Abstract
We have developed a rechargeable fetal micropacemaker in order to treat severe fetal bradycardia with comorbid hydrops fetalis, a life-threatening condition in pre-term non-viable fetuses for which there are no effective treatment options. The small size and minimally invasive form factor of our design limit the volume available for circuitry and a power source. The device employs a fixed-rate and fixed-amplitude relaxation oscillator and a tiny, rechargeable lithium ion power cell. For both research and clinical applications, it is valuable to monitor the electrode-myocardium interface in order to determine that adequate pacemaker output is being provided. This is typically accomplished by observing the minimal stimulus strength that achieves threshold for pacing capture. The output of our simple micropacemaker cannot be programmatically altered to determine this minimal capture threshold, but a safety factor can be inferred by determining the refractory period for ventricular capture at a given stimulus strength. This is done by measuring the minimal timing between naturally occurring QRS complexes and pacing stimuli that successfully generate a premature ventricular contraction. The method was tested in a pilot study in 4 fetal sheep and the data demonstrate that a relative measure of threshold is obtainable. This method provides valuable real-time information about the electrode-tissue interface.
Keywords: Pacemaker, strength-interval, electrode-tissue interface
1. Introduction
Progressive complete heart block with comorbid hydrops in the human fetus is a rare but life-threatening condition (Friedman et al., 2008; Lopes et al., 2008; Groves et al., 1996). Once hydrops develops as a result of heart block, fetal demise is nearly inevitable if the fetus cannot be delivered due to prematurity or other clinical concerns (Schmidt et al., 1991). Successfully pacing the fetus would be expected to restore adequate blood flow and theoretically allow resolution of hydrops fetalis within several weeks, permitting an otherwise normal gestation and delivery (Norton et al., 2015; Van Mieghem et al., 2013). After the birth, the infant could then be implanted with a standard pacemaker with epicardial leads. We have developed a fetal micropacemaker to meet this prenatal need (Loeb et al., 2013) and are currently performing pre-clinical studies in fetal sheep (Bar-Cohen et al., 2015). This paper describes a novel electrophysiological method that provides useful insights into the quality of the electrode-tissue interface during the course of such chronic experiments.
Previous attempts to use an adult pacemaker in the mother with a lead implanted in the fetus were unsuccessful, ending with fetal death (Carpenter Jr et al., 1986; Walkinshaw et al., 1994; Assad et al., 2003; Silverman et al., 1998; Eghtesady et al., 2011). The causes of death were not clearly identified in all cases, but were likely due to surgical complications from open surgery (Silverman et al., 1998; Eghtesady et al., 2011), lead placement complications (Walkinshaw et al., 1994; Assad et al., 2003), and/or lead dislodgement (Walkinshaw et al., 1994) or strangulation. The device that we have developed avoids these problems by utilizing a percutaneous approach through a fetal surgical cannula (3.8 mm i.d.) and a micropacemaker packaging scheme that can fit entirely inside the fetal chest wall. Power is obtained from a tiny lithium cell (2.9 mm o.d., 11.8 mm long) that must be recharged at regular intervals by inductive coupling from a coil outside the mother. Due to the limited number of patients with this condition, the technology must also be simple and inexpensive both to develop and to manufacture (Loeb et al., 2013).
The simple electronic circuit of our micropacemaker means that there is no data communication with the implant, so its nominal pacing rate and output strength must be pre-set during fabrication. It is desirable to set the stimulus strength as low as possible to conserve power in order to maximize the recharge interval, but it is also important to include a safety factor to ensure effective ventricular capture for somewhat unpredictable electrode placements and tissue conditions. Pacing rate is asynchronous with the intrinsic fetal heart rhythm, and although proarrhythmia is a risk associated with asynchronous pacing, this risk will be very low and possibly inconsequential for fetuses with complete heart block as the rate of the pacemaker (>100 bpm) is substantially above the intrinsic ventricular rate in fetal heart block (~40–60 bpm).
The asynchronous pacing of the normal fetal heart in our experiments can be used to infer threshold by measuring the minimal interval between an intrinsic myocardial contraction and a fixed stimulus that successfully captures the next contraction. In principle, the interval between naturally occurring contractions and the pacing stimuli could be varied systematically by detecting an intrinsic heart beat (QRS signature) and then commanding the stimulation to occur at a certain delay after that beat, but this is not possible with our simple micropacemaker. Instead we used a fixed pacing rate that was lower than the intrinsic fetal heart rate, which results in random time intervals between intrinsic beats and subsequent pacing stimuli, eventually ensuring that a large number of possible intervals have been sampled. By plotting successful versus non-successful intervals, an estimate of pacing refractory periods (and hence safety factor) can be determined.
Changes in safety factor may reflect changes in the electrode-myocardium interface such as from healing and formation of scar tissue around the electrode, mechanical stress on the tissue, and lead dislodgement. While these changes can be seen on necropsy and lead to some conclusions about the device at the end point of the study, interim outcomes provide important data regarding the electrical properties of the tissue, which cannot be determined post-mortem after cell death. In addition, these interim points allow us to determine when events occur in real time during implantation and follow up in order to better understand these changes over time.
2. Method
2.1. Animal Model
The animal study protocol was approved by and performed in accordance with the guidelines of the Institutional Animal Care and Use Committees at the University of Southern California and the Los Angeles Biomedical Research Institute. Fetal micropacemakers with rates between 100 bpm and 150 bpm were implanted into 7 fetal sheep (Ovis aries, Rambouillet, and Columbia mix breed) according to the procedure discussed in Bar-Cohen et al. (2015). In summary, a cannula and trocar were advanced to the apex of the fetal heart under ultrasound guidance. Once the tip of the cannula was confirmed to be orthogonal to the ventricular myocardium, the trocar was withdrawn and the fetal pacemaker delivery sheath was advanced through the cannula. The pacemaker, residing at the end of the delivery sheath, was anchored to the ventricular myocardium by rotating the sheath and pacemaker electrode. The instrumentation was then withdrawn. The optimal pacemaker placement required several turns of the corkscrew electrode engaged in myocardium, and the strain reliving coil spanning the diaphragm.
The device for sheep 1 failed, while the implantations for sheep 2 and 3 did not result in adequate ventricular capture. The implantation scheme was therefore revised with placement of an iatrogenic pericardial effusion at the time of implant (outlined in detail in Bar-Cohen, et al.). The latter 4 sheep experiments produced adequate data for the analysis presented here, and are alphabetized sheep A through D below, corresponding to sheep #4–7 respectively in Bar-Cohen et al. where histological results can be found.
2.2. Pacing Parameters
In 3 of 4 experiments (sheep A, B, and D), the micropacemakers had a 3 μC output pulse on the day of implant (3 V peak, 250 μs time constant). In sheep C, a higher output device (6.1 μC; 2-day recharging interval) was used to determine if a higher output device could result in a higher chance for ventricular capture success over time. Devices were implanted fully charged, but supply voltage and therefore output charge decreased over time. Devices were recharged in each case in order to test the recharging system, and this increased supply voltage and output charge when recharging was being performed (sheep D) or successful (sheep B and C).
2.3. Data Collection
Data was collected in the post-op acute phase and during follow up visits in the 3 days after surgery. The fetal electrocardiogram was detected by 3 trans-uterine electrodes attached directly to the fetus. The electrodes were sewn directly onto the chest surrounding the heart because this configuration appeared to result in larger ECG surface electrograms as compared to leads placed on the extremities. The fetal chest leads extended out of the maternal abdominal incision and could be placed into a pouch sewn on the maternal skin surface. During acute and follow up data collection sessions the electrodes were connected to an external biosignal monitoring device (BioRadio 150, Great Lakes NeuroTechnologies, sampling rate = 960 Hz, low gain) and the digitized signal was recorded by a personal computer and filtered to eliminate DC drift and high frequency noise (LPF=450 Hz, HPF=5 Hz).
2.4. Data Analysis
The collected signals were analyzed using Matlab, and each interval between the preceding intrinsic QRS signature and the subsequent stimulus artefact was measured and visually assessed for capture (figure 1). Successful capture was detected by the presence of a prematurely occurring ventricular contraction immediately after the pacing stimulus. Successful (X) vs. non-successful (O) stimuli were then plotted as a function of the interval between the previous QRS complex and the stimulus artefact (figure 2, A). When multiple instances of a particular interval were recorded, the frequency count increased. The boundary between the symbols denotes the minimal interval for successful capture and corresponds to the ventricular capture refractory period (figure 2, A). A clear boundary between successful and unsuccessful stimuli was not observed in every case; in those scenarios, the minimal interval was taken to be the earliest successful stimulus.
Figure 1.
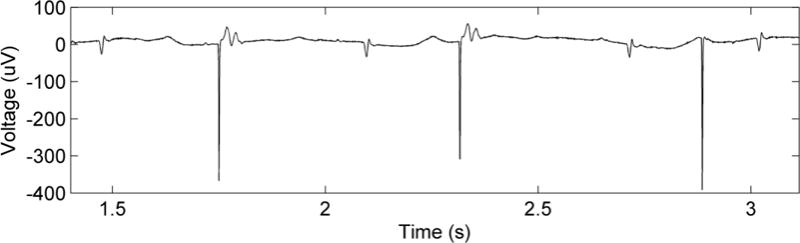
Characteristic electrical signals from fetal ECG leads (taken from sheep 4 on POD1). From left to right the complexes reflect normal sinus rhythm (NSR), pacing capture (PC), NSR, PC, NSR, and a pacing artefact without capture followed by NSR. The artefact without capture occurred at a slightly shorter interval from the previous QRS complex than those with PC.
Figure 2.
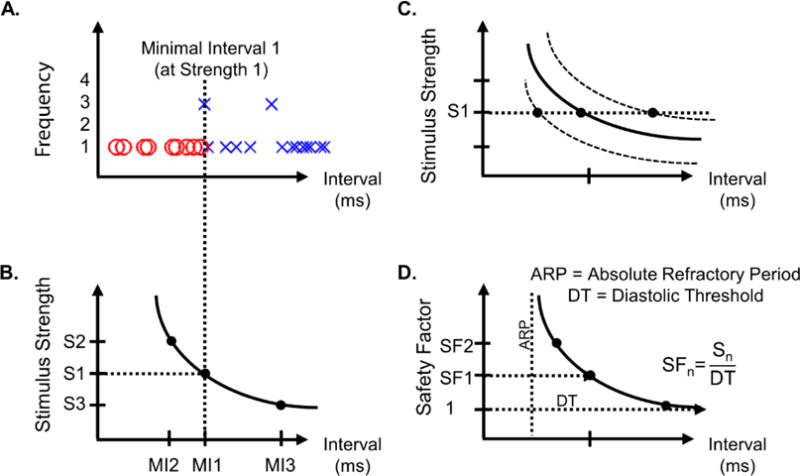
The boundary observed in (A) and its associated interval and strength make up a point on the strength- interval curve (B). Changes in the ability to elicit a contraction of the myocardial tissue would become evident in the strength-interval curve as an upwards or downwards shift (C) and would appear as a longer or shorter minimal interval (MI), respectively. If diastolic threshold is estimated, the safety factor of a given stimulus strength could be determined (D).
The duration of ECG analyzed varied from 15 s to 150 s, and depended on the availability of data and the length of data necessary to generate a clear minimal interval boundary. More data was available on follow up days when imaging and other time consuming observations were limited. Time with the unsedated animal was limited to two hours per the IACUC guidelines. The minimal duration of ECG necessary to generate a boundary of data varied based on the difference between the pacing rate of the pacemaker and the intrinsic rate of the fetal sheep.
2.5. Physiological Interpretation of Minimal Interval Data
The minimal interval was analyzed over every follow up day in which capture was seen. Observations were then made to determine shifts in minimal interval over time, which were taken as indications of shifts in safety factor. Each implantation built on the lessons learned from previous experiments, and therefore each was unique.
Interpretations of data generated utilized the concept of the strength-interval (SI) curve (figure 2, B). The minimal interval and the output strength used to generate it correspond to a point on that strength-interval SI curve. Changes in the ability to elicit a contraction of the myocardial tissue would become evident in changes to the strength-interval curve. It is well understood that pacing threshold tends to rise over time due to the normal inflammatory process of the foreign body reaction, rapidly in the acute phase and then gradually leveling out to a value somewhat higher than implant day (Danilovic and Ohm, 1999; Mond and Stokes, 1992). This apparent rise in threshold is a matter of increasing distance and lower charge density experienced by the nearest excitable myocytes. More strength is necessary to overcome these factors and the SI curve would appear to shift up (figure 2, C). A similar change would result from any increase in the distance between the electrode and the excitable myocytes, such as injury and cell death around the electrode tip or lead dislodgement. The shift up would be evident by a lengthening in the minimal interval measured at a given strength. A change in threshold of the individual myocytes would also shift the SI curve. If autonomic sympathetic tone increased, as it does during a stress response, the contractility of the myocytes would increase and the apparent threshold of the tissue would decrease, causing a shift in the strength-interval curve downwards. This would allow a given stimulus strength to capture at a shorter minimal interval. Because changes in autonomic tone generally result also in a change in intrinsic heart rate, it should be possible to distinguish these 2 mechanisms.
Minimal interval measurements by themselves provide a method to infer the relative threshold of the tissue over time, but they could also be used to generate a more complete picture of the threshold of the tissue and the safety factor if at least some variation of stimulus strength could be achieved. Our micropacemaker does not allow programmatic control of stimulus strength, but as the supply voltage from the lithium cell drops there is a corresponding drop in output charge, which can be measured accurately by calibration during manufacture. Allowing the supply voltage to drop, noting output charge, and making minimal interval observations can lead to the determination of many points on the strength-interval curve, assuming that the electrode interface and the autonomic tone are steady during this period. The supply voltage can be increased quite rapidly as well by recharging the device to the desired level; full recharge takes less than 3 hours with a favorable placement of the external transmitting coil (Zhou et al., 2014). The diastolic threshold (DT), the minimal energy needed to successfully capture the tissue given a very long interval well outside of the relative refractory period, can be estimated from such a calibrated strength-interval curve and the absolute safety factor of stimulation can be determined (figure 2, D). To determine the safety factor of a given stimulus, the strength of the stimulus would be divided by the strength of the diastolic threshold.
The resulting observations and interpretations will be presented for each case along with a summary of observations provided in table 1.
Table 1.
Summary of implants and devices.
| Sheep | Day (Device) | Battery Voltage (V) | Stimulus Output Charge (μC) | Dataset Length (s) | Minimal Interval (s) | FHR (bpm) | Summary |
|---|---|---|---|---|---|---|---|
| A | Op Day | 3.9 | 2.77 | 146 | 0.155 | 165–210 | The pacemaker was perfectly
located, with the nose wedged into the diaphragm, the flexible lead
partially extended and straight and the electrode buried in the left
ventricular wall. Fetus miscarried on POD5. |
| POD1 | 3.8 | 2.70 | 60 | 0.171 | 178 | ||
| POD3 | 3.6 | 2.56 | 60 | 0.109 | 262 | ||
| B | Op Day (Device I) | 3.9 | 2.73 | 36 | 0.151 | 242 | Device I was implanted first
and pushed into the myocardium with the insertion of device II. Xray and
histology confirmed that the electrodes were intertwined and the
electrode path of Device II extended approximately 3.7 mm into the
epicardial surface. Electively terminated on POD15 |
| Op Day (Device II) | 3.9 | 2.73 | 36 | 0.121 | 242 | ||
| POD2 (Device II) | 3.7 | 2.60 | 15 | 0.161 | 180–196 | ||
| C | OD | 3.9 | 6.10 | 110 | 0.175 | 157 | The electrode tip was implanted
adjacent to the epicardium, but not engaged in tissue (confirmed by
pathology); the location of the electrode outside the myocardium is
consistent with the high and increasing threshold. Experiment terminated at pre-mature labor on POD 5 |
| POD2 | 3.0 | 4.48 | 30 | 0.168 | 170–200 | ||
| D | OD Early | 3.7 | 2.67 | 36 | 0.200 | 177 | The electrode was implanted
adjacent to myocardium, but not fully engaged into it. Fetus miscarried on POD 5 |
| OD Recharging | 3.7 | 2.67 | 50 | 0.189 | 174 | ||
| OD Post-Charging | 3.7 | 2.67 | 16 | 0.194 | 178 |
3. Results
3.1. Sheep A
3.1.1. Observations
Measurements made on the operative day (OD) indicated a minimal interval between native QRS and stimulus resulting in capture of 0.155 s (figure 3, OD); however, significant overlap was seen over successfully versus unsuccessfully captured intervals. Measurements made on post-operative day (POD) 1 indicate that the minimal interval lengthened to 0.171 s (figure 3, POD1), the overlap region reduced, and the battery voltage dropped (3.9 V to 3.8 V). On POD3, measurements were again taken yielding a much lower minimal interval of 0.109 s (figure 3, POD3), even in the face of an even lower battery voltage of 3.6 V and lower stimulus charge (table 1). Upon necropsy, the pacemaker was observed to have been implanted in a nearly optimal orientation, with the battery case wedged into the diaphragm, the flexible lead partially extended in a straight alignment, and the electrode buried in the left ventricular myocardium (Bar-Cohen et al. figure 5 and figure 6).
Figure 3.
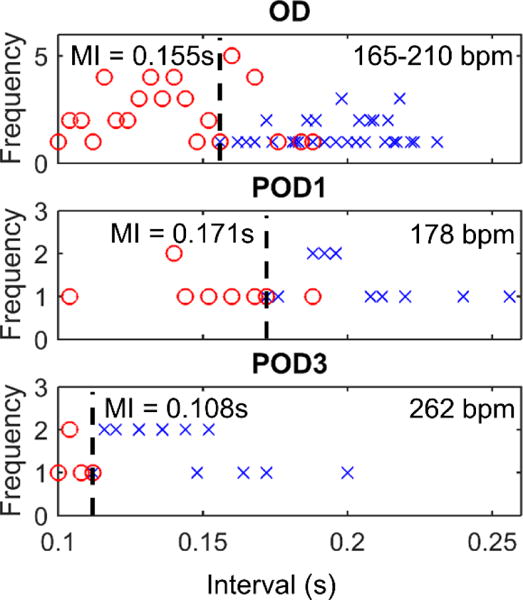
Thresholds for Sheep A were seen to lengthen on POD1 from OD, and then drastically shorten on POD3, possibly indicative of fetal distress.
3.1.2. Interpretation
Because the pacemaker was implanted in a very favorable location, the measured minimal interval of 0.155 s was most likely an indication of a low pacing threshold. The variability in threshold indicated by the substantial overlap region on OD could have been related to local and acute irritability from the electrode implantation, supported by the fact that overlap decreases in post-operative days. Because the stimulus output was nearly constant between OD and POD1 (2.77 μC vs 2.70 μC), and no obvious autonomic effects were evident in the stable heart rate, the threshold of the tissue likely increased slightly, a response consistent with the beginning stages of electrode stabilization and the inflammatory response.
On POD3, the much shorter minimal interval measured was unexpected, especially in the face of a lower strength stimulus output. It was interpreted to be due to changes in autonomic tone resulting from fetal stress, which was also suggested by a substantial increase in the intrinsic fetal heart rate (262 bpm vs 178 bpm). This fetal distress may have predicted the spontaneous abortion of the fetus just 2 days later on POD5 (table 1).
3.2. Sheep B
3.2.1. Observations
After the first micropacemaker (device I) was implanted in sheep B but did not show capture within 10 minutes, a second device was implanted (device II). Within several minutes of the implantation of the second device, both devices resulted in ventricular capture. The minimal intervals on OD measured for devices I and II were 0.151 s and 0.121 s, respectively (figure 4), and stimulus strengths were identical (battery voltage was equal to 3.9 V). On POD2, device I was no longer capturing the myocardium, but device II was still resulting in ventricular capture with a longer minimal interval period of 0.161 s (vs. 0.121 s on OD) and less overlap observed (figure 4, POD2). This measurement was in the presence of a lower heart rate (188 bpm instead of 242 bpm). Both devices had a battery voltage of 3.7 V.
Figure 4.
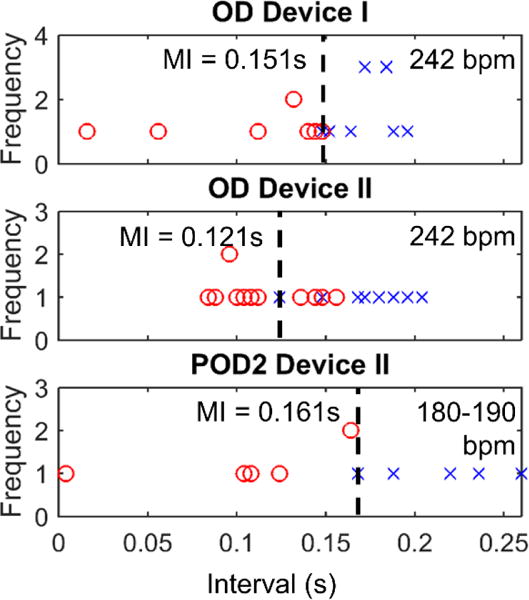
Implantation of 2 devices into sheep B complicated results, but it was shown that device II had a better electrode-tissue interface.
At necropsy, device II was observed to be well attached to the heart with about 2 turns of the electrode corkscrew embedded in the epicardium. Device I was lying adjacent to the myocardium with device II being screwed through the distal end of the first electrode, resulting in contact of the first electrode with the epicardium (figure 4).
3.2.2. Interpretation
Considering that capture for device I was seen after device II was advanced, it is thought that device II actually anchored device I to the myocardium, causing device I to capture the tissue. The lower minimal interval seen for the second device on OD suggests that this device had a more favorable electrode tissue interface. Considering that the minimal interval measurements from both devices were subject to the same autonomic effects, this difference between the measurements was entirely due to the electrode tissue interface. This interpretation is further confirmed by the necropsy results demonstrating that device II’s electrode was screwed through device I’s distal electrode and well anchored into the myocardium, while device I was pushed up against the epicardial surface without penetrating it (figure 5).
Figure 5.
This X-Ray image taken of the cardiac tissue blocked around the implanted electrodes in sheep B shows the orientation of the electrodes as they were implanted into the epicardium. Device II’s electrode is wound through the distal end of device I’s electrode.
Capture was seen on POD2 for only device II and a longer minimal interval was observed compared to OD (0.161 s vs 0.121 s). This change in minimal interval could be due to a reduction in autonomic sympathetic tone and an increase in threshold of the myocytes (indicated by a drop in heart rate). In addition, the stimulus strength also dropped from OD to POD2 (2.73 μC to 2.60 μC), which could also be a contributing factor to the lengthening of the minimal interval.
3.3. Sheep C
3.3.1. Observations
For sheep C, a pacemaker with a larger stimulus output (6.1 μC vs 2.7 μC) was used. This was chosen to counteract the loss of capture seen for Device I on POD2 in sheep B, the cause of which was not yet completely clear (although later suspected to be due to the intertwined electrode placements). The higher stimulus output resulted in a faster battery depletion, from 3.9 V on OD to 3.0 V on POD2, so recharging took place on POD2. Capture of the heart tissue was seen on OD after implant and on POD2 after recharging the device. On OD, the minimal interval observed was 0.175 s (figure 6, OD). The device continued to capture the myocardium on POD2, showing a slightly shorter minimal interval of 0.168 s (figure 6, POD2). The heart rate on POD2 fluctuated between 170 bpm and 200 bpm during measurement, as opposed to the stable heart rate of about 157 bpm seen on OD. Necropsy demonstrated that the electrode was in contact with the myocardial surface, but was not penetrating into the myocardium.
Figure 6.
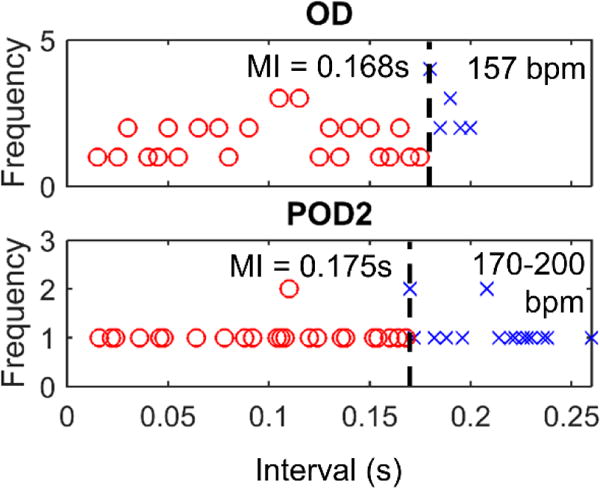
Minimal intervals for sheep C.
3.3.2. Interpretation
Due to the nearly double stimulus charge output used for sheep C as compared to the other 3 sheep, thresholds inferred in this animal are not able to be directly compared with sheep A, B, and D. However, we may be able to make a general conclusion that because the minimal intervals measured for sheep C were similar as previous implants while the output strength used was much higher, thresholds may have been much higher than sheep A and B. This would be consistent with the non-penetrating electrode tissue interface confirmed upon necropsy and histology. Nevertheless, the pacemaker continued to capture the myocardium on POD2, likely due to the higher output stimulus used. The slightly shorter minimal interval may have been related to transient adrenergic elevation on POD2, as suggested by a higher fetal heart rate (170 bpm to 200 bpm on POD2 vs 157 bpm on OD).
3.4. Sheep D
3.4.1. Observations
The standard lower output device was implanted for sheep D (battery voltage = 3.7 V, output stimulus = 2.67 μC). Capture was only confirmed on OD and was not seen on POD2. Recharging was performed on POD2 in an attempt to raise the output and the chance for capture of the tissue, but was not successful. Therefore, measurements are only included for OD (figure 7).
Figure 7.
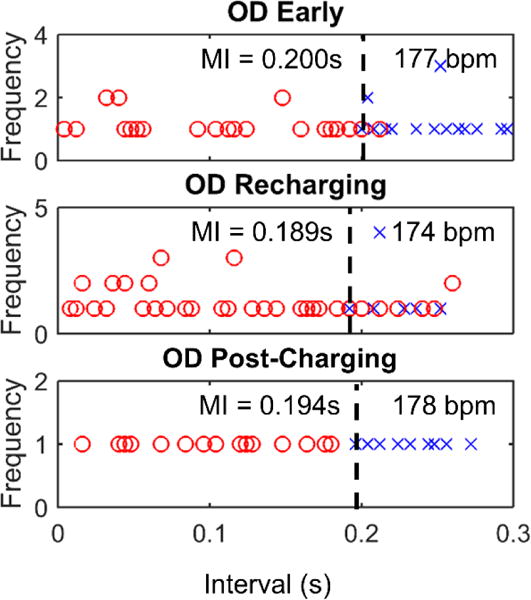
Minimal intervals for Sheep D.
The minimal interval early in the implant was 0.200 s (figure 7, OD Early) and settled to about 0.194 s at the end of data collection (figure 7, OD Post-Charging), indicating little change over time. The recharging equipment was tested during this implantation procedure and the minimal interval was measured during recharging to determine if the increased stimulus strength from the higher supply voltage during recharging affected the minimal capture interval. A minimal interval of 0.189 s was observed with recharging, but capture was intermittent at intervals longer than 0.189 s (figure 7, OD Post-Charging). At necropsy of the implant, the electrode was easily detached from the myocardium and the friction disk was seen to lie on the outside of the pericardium suggesting that the insertion cannula had not penetrated the pericardial membranes.
3.4.2. Interpretation
The evidence from necropsy indicated poor contact between the electrode and myocardium, which is supported by the very long minimal interval in comparison to earlier animals (sheep A and B).
The decreased minimum intervals during recharging (0.189 s vs 0.200 s) could be attributed to the higher charge delivered to the tissue during recharging. The fact that successful stimuli were not consistently seen above the minimal interval may have indicated that the effects of recharging on the supply voltage are not constant. This inconsistency in output strength could be due to movement of the device in relation to the recharging field or due to noise on the supply line from the high intensity electromagenetic field used to recharge.
On POD2, no device capture was seen, even during recharging. This indicates that the poor electrode tissue interface could not be overcome with the higher stimulus strength during recharging.
3.5. Maternal and Fetal Health and Mortality
Maternal sheep were observed to be in good health in the post-operative period and on each follow up day. No evidence was seen of infection and no notable changes in behavior were observed.
During the follow-up period, fetal sheep B was carried until euthanasia whereas fetal sheep A, C, and D died and were prematurely delivered. Histology for sheep A suggested the cause of death was infection related, but no cause was determined for sheep C or D. The only possible indication for fetal distress that was observed was a fetal heart rate increase in sheep A, from 178 bpm on POD 1 to 262 bpm on POD3. This change is still within the normal range of heart rates for fetal sheep, and did not cause alarm to the investigators. There is no evidence to suggest that the cardiac stimulation was linked to the premature deaths.
4. Discussion
The pilot study presented here of indirectly assessing the capture threshold of cardiac tissue showed that minimal interval observations convey valuable information about the status of each implant. The variety of electrode placements and clinical outcomes in these case studies provided discrete data points for this interpretation. The results of our minimal interval analysis were generally consistent with the conditions observed post mortem. A poor or deteriorating electrode tissue interface can be distinguished from an excellent one. For instance, the device implanted in sheep D had a much higher minimal interval than the device implanted in sheep A (0.155 s vs 0.200 s). Accordingly, necropsy showed that the electrode in sheep A was well embedded in the tissue while the electrode in sheep D was not.
Data obtained from the 4 animal implantations were not adequate to establish exact measures of safety factor, which would have required many follow up measurements after the implant and fetus stabilized. Premature animal deaths, possibly related to the lack of postoperative recovery period or the invasive implantation procedures required for fetal skin electrode placement, meant relatively short follow-up times for each animal. In addition, the need to minimize data collection times to minimize stress due to animal immobilization resulted in overall limited windows for exhaustive minimal interval measurements. Ultimately, comparing the safety factor used with these experiments with actual measurement of ventricular capture threshold by direct lead testing (not possible with our pacemaker) would allow for a precise understanding of the utility of our safety factor measurements.
Exact safety factor measurements would also be limited by the range of supply voltages achievable between the maximal safe voltage for the lithium cell (4.0 VDC) and the minimal supply voltage at which the relaxation oscillator generates output (1.8 VDC). Additional supply voltage is generated during the recharging process, and the newest version of the pacemaker can achieve an increase in the voltage supply of 1 V per applied mA in recharging current (up to 3 mA maximal safe charging rate). This enlarges the range of possible stimulus strengths, but the relationship is noisy as a result of the effects of the high field strength from the 6.78 MHz recharging coil (notably inconsistent minimal intervals in figure 7 during recharging).
The method employed in this paper to assess the capture of cardiac tissue has several other limitations, including the ability to control confounding variables, the time it takes to collect data, and the limited situations in which this is a viable measurement method. In order to determine if threshold changes are due to the electrode tissue interface, the effects of cardiac autonomic tone on threshold must be controlled or compensated. This can be done by using heart rate as a measure of autonomic tone and discarding data when heart rate is not constant. Drugs that affect sympathetic tone and fetal heart rate, such as positive or negative chronotropes, could also be administered via the maternal circulation in order to keep this variable constant during measurement; however, the utility of these medications would be confounded by their direct effects on tissue refractory periods. Data collection itself is a time-consuming process, and the length of ECG needed to produce interpretable data can range from a fraction of a minute to several minutes. This further compounds the problem of controlling for autonomic effects because they can vary from second to second, especially if the mother is not sedated. The stimulus strength in these experiments was not held constant from day to day, and therefore added a variable to consider when interpreting data. Stimulus strength could be controlled by starting each data acquisition session with a recharging step, so that the power supply, and therefore the charge delivered, from day to day is constant. The initial design of the study included this step, but technical difficulties with the recharging system limited its implementation in the studies presented here. Once this step is taken, interpreting shifts in minimal-interval in terms of the desired strength-interval curve will be more straightforward.
This method is only available as a measurement tool when the natural heart rate is above the rate of the pacemaker, therefore restricting its use to pre-clinical studies of healthy fetuses that have a high resting heart rate. A very good timing signal of the fetal ECG is also necessary, which we obtain here using chest leads directly on the fetus, a step that would not be practical or safe in human clinical studies. It has been shown that obtaining fetal ECG from the maternal abdomen is possible, but the fetal signals can at times be so attenuated or contaminated with other higher amplitude maternal signals (i.e. maternal ECG or EMG) that the fetal QRS signatures are unreadable (Crowe et al., 1995; Sameni and Clifford, 2010). The stimulus artefact, which is much larger in amplitude than the fetal ECG, should be readily detectable on the maternal abdomen. Although less precise than determining timing of ventricular electrical events, capture or non-capture can be readily determined by Doppler ultrasound, allowing extrapolation of the minimal interval (Kotas et al., 2011).
Observations on more implantations would need to be performed in order to more fully characterize this novel method of assessing threshold and safety factor. Additional data could include tracking a longer term device implant and then looking for the typical threshold changes expected with pacemaker leads: a low threshold during implantation, including short-term instability due to initial tissue injury, and then a stabilization of threshold at a somewhat higher value over the ensuing month (Mond and Stokes, 1992; Danilovic and Ohm, 1999). Future studies could further characterize the relationship between thresholds and minimal interval, and prove the effectiveness of the measurement by improving the quality and length of data collection.
In conclusion, this indirect method of measuring threshold is able to identify changes that result from autonomic effects and electrode-tissue interface. It yields a quantitative estimate of threshold when stimulus strength cannot be varied programmatically.
Acknowledgments
The authors would like to thank Drs. Ramen Chmait, Michael Silka, Jay Pruetz, and Catalina Guerra for implanting devices and their clinical expertise, and consultant Ray Peck for fabrication and engineering expertise. This work was generously funded by the Coulter Foundation, the NIH (grant #: 5R01HD075135-02), the Wright Foundation, and Southern California Clinical and Translational Science Initiative.
References
- Assad RS, Zielinsky P, Kalil R, Lima G, Aramayo A, Santos A, Costa R, Marcial MB, Oliveira SA. New lead for in utero pacing for fetal congenital heart block. The Journal of Thoracic and Cardiovascular Surgery. 2003;126:300–2. doi: 10.1016/s0022-5223(03)00220-4. [DOI] [PubMed] [Google Scholar]
- Bar-Cohen Y, Loeb GE, Pruetz JD, Silka MJ, Guerra C, Vest AN, Zhou L, Chmait RH. Preclinical testing and optimization of a novel fetal micropacemaker. Heart Rhythm. 2015;12:1683–90. doi: 10.1016/j.hrthm.2015.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter RJ, Jr, Strasburger JF, Garson A, Jr, Smith RT, Deter RL, Tristan Engelhardt H., Jr Fetal ventricular pacing for hydrops secondary to complete atrioventricular block. Journal of the American College of Cardiology. 1986;8:1434–6. doi: 10.1016/s0735-1097(86)80319-9. [DOI] [PubMed] [Google Scholar]
- Crowe J, Harrison A, Hayes-Gill B. The feasibility of long-term fetal heart rate monitoring in the home environment using maternal abdominal electrodes. Physiological Measurement. 1995;16:195–202. doi: 10.1088/0967-3334/16/3/006. [DOI] [PubMed] [Google Scholar]
- Danilovic D, Ohm O-J. Pacing threshold trends and variability in modern tined leads assessed using high resolution automatic measurements: Conversion of pulse width into voltage thresholds. Pacing and Clinical Electrophysiology. 1999;22:567–87. doi: 10.1111/j.1540-8159.1999.tb00498.x. [DOI] [PubMed] [Google Scholar]
- Eghtesady P, Michelfelder E, Knilans T, Witte D, Manning P, Crombleholme T. Fetal surgical management of congenital heart block in a hydropic fetus: lessons learned from a clinical experience. J Thorac Cardiovasc Surg. 2011:835–7. doi: 10.1016/j.jtcvs.2010.06.048. [DOI] [PubMed] [Google Scholar]
- Friedman DM, Kim MY, Copel JA, Davis C, Phoon CKL, Glickstein JS, Buyon JP, Investigators f t P Utility of Cardiac Monitoring in Fetuses at Risk for Congenital Heart Block. Circulation. 2008;117:485–93. doi: 10.1161/CIRCULATIONAHA.107.707661. [DOI] [PubMed] [Google Scholar]
- Groves AM, Allan LD, Rosenthal E. Outcome of isolated congenital complete heart block diagnosed in utero. Heart. 1996;75:190–4. doi: 10.1136/hrt.75.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotas M, Jezewski J, Horoba K, Matonia A. Application of spatio-temporal filtering to fetal electrocardiogram enhancement. Computer Methods and Programs in Biomedicine. 2011;104:1–9. doi: 10.1016/j.cmpb.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Loeb GE, Zhou L, Zheng K, Nicholson A, Peck RA, Krishnan A, Silka M, Pruetz J, Chmait R, Bar-Cohen Y. Design and Testing of a Percutaneously Implantable Fetal Pacemaker. Annals of Biomedical Engineering. 2013;41:17–27. doi: 10.1007/s10439-012-0631-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes LM, Tavares GMP, Damiano AP, Lopes MAB, Aiello VD, Schultz R, Zugaib M. Perinatal Outcome of Fetal Atrioventricular Block. Circulation. 2008;118:1268–75. doi: 10.1161/CIRCULATIONAHA.107.735118. [DOI] [PubMed] [Google Scholar]
- Mond H, Stokes K. The Electrode Tissue Interface: The Revolutionary Role of Steroid Elution. Pacing and Clinical Electrophysiology. 1992;15:95–107. doi: 10.1111/j.1540-8159.1992.tb02905.x. [DOI] [PubMed] [Google Scholar]
- Norton ME, Chauhan SP, Dashe JS. Society for Maternal-Fetal Medicine (SMFM) Clinical Guideline #7: nonimmune hydrops fetalis. American Journal of Obstetrics and Gynecology. 2015;212:127–39. doi: 10.1016/j.ajog.2014.12.018. [DOI] [PubMed] [Google Scholar]
- Sameni R, Clifford GD. A review of fetal ECG signal processing; issues and promising directions. The Open Pacing, Electrophysiology & Therapy Journal. 2010;3:4–20. doi: 10.2174/1876536X01003010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt KG, Ulmer HE, Silverman NH, Kleinman CS, Copel JA. Perinatal outcome of fetal complete atrioventricular block: A multicenter experience. Journal of the American College of Cardiology. 1991;17:1360–6. doi: 10.1016/s0735-1097(10)80148-2. [DOI] [PubMed] [Google Scholar]
- Silverman NH, Kohl T, Harrison MR, Hanley FL. Experimental fetal surgery in the animal model and in the human fetus. In: Imai Y, M K, editors. Second World Congress of Pediatric Cardiology and Cardiac Surgery. Honolulu, HI: Futura Publishing; 1998. pp. 622–3. [Google Scholar]
- Van Mieghem T, Martin A, Weber R, Barrea C, Windrim R, Hornberger L, Jaeggi E, Ryan G. Fetal cardiac function in recipient twins undergoing fetoscopic laser ablation of placental anastomoses for stage IV twin-twin transfusion syndrome. Ultrasound in Obstetrics & Gynecology. 2013;42:64–9. doi: 10.1002/uog.12454. [DOI] [PubMed] [Google Scholar]
- Walkinshaw SA, Welch CR, McCormack J, Walsh K. In utero Pacing for Fetal Congenital Heart Block. Fetal Diagnosis and Therapy. 1994;9:183–5. doi: 10.1159/000263929. [DOI] [PubMed] [Google Scholar]
- Zhou L, Vest AN, Chmait RH, Bar-Cohen Y, Pruetz J, Silka M, Zheng K, Peck R, Loeb GE. A percutaneously implantable fetal pacemaker. Engineering in Medicine and Biology Society (EMBC), 2014 36th Annual International Conference of the IEEE: IEEE. 2014:4459–63. doi: 10.1109/EMBC.2014.6944614. [DOI] [PMC free article] [PubMed] [Google Scholar]


