Abstract
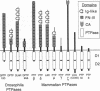
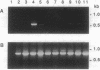
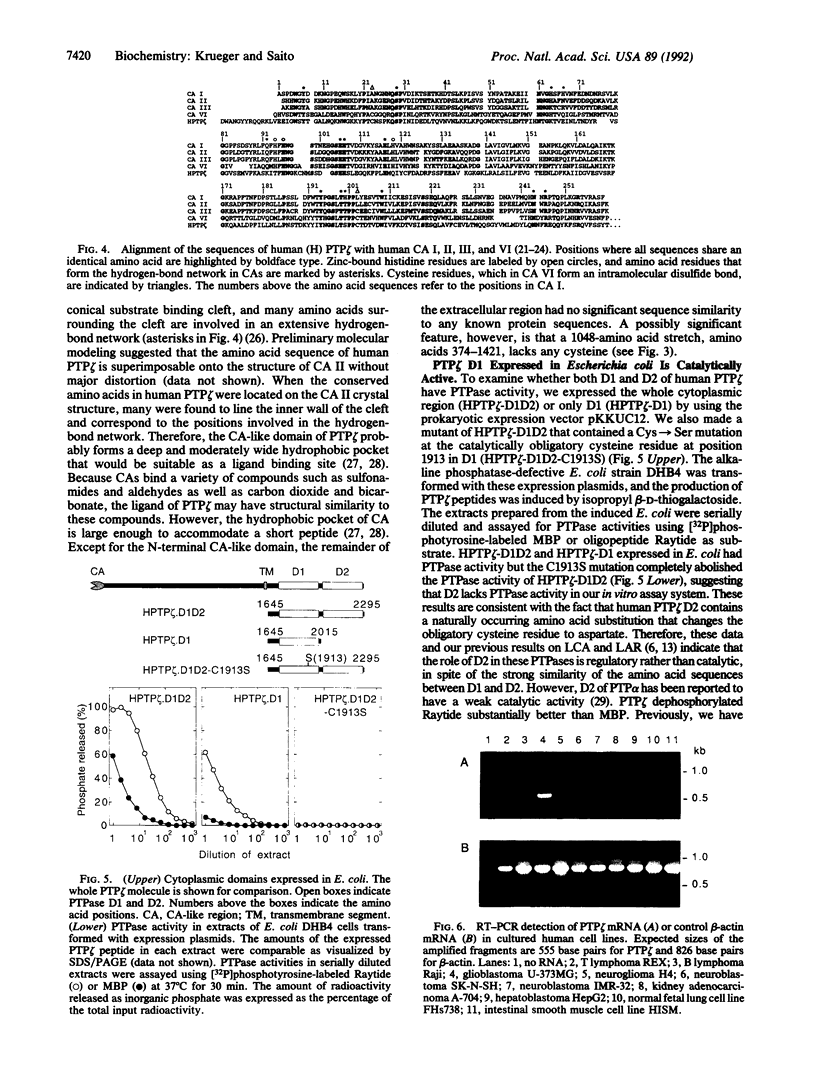
Protein-tyrosine-phosphatases (PTPases, EC 3.1.3.48) play a crucial role in the regulation of protein tyrosine phosphorylation. Recently, it was found that the PTPase gene family exhibits a large variety of different functional domains associated with the PTPase catalytic domains. In this paper, we report the complete cDNA sequence of a human transmembrane PTPase, PTP zeta, isolated from fetal brain cDNA libraries. The deduced amino acid sequence of human PTP zeta is composed of a putative signal peptide of 19 amino acids, a very large extracellular domain of 1616 amino acids, a transmembrane peptide of 26 amino acids, and a cytoplasmic domain of 653 amino acids. The extracellular portion of human PTP zeta contains two striking structural features: the N-terminal 280-amino acid sequence that is homologous to carbonic anhydrases (carbonate hydro-lyase, EC 4.2.1.1), and a sequence of 1048 amino acids without a cysteine residue. While it is unlikely that the carbonic anhydrase-like domain of PTP zeta has any carbonic anhydrase activity, its three-dimensional structure may be quite similar to that of carbonic anhydrases, a structure that appears ideal for binding a small soluble ligand. The cytoplasmic portion of human PTP zeta contains two repeated PTPase-like domains, which, when expressed in Escherichia coli, had PTPase activity in vitro. Mutational analyses indicate that only the membrane-proximal PTPase domain is catalytically active. Reverse transcription-polymerase chain reaction analyses indicate that human PTP zeta is highly expressed in a glioblastoma cell line.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldred P., Fu P., Barrett G., Penschow J. D., Wright R. D., Coghlan J. P., Fernley R. T. Human secreted carbonic anhydrase: cDNA cloning, nucleotide sequence, and hybridization histochemistry. Biochemistry. 1991 Jan 15;30(2):569–575. doi: 10.1021/bi00216a035. [DOI] [PubMed] [Google Scholar]
- Alexander R. S., Nair S. K., Christianson D. W. Engineering the hydrophobic pocket of carbonic anhydrase II. Biochemistry. 1991 Nov 19;30(46):11064–11072. doi: 10.1021/bi00110a008. [DOI] [PubMed] [Google Scholar]
- Barlow J. H., Lowe N., Edwards Y. H., Butterworth P. H. Human carbonic anhydrase I cDNA. Nucleic Acids Res. 1987 Mar 11;15(5):2386–2386. doi: 10.1093/nar/15.5.2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Eriksson A. E., Jones T. A., Liljas A. Refined structure of human carbonic anhydrase II at 2.0 A resolution. Proteins. 1988;4(4):274–282. doi: 10.1002/prot.340040406. [DOI] [PubMed] [Google Scholar]
- Fischer E. H., Charbonneau H., Tonks N. K. Protein tyrosine phosphatases: a diverse family of intracellular and transmembrane enzymes. Science. 1991 Jul 26;253(5018):401–406. doi: 10.1126/science.1650499. [DOI] [PubMed] [Google Scholar]
- Gebbink M. F., van Etten I., Hateboer G., Suijkerbuijk R., Beijersbergen R. L., Geurts van Kessel A., Moolenaar W. H. Cloning, expression and chromosomal localization of a new putative receptor-like protein tyrosine phosphatase. FEBS Lett. 1991 Sep 23;290(1-2):123–130. doi: 10.1016/0014-5793(91)81241-y. [DOI] [PubMed] [Google Scholar]
- Hariharan I. K., Chuang P. T., Rubin G. M. Cloning and characterization of a receptor-class phosphotyrosine phosphatase gene expressed on central nervous system axons in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11266–11270. doi: 10.1073/pnas.88.24.11266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M., Streuli M., Krueger N. X., Saito H. Purification and characterization of the catalytic domains of the human receptor-linked protein tyrosine phosphatases HPTP beta, leukocyte common antigen (LCA), and leukocyte common antigen-related molecule (LAR). J Biol Chem. 1992 Jun 15;267(17):12356–12363. [PubMed] [Google Scholar]
- Kaplan R., Morse B., Huebner K., Croce C., Howk R., Ravera M., Ricca G., Jaye M., Schlessinger J. Cloning of three human tyrosine phosphatases reveals a multigene family of receptor-linked protein-tyrosine-phosphatases expressed in brain. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7000–7004. doi: 10.1073/pnas.87.18.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger N. X., Streuli M., Saito H. Structural diversity and evolution of human receptor-like protein tyrosine phosphatases. EMBO J. 1990 Oct;9(10):3241–3252. doi: 10.1002/j.1460-2075.1990.tb07523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaForgia S., Morse B., Levy J., Barnea G., Cannizzaro L. A., Li F., Nowell P. C., Boghosian-Sell L., Glick J., Weston A. Receptor protein-tyrosine phosphatase gamma is a candidate tumor suppressor gene at human chromosome region 3p21. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):5036–5040. doi: 10.1073/pnas.88.11.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery J. C., Venta P. J., Tashian R. E., Hewett-Emmett D. Nucleotide sequence of human liver carbonic anhydrase II cDNA. Nucleic Acids Res. 1987 Jun 11;15(11):4687–4687. doi: 10.1093/nar/15.11.4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair S. K., Calderone T. L., Christianson D. W., Fierke C. A. Altering the mouth of a hydrophobic pocket. Structure and kinetics of human carbonic anhydrase II mutants at residue Val-121. J Biol Chem. 1991 Sep 15;266(26):17320–17325. [PubMed] [Google Scholar]
- Rothstein D. M., Saito H., Streuli M., Schlossman S. F., Morimoto C. The alternative splicing of the CD45 tyrosine phosphatase is controlled by negative regulatory trans-acting splicing factors. J Biol Chem. 1992 Apr 5;267(10):7139–7147. [PubMed] [Google Scholar]
- Saito H., Streuli M. Molecular characterization of protein tyrosine phosphatases. Cell Growth Differ. 1991 Jan;2(1):59–65. [PubMed] [Google Scholar]
- Streuli M., Hall L. R., Saga Y., Schlossman S. F., Saito H. Differential usage of three exons generates at least five different mRNAs encoding human leukocyte common antigens. J Exp Med. 1987 Nov 1;166(5):1548–1566. doi: 10.1084/jem.166.5.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli M., Krueger N. X., Hall L. R., Schlossman S. F., Saito H. A new member of the immunoglobulin superfamily that has a cytoplasmic region homologous to the leukocyte common antigen. J Exp Med. 1988 Nov 1;168(5):1523–1530. doi: 10.1084/jem.168.5.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli M., Krueger N. X., Thai T., Tang M., Saito H. Distinct functional roles of the two intracellular phosphatase like domains of the receptor-linked protein tyrosine phosphatases LCA and LAR. EMBO J. 1990 Aug;9(8):2399–2407. doi: 10.1002/j.1460-2075.1990.tb07415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli M., Krueger N. X., Tsai A. Y., Saito H. A family of receptor-linked protein tyrosine phosphatases in humans and Drosophila. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8698–8702. doi: 10.1073/pnas.86.22.8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. Selective inactivation of the exonuclease activity of bacteriophage T7 DNA polymerase by in vitro mutagenesis. J Biol Chem. 1989 Apr 15;264(11):6447–6458. [PubMed] [Google Scholar]
- Tashian R. E. The carbonic anhydrases: widening perspectives on their evolution, expression and function. Bioessays. 1989 Jun;10(6):186–192. doi: 10.1002/bies.950100603. [DOI] [PubMed] [Google Scholar]
- Tian S. S., Tsoulfas P., Zinn K. Three receptor-linked protein-tyrosine phosphatases are selectively expressed on central nervous system axons in the Drosophila embryo. Cell. 1991 Nov 15;67(4):675–685. doi: 10.1016/0092-8674(91)90063-5. [DOI] [PubMed] [Google Scholar]
- Tsai A. Y., Itoh M., Streuli M., Thai T., Saito H. Isolation and characterization of temperature-sensitive and thermostable mutants of the human receptor-like protein tyrosine phosphatase LAR. J Biol Chem. 1991 Jun 5;266(16):10534–10543. [PubMed] [Google Scholar]
- Wade R., Gunning P., Eddy R., Shows T., Kedes L. Nucleotide sequence, tissue-specific expression, and chromosome location of human carbonic anhydrase III: the human CAIII gene is located on the same chromosome as the closely linked CAI and CAII genes. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9571–9575. doi: 10.1073/pnas.83.24.9571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Pallen C. J. The receptor-like protein tyrosine phosphatase HPTP alpha has two active catalytic domains with distinct substrate specificities. EMBO J. 1991 Nov;10(11):3231–3237. doi: 10.1002/j.1460-2075.1991.tb04886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X. H., Seow K. T., Bahri S. M., Oon S. H., Chia W. Two Drosophila receptor-like tyrosine phosphatase genes are expressed in a subset of developing axons and pioneer neurons in the embryonic CNS. Cell. 1991 Nov 15;67(4):661–673. doi: 10.1016/0092-8674(91)90062-4. [DOI] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]