Abstract
The compatibility of the Negishi cross-coupling reaction with the versatile B–Cl functionality has been demonstrated in the context of late-stage functionalization of 1,2-azaborines. Alkyl-, aryl-, and alkenylzinc reagents have been utilized for the functionalization of the triply orthogonal precursor 3-bromo-1-(tert-butyldimethylsilyl)-2-chloro-1,2-dihydro-1,2-azaborine (2) to furnish new 2,3-substituted monocyclic 1,2-azaborines. This methodology has enabled the synthesis of previously elusive BN-naphthalene and BN-indenyl structures from a common intermediate.
Research into boron–nitrogen (BN) isosteres of classic organic molecules has garnered significant attention because of its potential to expand the chemical space of compounds in biomedical research and materials science.1 1,2-Dihydro-1,2-azaborines (abbreviated as 1,2-azaborines) are BN isosteres of the ubiquitous monocyclic arene motif.2 As an emerging heterocyclic structure, only limited synthetic methods are currently available for the generation of substituted monocyclic 1,2-azaborine derivatives. In addition to the earlier work by Dewar,3,4 White,5 Ashe,6,7 and our group,8 Yamaguchi9 recently prepared a 3,6-diaryl-1,2-azaborine from a N-Boc-protected bis(phenylpyrrolyl)borane using a ring-expansion rearrangement. Furthermore, Braunschweig developed two complementary synthetic approaches to highly substituted monocyclic 1,2-azaborines: (1) ring expansion of boroles with azides10 and (2) Rh-mediated cycloaddition of di-t-Bu-iminoborane and alkynes.11 Our group has recently focused on selective late-stage functionalization as a general approach to produce an array of derivatives from an assembled 1,2-azaborine core, and we have demonstrated this concept through a C6-selective borylation with a subsequent Suzuki cross-coupling.12 Despite the accomplishments made to date, the chemistry of 1,2-azaborines is still in its developing stages, and new, versatile synthetic strategies for monocyclic 1,2-azaborines in particular are needed to prepare previously inaccessible BN heterocycles.
In 2007, Ashe described the regioselective bromination of N-Et-B-Ph-1,2-azaborine at C3.13 This achievement should allow selective late-stage functionalization at C3 via cross-coupling technologies. Although not specifically demonstrated on a monocyclic 1,2-azaborine, Molander14 and Fang15 showed that a variety of cross-coupling methods, including Suzuki, Kumada, Sonogashira, and Heck reactions, can be performed on halogenated BN-naphthalenes.
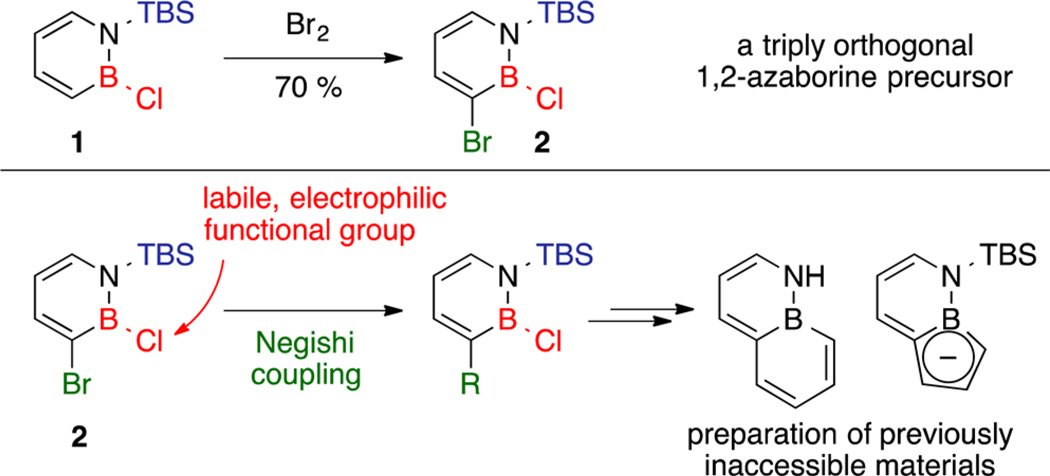
Since 1,2-azaborines contain the boron as an integral element of the heterocycle, a potential concern with the development of late-stage functionalization methods is compatibility with the reactivity associated with boron. This issue can be addressed by placing a relatively inert carbon-based substituent at boron (e.g., alkyl or aryl) to “protect” the boron prior to the late-stage functionalization process. The preinstallation of the B substituent, however, may limit the synthetic strategic options. A perhaps more general synthetic strategy is to develop a late-stage functionalization that is compatible with a labile boron substituent (e.g., B-X, X = Cl, Br) so that after initial “functionalization” the boron position is still available for derivatization. However, maintaining the reactive B–X bond while performing cross-coupling chemistry requires the nucleophilic reagent to couple preferentially with an electrophile (e.g., aryl halide) that is typically significantly less electrophilic than the B–X group. It is thus not surprising that the compatibility of the B–X (X = Cl, Br) bond—a functional group commonly involved in the synthesis of boron-containing materials16—with C–C bond-forming cross-coupling reactions has remained virtually unexplored.17 Here we show that Negishi cross-coupling at C3 of the triply orthogonal 1,2-azaborine precursor 2 (easily prepared from N-TBS-B-Cl-1,2-azaborine (1); Scheme 1, top) is compatible with the B-Cl functional group, adding a new strategic dimension in terms of the sequence of functionalizations of 1,2-azaborines. We also describe the synthesis of new BN isosteres of naphthalene and indenyl using our method (Scheme 1, bottom).
Scheme 1. Negishi Coupling in the Presence of a B–Cl Bond.
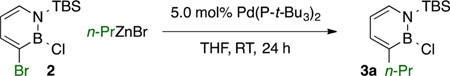
We considered the Negishi cross-coupling as an appealing method for the regioselective functionalization of 1,2-azaborine 2 because (1) it enables cross-coupling of aryl halides and alkyl-, aryl-, alkenyl-, alkynyl-, and heteroarylzinc halides with a reasonable functional group tolerance;18 (2) zinc reagents are nontoxic and readily available;19 and (3) unlike Suzuki coupling, it does not require borophilic additives as activating agents.20 To probe the viability of Negishi couplings in the presence of reactive boron centers in 1,2-azaborines, we treated 2 with stoichiometric diethylzinc and n-propylzinc bromide in tetrahydrofuran (THF). Gratifyingly, no background reactivity was observed at room temperature over 24 h. We then pursued optimization of the regioselective Negishi cross-coupling at C3 of 2 using n-propylzinc bromide as a model nucleophile because of the higher availability and lower reactivity of RZnX reagents.
Our optimization studies of Negishi cross-coupling of 2 identified Pd(P-t-Bu3)221 as an effective catalyst for the desired transformation, furnishing a benchmark yield of 87% (Table 1, entry 1). No reactivity was observed in the absence of a Pd catalyst (entry 2). Other Pd-based catalysts were active, but the yields were consistently lower than that with the Pd(P-t-Bu3)2 system (entries 3–5). Ni-based precatalysts gave poor yields and complex reaction mixtures (entries 6 and 7).22,23 A solvent switch to diethyl ether (entry 8) was not detrimental to the observed yield of 3a. However, the use of a nonpolar solvent such as toluene decreased the yield of 3a significantly (entry 9). Use of N-methyl-2 pyrrolidinone as an additive24 did not decrease the reaction yield (entry 10). The reaction time could be shortened to 3 h with no significant loss in yield (entry 11).
Table 1.
Survey of Catalysts and Solvents for the Regioselective Negishi Cross-Coupling of 1,2-Azaborine 2
 | ||
|---|---|---|
| entry | deviation from the standard conditionsa | yield (%)b |
| 1 | no deviation | 87 |
| 2 | no Pd catalyst | NR |
| 3 | catalyst: 5 mol % PdCl2(P-o-tol)3 | 75 |
| 4 | catalyst: 5 mol % (Xphos)PdG2 | 79 |
| 5 | catalyst: 5 mol % (PCy3)PdG2 | 63 |
| 6 | catalyst: 5 mol % NiCl2(PCy3)2 | 35 |
| 7 | catalyst: 5 mol % Ni(cod)2 + terpy | 31 |
| 8 | Et2O instead of THF | 80 |
| 9 | toluene instead of THF | 43 |
| 10 | additive: N-methyl-2-pyrrolidinone | 82 |
| 11 | 3 h instead of 24 h | 87 |
Abbreviations: Cy = cyclohexyl, PdG2 = chloro[2-(2′-amino-1,1′-biphenyl)]palladium(II), cod = cyclooctadiene, terpy = terpyridine.
Determined by 1H NMR spectroscopy against a calibrated internal standard; averages of two runs.
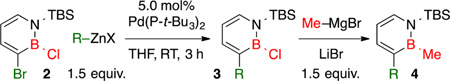
With the optimized conditions in hand, we investigated the reaction scope with respect to the organozinc nucleophile. The sensitivity of the Negishi cross-coupling products 3a–f toward moisture due to the presence of the B-Cl group made their isolation challenging. Nevertheless, we were able to determine the product yields of 3a–f by 1H NMR spectroscopy in the presence of an internal standard (see the Supporting Information (SI) for details). After a workup procedure following the Negishi coupling (see the SI for details), B-Cl compounds 3a–f could be reacted with LiBr-activated Me–MgBr25 to generate the readily isolable and chromatography-stable B-Me derivatives 4a–f (Table 2). Our model reaction with n-Pr–ZnBr gave 4a in 76% isolated yield (entry 1). Vinylzinc bromides were also suitable coupling partners (entries 2 and 3). An alkylzinc bromide bearing an acetal functional group was tolerated (entry 4). Halogenated arylzinc nucleophiles were also compatible with our optimized reaction conditions (entries 5 and 6).
Table 2.
Regioselective Negishi Cross-Coupling of 1,2-Azaborine 2: Scope of the Organozinc Nucleophile
 | |||
|---|---|---|---|
| entry | R–ZnX | 3, yield (%)a | 4, yield (%)b |
| 1 | n-Pr–ZnBr | 3a, 87 | 4a, 76 |
| 2 | 1-phenylvinyl–ZnBr | 3b, 97 | 4b, 97 |
| 3 | vinyl–ZnBr | 3c, 69c | 4c, 51d |
| 4 | 2-(1,3-dioxan-2-yl)ethyl–ZnBr | 3d, 69 | 4d, 66 |
| 5 | 4-chlorophenyl–ZnI | 3e, 83 | 4e, 50 |
| 6 | 3,4,5-trifluorophenyl–ZnBr | 3f, 91 | 4f, 89e |
Determined by 1H NMR spectroscopy against a calibrated internal standard in a separate experiment (0.098 mmol scale); averages of two runs.
Isolated yields (0.654 mmol scale); averages of two runs.
1.2 equiv of vinyl–ZnBr generated from 1.2 equiv of vinyl–MgBr and 2.4 equiv of ZnBr2, 24 h reaction time.
1.01 equiv of vinyl–ZnBr generated from 1.01 equiv of vinyl–MgBr and 2 equiv of ZnBr2, 22 h reaction time.
1.01 equiv of Me–MgBr instead of 1.5 equiv.
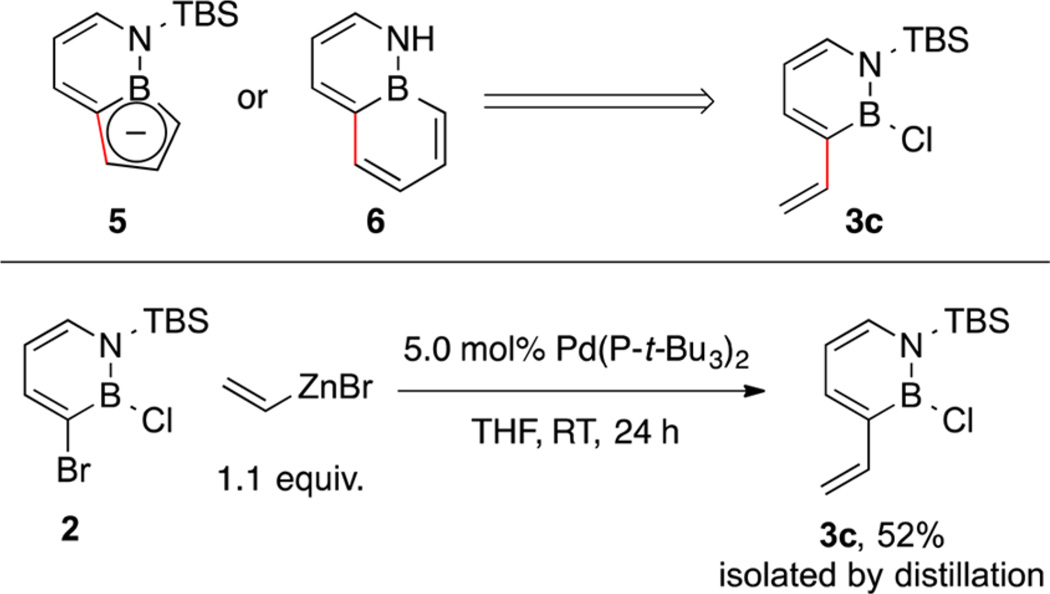
We identified compound 3c as a common intermediate for the synthesis of new BN-indenyl 5 and BN-naphthalene 6 using ring-closing metathesis (RCM) as a key strategy (Scheme 2, top). Thus, we scaled up the synthesis of intermediate 3c using our developed Negishi coupling procedure and isolated it as a colorless oil after distillation in 52% yield (Scheme 2, bottom).
Scheme 2. Compound 3c as a Versatile Common Intermediate for the Synthesis of BN Heterocycles 5 and 6.
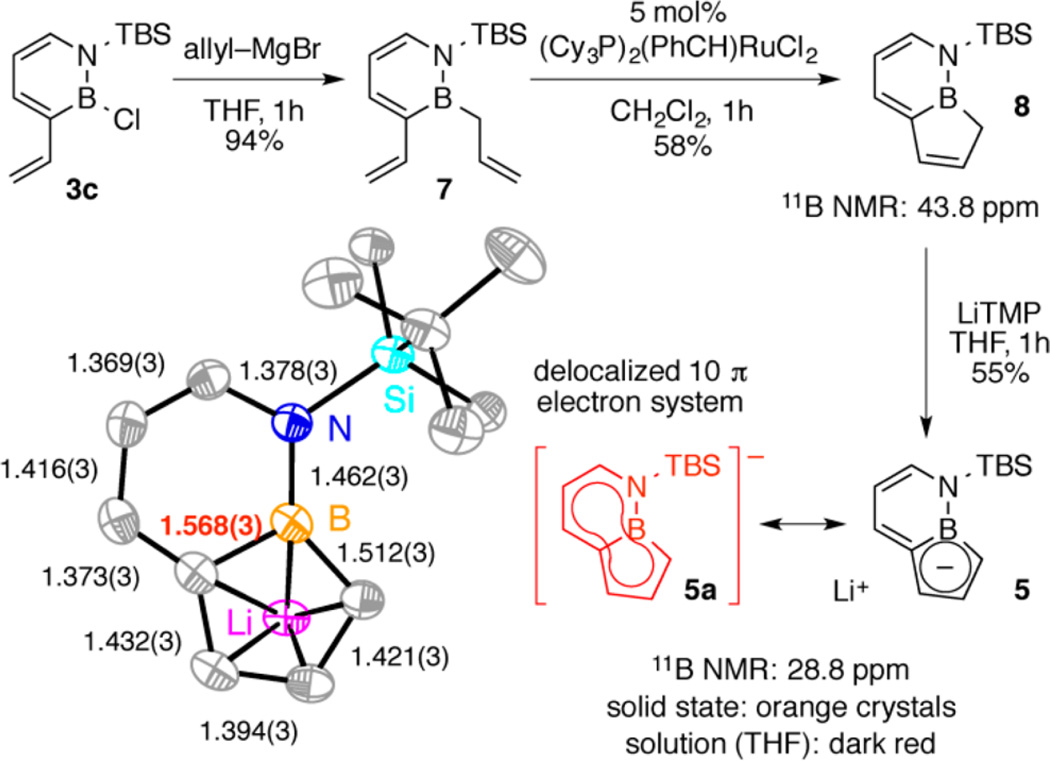
1,2-Azaborine 3c reacted with allyl–MgBr to produce intermediate 7 in excellent yield (Scheme 3). Subsequent RCM with Grubbs’ first-generation RCM catalyst provided 8 as a single isomer in moderate yield. Lithium tetramethylpiperidide (LiTMP) readily deprotonated 8 to generate the intensely colored BN-indenyl 5.26 It is worth noting that an approach involving initial installation of the allyl group at boron was not successful in our hands because the B-allyl substituent was prone to isomerization to the thermodynamically more stable internal B-alkenyl group under our cross-coupling conditions.
Scheme 3. Synthesis and ORTEP Plot of BN-Indenyl 5a.
aThermal ellipsoids at the 35% probability level; H atoms and two THF molecules (coordinated to Li+) omitted for clarity.
Single-crystal X-ray diffraction (XRD) analysis of 5 unambiguously confirmed the indenyl structure (Scheme 3). In the solid state, the molecule is planar and η5-bound to a lithium atom through the five-membered ring. The bond distances in the six-membered azaborine ring are consistent with previously reported bond lengths for monocyclic azaborines27 with the exception of the bridging B–C bond (bond distance highlighted in red), which is significantly longer than that found in typical monocyclic azaborine structures (1.568(3) Å vs ~1.52 Å).27 An elongation of the bridging C–C bond in the indene/indenyl system is also observed upon deprotonation, but to a somewhat lesser extent (~1.437 Å for Li indenyl28 vs ~1.40–1.41 Å for indene29). The bridging B–C distance of 1.568 Å approaches that of a B(sp2)–C(sp2) single bond (e.g., 1.574 Å observed for a B-Ph-substituted 1,2-azaborine8e). Thus, the observed geometric parameters for 5 are consistent with a significant contribution from the 10-π-electron delocalized resonance structure 5a.
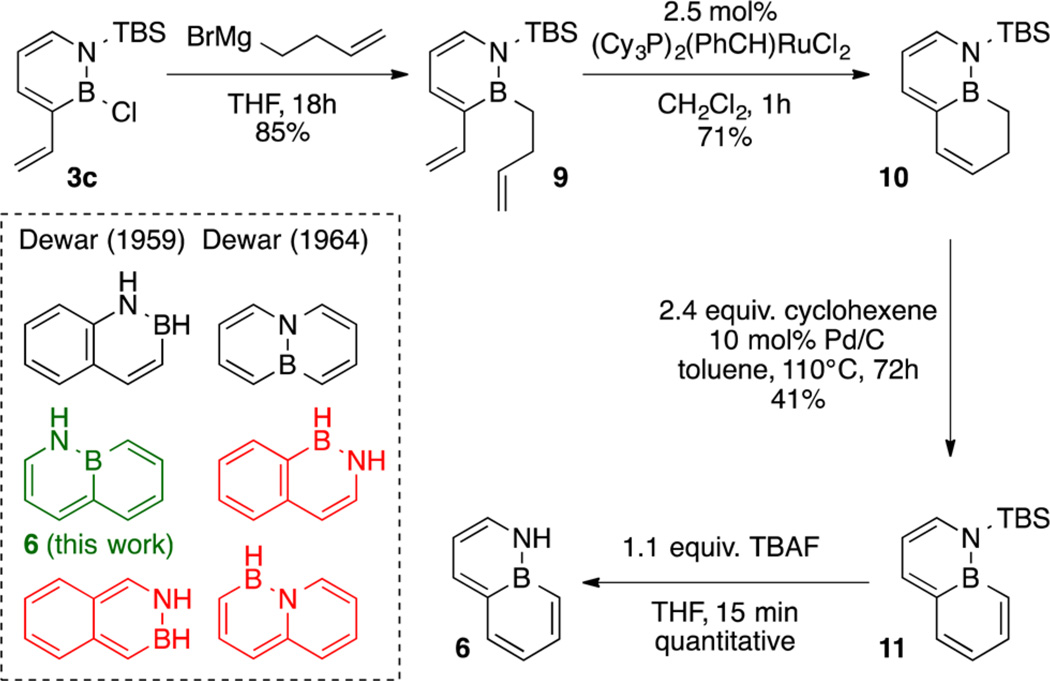
Scheme 4 illustrates the synthesis of BN-naphthalene 6. Treatment of precursor 3c with 3-butenylmagnesium bromide30 generated diene intermediate 9 in good yield. Compound 9 was competent inRCMto generate bicyclic compound 10. Oxidation of 10 using Pd/C as the catalyst with cyclohexene as a hydrogen scavenger in refluxing toluene furnished N-TBS-protected BN-naphthalene 11 in moderate yield.31 Removal of the N-TBS group with tetrabutylammonium fluoride (TBAF) proceeded in quantitative yield to generate the new parental BN-naphthalene isostere 6.32 It is worth noting that compound 6 represents the third parental BN isosterere of naphthalene (among the six possible isomers; see Scheme 4) that has been synthesized.33,34 We were able to grow single crystals of 6 suitable for XRD, but the structure was disordered because of the seemingly centrosymmetric structure of 6. The extended packing diagram shows an edge-to-face herringbone packing similar to that observed in carbonaceous naphthalene (see the SI for details).35
Scheme 4. Synthesis of the Parental BN-Naphthalene Isostere 6; Compounds Shown in Red Have Not Been Synthesized.
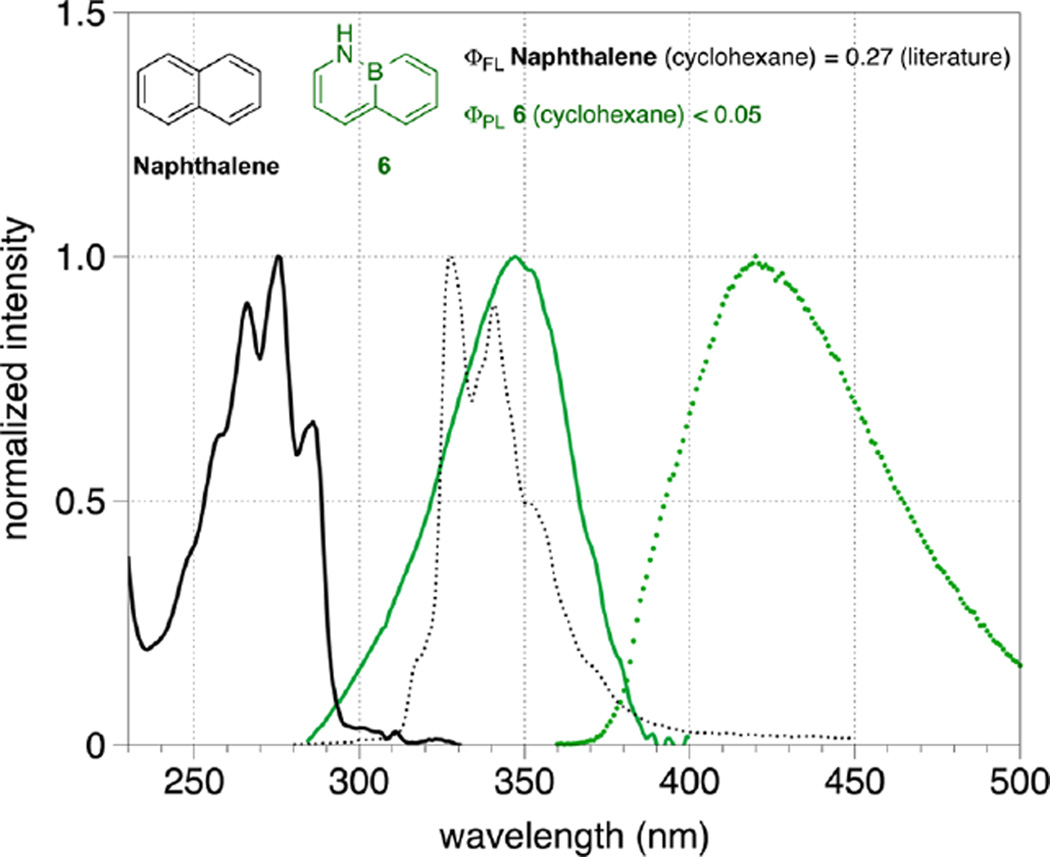
Figure 1 shows that both the absorption and emission maxima of BN-naphthalene 6 exhibit a large bathochromic shift relative to naphthalene (λabs = 347 nm and λem = 420 nm for 6 vs λabs = 275 nm and λem = 327 nm for naphthalene). The Stokes shift of parental naphthalene is 5782.6 cm−1, versus 5008.9 cm−1 for 6. This suggests that compared with naphthalene, the Franck–Condon excited state of 6 is somewhat closer in energy to the relaxed emissive state (S1), which is consistent with less spatial reorganization of atoms between the ground and excited states of BN-naphthalene 6 relative to naphthalene. Interestingly, the vibrational fine structure that is apparent in the absorption and emission spectra of naphthalene is not reproduced in the corresponding spectra for compound 6.
Figure 1.
Normalized absorption (solid green trace) and emission (dotted green trace) spectra of 6 (1 × 10−5 M in cyclohexane) overlaid with the normalized absorption (solid black trace) and emission (dotted black trace) spectra of naphthalene. The photoluminescence quantum yield of 6 was determined in cyclohexane at room temperature. The quantum yield of naphthalene was taken from the literature.36
In summary, we have developed in the context of the triply orthogonal 1,2-azaborine precursor 2 a Negishi cross-coupling protocol that is compatible with the versatile B-Cl functional group. The catalyst system selectively activates the C3–Br bond in 2 in the presence of the more electrophilic B–Cl bond to engage in C–C cross-coupling with an array of alkyl-, aryl-, and alkenylzinc nucleophiles. We have applied our method to the synthesis of a new BN isostere of indenyl (5) as well as a new BN isostere of naphthalene (6). BN-naphthalene 6 is the third parental BN isostere of naphthalene (out of six possible isomers) to be synthesized to date. Single-crystal XRD also revealed that the structure of BN-indenyl 5 is most consistent with a 10-π-electron delocalized bicyclic aromatic system. Many syntheses of boron-containing heterocycles/materials involve an intermediate bearing a labile B–X bond (X = Cl, Br). The availability of a regioselective functionalization method that leaves the B–X group untouched should broaden the strategic dimension for the synthesis of boron-containing compounds. Our current efforts are directed toward expanding the scope of the Negishi reaction in the context of 1,2-azaborines and accessing new “BN-doped” conjugated materials through a bottom-up approach from substituted monocyclic 1,2-azaborines.
Supplementary Material
Acknowledgments
This work was supported by NIH NIGMS (R01-GM094541) and NSF (CHE-1361618). S.-Y.L. thanks the Camille Dreyfus Teacher-Scholar Awards Program for a Teacher-Scholar Award.
Footnotes
ASSOCIATED CONTENT
Supporting Information
Procedures, spectroscopic data, and crystallographic data (CIF). The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.5b05879.
The authors declare no competing financial interest.
REFERENCES
- 1.For an overview of BN/CC isosterism, see: Bosdet MJD, Piers WE. Can. J. Chem. 2009;87:8. Campbell PG, Marwitz AJ, Liu S-Y. Angew. Chem. Int. Ed. 2012;51:6074. doi: 10.1002/anie.201200063. Wang X-Y, Wang J-Y, Pei J. Chem. - Eur. J. 2015;21:3528. doi: 10.1002/chem.201405627.
- 2.For the synthesis of the parental structure, see: Marwitz AJV, Matus MH, Zakharov LN, Dixon DA, Liu S-Y. Angew. Chem. Int. Ed. 2009;48:973. doi: 10.1002/anie.200805554.
- 3.Dewar MJS, Marr PA. J. Am. Chem. Soc. 1962;84:3782. [Google Scholar]
- 4.For an overview of Dewar’s work, see: Fritsch AJ. Chem. Heterocycl. Compd. (Hoboken NJ U. S.) 1977;30:381.
- 5.White DG. J. Am. Chem. Soc. 1963;85:3634. [Google Scholar]
- 6.(a) Ashe AJ, Fang Org. Lett. 2000;2:2089. doi: 10.1021/ol0001113. [DOI] [PubMed] [Google Scholar]; (b) Ashe AJ, Fang X, Fang X, Kampf JW. Organometallics. 2001;20:5413. [Google Scholar]
- 7.For an overview of Ashe’s work, see: Ashe AJ., III Organometallics. 2009;28:4236.
- 8.(a) Marwitz AJV, Abbey ER, Jenkins JT, Zakharov LN, Liu S-Y. Org. Lett. 2007;9:4905. doi: 10.1021/ol702383u. [DOI] [PubMed] [Google Scholar]; (b) Marwitz AJV, McClintock SP, Zakharov LN, Liu S-Y. Chem. Commun. 2010;46:779. doi: 10.1039/b919632c. [DOI] [PubMed] [Google Scholar]; (c) Lamm AN, Garner EB, Dixon DA, Liu S-Y. Angew. Chem. Int. Ed. 2011;50:8157. doi: 10.1002/anie.201103192. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Marwitz AJV, Lamm AN, Zakharov LN, Vasiliu M, Dixon DA, Liu S-Y. Chem. Sci. 2012;3:825. [Google Scholar]; (e) Rudebusch GE, Zakharov LN, Liu S-Y. Angew. Chem. Int. Ed. 2013;52:9316. doi: 10.1002/anie.201304443. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Abbey ER, Lamm AN, Baggett AW, Zakharov LN, Liu S-Y. J. Am. Chem. Soc. 2013;135:12908. doi: 10.1021/ja4073436. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Brown AN, Zakharov LN, Mikulas T, Dixon DA, Liu S-Y. Org. Lett. 2014;16:3340. doi: 10.1021/ol501362w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taniguchi T, Yamaguchi S. Organometallics. 2010;29:5732. [Google Scholar]
- 10.(a) Braunschweig H, Hörl C, Mailänder L, Radacki K, Wahler J. Chem. - Eur. J. 2014;20:9858. doi: 10.1002/chem.201403101. [DOI] [PubMed] [Google Scholar]; (b) Braunschweig H, Celik MA, Hupp F, Krummenacher I, Mailänder L. Angew. Chem. Int. Ed. 2015;54:6347. doi: 10.1002/anie.201500970. [DOI] [PubMed] [Google Scholar]
- 11.Braunschweig H, Geetharani K, Jimenez-Halla JOC, Schäfer M. Angew. Chem. Int. Ed. 2014;53:3500. doi: 10.1002/anie.201309707. [DOI] [PubMed] [Google Scholar]
- 12.Baggett AW, Vasiliu M, Li B, Dixon DA, Liu S-Y. J. Am. Chem. Soc. 2015;137:5536. doi: 10.1021/jacs.5b01916. [DOI] [PubMed] [Google Scholar]
- 13. Pan J, Kampf JW, Ashe AJ. Org. Lett. 2007;9:679. doi: 10.1021/ol062968r. For another report of regioselective halogenation of monocyclic 1,2-azaborines, see: Lamm AN, Liu S-Y. Mol. BioSyst. 2009;5:1303. doi: 10.1039/b904120f.
- 14.(a) Molander GA, Wisniewski SR. J. Org. Chem. 2014;79:6663. doi: 10.1021/jo5011894. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Molander GA, Wisniewski SR, Traister KM. Org. Lett. 2014;16:3692. doi: 10.1021/ol501495d. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Molander GA, Wisniewski SR. J. Org. Chem. 2014;79:8339. doi: 10.1021/jo501638q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun F, Lv L, Huang M, Zhou Z, Fang X. Org. Lett. 2014;16:5024. doi: 10.1021/ol502339h. [DOI] [PubMed] [Google Scholar]
- 16.For examples, see: Liu S-Y, Lo MMC, Fu GC. Angew. Chem. Int. Ed. 2002;41:174. doi: 10.1002/1521-3773(20020104)41:1<174::aid-anie174>3.0.co;2-7. Mercier LG, Piers WE, Parvez M. Angew. Chem. Int. Ed. 2009;48:6108. doi: 10.1002/anie.200902803. Caruso A, Jr, Siegler MA, Tovar JD. Angew. Chem. Int. Ed. 2010;49:4213. doi: 10.1002/anie.201000411. Weber L, Werner V, Fox MA, Marder TB, Schwedler S, Brockhinke A, Stammler H-G, Neumann B. Dalton Trans. 2009;8:1339. doi: 10.1039/b815931a. Biswas S, Oppel IM, Bettinger HF. Inorg. Chem. 2010;49:4499. doi: 10.1021/ic902436s. Braunschweig H, Kupfer T. Chem. Commun. 2011;47:10903. doi: 10.1039/c1cc13071d. Lorbach A, Bolte M, Li H, Lerner H-W, Holthausen MC, Jäkle F, Wagner M. Angew. Chem. Int. Ed. 2009;48:4584. doi: 10.1002/anie.200901226. Hübner A, Qu Z-W, Englert U, Bolte M, Lerner H-W, Holthausen MC, Wagner M. J. Am. Chem. Soc. 2011;133:4596. doi: 10.1021/ja110947k. Chen P, Jäkle F. J. Am. Chem. Soc. 2011;133:20142. doi: 10.1021/ja209602z. Bailey JA, Haddow MF, Pringle PG. Chem. Commun. 2014;50:1432. doi: 10.1039/c3cc49000a. Liu X, Wu P, Li J, Cui C. J. Org. Chem. 2015;80:3737. doi: 10.1021/jo5029437.
- 17.To the best of our knowledge, there is one published report (on the synthesis of a yellow scale pheromone) that describes a Negishi coupling of allylzinc reagents in the presence of a B–Br bond without alkylation at boron. See: Xu Z, Negishi E. Org. Lett. 2008;10:4311. doi: 10.1021/ol8017566.
- 18.Nicolaou KC, Bulger PG, Sarlah D. Angew. Chem. Int. Ed. 2005;44:4442. doi: 10.1002/anie.200500368. [DOI] [PubMed] [Google Scholar]
- 19.Sigma-Aldrich offers 175 Rieke and organozinc reagents in their online catalog (accessed March 2015).
- 20.For recent reviews of modern metal-catalyzed cross-coupling, see: Jana R, Pathak TP, Sigman MS. Chem. Rev. 2011;111:1417. doi: 10.1021/cr100327p. Johansson Seechurn CCC, Kitching MO, Colacot TJ, Snieckus V. Angew. Chem. Int. Ed. 2012;51:5062. doi: 10.1002/anie.201107017.
- 21.(a) Dai C, Fu GC. J. Am. Chem. Soc. 2001;123:2719. doi: 10.1021/ja003954y. [DOI] [PubMed] [Google Scholar]; (b) Fu GC. Acc. Chem. Res. 2008;41:1555. doi: 10.1021/ar800148f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.(a) Huo S. Org. Lett. 2003;5:423. doi: 10.1021/ol0272693. [DOI] [PubMed] [Google Scholar]; (b) Smith SW, Fu GC. Angew. Chem. Int. Ed. 2008;47:9334. doi: 10.1002/anie.200802784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.For a review of Ni-catalyzed cross-coupling, see: Adhikary A, Guan H. In: Pincer and Pincer-Type Complexes. Szabó KJ, Wendt OF, editors. Weinheim, Germany: Wiley-VCH; 2014. pp. 117–148.
- 24.THF/NMP mixtures are common solvents in Negishi couplings. See: Jana R, Pathak TP, Sigman MS. Chem. Rev. 2011;111:1417. doi: 10.1021/cr100327p.
- 25.(a) Tilly D, Chevallier F, Mongin F, Gros PC. Chem. Rev. 2014;114:1207. doi: 10.1021/cr400367p. [DOI] [PubMed] [Google Scholar]; (b) Bao RL-Y, Zhao R, Shi L. Chem. Commun. 2015;51:6884. doi: 10.1039/c4cc10194d. [DOI] [PubMed] [Google Scholar]
- 26.LiHMDS (pKa ~ 30) failed to deprotonate 8, suggesting a much higher pKa for 8 than for indene (pKa ~ 20)
- 27.For a structural analysis of 1,2-azaborines, see: Abbey ER, Zakharov LN, Liu S-Y. J. Am. Chem. Soc. 2008;130:7250. doi: 10.1021/ja8024966.
- 28.(a) Jones JN, Cowley AH. Chem. Commun. 2005:1300. doi: 10.1039/b410549d. [DOI] [PubMed] [Google Scholar]; (b) Michel R, Herbst-Irmer R, Stalke D. Organometallics. 2011;30:4379. [Google Scholar]
- 29.(a) McGonigal PR, de Leon C, Wang Y, Homs A, Solorio-Alvarado CR, Echavarren AM. Angew. Chem. Int. Ed. 2012;51:13093. doi: 10.1002/anie.201207682. [DOI] [PubMed] [Google Scholar]; (b) Kinoshita H, Hirai N, Miura K. J. Org. Chem. 2014;79:8171. doi: 10.1021/jo501383v. [DOI] [PubMed] [Google Scholar]
- 30.With LiBr, the terminal alkene isomerized to an internal alkene.
- 31.The major side product of this reaction comes from competitive reduction of the bicylic starting material.
- 32.Compound 6 exhibits a strong odor similar to that of naphthalene.
- 33.For Dewar’s work on 2,1- and 10,9-borazaronaphthalenes, see: Dewar MJS, Dietz R. J. Chem. Soc. 1959:2728. Dewar MJS, Gleicher GJ, Robinson BP. J. Am. Chem. Soc. 1964;86:5698.
- 34.The synthesis of substituted 1,2-borazaronaphthalene has recently been reported (see ref 16k).
- 35.Capelli SC, Albinati A, Mason SA, Willis BTM. J. Phys. Chem. A. 2006;110:11695. doi: 10.1021/jp062953a. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki K, Kobayashi A, Kaneko S, Takehira K, Yoshihara T, Ishida H, Shiina Y, Oishi S, Tobita S. Phys. Chem. Chem. Phys. 2009;11:9850. doi: 10.1039/b912178a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.