer (2).
Fig. 4 .
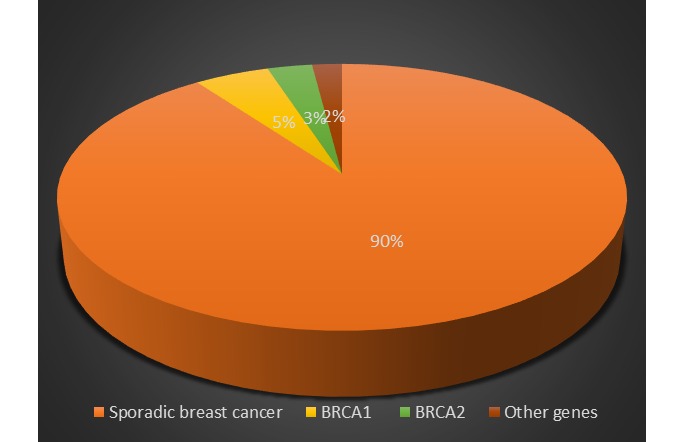
The frequency of different causes of breast cancer
BRCA1 and BRCA2 genes mutations
BRCA1 gene mutations cause breast cancer in women with a possibility of 60% to 80% and also cause increased risk of ovarian cancer development in women and prostate cancer in men. Germ-line mutations in BRCA2 gene are seen in approximately 35% of families with early-onset breast cancer in women and also lead to an increased risk of ovarian cancer development in women and breast cancer in men (2). Breast cancer caused by a mutation in BRCA1 gene has a higher incidence, higher mitotic rate, and more lymphatic penetrance than sporadic breast cancer (62). It is also more likely to lack expression of ER and PR and HER-2nue receptors (25,62-64) and to have a somatic mutation in P53 gene (62,64). In addition to having an increased risk of breast cancer development, BRCA1 or BRCA2 genes mutation carriers have an increased risk for other cancers like colon, prostate, pancreatic, melanoma, and gastric cancers (38,45). Novel mutations in BRCA1 and BRCA2 genes are very rare because 2000 mutations have been discovered in these two genes so far. The most common mutation forms are a small insertion, small deletion, nonsense mutation, missense mutation, premature transcription termination, and splicing troubles. Deletion and insertion mutation lead to a frame shift. Mutation in splicing spots leads to produce a non-functional protein (6,9,65,66). According to BIC (Breast Information Core), most of the breast cancer-causing mutations in BRCA1 and BRCA2 genes lead to produce truncated protein through the nonsense, frame shift, and splicing mutations (21).
Conclusion
According to conducted worldwide studies investigated in this review, the following important items can be concluded. In this study, about 30 mutations in BRCA1 gene and 35 mutations in BRCA2 gene in relation to breast cancer and ovarian cancer caused by breast cancer are reported with mutation location.
BRCA1 gene was analyzed first. In these studies, many mutations were reported in this gene, most of which led to producing truncated protein. The mutation that produces this type of protein can affect one or more nucleotides deletion, duplication, frame shift mutation, or intervention in the splicing process. One striking point in this study is that most of these mutations are located in BRCA1 gene exon 11. Therefore, among 30 mutations in this gene, 12 mutations are located in exon 11. This means that 40% of all mutations with any mutations in this gene are in exon 11. (See the BRCA1 mutation table) This result conforms to the study that was conducted in the United States. In the study, about 50% of all mutations that produced truncated protein in BRCA1 gene were found in exon 11 (67). This finding was somewhat surprising because this exon size is small and has 89 bps. Despite its small size, however, a large number of mutations occur in this exon. Another interesting point about most mutations in this exon is their novelty. Among 12 mutations listed in this article for exon 11, 9 mutations that have not been reported before in BIC were novel. This finding can mean that for discovering novel mutations in BRCA1 gene, exon 11 can be an appropriate and prone exon, and the possibility of discovering a novel mutation in it is high. In this study, the most mutated exon in BRCA1 gene after exon 11 was exon 10. This exon size is 33 times larger than exon 11, but a lower ratio of deleterious mutations are in exon 10 than exon 11. Among 30 BRCA1 gene mutations reported in the 76 investigated articles, 3 mutations were related to exon 10, meaning that 10% of mutations are in this exon. An interesting point about this exon is that all of its reported mutations are novel, and so this exon can be a prone exon for more novel mutations in the future. However, the last category of mutations that this article reports includes 4 mutations in exons 20 to the next. These mutations constitute 13.33% of all mutations reported for BRCA1 gene in this article. Among these 4 mutations, 3 mutations were novel.
In the following, we analyze mutations in BRCA1 gene on the basis of mutation type. Among 30 mutations reported for BRCA1 gene in this article, the mutation type for 19 mutations is determined exactly. Among these 19 mutations, 9 mutations are deletion-one or more nucleotides-, and it means about 50% of all deleterious mutations are deletion type. Another mutation type (with lower frequency) which can lead to disease, is splicing mutations. 5 mutations of these 19 mutations are splicing type. The last mutation type worth comparing is duplication mutation type-one or more nucleotides. This type includes 4 mutations among 19 reported in this study. However, among disease-causing mutations, large rearrangements can be seen, although its frequency is low. It is noteworthy that the result of a study conducted in Turkey shows that large rearrangement rate in BRCA1 gene is very low (68).
BRCA2 gene was also investigated. In these 76 investigated articles, many mutations were reported in this gene. Most of them, like BRCA1 gene mutations, led to producing truncated protein (37). This fact is consistent with the finding that more than 500 mutations in BRCA2 gene are reported and most of them lead to produce truncated protein. The mutations that produce this type of protein can affect one or more nucleotides deletion, duplication, frame shift, or missense mutations, or intervene in the splicing process. One of the interesting points in this study is that most of these mutations are located in exon 11. In fact, from the 35 mutations reported, 23 (or 65/7%) are located in exon 11. This finding conforms to a study that was conducted in the United States, the result of which showed that mutations which produced truncated protein in exon 11 of BRCA2 gene constituted about 50% of all mutations in this gene (67). However, this finding is not completely unexpected as this exon is the largest one in BRCA2 gene and therefore expected to be the most mutated among all exons in BRCA2 gene. Another interesting point about this exon is that most of its mutations are novel. In studies collected in this article, among 23 mutations reported in exon 11, 16 were novel and had not been reported in BIC before. This finding suggests that for discovering novel mutation linked to breast cancer, exon 11 in BRCA2 gene is an appropriate exon that has a high possibility to include more novel mutations. Exons 20 to the next had 4 mutations (11/42%) of 35 mutations reported for BRCA2 gene in this study. One mutation of 5 was novel.
In the following, we analyze deleterious mutation types in BRCA2 gene. Among 35 mutations reported in this gene in this study, the mutation type for 16 mutations is determined exactly. Among these 16, 10 are deletion-one or more nucleotides-, which suggests that about 62/5% of all deleterious mutations are deletion type.
As shown, exon 11 is the most mutated exon in both BRCA1 and BRCA2 genes. Therefore, this exon can be considered as the most prone exon for having the mutation-causing disease in both BRCA1 and BRCA2 genes. This result came from findings in the 76 investigated articles in this review. However, to prove this hypothesis, we can cite one of the results of a study conducted by Merge et al. which showed that patients 35 years old or younger with 35 mutations have mutations in exon 11 of BRCA1 gene primarily (69). This result solely considered BRCA1 gene, but in this review article which considered the results of 76 articles, we generated this result to BRCA2 gene. Another point that should be considered is whether there is a family history of breast cancer and/or ovarian cancer. This issue has an effect on mutation frequency in exon 11 in BRCA1 gene. According to another study result conducted by Merge et al., the frequency of these mutations in people with a family history is more than people without one (69). With generalization of this result to BRCA2 gene, screening of BRCA1 and BRCA2 genes should be proposed to all patients with a family history of breast or ovarian cancer (44). One of the problems about mutations detection is an excessive focus on people with family history because usually people with a family history are considered for BRCA1 and BRCA2 gene mutation screening. On the other hand, a person with no family history but with a mutation in BRCA1 gene or BRCA2 gene does not take part in this process (6,70). Another issue that should be considered is large rearrangements even though the frequency for BRCA1 gene is low (68). However even in the absence of family history, large rearrangements should be considered for a complete screening for BRCA1 and BRCA 2 genes in addition to whole gene sequencing (6). This solution is not as easy as it looks because screening BRCA1 and BRCA2 genes is a tedious process due to gene size, high mutation frequency, high rearrangement frequency in BRCA1 gene, and due to the need of special methods. Furthermore, because of its high cost, it is not routinely feasible, especially in developing countries (1).
Cite this article as: Mehrgou A, Akouchekian M. The importance of BRCA1 and BRCA2 genes mutations in breast cancer development. Med J Islam Repub Iran 2016 (15 May?). Vol. 30:369.
References
- 1.Armaou S, Pertesi M, Fostira F, Thodi G, Athanasopoulos PS, Kamakari S. et al. Contribution of BRCA1 germ-line mutations to breast cancer in Greece: a hospital-based study of 987 unselected breast cancer cases. British journal of cancer. 2009;101(1):32–7. doi: 10.1038/sj.bjc.6605115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayub SG, Rasool S, Ayub T, Khan SN, Wani KA, Andrabi KI. Mutational analysis of the BRCA2 gene in breast carcinoma patients of Kashmiri descent. Molecular medicine reports. 2014;9(2):749–53. doi: 10.3892/mmr.2013.1862. [DOI] [PubMed] [Google Scholar]
- 3.Balraj P, Khoo AS, Volpi L, Tan JA, Nair S, Abdullah H. Mutation analysis of the BRCA1 gene in Malaysian breast cancer patients. Singapore medical journal. 2002;43(4):194–7. [PubMed] [Google Scholar]
- 4.Calderon-Garciduenas AL, Ruiz-Flores P, Cerda-Flores RM, Barrera-Saldana HA. Clinical follow up of mexican women with early onset of breast cancer and mutations in the BRCA1 and BRCA2 genes. Salud publica de Mexico. 2005;47(2):110–5. doi: 10.1590/s0036-36342005000200004. [DOI] [PubMed] [Google Scholar]
- 5.Juwle A, Saranath D. BRCA1/BRCA2 gene mutations/SNPs and BRCA1 haplotypes in early-onset breast cancer patients of Indian ethnicity. Medical oncology (Northwood, London, England) 2012;29(5):3272–81. doi: 10.1007/s12032-012-0294-9. [DOI] [PubMed] [Google Scholar]
- 6.Kwong A, Ng EK, Tang EY, Wong CL, Law FB, Leung CP. et al. A novel de novo BRCA1 mutation in a Chinese woman with early onset breast cancer. Familial cancer. 2011;10(2):233–7. doi: 10.1007/s10689-011-9429-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loizidou M, Marcou Y, Anastasiadou V, Newbold R, Hadjisavvas A, Kyriacou K. Contribution of BRCA1 and BRCA2 germline mutations to the incidence of early-onset breast cancer in Cyprus. Clinical genetics. 2007;71(2):165–70. doi: 10.1111/j.1399-0004.2007.00747.x. [DOI] [PubMed] [Google Scholar]
- 8.Martinez-Ferrandis JI, Vega A, Chirivella I, Marin-Garcia P, Insa A, Lluch A. et al. Mutational analysis of BRCA1 and BRCA2 in Mediterranean Spanish women with early-onset breast cancer: identification of three novel pathogenic mutations. Human mutation. 2003;22(5):417–8. doi: 10.1002/humu.9188. [DOI] [PubMed] [Google Scholar]
- 9.Neamatzadeh H, Shiryazdi SM, Kalantar SM. BRCA1 and BRCA2 mutations in Iranian breast cancer patients: A systematic review. Journal of research in medical sciences : the official journal of Isfahan University of Medical Sciences. 2015;20(3):284–93. [PMC free article] [PubMed] [Google Scholar]
- 10.Toh GT, Kang P, Lee SS, Lee DS, Lee SY, Selamat S. et al. BRCA1 and BRCA2 germline mutations in Malaysian women with early-onset breast cancer without a family history. PloS one. 2008;3(4):e2024. doi: 10.1371/journal.pone.0002024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdulrahman GOJr, Rahman GA. Epidemiology of breast cancer in europe and Africa. Journal of cancer epidemiology. 2012;2012:915610. doi: 10.1155/2012/915610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beebe-Dimmer JL, Yee C, Cote ML, Petrucelli N, Palmer N, Bock C. et al. Familial clustering of breast and prostate cancer and risk of postmenopausal breast cancer in the Women's Health Initiative Study. Cancer. 2015;121(8):1265–72. doi: 10.1002/cncr.29075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee J, Park S, Kim S, Kim J, Ryu J, Park HS. et al. Characteristics and Survival of Breast Cancer Patients with Multiple Synchronous or Metachronous Primary Cancers. Yonsei medical journal. 2015;56(5):1213–20. doi: 10.3349/ymj.2015.56.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Obrist M, Osei-Bonsu E, Awuah B, Watanabe-Galloway S, Merajver SD, Schmid K. et al. Factors related to incomplete treatment of breast cancer in Kumasi, Ghana. Breast (Edinburgh, Scotland) 2014;23(6):821–8. doi: 10.1016/j.breast.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parkin DM. International variation. Oncogene. 2004;23(38):6329–40. doi: 10.1038/sj.onc.1207726. [DOI] [PubMed] [Google Scholar]
- 16. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA: a cancer journal for clinicians 2005;55(2):74-108. [DOI] [PubMed]
- 17.Poole VL, McCabe CJ. Iodide transport and breast cancer. The Journal of endocrinology. 2015;227(1):R1–r12. doi: 10.1530/JOE-15-0234. [DOI] [PubMed] [Google Scholar]
- 18.Xing W, Li Q, Cao R, Xu Z. Neogenin expression is inversely associated with breast cancer grade in ex vivo. World journal of surgical oncology. 2014;12:352. doi: 10.1186/1477-7819-12-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dumitrescu RG, Cotarla I. Understanding breast cancer risk -- where do we stand in 2005? Journal of cellular and molecular medicine. 2005;9(1):208–21. doi: 10.1111/j.1582-4934.2005.tb00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olopade OI, Grushko TA, Nanda R, Huo D. Advances in breast cancer: pathways to personalized medicine. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14(24):7988–99. doi: 10.1158/1078-0432.CCR-08-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pohlreich P, Zikan M, Stribrna J, Kleibl Z, Janatova M, Kotlas J. et al. High proportion of recurrent germline mutations in the BRCA1 gene in breast and ovarian cancer patients from the Prague area. BCR. 2005;7(5):BCR 2005;7(5). doi: 10.1186/bcr1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50302 women with breast cancer and 96973 women without the disease. Lancet (London, England) 2002;360(9328):187–95. doi: 10.1016/S0140-6736(02)09454-0. [DOI] [PubMed] [Google Scholar]
- 23.Bray F, McCarron P, Parkin DM. The changing global patterns of female breast cancer incidence and mortality. Breast cancer research: BCR. 2004;6(6):229–39. doi: 10.1186/bcr932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. The New England journal of medicine. 2003;348(17):1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 25.Chen W, Pan K, Ouyang T, Li J, Wang T, Fan Z. et al. BRCA1 germline mutations and tumor characteristics in Chinese women with familial or early-onset breast cancer. Breast cancer research and treatment. 2009;117(1):55–60. doi: 10.1007/s10549-008-0066-6. [DOI] [PubMed] [Google Scholar]
- 26.Datta K, Biswas J. Influence of dietary habits, physical activity and affluence factors on breast cancer in East India: a case-control study. Asian Pacific journal of cancer prevention: APJCP. 2009;10(2):219–22. [PubMed] [Google Scholar]
- 27.Hamajima N, Hirose K, Tajima K, Rohan T, Calle EE, Heath CWJr. et al. Alcohol, tobacco and breast cancer--collaborative reanalysis of individual data from 53 epidemiological studies, including 58,515 women with breast cancer and 95,067 women without the disease. British journal of cancer. 2002;87(11):1234–45. doi: 10.1038/sj.bjc.6600596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lord SJ, Bernstein L, Johnson KA, Malone KE, McDonald JA, Marchbanks PA. et al. Breast cancer risk and hormone receptor status in older women by parity, age of first birth, and breastfeeding: a case-control study. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2008;17(7):1723–30. doi: 10.1158/1055-9965.EPI-07-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schulz M, Hoffmann K, Weikert C, Nothlings U, Schulze MB, Boeing H. Identification of a dietary pattern characterized by high-fat food choices associated with increased risk of breast cancer: the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. The British journal of nutrition. 2008;100(5):942–6. doi: 10.1017/S0007114508966149. [DOI] [PubMed] [Google Scholar]
- 30.Beral V. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet (London, England) 2003;362(9382):419–27. doi: 10.1016/s0140-6736(03)14065-2. [DOI] [PubMed] [Google Scholar]
- 31.Huo D, Adebamowo CA, Ogundiran TO, Akang EE, Campbell O, Adenipekun A. et al. Parity and breastfeeding are protective against breast cancer in Nigerian women. British journal of cancer. 2008;98(5):992–6. doi: 10.1038/sj.bjc.6604275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tereschenko IV, Basham VM, Ponder BA, Pharoah PD. BRCA1 and BRCA2 mutations in Russian familial breast cancer. Human mutation. 2002;19(2):184. doi: 10.1002/humu.9008. [DOI] [PubMed] [Google Scholar]
- 33.Antoniou AC, Gayther SA, Stratton JF, Ponder BA, Easton DF. Risk models for familial ovarian and breast cancer. Genetic epidemiology. 2000;18(2):173–90. doi: 10.1002/(SICI)1098-2272(200002)18:2<173::AID-GEPI6>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 34.Malone KE, Daling JR, Doody DR, Hsu L, Bernstein L, Coates RJ. et al. Prevalence and predictors of BRCA1 and BRCA2 mutations in a population-based study of breast cancer in white and black American women ages 35 to 64 years. Cancer research. 2006;66(16):8297–308. doi: 10.1158/0008-5472.CAN-06-0503. [DOI] [PubMed] [Google Scholar]
- 35.Antoniou AC, Pharoah PD, McMullan G, Day NE, Stratton MR, Peto J. et al. A comprehensive model for familial breast cancer incorporating BRCA1, BRCA2 and other genes. British journal of cancer. 2002;86(1):76–83. doi: 10.1038/sj.bjc.6600008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nanda R, Schumm LP, Cummings S, Fackenthal JD, Sveen L, Ademuyiwa F. et al. Genetic testing in an ethnically diverse cohort of high-risk women: a comparative analysis of BRCA1 and BRCA2 mutations in American families of European and African ancestry. Jama. 2005;294(15):1925–33. doi: 10.1001/jama.294.15.1925. [DOI] [PubMed] [Google Scholar]
- 37.Armakolas A, Ladopoulou A, Konstantopoulou I, Pararas B, Gomatos IP, Kataki A. et al. BRCA2 gene mutations in Greek patients with familial breast cancer. Human mutation. 2002;19(1):81–2. doi: 10.1002/humu.9003. [DOI] [PubMed] [Google Scholar]
- 38.Jancarkova N, Zikan M, Pohlreich P, Freitag P, Matous B, Zivny J. [Detection and occurrence of BRCA 1 gene mutation in patients with carcinoma of the breast and ovary] Ceska gynekologie / Ceska lekarska spolecnost J Ev Purkyne. 2003;68(1):11–6. [PubMed] [Google Scholar]
- 39.Martin SE, Sausen M, Joseph A, Biggs DD, Kingham BF, Martin ES. BRCA1 E1644X: a deleterious mutation in an African American individual with early onset breast cancer. Breast cancer research and treatment. 2009;113(2):393–5. doi: 10.1007/s10549-008-9928-1. [DOI] [PubMed] [Google Scholar]
- 40.Marchina E, Fontana MG, Speziani M, Salvi A, Ricca G, Di Lorenzo D. et al. BRCA1 and BRCA2 genetic test in high risk patients and families: counselling and management. Oncology reports. 2010;24(6):1661–7. doi: 10.3892/or_00001031. [DOI] [PubMed] [Google Scholar]
- 41.Patmasiriwat P, Bhothisuwan K, Sinilnikova OM, Chopin S, Methakijvaroon S, Badzioch M. et al. Analysis of breast cancer susceptibility genes BRCA1 and BRCA2 in Thai familial and isolated early-onset breast and ovarian cancer. Human mutation. 2002;20(3):230. doi: 10.1002/humu.9049. [DOI] [PubMed] [Google Scholar]
- 42.Schoumacher F, Glaus A, Mueller H, Eppenberger U, Bolliger B, Senn HJ. BRCA1/2 mutations in Swiss patients with familial or early-onset breast and ovarian cancer. Swiss medical weekly. 2001;131(15-16):223–6. doi: 10.4414/smw.2001.09677. [DOI] [PubMed] [Google Scholar]
- 43.Seong MW, Cho S, Noh DY, Han W, Kim SW, Park CM. et al. Comprehensive mutational analysis of BRCA1/BRCA2 for Korean breast cancer patients: evidence of a founder mutation. Clinical genetics. 2009;76(2):152–60. doi: 10.1111/j.1399-0004.2009.01202.x. [DOI] [PubMed] [Google Scholar]
- 44.Tazzite A, Jouhadi H, Nadifi S, Aretini P, Falaschi E, Collavoli A. et al. BRCA1 and BRCA2 germline mutations in Moroccan breast/ovarian cancer families: novel mutations and unclassified variants. Gynecologic oncology. 2012;125(3):687–92. doi: 10.1016/j.ygyno.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 45.Dutil J, Colon-Colon JL, Matta JL, Sutphen R, Echenique M. Identification of the prevalent BRCA1 and BRCA2 mutations in the female population of Puerto Rico. Cancer genetics. 2012;205(5):242–8. doi: 10.1016/j.cancergen.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mousavi SM, Gouya MM, Ramazani R, Davanlou M, Hajsadeghi N, Seddighi Z. Cancer incidence and mortality in Iran. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2009;20(3):556–63. doi: 10.1093/annonc/mdn642. [DOI] [PubMed] [Google Scholar]
- 47.Davies AA, Masson JY, McIlwraith MJ, Stasiak AZ, Stasiak A, Venkitaraman AR. et al. Role of BRCA2 in control of the RAD51 recombination and DNA repair protein. Molecular cell. 2001;7(2):273–82. doi: 10.1016/s1097-2765(01)00175-7. [DOI] [PubMed] [Google Scholar]
- 48.Moynahan ME, Pierce AJ, Jasin M. BRCA2 is required for homology-directed repair of chromosomal breaks. Molecular cell. 2001;7(2):263–72. doi: 10.1016/s1097-2765(01)00174-5. [DOI] [PubMed] [Google Scholar]
- 49.Pellegrini L, Yu DS, Lo T, Anand S, Lee M, Blundell TL. et al. Insights into DNA recombination from the structure of a RAD51-BRCA2 complex. Nature. 2002;420(6913):287–93. doi: 10.1038/nature01230. [DOI] [PubMed] [Google Scholar]
- 50.Ahn SH, Son BH, Yoon KS, Noh DY, Han W, Kim SW. et al. BRCA1 and BRCA2 germline mutations in Korean breast cancer patients at high risk of carrying mutations. Cancer letters. 2007;245(1-2):90–5. doi: 10.1016/j.canlet.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 51. Prevalence and penetrance of BRCA1 and BRCA2 mutations in a population-based series of breast cancer cases . Anglian Breast Cancer Study Group. British journal of cancer. 2000;83(10):1301–8. doi: 10.1054/bjoc.2000.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hamann U, Liu X, Bungardt N, Ulmer HU, Bastert G, Sinn HP. Similar contributions of BRCA1 and BRCA2 germline mutations to early-onset breast cancer in Germany. European journal of human genetics: EJHG. 2003;11(6):464–7. doi: 10.1038/sj.ejhg.5200988. [DOI] [PubMed] [Google Scholar]
- 53.Loman N, Johannsson O, Kristoffersson U, Olsson H, Borg A. Family history of breast and ovarian cancers and BRCA1 and BRCA2 mutations in a population-based series of early-onset breast cancer. Journal of the National Cancer Institute. 2001;93(16):1215–23. doi: 10.1093/jnci/93.16.1215. [DOI] [PubMed] [Google Scholar]
- 54.Malone KE, Daling JR, Neal C, Suter NM, O'Brien C, Cushing-Haugen K. et al. Frequency of BRCA1/BRCA2 mutations in a population-based sample of young breast carcinoma cases. Cancer. 2000;88(6):1393–402. doi: 10.1002/(sici)1097-0142(20000315)88:6<1393::aid-cncr17>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 55.John EM, Hopper JL, Beck JC, Knight JA, Neuhausen SL, Senie RT. et al. The Breast Cancer Family Registry: an infrastructure for cooperative multinational, interdisciplinary and translational studies of the genetic epidemiology of breast cancer. Breast cancer research: BCR. 2004;6(4):R375–89. doi: 10.1186/bcr801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith LD, Tesoriero AA, Wong EM, Ramus SJ, O'Malley FP, Mulligan AM. et al. Contribution of large genomic BRCA1 alterations to early-onset breast cancer selected for family history and tumour morphology: a report from The Breast Cancer Family Registry. Breast cancer research: BCR. 2011;13(1):R14. doi: 10.1186/bcr2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brose MS, Rebbeck TR, Calzone KA, Stopfer JE, Nathanson KL, Weber BL. Cancer risk estimates for BRCA1 mutation carriers identified in a risk evaluation program. Journal of the National Cancer Institute. 2002;94(18):1365–72. doi: 10.1093/jnci/94.18.1365. [DOI] [PubMed] [Google Scholar]
- 58.Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25(11):1329–33. doi: 10.1200/JCO.2006.09.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eeles RA. Future possibilities in the prevention of breast cancer: intervention strategies in BRCA1 and BRCA2 mutation carriers. Breast cancer research: BCR. 2000;2(4):283–90. doi: 10.1186/bcr70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thompson D, Easton DF. Cancer Incidence in BRCA1 mutation carriers. Journal of the National Cancer Institute. 2002;94(18):1358–65. doi: 10.1093/jnci/94.18.1358. [DOI] [PubMed] [Google Scholar]
- 61.Walsh T, King MC. Ten genes for inherited breast cancer. Cancer cell. 2007;11(2):103–5. doi: 10.1016/j.ccr.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 62.Musolino A, Naldi N, Michiara M, Bella MA, Zanelli P, Bortesi B. et al. A breast cancer patient from Italy with germline mutations in both the BRCA1 and BRCA2 genes. Breast cancer research and treatment. 2005;91(2):203–5. doi: 10.1007/s10549-004-7704-4. [DOI] [PubMed] [Google Scholar]
- 63.Lakhani SR, Van De Vijver MJ, Jacquemier J, Anderson TJ, Osin PP, McGuffog L. et al. The pathology of familial breast cancer: predictive value of immunohistochemical markers estrogen receptor, progesterone receptor, HER-2, and p53 in patients with mutations in BRCA1 and BRCA2. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2002;20(9):2310–8. doi: 10.1200/JCO.2002.09.023. [DOI] [PubMed] [Google Scholar]
- 64.Phillips KA. Immunophenotypic and pathologic differences between BRCA1 and BRCA2 hereditary breast cancers. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2000;18(21 Suppl):107s–12s. [PubMed] [Google Scholar]
- 65.Pylkas K, Erkko H, Nikkila J, Solyom S, Winqvist R. Analysis of large deletions in BRCA1, BRCA2 and PALB2 genes in Finnish breast and ovarian cancer families. BMC cancer. 2008;8:146. doi: 10.1186/1471-2407-8-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Walsh T, Casadei S, Coats KH, Swisher E, Stray SM, Higgins J. et al. Spectrum of mutations in BRCA1, BRCA2, CHEK2, and TP53 in families at high risk of breast cancer. Jama. 2006;295(12):1379–88. doi: 10.1001/jama.295.12.1379. [DOI] [PubMed] [Google Scholar]
- 67.Pal T, Vadaparampil S, Betts J, Miree C, Li S, Narod SA. BRCA1/2 in high-risk African American women with breast cancer: providing genetic testing through various recruitment strategies. Genetic testing. 2008;12(3):401–7. doi: 10.1089/gte.2007.0108. [DOI] [PubMed] [Google Scholar]
- 68.Manguoglu E, Guran S, Yamac D, Simsek M, Akdeniz S, Colak T. et al. Genomic large rearrangement screening of BRCA1 and BRCA2 genes in high-risk Turkish breast/ovarian cancer patients by using multiplex ligation-dependent probe amplification assay. Cancer investigation. 2011;29(1):73–7. doi: 10.3109/07357907.2010.512599. [DOI] [PubMed] [Google Scholar]
- 69.Meng J, Shi YR, Niu RF, Fu L. [Relationship between mutation of BRCA1 and susceptibility to early onset of breast cancer] Zhonghua yi xue za zhi. 2009;89(2):79–82. [PubMed] [Google Scholar]
- 70.Edwards E, Yearwood C, Sillibourne J, Baralle D, Eccles D. Identification of a de novo BRCA1 mutation in a woman with early onset bilateral breast cancer. Familial cancer. 2009;8(4):479–82. doi: 10.1007/s10689-009-9270-8. [DOI] [PubMed] [Google Scholar]
- 71.Song CG, Hu Z, Yuan WT, Di GH, Shen ZZ, Huang W. et al. [Mutational analysis of BRCA1 and BRCA2 genes in early-onset breast cancer patients in Shanghai] Zhonghua yi xue za zhi. 2005;85(43):3030–4. [PubMed] [Google Scholar]
- 72.Ozdag H, Tez M, Sayek I, Muslumanoglu M, Tarcan O, Icli F. et al. Germ line BRCA1 and BRCA2 gene mutations in Turkish breast cancer patients. European journal of cancer (Oxford, England: 1990) 2000;36(16):2076–82. doi: 10.1016/s0959-8049(00)00277-x. [DOI] [PubMed] [Google Scholar]
- 73.Ellis D, Patel Y, Yau SC, Hodgson SV, Abbs SJ. Low prevalence of BRCA1 exon rearrangements in familial and young sporadic breast cancer patients. Familial cancer. 2006;5(4):323–6. doi: 10.1007/s10689-006-0001-0. [DOI] [PubMed] [Google Scholar]
- 74.Phelan CM, Kwan E, Jack E, Li S, Morgan C, Aube J. et al. A low frequency of non-founder BRCA1 mutations in Ashkenazi Jewish breast-ovarian cancer families. Human mutation. 2002;20(5):352–7. doi: 10.1002/humu.10123. [DOI] [PubMed] [Google Scholar]
- 75.Marino M, Rabacchi C, Simone ML, Medici V, Cortesi L, Calandra S. A novel deletion of BRCA1 gene that eliminates the ATG initiation codon without affecting the promoter region Clinica chimica acta; international journal of clinical chemistry. 2009;403(1-2):249–53. doi: 10.1016/j.cca.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 76.Soares R, Amendoeira I, Monteiro P, Lopes CS, Schmitt FC. [Analysis of mutations in the BRCA1 gene in patients with cancer of the breast and/or the ovary in Portugal] Acta medica portuguesa. 2000;13(5-6):273–6. [PubMed] [Google Scholar]


