ABSTRACT
miR-34a-mediated asymmetric cell division reins in excessive stem cell expansion during tissue regeneration in the intestine and colon. Loss of miR-34a switches asymmetric division to symmetric division and enhances stem cell proliferation. Asymmetric division also occurs in the early stages of colon cancer stem cells. Mechanistically, miR-34a, Numb, and Notch form a feed-forward loop that specifies cell fate when stem cells divide.
Stem cells can symmetrically divide into two daughter cells with stem cell characteristics (self-renewal) or differentiate. A stem cell can also undergo asymmetric division to produce one daughter cell like itself and another daughter cell that differentiates.1 Symmetric self-renewal commonly occurs during development to increase the stem cell pool. Asymmetric division is useful for adult tissues because it maintains stem cell number and produces a differentiated cell during one single division.
Some mammalian stem cells seem to switch between symmetric and asymmetric cell divisions. For example, stem cells divide symmetrically to expand their number in early development of the embryo and later switch to asymmetric division to keep the stem cell number constant. Regulated by the niche signals at the base of the crypt, Lgr5+ intestine stem cells (ISCs) divide symmetrically and compete with each other in a neutral drift process.2 It is unclear whether intestine and colon stem cells are capable of asymmetric division and whether they can switch cell fate under pathologic conditions such as inflammation-induced tissue injury.
Exposure to food, acids, and bacteria can cause inflammation and damage in the intestine that requires tissue regeneration and repair. Although an increase in stem cell self-renewal promotes tissue regeneration, the stem cell pool needs to be tightly controlled to prevent dysplasia and tumorigenesis.3 What is the mechanism patrolling stem cell proliferation during regeneration and repair?
Our recent study showed that ISCs could divide asymmetrically when trigged by inflammation injury. Inflammation promotes ISC self-renewal that expands the stem cell pool for regeneration; however, miR-34a-mediated asymmetric division limits excessive ISC accumulation, hence serving as a safeguard. Loss of miR-34a abolishes ISC asymmetric division and further induces ISC proliferation 4 (Fig. 1).
Figure 1.
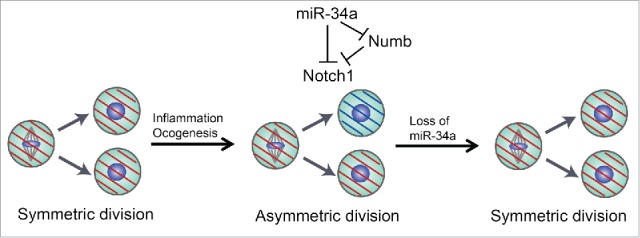
miR-34a-mediated asymmetric divisions in stem cells. Asymmetric division mechanisms are silenced in normal intestine or colon stem cells. The asymmetric division rate increases to rein in excessive stem cell expansion during tissue regeneration caused by inflammation. Loss of miR-34a enhances symmetric division and stem cell proliferation.
miR-34a was originally identified as a target of p53 that plays an important role in regulation of the cell cycle and apoptosis.5 Recent studies indicated that miR-34a also contributes to stem cell differentiation and reprogramming.6,7 miR-34a-mediated asymmetric division is active in the early stage of colon cancer stem cells (CCSCs).4,8 The decision of whether a CCSC will perform symmetric or asymmetric division is tightly controlled by the miR-34a level: high miR-34a levels promote CCSC self-renewal, while low miR-34a levels lead to differentiation. Mechanistically, miR-34a inhibits Notch signaling by directly binding to the 3´untranslated region (3´UTR) of the Notch receptor mRNA to silence its expression. Notch signaling is essential for CCSC self-renewal: activation of Notch signaling enhances CCSC populations and suppression of Notch signaling leads to CCSC differentiation. In mouse intestine, high Notch activity helps ISCs maintain stemness and disruption of Notch signaling promotes ISC differentiation into goblet cells.4,9 When miR-34a binds to the 3´UTR on Notch mRNA, mutual sequestration enables miR-34a to regulate Notch as a bimodal switch. Notch bimodality is important for robust cell fate decision because bimodal signals allow the majority of daughter cells to specify their CCSC versus non-CCSC identity unequivocally, whereas non-bimodal signals leave a substantial portion of the population undecided and subject to stochastic variations.
Besides miR-34a, Numb is another well-established suppressor of Notch signaling by inducing Notch receptor endocytosis and degradation. Furthermore, Numb is a cell fate determinant for various cancer stem cells and has been used as a marker for distinguishing symmetric versus asymmetric division.10
Our recent study showed that miR-34a directly targets Numb and suppress Numb expression, such that miR-34a, Numb, and Notch form an incoherent feed-forward loop (IFFL).4 Since Notch signaling is critical for CCSC fate, it is counterintuitive that miR-34a targets Numb for suppression considering that both cell fate determinants suppress Notch and promote differentiation. By combining computational analysis and quantitative experiments, we revealed that the IFFL buffers Notch expression from miR-34a copy number variation so that the Notch level is steady and insensitive to the precise miR-34a level except for a sharp transition region. The switch enforces bimodality and cell fate bifurcation in the population. Subversion of this IFFL via Numb knockdown degrades Notch bimodality and gives rise to an intermediate subpopulation of cells with ambiguous and plastic cell fate.
Our data demonstrate how tumor suppressors can influence tumor initiation or progression through modulation of stem cell division. The mechanism of asymmetric division is largely silent during normal tissue homeostasis, and the rate increases to rein in the number of proliferating stem cells during tissue regeneration after inflammatory damages. It is plausible that asymmetric division may be activated to counter stem cell proliferation at the onset of oncogenesis and remains active in early-stage CCSCs until it is eventually silenced (e.g., through silencing miR-34a) during tumor progression (Fig. 1). Indeed, loss of miR-34a in CCSCs from late-stage CRCs promotes symmetric self-renewal.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by NIH R01GM95990, NIH R01GM114254, NSF 1350659 career award, NSF 1137269, NYSTEM C029543, and DARPA 19-1091726.
References
- 1.Clevers H. Stem cells, asymmetric division and cancer. Nat Genet 2005; 37:1027-8; PMID:16195718; http://dx.doi.org/ 10.1038/ng1005-1027 [DOI] [PubMed] [Google Scholar]
- 2.Snippert HJ, van der Flier LG, Sato T, van Es JH, van den Born M, Kroon-Veenboer C, Barker N, Klein AM, van Rheenen J, Simons BD, et al.. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 2010; 143:134-44; PMID:20887898; http://dx.doi.org/ 10.1016/j.cell.2010.09.016 [DOI] [PubMed] [Google Scholar]
- 3.Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 2009; 457:608-11; PMID:19092804; http://dx.doi.org/ 10.1038/nature07602 [DOI] [PubMed] [Google Scholar]
- 4.Bu P, Wang L, Chen KY, Srinivasan T, Murthy PK, Tung KL, Varanko AK, Chen HJ, Ai Y, King S, et al.. A miR-34a-Numb Feedforward Loop Triggered by Inflammation Regulates Asymmetric Stem Cell Division in Intestine and Colon Cancer. Cell Stem Cell 2016; 18:189-202; PMID:26849305; http://dx.doi.org/ 10.1016/j.stem.2016.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hermeking H. p53 enters the microRNA world. Cancer Cell 2007; 12:414-8; PMID:17996645; http://dx.doi.org/ 10.1016/j.ccr.2007.10.028 [DOI] [PubMed] [Google Scholar]
- 6.Choi YJ, Lin CP, Ho JJ, He X, Okada N, Bu P, Zhong Y, Kim SY, Bennett MJ, Chen C, et al.. miR-34 miRNAs provide a barrier for somatic cell reprogramming. Nat Cell Biol 2011; 13:1353-60; PMID:22020437; http://dx.doi.org/ 10.1038/ncb2366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu C, Kelnar K, Liu B, Chen X, Calhoun-Davis T, Li H, Patrawala L, Yan H, Jeter C, Honorio S, et al.. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat Med 2011; 17:211-5; PMID:21240262; http://dx.doi.org/ 10.1038/nm.2284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bu P, Chen KY, Chen JH, Wang L, Walters J, Shin YJ, Goerger JP, Sun J, Witherspoon M, Rakhilin N, et al.. A microRNA miR-34a-regulated bimodal switch targets Notch in colon cancer stem cells. Cell Stem Cell 2013; 12:602-15; PMID:23642368; http://dx.doi.org/ 10.1016/j.stem.2013.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, Cozijnsen M, Robine S, Winton DJ, Radtke F, et al.. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature 2005; 435:959-63; PMID:15959515; http://dx.doi.org/ 10.1038/nature03659 [DOI] [PubMed] [Google Scholar]
- 10.Hwang WL, Jiang JK, Yang SH, Huang TS, Lan HY, Teng HW, Yang CY, Tsai YP, Lin CH, Wang HW, et al.. MicroRNA-146a directs the symmetric division of Snail-dominant colorectal cancer stem cells. Nat Cell Biol 2014; 16:268-80; PMID:24561623; http://dx.doi.org/ 10.1038/ncb2910 [DOI] [PubMed] [Google Scholar]


